Introduction
In March 2016, a landmark publication by Margolin
et al (1) showed the presence
of a highly conserved hypermethylation signature in circulating
nucleic acids (CNAs) present in blood across a multitude of cancer
types. Such a pan-cancer biomarker, if validated, may present as a
strong diagnostic tool in the fight against cancer. However, our
knowledge of CNAs and their resurgence as important factors in
cancer pathogenesis will determine their development as an
effective diagnostic tool.
The term CNAs refers to segments of genomic,
mitochondrial or viral DNA, RNA and microRNA (miRNA) found in the
bloodstream (2). The history of CNAs
dates back to the 1940s, when Metais and Mandel reported the
presence of free nucleic acids in plasma (3). Over the past decade, the unequivocal
proof that certain CNAs are of tumor origin (4) has initiated a surge of studies. This has
led to a wealth of information indicating the diagnostic potential
of these CNAs, particularly in cancer screening and monitoring of
the efficacy of anticancer therapeutic strategies (5).
There are various mechanisms by which these nucleic
acids are proposed to be released into circulation. These include
passive mechanisms, including apoptosis and necrosis (6) or active mechanisms, such as spontaneous
release of nucleic acids from cells (7). While numerous studies have been conducted
to determine the mechanism by which circulating DNA/RNA fragments
are released into the blood, the sources of these CNAs remain
elusive. Certain theories include circulating tumor cells (8), stem cells (9), blood cells/lymphocytes (10), and viruses [ebstein barr virus (EBV)
and human papilloma virus (HPV)] (11,12) as
potential origins of these cell-free nucleic acids in
circulation.
Information regarding the source and mechanisms of
the release of these CNAs may present an insight into their
possible roles or functions in the body. A clear understanding of
all the potential functions and influences of CNAs would add marked
prognostic value to the assessment of CNAs, in addition to an
already established role in disease diagnosis and monitoring.
Origins and mechanisms of CNA release
While the existence of cell-free nucleic acids in
the bloodstream has been shown by a number of studies, there is a
lack of clarity regarding their source(s) (13). In 1998, it was shown that plasma or
serum DNA often presents a ladder pattern when subjected to
electrophoresis (7), which is similar
to that produced by degraded DNA fragments from apoptotic cells
(14–17). Since then, numerous reports on
circulating DNA have indicated apoptosis or necrosis as the main
phenomenon responsible for cell-free CNAs (6,7,18,19). While
certain nucleic acids in plasma/serum maybe released through
apoptosis or necrosis, these are certainly not the only mechanisms
responsible for their presence in circulation. This is evident from
studies in cancer patients, showing decreased quantities of
circulating DNA following chemotherapy/radiotherapy-induced
apoptosis (20).
In addition to apoptosis and necrosis, the
phenomenon of active release of DNA from cells is expected to be a
mechanism of CNA's release into plasma (10). In 1975, Anker et al (21) showed that living cells are capable of
spontaneously releasing newly synthesized DNA in a preferential
manner. The study demonstrated that non-dividing cells, such as
lymphocytes, frog auricles and cultured cell lines, including
HL-60, spontaneously discharged a nucleoprotein complex within a
homeostatic system in which newly synthesized DNA was released
preferentially (21). Further evidence
for preferential release of DNA by viable cells was demonstrated by
Stroun et al (22). In their
study, the proportion of Alu repeat sequences to β-globin
gene in the serum and lymphocytes of 27 cancer patients and 22
healthy controls was compared. The proportion of Alu gene to
β-globin gene was observed to be significantly higher in
serum DNA than in the DNA from lymphocytes. This was observed in
the control subjects (P=0.003) and cancer patients (P<0.001)
(22). This leads to the hypothesis
that active DNA release may be significantly involved in marking
the presence of extracellular nucleic acids. Another study
indicated that active release may also occur during division of
normal and malignant cells in the body (10).
Thus far, the origin of CNAs has been attributed to
the known mechanism of dying cells releasing their cell contents
and to the unknown mechanism of living cells releasing newly
synthesized DNA in a preferential manner. Through mechanisms, which
remain unclear, circulating tumor cells (CTCs) have been
hypothesized to also contribute to the pool of CNAs in the
bloodstream (23–25). This theory is supported by studies
involving lung (26), prostate
(27) and breast carcinomas (28), where clear correlations between CTCs
and tumor-derived CNAs (methylated DNA, miRNA and microsatellite
alterations) are demonstrated. However, even in the case of
advanced disease, the mass of CTCs does not account for the
quantity of tumor-derived CNAs present (5,13). This
indicates that CTCs may contribute to only a small percentage of
the total CNAs observed in plasma/serum.
Another possible origin of CNAs in plasma/serum is
from viruses, such as EBV, HPV and hepatitis B virus. The presence
of these circulating viral DNAs is observed in cases of healthy
individuals, and in cases of malignancies associated with viral
infection, such as nasopharyngeal carcinoma (11), cervical carcinoma (12) and hepatocellular carcinoma (29).
Although a variety of unrelated conditions mark the
presence of these CNAs in the bloodstream, there may be a
correlation between their source and functions. Evidences
suggesting the role of CNAs in regular functioning (30,31) and the
pathophysiology of cancers (13) leads
to the evaluation of whether the origins and mechanisms of CNA
release differ between normal and tumor-specific microenvironments.
