Introduction
Crimean-Congo hemorrhagic fever (CCHF) is a lethal
viral infection of medical significance. It is widespread
throughout the world and is most common among tick-borne viral
diseases. Ticks of the genus Hyalomma are transmission
agents of the CCHF virus (CCHFV) in humans. The virus is maintained
in tick species through horizontal and vertical transmission and
spreads to domestic animals, which further carry the disease to
humans. Therefore, it is a zoonotic disease (1–4). CCHFV
evolved 3,100–3,500 years ago (5). In
1944, the disease was first reported in Crimea, and was therefore
assigned as Crimean hemorrhagic fever. In 1969, the same disease
was reported in the Congo region, resulting in its current name:
‘Crimean-Congo hemorrhagic fever.’ Previous studies have observed
that the disease is widely distributed in different regions of the
world, including Africa; Asia; central southern Europe; eastern
Europe, particularly in the former Soviet Union; throughout the
Mediterranean; in north-western China; the Middle East; and the
Indian subcontinent (2,6). Since 2002, the virus has also shown its
emergence in several countries of the Balkans, leading to the
concern that CCHF is expanding in its current geographical
distribution (7). The virus is
clustered among seven genotypes; Africa-1, Africa-2, Africa-3,
Europe-1, Europe-2, Asia-1 and Asia-2. These seven genotypes are
characterized on the basis of genetic variation in small segments
of RNA (8,9). The CCHF is severe as it causes serious
medical problems and also results in fatalities when not treated.
The disease can be described mainly as the presence of blood in
sputum, gums, rectum and urine (10).
Another cause for concern is that CCHFV is highly pathogenic in
nature, easily transmissible and has a high case-fatality rate of
10–40%. Due to the highly pathogenic nature of CCHFV, the culture
of the virus is only permitted in biosafety level four (BSL-4) and
in maximum secured laboratories; there is a possible risk of this
virus being used as an agent of bioterrorism or as biological
warfare (7,11–14).
As the virus has a widespread geographical
distribution, it must be recognized as a global health threat.
Pakistan has also been experiencing this epidemic disease, covering
almost all four of the Punjab, Baluchistan, Khyber Pakhtunkhwan
(KPK) and Sindh provinces. Administering preventive measures is
urgently required to eradicate the virus from the country, as
subsequent to poliovirus, CCHFV may become a serious challenge for
the country (15).
In the present review, virology, vector,
transmission pathway of the virus, risk factors and control
measures are discussed with regards to the current literature to
minimize the impact of infection in Pakistan. In additional, the
clinical symptoms, diagnostic tests and treatment for this epidemic
disease are briefly described.
Virology
CCHFV belongs to the genus Nairovirus and
family Bunyaviridae (7). The
genus Nairovirus contains ~34 tick-borne viruses and these
are categorized into seven serogroups (16). The circulation of CCHFV is dependent
upon the distribution of ticks, mainly of the Hyalomma genus
(1,17).
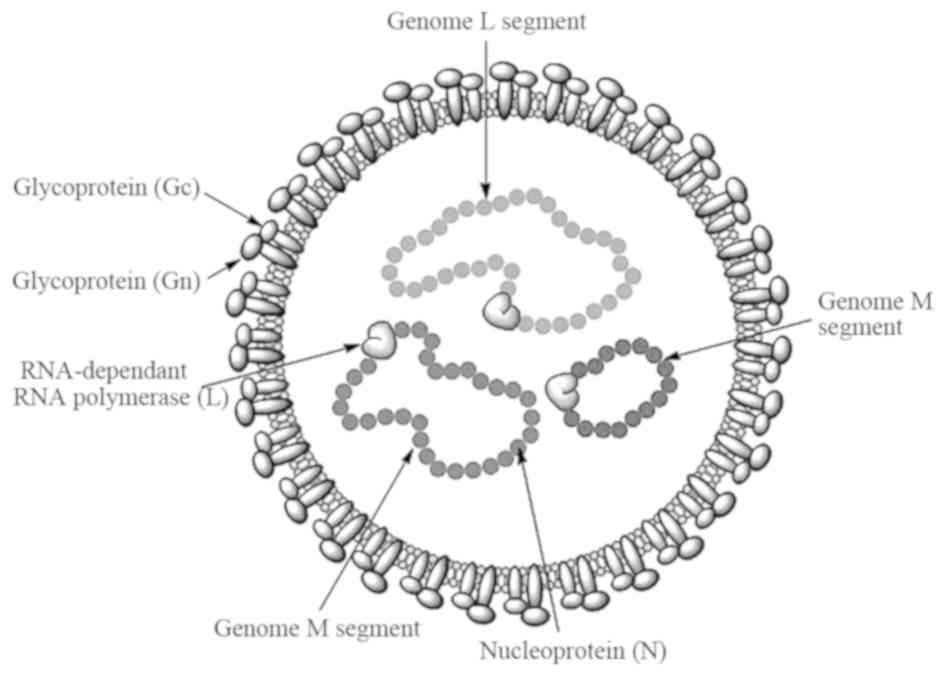
The virus is spherical in shape with a diameter of 80–100 nm, the
lipid envelope is 5–7 nm thick and glycoprotein spikes are 8–10 nm
in length (18). The genome consists
of single-stranded RNA with negative polarity, divided into three
segments: Small, medium and large segments. These three segments
form a complex with nucleocapsid proteins to become a
ribo-nucleocapsid. The virion contains three structural proteins:
i) A nucleocapsid protein, ii) glycoproteins (Gn and Gc) and iii) a
large polypeptide protein, which is a virion-associated
RNA-dependent RNA polymerase with a size of 200 kDa (13,19). The
structural features of the virus are shown in Fig. 1.
