Introduction
Insulin is a peptide hormone, which is stored in
cells in large dense-core secretory granules and secreted by the
islets β-cells, released through granule exocytosis, upon
stimulation (1). Exocytosis, a key
process in insulin secretion, includes two steps; intracellular
insulin secretory vesicle transport and insulin secretory vesicle
fusion with the plasma membrane (2).
Fusion of the insulin secretory vesicle and the plasma membrane
involves the formation of an array of soluble
N-ethylmaleimide-sensitive factor attachment protein receptor
(SNARE) complex proteins (3), as well
as many other important proteins. The trafficking and fusion of
secretory vesicles involve multiple steps and complex proteins
interactions, and are subject to strict regulation. Currently, the
specific mechanisms of the biosynthesis and maturation of insulin
particles, as well as the specific mechanisms by which it is
released from cells remains unclear. Therefore, understanding the
molecular secretion process will improve the understanding of the
pathogenesis of diabetes mellitus (DM) and provide information for
the treatment of diabetes. The current study aims to demonstrate
the mechanism of insulin secretory vesicle exocytosis and the
regulation of insulin secretion.
Role of SNARE proteins in exocytosis
Types and construction of SNARE
proteins
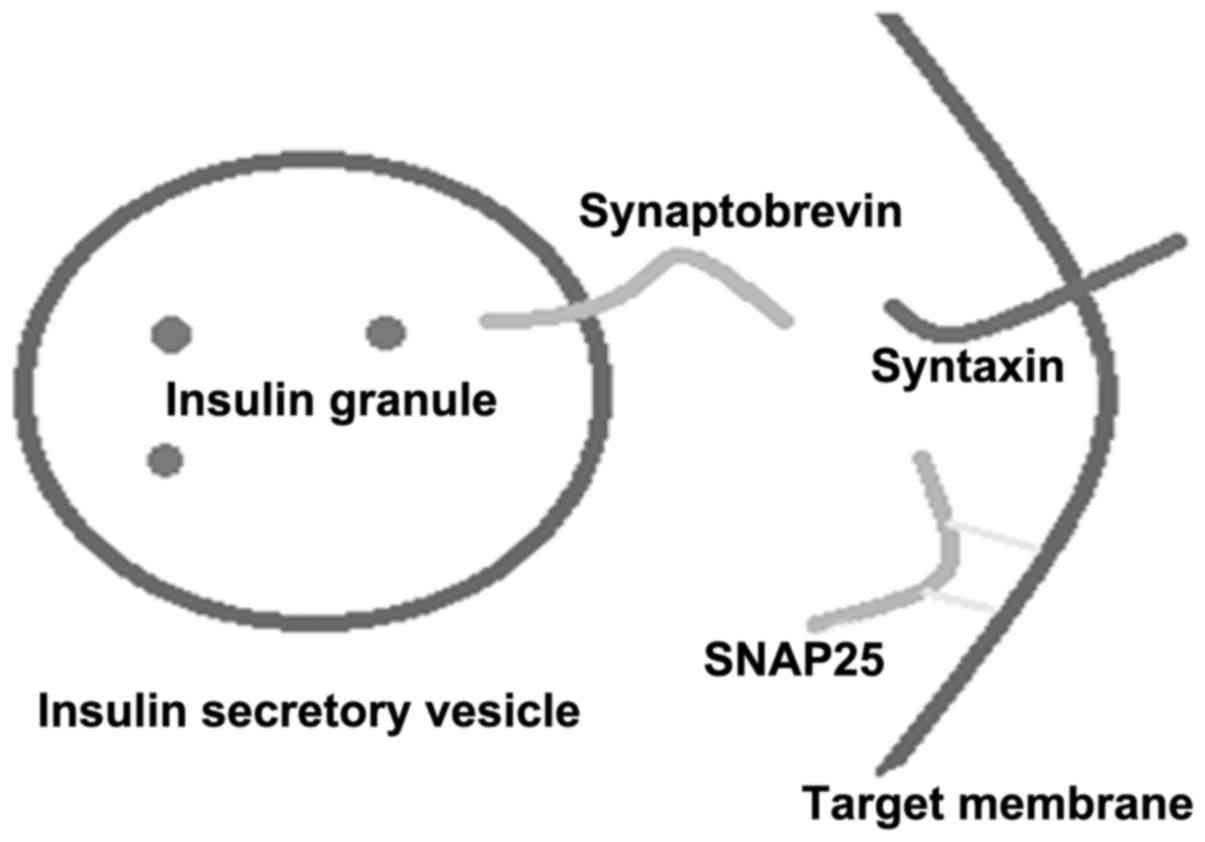
SNAREs are content-rich transmembrane proteins. The
SNARE protein is a transmembrane protein family, which is located
in cell organelles and vesicles. There are >30 types of SNARE
family protein in mammalian cells, each of which is in a separate
sub cellular component. Although the size of SNAREs varies, their
structure contains a common SNARE domain, which consists of 60–70
amino acids, in a coiled-coil structure containing seven repeats.