If this is the case, it strengthens the hypothesis that CNAs are
significantly involved in modulating the microenvironment to either
maintain normal cellular functioning or drive the cells towards
tumorigenesis/metastasis. However, if no such difference in release
mechanisms actually exists, the observation of nucleic acids
circulating in the bloodstream will remain primarily significant
for diagnostic screening purposes.
Applications of CNAs
In recent years, there has been a considerable focus
on the requirement for a non-invasive blood-based biomarker for
cancer treatment. CNAs have become prominent due to their potential
use as surrogate indicators for disease monitoring by early
identification of disease progression and recurrence (32–35). In
addition, CNAs have been identified as useful for monitoring tumor
burden (36), are associated with
minimal residual disease (37), tumor
heterogeneity (38), detecting
resistance to therapy (39), and also
proven to be effective in the early diagnosis of different types of
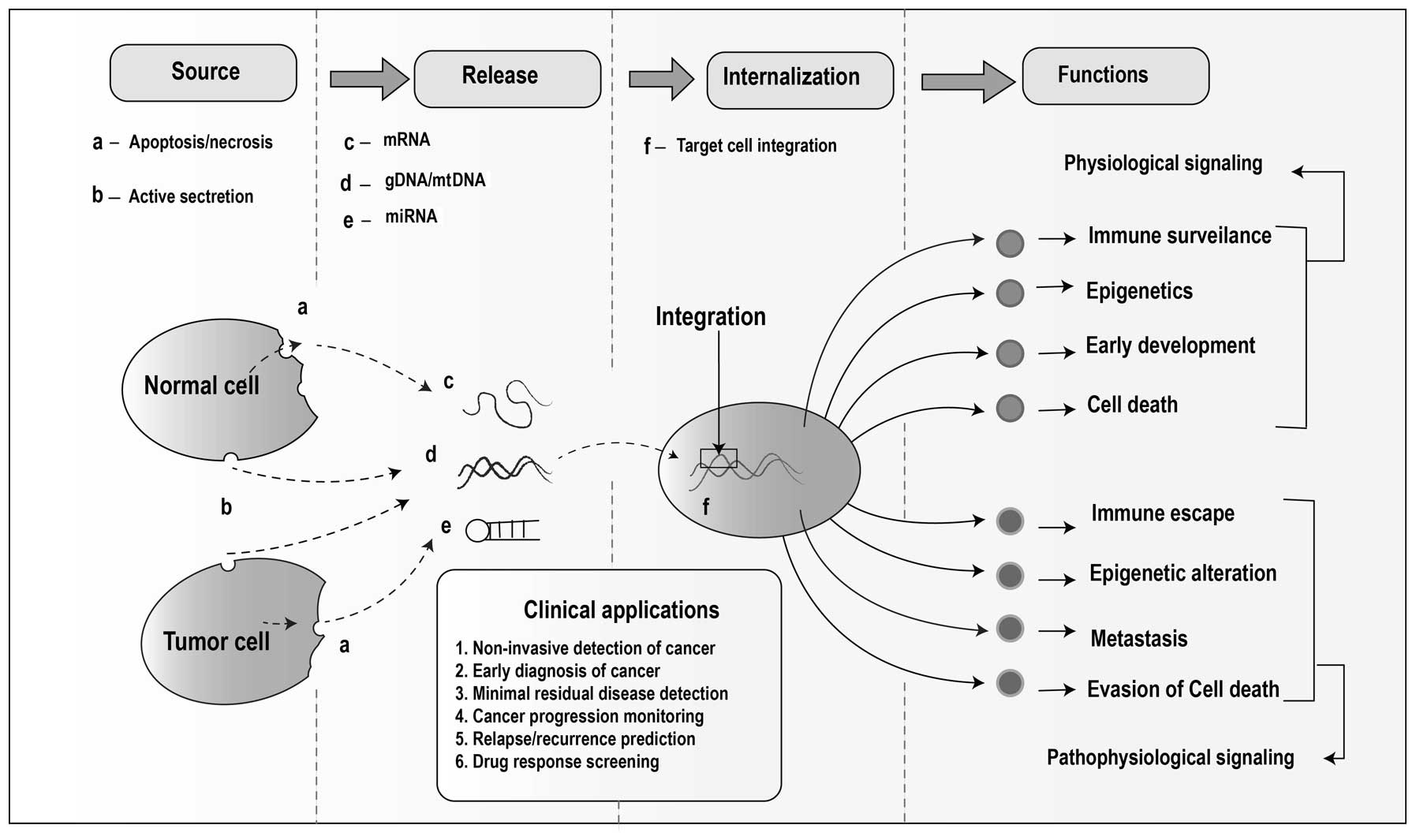
cancer (35), as schematically
represented in Fig. 1.
Quantification of CNAs
It has now been established that higher
concentrations of free CNAs are found in the blood of patients with
malignant diseases when compared with healthy subjects (10,40,41). Furthermore, a correlation has been
established between the concentrations of specific sequences of
nucleic acids in circulation and the disease stage (2,42). Numerous
studies have confirmed the use of measuring the quantity of CNAs in
plasma for monitoring colon (43),
breast (44), lung (45), stomach (46) and esophagus (47) cancer. While this method of quantifying
CNAs has shown promise in certain studies for disease screening and
monitoring, no direct association between these features has been
demonstrated. Although, theoretically, the levels of cell free DNA
(cfDNA) in plasma are affected by various clinicopathological
features, such as tumor size, tumor stage or metastasis, there is
currently a lack of studies that confirm this in human cohorts.