Vector
CCHFV is mainly tick borne and is also found in one
biting midge species (Culicoides spp.). The virus has been
isolated from two different families of tick: Argasidae
(soft ticks) and Ixodidae (hard ticks) (20). The transmission cycle runs between tick
to vertebrate and again to tick. Vertical and horizontal
transmissions involve tick and domestic/wild-live stocks causing
them to become viremic without any disease symptoms. Migrating
birds can easily carry infected ticks and act as a source of virus
dispersal (21). CCHFV occurrence has
been reported in >30 species of ticks belonging to different
genera. Ticks of Hyalomma genus are considered as the major
vector for human infection; however, in Kazakhstan, the
Dermatocentor niveus ticks are also considered as vectors
(22).
Transmission
Transmission can be from person to person, through
contact with infectious body fluids of the infected person and
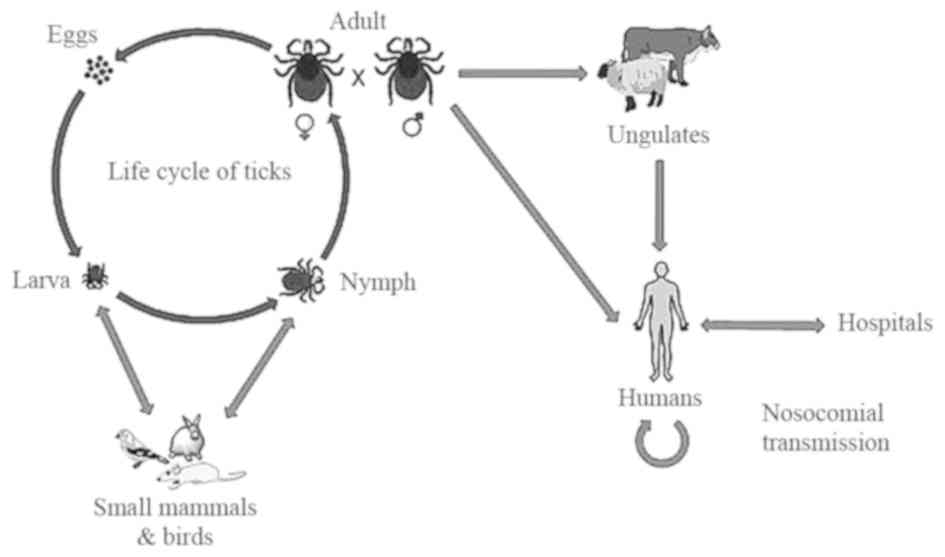
contact with animal blood or products. The life cycle of ticks and
the transmission pathway of CCHFV is shown in Fig. 2. The Hyalomma genus ticks are
the reservoirs and vectors of the CCHFV. The larvae and nymphs of
two-host ticks of this genus feed on hares and small birds feeding
on the ground, while at the adult stage they obtain their
nutritional requirements from cattle, sheep and certain large
mammals. There are certain other Hyalomma species, which are
three-host ticks as they drop off their host following each molt
(3). Another factor responsible for
the transmission of CCHF is the migration of infected livestock
populations from infected areas to new areas (23).
Vertical transmission
While shifting from larva to the adult stage during
metamorphosis, tick vectors support the replication of the virus
present inside their body tissues. Following this, the virus is
transmitted to eggs and adult females from adult females and adult
males, respectively (24–27). In the mid-gut lining of the tick, the
virus replicates and finally disseminates to different body
tissues, for example, reproductive organs and salivary glands
(28). Therefore, through transovarian
transmission, thousands of infected eggs are being produced, which
are sufficient enough to maintain a large population of infected
ticks (29).
Horizontal transmission
During the summer and spring months (June-October),
the spread of CCHFV between ticks and animals is higher when larvae
and nymphs develop into the adult form by taking the blood meal for
their growth. A bite of the infected tick to their host, i.e.,
small vertebrates, results in transmission of the virus from the
tick to their host, and subsequently, healthy ticks feeding on the
same host followed by virus replication in host tissue and its
circulation in the bloodstream. Previous studies have revealed that
all the mammals are not susceptible to infection by CCHFV (27,30).
Non-viremic transmission
This is another type of viral transmission that does
not require an animal to be viremic, but directly transfer from the
infected to healthy ticks feeding together. During co-feeding,
viral substances present in the saliva of ticks accelerate the
viral transmission (31–33).