This domain determines all the characteristics of SNAREs and
mediates the formation of SNARE complexes; therefore it is
significant to SNARE function. In addition, SNAREs contain two
domains as follows: One that is located in the transmembrane domain
of the C-terminal end, responsible for anchoring the bubble film
(the fusion of vesicle membrane proteins and target membrane
proteins) of SNAREs in the target capsule. The synaptobrevin
protein promotes the SNARE zipper-like binding reaction through the
transmembrane domain of the C-terminal, resulting in fusion of the
vesicles. The second is the regulating domain of the N-terminal,
which is responsible for regulating SNARE activity (4).
There are two classifications of SNAREs; the first
is based on the different subcellular localization, divided into
vesicle (v)-SNAREs and target (t)-SNAREs from the target membrane.
The second classification is based on the amino acid located in the
center of the SNARE domain, arginine (Arg) or glutamine (Glu),
which is divided into R-SNAREs and Q-SNAREs, respectively (5). Under normal circumstances, there are more
t-SNAREs than Q-SNAREs and more v-SNAREs than R-SNAREs (4). In addition, Q-SNAREs are further divided
into Qa-SNAREs (syntaxin), Qb-SNAREs and Qc-SNAREs (5).
t-SNARES
t-SNARE is distributed on the target membrane,
including the syntaxin and SNAP-25 families (5). Various studies have confirmed that
syntaxin-1a and synaptosome associated protein 25 (SNAP-25) have
formed a specific t-SNARE two element complex (6). In vitro studies have shown that
the interaction of the syntaxin-1a/SNAP-25 complex with
vesicle-associated membrane protein 2 (VAMP2) produces a coiled
coil, which provides sufficient energy for driving the fusion
reaction (4). Prior to formation of
the SNARE complex, Sec1/Munc18 (SM) protein and Qa-SNARE binding
occurred, so that the space between membranes was closed. When
membrane fusion begins, the SM protein binds with the N-terminal
end of the Qa-SNARE, so that it is exposed to the SNARE domain
(7). First, the Qa-SNARE binds with
the Qc-SNARE and Qb-SNARE to form a three element complex. When the
vesicle membrane docks in the target membrane, the three element
complex of the SNAREs complex and R-SNARE forms a specific four
element complex (loose complex). Subsequently, assembly of the
SNAREs core complex. SNAREs connect the vesicle membrane and cell
membrane via three molecular structures. One of which is composed
of synaptobrevin and syntaxin proteins, and SNAP-25 forms the two
other molecular structures. The SNAREs core complex is composed of
relational twisting of numerous parallel protein spiral structures,
forming a leucine zipper embedded structure, the leucine zipper
enables the connection of two domains between adjacent vesicles and
cell membranes. The SNARE transmembrane domain is anchored during
the fusion of the two films, while t-SNARE and v-SNARE mediate
membrane fusion to connect t-SNARE and v-SNARE at a certain
position (8). The t-SNARE complex
contains a Glu relatively conserved sequence, which is positioned
in the center of the helix bundle (termed Q-SNARE protein). The
v-SNARE contributes an Arg sequence (termed R-SNARE protein) and is
also in this position. Certain proteins [such as RAB3A, member RAS
oncogene family (Rab3A)] coexist on the vesicle membrane and the
target membrane, establishing the Q-R-SNARE classification model,
which is an increasingly prevalent model. This model is based on
two conserved amino acid sequences of the cytoplasmic domain of
SNARE protein, Glu (Q) is a conserved sequence, Arg (R) is another