These may be due to overlapping concentrations of cfDNA, found in
healthy individuals under physiological stress (for example during
physical exercise) (48) or in
patients affected by other pathological processes, such as
inflammation, trauma or sepsis (49).
It has been reported that plasma levels of CNAs
decreased in cancer patients following surgical treatments and/or
chemoradiotherapy (20,43,44,47), which was probably due to the inhibitory
effect of treatment on the proliferation of cancer cells. These
studies also indicated that patients who maintained high levels of
cfDNA in the plasma either did not respond to the treatment or were
at high risk of a possible relapse. This indicates that, with
further investigations and establishment of a statistical standard,
the quantification of cfDNA in plasma will be of particular
clinical value in disease monitoring.
Cancer specific biomarkers
Characteristics of tumor DNA have been found in
genetic material extracted from the plasma of cancer patients
(50). These include decreased strand
stability (Integrity Index) (51–53),
microsatellite alterations (indicating loss of heterozygosity or
microsatellite instability) (54,55),
epigenetic alterations (gene hypermethylations) (56,57) and
presence of specific mutations in oncogenes/tumor suppressor genes
[including APC, WNT signaling pathway regulator, KRAS
proto-oncogene (KRAS), GTPase, tumor protein p53,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit
αand B-Raf proto-oncogene, serine/threonine kinase (BRAF)]
(2,28,41). A
recent study on circulating cell free-DNA in colorectal cancer
(CRC) showed 100% specificity and sensitivity for the BRAF
V600E mutation and 98% specificity and 92 sensitivity for
detection of KRAS point mutations (58). The same study indicated that overall,
100% of patients with CRC expressed detectable levels of
tumor-specific cfDNA.
As mentioned above, one study showed that detection
of hypermethylation of the CpG island of Zinc Finger Protein 154 in
circulating tumour DNA is a strong candidate for a future
pan-cancer biomarker (1). The study
demonstrated that this hypermethylation signature was consistent in
colon, lung, breast, stomach and endometrial tumors. The detection
of these alterations represents one of the most promising advances
in improved diagnosis and monitoring of various cancer types.
Non-invasive tests such as these, may potentially replace
tumor-section analysis, thereby expanding the scope of personalized
medicine for patients with cancer.
In addition to cfDNA, specific mRNAs have been
subsequently reported to be present in the plasma (59) of patients with various types of cancer,
including colon (60,61), breast (62), prostate (63), melanoma (64), lung (65)
and thyroid (66). As free RNA
molecules are likely to be degraded by RNase outside the cell, it
is likely to be packaged, either through lipid vesicles (59), or RNA-binding proteins (67), for protection. Furthermore, it has been
demonstrated that miRNA are significantly involved in control of
cell proliferation, cell differentiation and apoptosis (68); thus, their dysregulation is known to
contribute towards tumor development. Levels of circulating miRNA,
as well as various mRNAs, have been shown to be associated with the
different stages and types of cancer (69,70).
Although the investigation of genetic and epigenetic
alterations of genomic DNA is predominantly adopted in cancer
research, the identification of cancer-specific alterations in
mitochondrial DNA (mtDNA) has helped to expand the search for
clinical tools (71). The alterations
in mtDNA, which are point mutations, deletions, insertions and
quantitative changes, have been detected in a wide range of tumor
types, such as breast, colon, liver, head and neck, and lung
(72,73).
In addition to the circulating endogenous DNA, RNA,
miRNA and mtDNAs, exogenous viral DNA in plasma has been
demonstrated as a powerful diagnostic tool and its quantification
may present marked prognostic value. A study on cervical cancer
indicated 81% sensitivity (13/16) in detecting circulating
(c-)HPVDNA in patients who have progressed beyond stage 1B
(74). The same study showed
correlations between c-HPV, and response to chemotherapy and
disease progression. Notably, this study demonstrated increasing
quantities of c-HPV DNA, which was observed 6 months prior to a
patient's relapse. This illustrates the potential of circulating
viral DNA in predicting disease recurrences.
A recent study by Bettegowda et al (35) indicated that in patients with localized
tumors, ctDNA was detectable in 73, 57, 48 and 50% of patients with
colorectal cancer, gastroesophageal cancer, pancreatic cancer, and
breast adenocarcinoma, respectively. It was also found that ctDNA
was detectable in >75% of patients with advanced malignancies in
pancreatic, ovarian, colorectal, bladder, gastroesophageal, breast,
melanoma, hepatocellular, and head and neck cancers. The same
study, however, indicated that while the sensitivity of detection
of disease-specific CNAs was considerably high in certain
malignancies, it was detected in <50% of primary brain, renal,
prostate, or thyroid cancers (35).
This selective sensitivity in detection of circulating tumor
nucleic acids presents an insight into the role of CNAs in
establishing a malignant physiology.