Transmission to birds
Birds are commonly resistant to becoming viremic. No
specific antibodies are detected in 37 different species of birds
infected with the virus, as CCHFV do not rely on birds as a host
for its replication (34).
Transmission to humans
Humans are considered as the dead-end host of CCHFV.
CCHFV infection is most common in rural areas where exposure to
ticks is high and people become infected when bitten by infected
ticks. Physical contact with infected bodily fluids or blood can
transmit the virus from person to person within 7–10 days of
illness. Transmission can also occur by contact with infected
animal blood. This type of transmission is extremely common in
butchers' shops (3).
Clinical symptoms
There are mainly four different phases that are
involved in the infection of the CCHFV: Incubation period
(non-symptomatic phase), pre-hemorrhagic, hemorrhagic and
convalescent (symptomatic phases). The incubation period lasts from
3–7 days of infection. The disease starts with the pre-hemorrhagic
period for 4–5 days. The major symptoms include headache, high
fever, abdominal pain, myalgia, hypotension and flushed face
(10). As the disease progresses,
severe symptoms starts appearing including petechiae (red spots on
skin), ecchymosis (extravasation of blood), epistaxis (nose
bleeding), gum bleeding and emesis (35–37). Nausea,
diarrhea, emesis, neuropsychiatric and cardiovascular changes can
be additional symptoms (20). When the
disease is not treated, patients may succumb due to multiorgan
failure. The convalescent period begins in survivors after 10–20
days of illness (16). Full recovery
can take a complete year in survivors of CCHF (1).
Scenario of CCHF in Pakistan
Due to its name, there is confusion surrounding the
prevalance of the virus outside of the Congo; however, it has been
reported in Pakistan and the ‘Congo virus is a reality in the
country’, as stated by Dr Muhammad Najeeb Khan Durrani, a Senior
Surveillance Coordinator of Communicable Diseases in Islamabad
(38). In Pakistan, the virus was
first isolated from the Hyalomma tick species in 1960
(39). Since then, sporadic cases and
repeated outbreaks have been observed mainly in people who deal
with livestock (40). The most
prevalent genotype of CCHFV in Pakistan is Asia-1; however, in
Baluchistan the Asia-1 and Asia-2 genotypes have been reported
(8). In 1976, at a general hospital of
Rawalpindi, a person suffering from abdominal pain and hematemesis
(blood vomiting) was reported to be the first case of CCHF in
Pakistan (41,42). According to published studies of CCHF
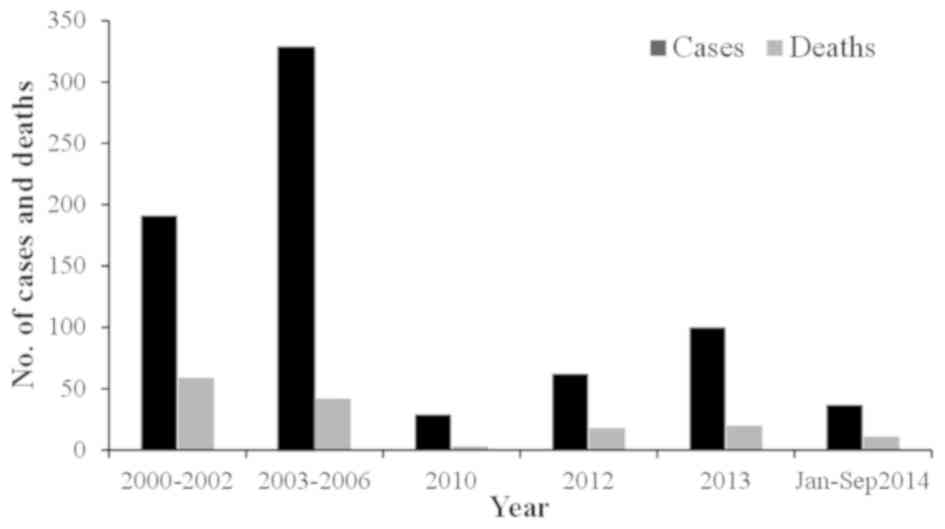
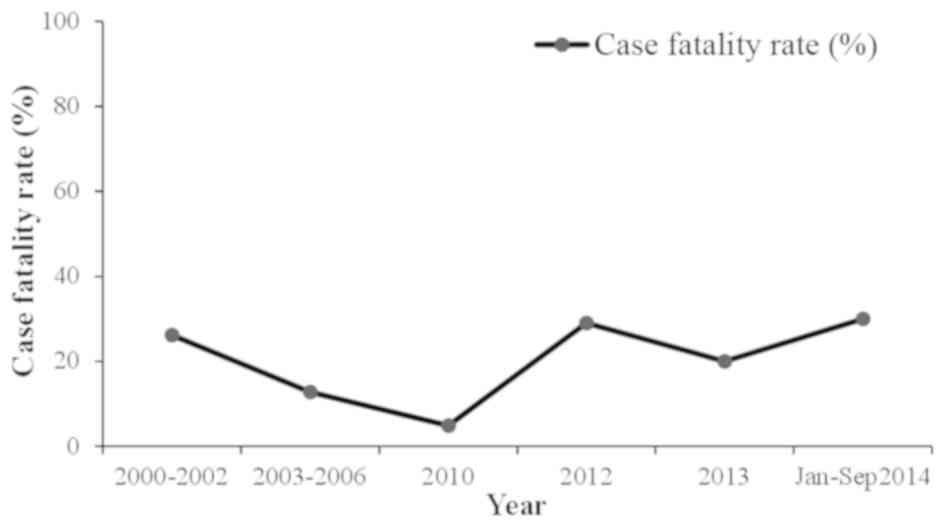
between 1976 and 2000, there were 23 cases of CCHF in Pakistan with
a case fatality rate of 39% (12,43). From
2000, a significant increase in CCHF cases was observed, with 50–60
cases reported annually. In general, there is usually a biannual
surge of cases in the country appearing in June and October, at its
peak in association with the life cycle of the tick (44). The country experienced an outbreak of
CCHF in 2012 when there were 61 suspected cases with 17 fatalities
and a 27.8% case-fatality rate. The disease was mainly prevalent in
the province of Baluchistan; however, cases were also reported in
Sindh, KPK and Punjab (45). Another
outbreak of the virus was reported on September 7, 2013 in Haripur
when four butchers succumbed due to working with the meat of an
infected sheep (38). In 2014,
confirmation of the virus in Baluchistan triggered a further
requirement to improve the management of the virus (46). The annual suspected cases and the case
fatality rate are shown in Figs. 3 and
4, respectively.