conserved sequence. Those cytoplasmic domain contains Glu conserved
sequence of SNARE protein (such as Syntaxin, SNAP25) and is
classified as Q-SNARE, whereas SNARE protein (such as VAMP and
Rab3A) containing an Arg conserved sequence is referred to as
R-SNARE. The next R-SNARE and Qa-SNARE transmembrane domain
rearrangement forms cis-SNAREs, prompting membrane distal binding
and, thus, the formation of fusion pores and fusion pore expansion,
which releases the material from inside the vesicle (9).
v-SNAREs (the role of VAMP proteins in
exocytosis)
VAMP is a v-SNARE anchored to synaptic vesicles and
secretory granules. v-SNARE is distributed on the vesicle membrane,
along with synaptobrevin/VAMPs and associated proteins (10). The v-SNARE complex is formed during a
membrane fusion process, which requires a series of molecules, such
as synaptobrevins, syntaxins and SNAREs core proteins (such as
synaptic-associated proteins) to achieve synergy. According to
recent analysis of VAMP (11), the
detailed proteomic characterizations of granule secretory vesicles
were identified. There are four identified VAMPs, including VAMP2,
VAMP3, VAMP7 and VAMP8. VAMP2, VAMP3 and VAMP8 are known to
participate in exocytosis in various cell types and to form a
complex substance with syntaxin 4/SNAP-23 (12). VAMP2, a v-SNARE granule protein, is
important; it is a structural protein of the synaptic vesicle
membrane, which is closely associated with all of the neurons and
endocrine cells, which are closely associated with
neurotransmitter, hormone release and synaptic plasticity.
For example, in the compound of syntaxin 1a,
following dissociation, VAMP2 or SNAP-25 toxins cause β-cell
insulin secretion. Notably, in vitro studies have shown that
the interaction of the syntaxin 1/SNAP-25 complex and VAMP2
produces a spiral beam, which provides enough energy to drive the
fusion reaction (13). VAMP7 is
predominantly located in the inner body, lysosomes and the cell
membrane, which is involved in vesicle transport between the inner
body and the lysosome. VAMP5, located in the cell membrane, is
highly expressed in muscle cells, and is associated with the cell
membrane and vesicle structure, and myotubes. VAMP5 is usually
considered to be an adjustable vesicle that mediates docking and
fusion of vesicles to target membranes, and is important for
membrane transport of skeletal and cardiac muscle. VAMP8 is located
in the cell membrane, and early and late endosomes found in the
trans Golgi network and other organelles, suggest its extensive
role. A previous study indicates that VAMP8 mediated endocytosis of
glucose transporter type 4 (14).
R-SNARE (synaptobrevin)
The SNARE protein is formed according to the zipper
hypothesis; a zipper exists at the interface of the v-/t-SNARE
interaction and, therefore, is equivalent to providing a fusion
reaction to an inward tension (15).
t-SNARE is distributed on the target membrane, including the
syntaxin and SNAP-25 families. v-SNAREs are distributed on the
vesicle membrane, and include synaptobrevins/VAMPS and associated
proteins. Synaptobrevins, syntaxins and SNAP-25 form a stable
melamine according to the proportion of 1:1:1 (Fig. 1) (4). The
SNARE motif in the three classes of the SNARE protein is formed in
parallel with the α helical region of the N-motif, which forms the
core complex (N-ethylmaleimide-sensitive factor; NSF). Under the
assistance of the α/β-soluble N-ethylmaleimide-sensitive attachment
proteins (SNAPs), the hydrolysis of ATP results in SNARE polymer
dissociation (16).