Physiology/pathophysiology of CNAs
While the role of CNAs in diagnostics has been
investigated extensively, the reason for their presence remains
uncertain. The fact that normal blood cells (lymphocytes) and many
other types of living cells release nucleic acids into circulation
(21,75)
indicates the possibility of these CNAs being involved in the
normal functioning of cells. A theory of intercommunication between
cells via release of nucleic acids acting as signaling molecules
was proposed by Gardiner et al (31). Recent evidence from different fields
indicate active trafficking of nucleic acids between cells through
carrier mechanisms on the cell membrane (30). Nucleic acid trafficking may be involved
in intercellular signaling during development, epigenetic
remodelling, tissue regeneration and fine-tuning of the adaptive
immune system. In addition, it may be involved in cancer
development and immune surveillance (30).
The role of the immune system in cancer has become
increasingly evident over the past decade. The immune system is
widely known to interact closely with tumors over the entire
process of disease development and progression to metastasis
(76), and is now classified as a
hallmark of cancer (77). The
mechanisms by which different types of cancer evade the immune
response has been attributed to higher suppressive activity of T
regulatory cells (78), defective
antigen presentation (79), immune
suppressive mediators (80),
immunoediting (81) and selective
deletion of tumor-specific cytotoxic T-lymphocytes (82). It is possible that CNAs act as
modulators of the immune system. In studies regarding fetal-derived
CNAs in the bloodstream (83), it has
been shown that foreign DNA is recognized by Toll-like receptor
(TLR) 9, and RNA is recognized by TLR3 on immune cells, which
activate inflammatory processes (84,85). This
CNA-TLR signaling has been shown to be significant for the
reactivation of immunity in pregnancy during labor (83). Similar interaction between CNAs and
immune receptors is hypothesized to contribute towards cancer
(20).
Immune responses are tightly regulated; just as TLR
stimulation will activate responses it will also suppress them so
as to prevent autoimmunity (86), thus
indicating the possible role of CNAs in cancer and normal
environments, respectively. Therefore, an improved understanding of
the role of CNAs in modulating immune responses may provide
insights for the development of novel therapeutic strategies, which
may facilitate with overcoming the problem of immune resistance and
prevent/hinder cancer progression.
In addition to the role of circulating miRNAs in
gene silencing, recent studies have shown their contribution in
promoting gene expression via epigenetic modulation. Place et
al (87) have shown that miR-373
induced expression of E-cadherin and cold-shock domain-containing
protein C2 genes with complementary sequences in their promoter
regions. Furthermore, one study by Margolin et al (1) demonstrated that there exists
differentially methylated DNA/miRNA in circulation. It may be
possible that the release of CNAs from the dying human cancer cells
is involved in modulating epigenetic mechanisms.
As it is known that cell free nucleic acids may
affect the ability of a cell to evade cell death, whether cell free
nucleic acids aid in the process of metastasis remains to be
elucidated. A study conducted in mice showed that these CNAs, when
injected intravenously, integrate into normal cells of vital organs
and greatly alter the normal apoptotic pathways (88). This indicates that stretches of nucleic
acids may change the expression levels of certain genes in
otherwise healthy cells, thereby promoting invasion and metastasis
(20). This may lead to mutations and
subsequent malignant transformation in these cells, a theory termed
as genometastasis (9,88,89). The
mechanism of cancer dissemination in the context of CNAs is not
fully understood. If the role of CNAs in metastasis is validated,
monitoring their levels may lead to a predictive test for
metastasis and micrometastasis. Understanding the role of CNAs in
metastasis may elucidate potential ‘druggable’ signaling pathways,
which would lead to a specific treatment strategy for preventing
metastasis (Fig. 1).
Conclusion
Thus far, the utility of CNAs has been evaluated in
various diagnostic assays. Simple quantification of CNAs in the
blood has been identified as a useful tool in determining patient
prognoses. Differential methylation patterns in specific genes have
the potential to turn into pan-cancer biomarkers and may serve as
an effective screening tool. CNAs, right from their origin, appear
to exert orchestrated activity in the pathophysiology of cancer.
Conclusive proof of the influence of CNAs in immune escape
mechanisms, epigenetic modulations or even as a mediator of
metastasis cannot be ascertained from existing studies.
There is an ambiguity in the current understanding
on CNAs regarding their possible roles in establishing a
disease-specific microenvironment or their role in maintaining the
regular framework of functioning. As a result of evaluating the
current literature on possible functions of CNAs, we hypothesize
that CNAs have an established system for modulating the
microenvironment for regular cell functioning and signaling that,
when altered, may lead to a particular disease pathophysiology.
Further research is required to fully understand the
role of CNAs in the initiation and progression of different types
of cancer. Research in this direction, however, poses its own set
of challenges. Mutant CNAs of tumour origin are present in
miniscule quantities as compared to the wild-type copies,
particularly in early stage tumours, making detection a challenge.
This is being addressed by novel technological advancements with
high sensitivity, such as droplet digital polymerase chain
reaction, Beads Emulsions Amplification and Magnetics (BEAMING) and
cancer personalized profiling by deep sequencing. However, the high
cost associated with these technologies hinders their utility in
affordable and continued patient care. This presents a requirement
for the development of low-cost alternatives, which will facilitate
cancer diagnostics and screening.
Acknowledgements
The authors would like to thank Dr Cecil Ross and
Professor Sudhir Krishna for their scientific discussions and
inputs, and Ms. Annapurna Pranatharthi, Ms. Mugdha Sharma and Ms.
Pavana Thomas for their critical comments.