Risk factors
The virus can be transmitted from person to person
through contact with animal blood or products, contact with
infectious body fluids of an infected person and by handling the
infected ticks (47). The areas
outside the range of tick distribution are at little or no risk of
exposure to ticks. Crushing and rubbing the infected tick on skin
or slaughtering the infected animal is also one of the main risk
factors towards the exposure of CCHFV. Another well-documented risk
factor is nosocomial infection. This is most common among health
care workers, particularly during the hemorrhagic period of the
disease (48,49). As previously reported, this factor was
exemplified in January 1976, when at Central Government Hospital
Rawalpindi (Pakistan), a nosocomial incident occurred (10,43), in
which the infection was transmitted from a shepherd to a female
physician, a surgeon, an assistant surgeon and other health
workers. In South Africa at the Tygerberg Hospital, another
nosocomial outbreak occurred in which 33% of the health workers
developed CCHF by accidental contact with the patient through a
needle prick, and 8.7% were infected through contact with the blood
or other body fluids of the patient. Droplet-respiratory route of
infection is also counted as one of the risk factors of CCHF
(13). This is supported by several
cases of laboratory-acquired CCHF in Africa. Laboratory personnel
dealing with viral samples are also highly likely to develop the
disease, as supported by numerous cases of CCHF acquired from a
laboratory in Africa (50). For all
these reasons, CCHFV has been characterized as a BSL-4 pathogen in
the United States by the Center for Disease Control and Prevention
(51).
Control measures
As the life cycle of the tick remains unnoticed in
animals, control of CCHF infection in animals and ticks is
difficult. The infection is not usually apparent in animals, and
only viremia occurs (20). There is no
vaccine available, the only way to reduce the infection is by
creating public awareness regarding the risk factors of the disease
and possible preventive measures, which aid in reducing the
exposure to the virus and controlling the spread of the
disease.
The risk of the tick to human transmission can be
minimized by avoiding areas with a high prevalence of ticks and
undertaking special precautions in the most active season of ticks.
People who are in high-risk occupations (such as butchers,
veterinarians and shepherds) should undergo every possible measure
to avoid exposure to virus-infected ticks or virus-contaminated
animal blood and other tissues. For instance, the use of gloves and
minimal exposure of naked skin to fresh blood animal and other
tissues are effective control measures. Similarly, medical workers
caring for suspected patients of CCHF should adopt standard barrier
nursing techniques. Unpasteurized milk should not be utilized. Only
properly cooked food should be consumed, as this kills the viruses.
Treating the livestock with acaricides is effective in decreasing
the population of infected ticks. The use of commercially available
insect repellents, including diethyl toluamide on naked skin is
also protective against tick bites. Clothes should be treated with
permethrin spray, as it also shields against tick bites (20).
For reducing the risk of animal to human
transmission, quarantine measures should be taken while importing
animals and they should be treated with pesticides regularly.
Maintenance of hygienic conditions during slaughtering, butchering
and culling procedures in slaughterhouses or at home is mandatory.
Gloves should be worn during the handling of meat. Following the
slaughter of an animal, the utensils and other equipment should be
washed prior to reuse (14).
To reduce the risk of human-human transmission,
close physical contact with the infected person should be avoided.
Hands should be washed properly and regularly subsequent to
visiting and caring for ill people (14). In certain developed countries, it is
recommended that health care workers must use high efficiency air
respirators (52); however, this
practice is not feasible in a country such as Pakistan (53). Face shields, safety goggles and
surgical masks should be used when coming into contact with the
patient from three feet away (54,55).