Role of vacuolar-type H+-ATPase
(V-ATPase) in exocytosis
Molecular characteristic of
V-ATPase
A protein that is widely distributed in the
eukaryotic cell membrane and the cell membrane of the cell
membrane, which is associated with a type of V-type H+
ATPase. The structure of V-ATPase is composed of two subunits, V1
and V0 in the cytoplasm. The former provides a channel for
H+ and the latter decomposes ATP, and provides energy
for the transfer of H+ against the concentration
gradient. V0 and V1 are only involved in polymeriztion (17). Tonoplast V-ATPase is a particularly
important type of proton pump, involved in ion homeostasis in the
cytoplasm and in cell metabolism. This enzyme is a large multimeric
enzyme composed of cytoplasmic and membrane surface V1 and V0
complexes. The V1 domain, located in the cytoplasm, contains eight
subunits (A3, B3, C, D, E, F, G2 and H), which are responsible for
the hydrolysis of ATP, with subunits A and B being the most
important. Subunit A catalyzes the hydrolysis of ATP (subunit B is
not conserved in the glycine rich region; therefore, has no
catalytic function, but has the ability to regulate). ATP is
involved in the hydrolysis of the A3 and B subunits, which function
in a similar way to the F-ATPase β and α subunits (18). There are three copies of each of the A
and B subunits, to form the six polymer units. The A subunit is the
catalytic subunit of the hydrolysis of ATP and the B subunit is a
non-catalytic subunit, which is a site of ATP binding. Certain
residues of the A subunit are essential for enzyme activity, such
as E286, which is involved in proton uptake. Negatively charged
phosphate groups, located in the glycine rich region, may be
associated with the ATP phase of K263. The B subunit is the
regulatory subunit, and each of the six polymer units contains
three sites for the catalytic hydrolysis of ATP (19,20). The V0
domain is composed of five subunits (a, c, c′′, d and e), which are
responsible for proton transport.
Function of V-ATPase in
exocytosis
V-ATPase activity in neuronal synaptic vesicles
produced a large number of proton electrochemical gradients
(21). This electrochemical proton
gradient is a low pH condition for the accumulation of
neurotransmitter in the synaptic vesicles or catecholamine in the
granules of the granule (22). The V0
region of V-ATPase was found to interact with proteins involved in
the extracellular region of the protein (Fig. 2). The interaction of V0 and VAMP2 may
be expressed in the cis complex of the same insulin secretory
vesicles (15). The interaction
between V-ATPase and syntaxin-1 is also important for the
regulation of the cell, and these interactions are regulated by
calmodulin and Ca2+ (21).