Glossary
Abbreviations
Abbreviations:
|
CNAs
|
circulating nucleic acids
|
|
cfDNA
|
cell free deoxyribonucleic acid
|
|
ctDNA
|
circulating tumour deoxyribonucleic
acid
|
|
RNA
|
ribonucleic acid
|
|
DNA
|
deoxyribonucleic acid
|
|
HPV
|
human papilloma virus
|
|
EBV
|
ebstein barr virus
|
|
HBV
|
hepatitis B virus
|
|
miRNA
|
micro ribonucleic acids
|
|
mtDNA
|
mitochondrial deoxyribonucleic
acid
|
References
|
1
|
Margolin G, Petrykowska HM, Jameel N, Bell
DW, Young AC and Elnitski L: Robust Detection of DNA
Hypermethylation of ZNF154 as a Pan-Cancer Locus with in Silico
Modeling for Blood-Based Diagnostic Development. J Mol Diagn.
18:283–298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
González-Masiá JA, García-Olmo D and
García-Olmo DC: Circulating nucleic acids in plasma and serum
(CNAPS): Applications in oncology. Onco Targets Ther. 6:819–832.
2013.PubMed/NCBI
|
|
3
|
Metais P and Mandel P: Les acides
necleiques du plasma sanguin chez l'Homme. C R Acad Sci Paris.
142:241–243. 1948.
|
|
4
|
Fleischhacker M and Schmidt B: Circulating
nucleic acids (CNAs) and cancer - a survey. Biochim Biophys Acta.
1775:181–232. 2007.PubMed/NCBI
|
|
5
|
Crowley E, Di Nicolantonio F, Loupakis F
and Alberto Bardelli A: Liquid biopsy: monitoring cancer-genetics
in the blood. Nature Reviews Clinical Oncology. 10:472–484. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwarzenbach H, Hoon DS and Pantel K:
Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev
Cancer. 11:426–437. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stroun M, Lyautey J, Lederrey C,
Olson-Sand A and Anker P: About the possible origin and mechanism
of circulating DNA apoptosis and active DNA release. Clin Chim
Acta. 313:139–142. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stroun M, Anker P, Maurice P, Lyautey J,
Lederrey C and Beljanski M: Neoplastic characteristics of the DNA
found in the plasma of cancer patients. Oncology. 46:318–322.
1989.PubMed/NCBI
|
|
9
|
García-Olmo DC, Ruiz-Piqueras R and
García-Olmo D: Circulating nucleic acids in plasma and serum
(CNAPS) and its relation to stem cells and cancer metastasis: State
of the issue. Histol Histopathol. 19:575–583. 2004.PubMed/NCBI
|
|
10
|
van der Vaart M and Pretorius PJ:
Circulating DNA. Its origin and fluctuation. Ann N Y Acad Sci.
1137:18–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lo YM, Chan LY, Lo KW, Leung SF, Zhang J,
Chan AT, Lee JC, Hjelm NM, Johnson PJ and Huang DP: Quantitative
analysis of cell-free Epstein-Barr virus DNA in plasma of patients
with nasopharyngeal carcinoma. Cancer Res. 59:1188–1191.
1999.PubMed/NCBI
|
|
12
|
Yang HJ, Liu VW, Tsang PC, Yip AM, Tam KF,
Wong LC, Ng TY and Ngan HY: Quantification of human papillomavirus
DNA in the plasma of patients with cervical cancer. Int J Gynecol
Cancer. 14:903–910. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anker P, Mulcahy H, Chen XQ and Stroun M:
Detection of circulating tumour DNA in the blood (plasma/serum) of
cancer patients. Cancer Metastasis Rev. 18:65–73. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giacona MB, Ruben GC, Iczkowski KA, Roos
TB, Porter DM and Sorenson GD: Cell-free DNA in human blood plasma:
Length measurements in patients with pancreatic cancer and healthy
controls. Pancreas. 17:89–97. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagata S, Nagase H, Kawane K, Mukae N and
Fukuyama H: Degradation of chromosomal DNA during apoptosis. Cell
Death Differ. 10:108–116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holdenrieder S and Stieber P: Apoptotic
markers in cancer. Clin Biochem. 37:605–617. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagata S: DNA degradation in development
and programmed cell death. Annu Rev Immunol. 23:853–875. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Atamaniuk J, Ruzicka K, Stuhlmeier KM,
Karimi A, Eigner M and Mueller MM: Cell-free plasma DNA: A marker
for apoptosis during hemodialysis. Clin Chem. 52:523–526. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fournié GJ, Courtin JP, Laval F, Chalé JJ,
Pourrat JP, Pujazon MC, Lauque D and Carles P: Plasma DNA as a
marker of cancerous cell death. Investigations in patients
suffering from lung cancer and in nude mice bearing human tumours.
Cancer Lett. 91:221–227. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Z, Fadiel A, Naftolin F, Eichenbaum
KD and Xia Y: Circulation DNA: Biological implications for cancer
metastasis and immunology. Med Hypotheses. 65:956–961. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anker P, Stroun M and Maurice PA:
Spontaneous release of DNA by human blood lymphocytes as shown in
an in vitro system. Cancer Res. 35:2375–2382. 1975.PubMed/NCBI
|
|
22
|
Stroun M, Lyautey J, Lederrey C, Mulcahy
HE and Anker P: Alu repeat sequences are present in increased
proportions compared to a unique gene in plasma/serum DNA: Evidence
for a preferential release from viable cells? Ann N Y Acad Sci.