Isolation of the patient and barrier nursing is also
recommended.
Disposal of used instruments and equipment,
including needles, syringes and employing safe burial practices,
should be implemented (56).
Disinfectants, including 2% glutaraldehyde and 1% hypochlorite, can
inactivate the CCHFV by heating at 56°C for 30 min (16).
Diagnostic tests
Diagnosis at an early stage is indispensable to
prevent further transmission of the infection. There are different
techniques for the infection diagnosis, including enzyme-linked
immunosorbent assay (ELISA), quantitative polymerase chain reaction
(qPCR), antigen detection, serum neutralization and isolation of
the virus by cell culture (14,57). At the
Bernhard-Nocht-Institute for Tropical Medicine (Hamburg, Germany),
scientists have experimented with certain test systems for the
detection of infection with CCHFV. All the tests use ELISA for
detecting pathogen-specific immunoglobulins (Ig); human IgM or IgG
blood serum antibodies. Tests were based on specific monoclonal
antibodies. The hybridoma cell lines were offered for
co-development of diagnostic test systems using ELISA or other
technology platforms (58). The
patients of CCHF are viremic in 7–10 days of disease, and by the
end of the first day, weak IgM becomes detectable followed by IgG
(3). The viral antigen can also be
visualized in formalin-fixed tissues by immunohistochemical
staining (59). The screening tests
are available in the majority of diagnostic labs in Pakistan,
including the Islamabad Diagnostic Center, Chughtais Lahore lab,
Centre of Excellence in Molecular Biology, Lahore and Shoukat
Khanum Memorial Cancer Hospital. Reliable and sensitive diagnostic
tests, including ELISA and qPCR, provide an essential tool for
viral detection and are helpful in minimizing the impact of
infection.
Treatment
The treatment for CCHF viral infection mainly
depends on the severity and symptoms of disease. Currently, there
is no antiviral drug against CCHFV that is approved by the Food and
Drug Administration (60). However,
ribavirin (Virazole) is the only antiviral drug used against CCHFV,
which is only effective in certain cases (61). Despite the verified insufficient
efficacy of ribavirin for CCHF patients by two systematic reviews
and meta-analyses, the World Health Organization has approved
antiviral ribavirin for treatment of CCHFV infection based on in
vitro data (7,60,62,63). Ribavirin can be taken orally and
intravenously. For effective results, ribavirin is used along with
supportive therapy, such as interferons (16). In numerous in vitro studies,
interferon type-I is shown to have antiviral activity, however, no
clinical data is available on interferon use (60,64). A
recent study utilized modified vaccinia virus Ankara (attenuated
poxvirus vector) to develop a recombinant vaccine that expresses
glycoproteins of CCHFV in two mouse strains. A cellular and humoral
immune response was confirmed against this vaccine, which protected
the recipient model animals from developing the lethal disease
(61). Studies were also conducted to
determine the role of immunotherapy in the treatment of CCHF. A new
immunoglobulin, Venin, which is specific to CCHFV, has been
prepared from the plasma pool of boosted donors through
ethanol-polyethylene glycol fractionation and an ion-exchange
purification step (65). However, in
the case of CCHFV, the beneficial effects of immunotherapy are
extremely limited (20,66).
Conclusion
CCHF is harmful in the sense that it does not have
any specific treatment. The only way to avoid this widespread
infection is prevention. In a developing country such as Pakistan,
the disease poses more serious effects due to inadequate resources.
Due to the risks of disease in Pakistan, cross-border surveillance
needs to be strengthened. Reinforcing the control measures to
prevent the transmission of the disease to new areas is necessary.
The animal and health sectors, by taking solid steps, can
contribute to reduce the spread of this disease across the country.
Awareness campaigns regarding risk factors and control measures can
aid in apprising the public of the ill effects of this virus.
Glossary
Abbreviations
Abbreviations:
|
CCHF
|
Crimean-Congo hemorrhagic fever
|
|
CCHFV
|
CCHF virus
|
|
BSL
|
biosafety level
|
|
KPK
|
Khyber Pakhtunkhwan
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
Ig
|
immunoglobulin
|
References
|
1
|
Ergönül O: Crimean-Congo haemorrhagic
fever. Lancet Infect Dis. 6:203–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mahzounieh M, Dincer E, Faraji A, Akin H,
Akkutay AZ and Ozkul A: Relationship between Crimean-Congo
hemorrhagic fever virus strains circulating in Iran and Turkey:
Possibilities for transborder transmission. Vector Borne Zoonotic
Dis. 12:782–785. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bente DA, Forrester NL, Watts DM, McAuley
AJ, Whitehouse CA and Bray M: Crimean-Congo hemorrhagic fever:
History, epidemiology, pathogenesis, clinical syndrome and genetic
diversity. Antiviral Res. 100:159–189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Papa A, Sidira P, Larichev V, Gavrilova L,
Kuzmina K, Mousavi-Jazi M, Mirazimi A, Ströher U and Nichol S:
Crimean-Congo hemorrhagic fever virus, Greece. Emerg Infect Dis.