Other proteins participate in insulin
secretory granules exocytosis
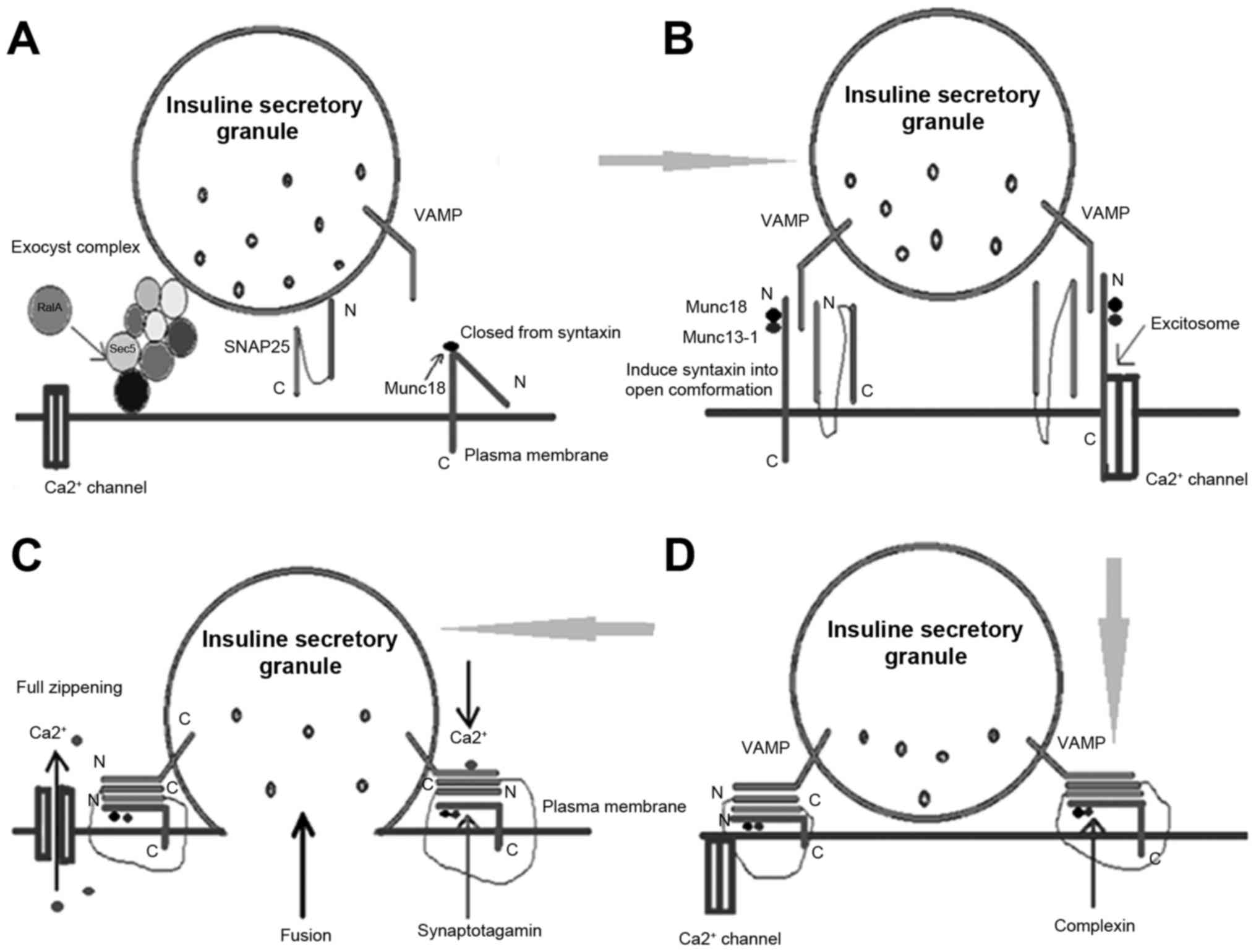
SM proteins participate in insulin
secretory granule exocytosis
The majority of transport pathways require a SNARE
protein complex that mediates the fusion of transport vesicles and
target membranes. Simultaneously, another protein family, SM
proteins, is significant to vesicle transport (4). The SM protein is a type of hydrophilic
protein, which is composed of ~600 amino acids. The homology
between the family members is high. The spatial structure of the
family is roughly the ‘bow’ structural protein, which is closely
associated with secretion (5). It has
been identified that integration of the cell membrane in the
vesicle transport pathway requires SNARE protein family involvement
(4). The family is predominantly
composed of two subtypes of the target membrane, t-SNARE and
v-SNARE vesicles. v- and t-SNAREs are assembled into SNARE
complexes, which is promoted by the fusion of the vesicle membrane
and the target membrane, thus is key in the membrane transport
process. However, v- and t-SNARE do not spontaneously assemble into
a complex, the assembly of the anterior vesicle anchored target
membrane and the SNARE complex requires the involvement of other
proteins. This includes SM protein family members, which are
significant in SNARE-mediated membrane fusion and vesicle
trafficking events (3,22). Previous studies suggest that SM
proteins may regulate the process of vesicle anchoring to the
target membrane, and may also be involved in the initiation, fusion
and other steps in the regulation (23). The interaction between SM proteins and
the SNARE complex has been identified to occur via four primary
mechanisms (24) as follows: i) The SM
proteins bind to the closed conformation of the syntaxin protein.