945:258–264. 2001.PubMed/NCBI
|
|
23
|
Alix-Panabières C, Schwarzenbach H and
Pantel K: Circulating tumor cells and circulating tumor DNA. Annu
Rev Med. 63:199–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bidard FC, Weigelt B and Reis-Filho JS:
Going with the flow: From circulating tumor cells to DNA. Sci
Transl Med. 5:207ps14. 2013.
|
|
25
|
Ignatiadis M and Dawson SJ: Circulating
tumor cells and circulating tumor DNA for precision medicine: Dream
or reality? Ann Oncol. 25:2304–2313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roth C, Kasimir-Bauer S, Pantel K and
Schwarzenbach H: Screening for circulating nucleic acids and
caspase activity in the peripheral blood as potential diagnostic
tools in lung cancer. Mol Oncol. 5:281–291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Altimari A, Grigioni AD, Benedettini E,
Gabusi E, Schiavina R, Martinelli A, Morselli-Labate AM, Martorana
G, Grigioni WF and Fiorentino M: Diagnostic role of circulating
free plasma DNA detection in patients with localized. Am J Clin
Pathol. 129:756–762. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schwarzenbach H: Circulating nucleic acids
as biomarkers in breast cancer. Breast Cancer Res. 15:2112013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ono A, Fujimoto A, Yamamoto Y, Akamatsu S,
Hiraga N, Imamura M, Kawaoka T, Tsuge M, Abe H, Hayes CN, et al:
Circulating Tumor DNA Analysis for Liver Cancers and Its Usefulness
as a Liquid Biopsy. Cellular and Molecular Gastroenterology and
Hepatology. 1:516–534. 2015. View Article : Google Scholar
|
|
30
|
Ho MW: Intercommunication via circulating
nucleic acids. Science in Society. 42:46–48. 2009.
|
|
31
|
Gardiner C, Harrison P, Belting M, Böing
A, Campello E, Carter BS, Collier ME, Coumans F, Ettelaie C, van Es
N, et al: Extracellular vesicles, tissue factor, cancer and
thrombosis - discussion themes of the ISEV 2014 Educational Day. J
Extracell Vesicles. 4:269012015.PubMed/NCBI
|
|
32
|
Garcia-Murillas I, Schiavon G, Weigelt B,
Ng C, Hrebien S, Cutts RJ, Cheang M, Osin P, Nerurkar A, Kozarewa
I, et al: Mutation tracking in circulating tumor DNA predicts
relapse in early breast cancer. Sci Transl Med. 7:302ra133.
2015.
|
|
33
|
Umetani N, Giuliano AE, Hiramatsu SH,
Amersi F, Nakagawa T, Martino S and Hoon DS: Prediction of breast
tumor progression by integrity of free circulating DNA in serum. J
Clin Oncol. 24:4270–4276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hamakawa T, Kukita Y, Kurokawa Y, Miyazaki
Y, Takahashi T, Yamasaki M, Miyata H, Nakajima K, Taniguchi K and
Takiguchi S: Monitoring gastric cancer progression with circulating
tumour DNA. Br J Cancer. 112:352–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bettegowda C, Sausen M, Leary RJ, Kinde I,
Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, et al:
Detection of circulating tumor DNA in early- and late-stage human
malignancies. Sci Transl Med. 6:224ra24. 2014.
|
|
36
|
Figg WD II and Reid J: Monitor tumor
burden with circulating tumor DNA. Cancer Biol Ther. 14:697–698.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Roschewski M, Dunleavy K, Pittaluga S,
Kong K, Shovlin M, Jaffe ES, Staudt LM, Lai C, Chen CC, Zheng J, et
al: Monitoring of Circulating Tumor DNA As Minimal Residual Disease
in Diffuse Large B-Cell Lymphoma. Blood. 124:1392014.
|
|
38
|
De Mattos-Arruda L, Weigelt B, Cortes J,
Won HH, Ng CKY, Nuciforo P, Bidard FC, Aura C, Saura C, Peg V, et
al: Capturing intra-tumor genetic heterogeneity by de novo mutation
profiling of circulating cell-free tumor DNA: A proof-of-principle.
Ann Oncol. 25:1729–1735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Siravegna G and Bardelli A: Genotyping
cell-free tumor DNA in the blood to detect residual disease and
drug resistance. Genome Biol. 15:4492014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yoon KA, Park S, Lee SH, Kim JH and Lee
JS: Comparison of circulating plasma DNA levels between lung cancer
patients and healthy controls. J Mol Diagn. 11:182–185. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Diehl F, Schmidt K, Choti MA, Romans K,
Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, et al:
Circulating mutant DNA to assess tumor dynamics. Nat Med.