20:288–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carroll SA, Bird BH, Rollin PE and Nichol
ST: Ancient common ancestry of Crimean-Congo hemorrhagic fever
virus. Mol Phylogenet Evol. 55:1103–1110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ergönül O: Crimean-Congo hemorrhagic fever
virus: New outbreaks, new discoveries. Curr Opin Virol. 2:215–220.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burt FJ and Goedhals D: Crimean-Congo
haemorrhagic fever virus, an emerging and re-emerging pathogen.
Zoonoses Infections Affecting Humans and Animals. Sing A:
(Netherlands). Springer. 977–996. 2015.
|
|
8
|
Alam MM, Khurshid A, Sharif S, Shaukat S,
Suleman RM, Angez M and Zaidi SS: Crimean-Congo hemorrhagic fever
Asia-2 Genotype, Pakistan. Emerging. Infect Dis J. 19:1017–1019.
2013.
|
|
9
|
Mild M, Simon M, Albert J and Mirazimi A:
Towards an understanding of the migration of Crimean-Congo
hemorrhagic fever virus. J Gen Virol. 91:199–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoogstraal H: The epidemiology of
tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and
Africa. J Med Entomol. 15:307–417. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Institute of Health, World Health
Organization Paistan: Seasonal awareness and alert letter (SAAL)
for epidemic prone infectious diseases in Pakistan winter season.
http://www.nih.org.pk/files/Newsletter/Seasonal%20Awarness%20and%20Alert%20Letter%20%28SAAL%29%2029th%20Issue.pdfAccessed.
October 08–2014
|
|
12
|
Smego RA Jr, Sarwari AR and Siddiqui AR:
Crimean-Congo hemorrhagic fever: Prevention and control limitations
in a resource-poor country. Clin Infect Dis. 38:1731–1735. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Whitehouse C: Risk groups and control
measures for Crimean-Congo hemorrhagic fever. Crimean-Congo
Hemorrhagic Fever. Ergonul O and Whitehouse C: (Netherlands).
Springer. 273–280. 2007. View Article : Google Scholar
|
|
14
|
WHO: Crimean Congo haemorrhagic fever.
Fact Sheet No. 208. January;2013.http://www.who.int/mediacentre/factsheets/fs208/en/Accessed.
March. 2015
|
|
15
|
Baloch S: Congo virus a fresh challenge
for Balochistan. The Express Tribune. August 23–2014.
|
|
16
|
Appannanavar SB and Mishra B: An update on
Crimean-Congo hemorrhagic fever. J Glob Infect Dis. 3:285–292.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hoogstraal H: African Ixodoidea.
Ticks of the Sudan (with special reference to Equatoria Province
and with preliminary reviews pf the genera Boophilus,
Margaropus, and Hyalomma). Research Report. NM
005.050.29.27. Department of the Navy, Bureau of Medicine and
Surgery. (Washington, DC). 11011956.
|
|
18
|
Marriott AC and Nuttall PA: Molecular
biology of nairoviruses. The Bunyaviridae. Elliott R: (US).
Springer. 91–104. 1996. View Article : Google Scholar
|
|
19
|
Marriott AC and Nuttall PA: Large RNA
segment of Dugbe nairovirus encodes the putative RNA polymerase. J
Gen Virol. 77:1775–1780. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whitehouse CA: Crimean-Congo hemorrhagic
fever. Antiviral Res. 64:145–160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palomar AM, Portillo A, Santibáñez P,
Mazuelas D, Arizaga J, Crespo A, Gutiérrez Ó, Cuadrado JF and Oteo
JA: Crimean-Congo hemorrhagic fever virus in ticks from migratory
birds, Morocco. Emerg Infect Dis. 19:260–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Onishchenko GG, Tumanova IIU, Vyshemirskiĭ
OI, Kuhn J, Seregin SV, Tiunnikov GI, Petrova ID, Tishkova FKH,
Ospanov KS, Kazakov SV, et al: Study of virus contamination of
Ixodes ticks in the foci of Crimean-Congo hemorrhagic fever in
Kazakhstan and Tajikistan. Zh Mikrobiol Epidemiol Immunobiol.