14:985–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
García-Olmo DC, Samos J, Picazo MG,
Asensio AI, Toboso I and García-Olmo D: Release of cell-free DNA
into the bloodstream leads to high levels of non-tumor plasma DNA
during tumor progression in rats. Cancer Lett. 272:133–140. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Frattini M, Gallino G, Signoroni S,
Balestra D, Battaglia L, Sozzi G, Leo E, Pilotti S and Pierotti MA:
Quantitative analysis of plasma DNA in colorectal cancer patients:
a novel prognostic tool. Ann N Y Acad Sci. 1075:185–190. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Catarino R, Ferreira MM, Rodrigues H,
Coelho A, Nogal A, Sousa A and Medeiros R: Quantification of free
circulating tumor DNA as a diagnostic marker for breast cancer. DNA
Cell Biol. 27:415–421. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sozzi G, Conte D, Leon M, Ciricione R, Roz
L, Ratcliffe C, Roz E, Cirenei N, Bellomi M, Pelosi G, et al:
Quantification of free circulating DNA as a diagnostic marker in
lung cancer. J Clin Oncol. 21:3902–3908. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sai S, Ichikawa D, Tomita H, Ikoma D, Tani
N, Ikoma H, Kikuchi S, Fujiwara H, Ueda Y and Otsuji E:
Quantification of plasma cell-free DNA in patients with gastric
cancer. Anticancer Res 27 (4C). 2747–2751. 2007.
|
|
47
|
Tomita H, Ichikawa D, Ikoma D, Sai S, Tani
N, Ikoma H, Fujiwara H, Kikuchi S, Okamoto K, Ochiai T, et al:
Quantification of circulating plasma DNA fragments as tumor markers
in patients with esophageal cancer. Anticancer Res 27 (4C).
2737–2741. 2007.
|
|
48
|
Atamaniuk J, Vidotto C, Tschan H, Bachl N,
Stuhlmeier KM and Müller MM: Increased concentrations of cell-free
plasma DNA after exhaustive exercise. Clin Chem. 50:1668–1670.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lo YM, Rainer TH, Chan LY, Hjelm NM and
Cocks RA: Plasma DNA as a prognostic marker in trauma patients.
Clin Chem. 46:319–323. 2000.PubMed/NCBI
|
|
50
|
Jacobs EL and Haskell CM: Clinical use of
tumor markers in oncology. Curr Probl Cancer. 15:299–360.
1991.PubMed/NCBI
|
|
51
|
Wang BG, Huang HY, Chen YC, Bristow RE,
Kassauei K, Cheng CC, Roden R, Sokoll LJ, Chan DW and Shih IeM:
Increased plasma DNA integrity in cancer patients. Cancer Res.
63:3966–3968. 2003.PubMed/NCBI
|
|
52
|
Umetani N, Kim J, Hiramatsu S, Reber HA,
Hines OJ, Bilchik AJ and Hoon DS: Increased integrity of free
circulating DNA in sera of patients with colorectal or
periampullary cancer: Direct quantitative PCR for ALU repeats. Clin
Chem. 52:1062–1069. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Umetani N, Giuliano AE, Hiramatsu SH,
Amersi F, Nakagawa T, Martino S and Hoon DS: Prediction of breast
tumor progression by integrity of free circulating DNA in serum. J
Clin Oncol. 24:4270–4276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Schwarzenbach H, Müller V, Stahmann N and
Pantel K: Detection and characterization of circulating
microsatellite-DNA in blood of patients with breast cancer. Ann N Y
Acad Sci. 1022:25–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sunami E, Shinozaki M, Higano CS, Wollman
R, Dorff TB, Tucker SJ, Martinez SR, Mizuno R, Singer FR and Hoon
DS: Multimarker circulating DNA assay for assessing blood of
prostate cancer patients. Clin Chem. 55:559–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Esteller M, Sanchez-Cespedes M, Rosell R,
Sidransky D, Baylin SB and Herman JG: Detection of aberrant
promoter hypermethylation of tumor suppressor genes in serum DNA
from non-small cell lung cancer patients. Cancer Res. 59:67–70.
1999.PubMed/NCBI
|
|
57
|
Wong IH, Lo YM, Zhang J, Liew CT, Ng MH,
Wong N, Lai PB, Lau WY, Hjelm NM and Johnson PJ: Detection of
aberrant p16 methylation in the plasma and serum of liver cancer
patients. Cancer Res. 59:71–73. 1999.PubMed/NCBI
|
|
58
|
Thierry AR, Mouliere F, El Messaoudi S,
Mollevi C, Lopez-Crapez E, Rolet F, Gillet B, Gongora C, Dechelotte
P and Robert B: Clinical validation of the detection of KRAS and
BRAF. Nat Med. 20:430–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Etheridge A, Gomes CP, Pereira RW, Galas D
and Wang K: The complexity, function and applications of RNA in
circulation. Front Genet. 4:1152013.PubMed/NCBI
|
|
60
|
Dasí F, Lledó S, García-Granero E, Ripoll
R, Marugán M, Tormo M, García-Conde J and Aliño SF: Real-time
quantification in plasma of human telomerase reverse transcriptase
(hTERT) mRNA: A simple blood test to monitor disease in cancer
patients. Lab Invest. 81:767–769. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lledó SM, Garcia-Granero E, Dasí F, Ripoli
R, García SA, Cervantes A and Aliño SF: Real time quantification in
plasma of human telomerase reverse transcriptase (hTERT) mRNA in
patients with colorectal cancer. Colorectal Dis. 6:236–242. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Silva JM, Dominguez G, Silva J, Garcia JM,
Sanchez A, Rodriguez O, Provencio M, España P and Bonilla F:
Detection of epithelial messenger RNA in the plasma of breast
cancer patients is associated with poor prognosis tumor
characteristics. Clin Cancer Res. 7:2821–2825. 2001.PubMed/NCBI
|
|
63
|
Dasí F, Martínez-Rodes P, March JA,
Santamaría J, Martínez-Javaloyas JM, Gil M and Aliño SF: Real-time
quantification of human telomerase reverse transcriptase mRNA in
the plasma of patients with prostate cancer. Ann N Y Acad Sci.