1:27–31. 2005.(In Russian). PubMed/NCBI
|
|
23
|
Alam MM, Khurshid A, Sharif S, Shaukat S,
Rana MS, Angez M and Zaidi SS: Genetic analysis and epidemiology of
Crimean-Congo hemorrhagic fever viruses in Baluchistan province of
Pakistan. BMC Infect Dis. 13:2012013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dohm DJ, Logan TM, Linthicum KJ, Rossi CA
and Turell MJ: Transmission of Crimean-Congo hemorrhagic fever
virus by Hyalomma impeltatum (Acari: Ixodidae) after
experimental infection. J Med Entomol. 33:848–851. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gonzalez JP, Camicas JL, Cornet JP, Faye O
and Wilson ML: Sexual and transovarian transmission of
Crimean-Congo haemorrhagic fever virus in Hyalomma truncatum
ticks. Res Virol. 43:23–28. 1992. View Article : Google Scholar
|
|
26
|
Logan TM, Linthicum KJ, Bailey CL, Watts
DM, Dohm DJ and Moulton JR: Replication of Crimean-Congo
hemorrhagic fever virus in four species of ixodid ticks (Acari)
infected experimentally. J Med Entomol. 27:537–542. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shepherd AJ, Swanepoel R, Shepherd SP,
Leman PA and Mathee O: Viraemic transmission of Crimean-Congo
haemorrhagic fever virus to ticks. Epidemiol Infect. 106:373–382.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dickson DL and Turell MJ: Replication and
tissue tropisms of Crimean-Congo hemorrhagic fever virus in
experimentally infected adult Hyalomma truncatum (Acari:
Ixodidae). J Med Entomol. 29:767–773. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Labuda M and Nuttall PA: Tick-borne
viruses. Parasitology. 129:S221–S245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shepherd AJ, Leman PA and Swanepoel R:
Viremia and antibody response of small African and laboratory
animals to Crimean-Congo hemorrhagic fever virus infection. Am J
Trop Med Hyg. 40:541–547. 1989.PubMed/NCBI
|
|
31
|
Nuttall PA and Labuda M: Tick-host
interactions: Saliva-activated transmission. Parasitology.
129:S177–S189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nuttall PA and Labuda M: Dynamics of
infection in tick vectors and at the tick-host interface. Adv Virus
Res. 60:233–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jones LD, Davies CR, Steele GM and Nuttall
PA: A novel mode of arbovirus transmission involving a nonviremic
host. Science. 237:775–777. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shepherd AJ, Swanepoel R, Leman PA and
Shepherd SP: Field and laboratory investigation of Crimean-Congo
haemorrhagic fever virus (Nairovirus, family Bunyaviridae)
infection in birds. Trans R Soc Trop Med Hyg. 81:1004–1007. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ergönül O, Celikbaş A, Dokuzoguz B, Eren
S, Baykam N and Esener H: Characteristics of patients with
Crimean-Congo hemorrhagic fever in a recent outbreak in Turkey and
impact of oral ribavirin therapy. Clin Infect Dis. 39:284–287.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bakir M, Ugurlu M, Dokuzoguz B, Bodur H,
Tasyaran MA and Vahaboglu H: Turkish CCHF Study Group:
Crimean-Congo haemorrhagic fever outbreak in Middle Anatolia: A
multicentre study of clinical features and outcome measures. J Med
Microbiol. 54:385–389. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ozkurt Z, Kiki I, Erol S, Erdem F, Yilmaz
N, Parlak M, Gundogdu M and Tasyaran MA: Crimean-Congo hemorrhagic
fever in Eastern Turkey: Clinical features, risk factors and
efficacy of ribavirin therapy. J Infect. 52:207–215. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Butt Q: Another outbreak?: Congo virus
threatens lives, warn doctors. The Express Tribune. October
07–2013.
|
|
39
|
Begum F, Wisseman CL Jr and Casals J:
Tick-borne viruses of West Pakistan. IV. Viruses similar to or
identical with, Crimean hemorrhagic fever (Congo-Semunya), Wad
Medani and Pak Argas 461 isolated from ticks of the Changa Manga
Forest, Lahore District, and of Hunza, Gilgit Agency, W. Pakistan.
Am J Epidemiol. 92:197–202. 1970.PubMed/NCBI
|
|
40
|
Jamil B, Hasan RS, Sarwari AR, Burton J,
Hewson R and Clegg C: Crimean-Congo hemorrhagic fever: Experience
at a tertiary care hospital in Karachi, Pakistan. Trans R Soc Trop
Med Hyg. 99:577–584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Malik S, Diju IU and Naz F: Crimean Congo
hemorrhagic fever in Hazara division. J Ayub Med Coll Abbottabad.
23:90–92. 2011.PubMed/NCBI
|
|
42
|
Athar MN, Baqai HZ, Ahmad M, Khalid MA,
Bashir N, Ahmad AM, Balouch AH and Bashir K: Short report:
Crimean-Congo hemorrhagic fever outbreak in Rawalpindi, Pakistan,
February 2002. Am J Trop Med Hyg. 69:284–287. 2003.PubMed/NCBI
|
|
43
|
Burney MI, Ghafoor A, Saleen M, Webb PA
and Casals J: Nosocomial outbreak of viral hemorrhagic fever caused
by Crimean Hemorrhagic fever-Congo virus in Pakistan, January 1976.
Am J Trop Med Hyg. 29:941–947. 1980.PubMed/NCBI
|
|
44
|
NIH and WHO: Guidelines for Crimean-Congo
hemorrhagic fever (CCHF). September;2013.http://www.nih.org.pk/files/Guidelines/CCHF%20guidelines%20September%202013.pdfAccessed.
March. 2015
|
|
45
|
WHO: Crimean-Congo haemorrhagic fever
(CCHF) in Pakistan. 14–June;2013.Surveillance, Forecasting and
Response. 2013. http://www.emro.who.int/index.htmlAccessed.