1075:204–210. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kopreski MS, Benko FA, Kwak LW and Gocke
CD: Detection of tumor messenger RNA in the serum of patients with
malignant melanoma. Clin Cancer Res. 5:1961–1965. 1999.PubMed/NCBI
|
|
65
|
Kopreski MS, Benko FA and Gocke CD:
Circulating RNA as a tumor marker: detection of 5T4 mRNA in breast
and lung cancer patient serum. Ann N Y Acad Sci. 945:172–178.
2001.PubMed/NCBI
|
|
66
|
Chinnappa P, Taguba L, Arciaga R, Faiman
C, Siperstein A, Mehta AE, Reddy SK, Nasr C and Gupta MK: Detection
of thyrotropin-receptor messenger ribonucleic acid (mRNA) and
thyroglobulin mRNA transcripts in peripheral blood of patients with
thyroid disease: Sensitive and specific markers for thyroid cancer.
J Clin Endocrinol Metab. 89:3705–3709. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Turchinovich A, Weiz L and Burwinkel B:
Isolation of circulating microRNA associated with RNA-binding
protein. Methods Mol Biol. 1024:97–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yu M: Somatic mitochondrial DNA mutations
in human cancers. Adv Clin Chem. 57:99–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chiu RW, Chan LY, Lam NY, Tsui NB, Ng EK,
Rainer TH and Lo YM: Quantitative analysis of circulating
mitochondrial DNA in plasma. Clin Chem. 49:719–726. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Copeland WC, Wachsman JT, Johnson FM and
Penta JS: Mitochondrial DNA alterations in cancer. Cancer Invest.
20:557–569. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Campitelli M, Jeannot E, Peter M,
Lappartient E, Saada S, de la Rochefordière A, Fourchotte V, Alran
S, Petrow P, Cottu P, et al: Human papillomavirus mutational
insertion: Specific marker of circulating tumor DNA in cervical
cancer patients. PLoS One. 7:e433932012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
van der Vaart M and Pretorius PJ: The
origin of circulating free DNA. Clin Chem. 53:22152007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Vinay DS, Ryan EP, Pawelec G, et al:
Immune evasion in cancer: Mechanistic basis and therapeutic
strategies. Semin Cancer Biol. 35:S185–S198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zou W: Regulatory T cells, tumour immunity
and immunotherapy. Nat Rev Immunol. 6:295–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Johnsen AK, Templeton DJ, Sy M and Harding
CV: Deficiency of transporter for antigen presentation (TAP) in
tumor cells allows evasion of immune surveillance and increases
tumorigenesis. J Immunol. 163:4224–4231. 1999.PubMed/NCBI
|
|
80
|
Gabrilovich D: Mechanisms and functional
significance of tumour-induced dendritic-cell defects. Nat Rev
Immunol. 4:941–952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: From immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lauritzsen GF, Hofgaard PO, Schenck K and
Bogen B: Clonal deletion of thymocytes as a tumor escape mechanism.
Int J Cancer. 78:216–222. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Enninga EA, Nevala WK, Holtan SG and
Markovic SN: Immune reactivation by cell-free fetal DNA in healthy
pregnancies re-purposed to target tumors: Novel checkpoint
inhibition in cancer therapeutics. Front Immunol.
6:4242015.PubMed/NCBI
|
|
84
|
Krieg AM, Yi AK, Matson S, Waldschmidt TJ,
Bishop GA, Teasdale R, Koretzky GA and Klinman DM: CpG motifs in
bacterial DNA trigger direct B-cell activation. Nature.
374:546–549. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang SY, Jouanguy E, Ugolini S, Smahi A,
Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, et
al: TLR3 deficiency in patients with herpes simplex encephalitis.
Science. 317:1522–1527. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wölfle SJ, Strebovsky J, Bartz H, Sähr A,
Arnold C, Kaiser C, Dalpke AH and Heeg K: PD-L1 expression on
tolerogenic APCs is controlled by STAT-3. Eur J Immunol.
41:413–424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Place RF, Li LC, Pookot D, Noonan EJ and
Dahiya R: MicroRNA-373 induces expression of genes with
complementary promoter sequences. Proc Natl Acad Sci USA.
105:1608–1613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
García-Olmo DC, Domínguez C, García-Arranz
M, Anker P, Stroun M, García-Verdugo JM and García-Olmo D:
Cell-free nucleic acids circulating in the plasma of colorectal
cancer patients induce the oncogenic transformation of susceptible
cultured cells. Cancer Res. 70:560–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mittra I, Khare NK, Raghuram GV, Chaubal
R, Khambatti F, Gupta D, Gaikwad A, Prasannan P, Singh A, Iyer A,
et al: Circulating nucleic acids damage DNA of healthy cells by
integrating into their genomes. J Biosci. 40:91–111. 2015.
View Article : Google Scholar : PubMed/NCBI
|