April. 2015
|
|
46
|
Abbas T, Younus M and Muhammad SA: Spatial
cluster analysis of human cases of Crimean-Congo hemorrhagic fever
reported in Pakistan. Infect Dis Poverty. 4:92015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gozel MG, Bakir M, Oztop AY, Engin A,
Dokmetas I and Elaldi N: Investigation of Crimean-Congo hemorrhagic
fever virus transmission from patients to relatives: a prospective
contact tracing study. Am J Trop Med Hyg. 90:160–162. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
van de Wal BW, Joubert JR, van Eeden PJ
and King JB: A nosocomial outbreak of Crimean-Congo haemorrhagic
fever at Tygerberg Hospital. Part IV. Preventive and prophylactic
measures. S Afr Med J. 68:729–732. 1985.PubMed/NCBI
|
|
49
|
Fisher-Hoch SP, Khan JA, Rehman S, Mirza
S, Khurshid M and McCormick JB: Crimean Congo-haemorrhagic fever
treated with oral ribavirin. Lancet. 346:472–475. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Simpson DI, Knight EM, Courtois G,
Williams MC, Weinbren MP and Kibukamusoke JW: Congo virus: A
hitherto undescribed virus occurring in Africa. I. Human isolations
- clinical notes. East Afr Med J. 44:86–92. 1967.PubMed/NCBI
|
|
51
|
Richmond JY and McKinney RW: Biosafety in
microbiological and biomedical laboratories (4th). Washington: U.S.
Government Printing Office. April;1999.
|
|
52
|
Fisher-Hoch SP, Price ME, Craven RB, Price
FM, Forthall DN, Sasso DR, Scott SM and McCormick JB: Safe
intensive-care management of a severe case of Lassa fever with
simple barrier nursing techniques. Lancet. 2:1227–1229. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Athar MN, Khalid MA, Ahmad AM, Bashir N,
Baqai HZ, Ahmad M, Balouch AH and Bashir K: Crimean-Congo
hemorrhagic fever outbreak in Rawalpindi, Pakistan, February 2002:
Contact tracing and risk assessment. Am J Trop Med Hyg. 72:471–473.
2005.PubMed/NCBI
|
|
54
|
Centers for Disease Control and Prevention
(CDC): Update: Management of patients with suspected viral
hemorrhagic fever - United States. MMWR Morb Mortal Wkly Rep.
44:475–479. 1995.PubMed/NCBI
|
|
55
|
Leblebicioglu H, Bodur H, Dokuzoguz B,
Elaldi N, Guner R, Koksal I, Kurt H and Senturk GC: Case management
and supportive treatment for patients with Crimean-Congo
hemorrhagic fever. Vector Borne Zoonotic Dis. 12:805–811. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lloyd E and Perry H: Infection control for
viral haemorrhagic fevers in the African health care setting.
Centers for Disease Control and Prevention and World Health
Organization, Infection Control for Viral Haemorrhagic Fevers in
the African Health Care Setting. (Atlanta). Centers for Disease
Control and Prevention. 1–198. 1998.
|
|
57
|
Vanhomwegen J, Alves MJ, Zupanc TA, Bino
S, Chinikar S, Karlberg H, Korukluoğlu G, Korva M, Mardani M,
Mirazimi A, et al: Diagnostic assays for Crimean-Congo hemorrhagic
fever. Emerg Infect Dis. 18:1958–1965. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Stachelhaus DT: Efficient tools for
sensitive and specific detection of infections with
Chikungunya virus, Crimean-Congo hemorrhagic fever virus and
other viruses causing emerging diseases. Ascenion reference number
TO 12–00042.
|
|
59
|
CDC. http://www.cdc.gov/vhf/crimean-congo/diagnosis/index.htmlAccessed.
February 21–2015
|
|
60
|
Oncü S: Crimean-Congo hemorrhagic fever:
An overview. Virol Sin. 28:193–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Buttigieg KR, Dowall SD, Findlay-Wilson S,
Miloszewska A, Rayner E, Hewson R and Carroll MW: A novel vaccine
against Crimean-Congo haemorrhagic fever protects 100% of animals
against lethal challenge in a mouse model. PLoS One. 9:e915162014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Soares-Weiser K, Thomas S, Thomson G and
Garner P: Ribavirin for Crimean-Congo hemorrhagic fever: Systematic
review and meta-analysis. BMC Infect Dis. 10:2072010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ascioglu S, Leblebicioglu H, Vahaboglu H
and Chan KA: Ribavirin for patients with Crimean-Congo haemorrhagic
fever: A systematic review and meta-analysis. J Antimicrob
Chemother. 66:1215–1222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ergonul O: Treatment of Crimean-Congo
hemorrhagic fever. Antiviral Res. 78:125–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Vassilenko SM, Vassilev TL, Bozadjiev LG,
Bineva IL and Kazarov GZ: Specific intravenous immunoglobulin for
Crimean-Congo haemorrhagic fever. Lancet. 335:791–792. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Flick R and Whitehouse CA: Crimean-Congo
hemorrhagic fever virus. Curr Mol Med. 5:753–760. 2005. View Article : Google Scholar : PubMed/NCBI
|