This combination makes the syntaxin-1 and munc18-1 more stable in
the body relative to their respective monomer forms (6). ii) Certain SM proteins interact in other
ways with the syntaxin protein family; the SM proteins combine with
the N terminal sequence of the conserved syntaxin protein, which is
termed the open state. This interaction is more common than the
aforementioned interaction (6,25). iii) The direct combination of SM
proteins and the SNARE complex. SM proteins directly bind to the
SNARE complex, which indicates that the SM protein is involved in
the regulation of the fusion of vesicles and target membranes
(26). iv) A small number of SM
proteins do not interact directly with syntaxin, but first bind to
other soluble proteins and form a complex, and subsequently bind to
the syntaxin protein. Numerous studies demonstrate that the correct
assembly of the SNARE complex predominantly depends on the
regulation of SM proteins, and the binding of SM proteins and the
SNARE complex ensures the stability of the SNARE complex (5).
Rab proteins and insulin secretory
granule exocytosis
Rab protein is the largest family of the small
molecule guanosine-5′-triphosphate (GTP) binding protein family
(small GTP-binding proteins). Composed of 200 amino acids, it
comprises a conserved G domain with highly variable N and C ends.
The Rab protein serves as a vesicle trafficking molecular switch,
and its upstream regulator and downstream effector specific
interactions, coupled with the GTP binding and hydrolysis process,
are involved in the different stages of vesicular transport
(27). The Rab protein is ubiquitous
in vesicular transport components contributing to the basic
functions, as well as the regulatory functions of vesicle
transport. Rab proteins facilitate vesicle aggregation close to the
target membrane, triggering SNARE to release inhibiting factor. Rab
protein is a GTP binding protein and Rab-GTP guides transport
vesicles from the donor area to the target area of the plasma
membrane, with assistance from SNAP25. The Rab-GTP complex combines
with the vesicle SNAP receptor (t-SNARE) to form the anterior
fusion complex. The Rab protein hydrolyzes GTP to generate
guanosine-5′-triphosphate (GDP), and the Rab-GDP complex separates
from the vesicles and binds with the GDP dissociation inhibitor
(GDI) in the cytoplasm. This is then transferred to the plasma
membrane of the donor site. Rab-GDP separates from GDI and combines
with guanine nucleotide exchange factor (GEF), stimulating GTP
replacement of GDP. Rab-GTP may then recombine with transport
vesicles; simultaneously, Three classes of SNAP combine with the
anterior fusion complex and combines with the ATP enzyme, NSF. NSF
hydrolyses ATP, which triggers the fusion of vesicles and target
membranes. Transport vesicles are only able to form when they
contain specific Rabs and SNAREs. Rab proteins connect or activate
specific effectors, and regulate vesicle transport during vesicle
formation, transport, adhesion, anchoring, and fusion at different
transport phases (28). Furthermore,
vesicle formation requires interactions between multiple proteins,
such as ADP-ribosylation factor GTPases, coatmer protein subunits,
cohesion and grid proteins. Thus, the Rab protein is essential for
vesicle formation (29). In the
cytosol, vesicular transport must occur over a long distance. Rab
proteins interact with actin and tubulin in the cytoskeleton to
regulate vesicle transport. For example Rab-6 (Rabkinesin-6), a
kinesin effector, is a member of the kinesin family; it has the
traditional structural characteristics of kinesin. Rabkinesin-6
interacts with Rab6-GTP, and uses the microtubule-based
cytoskeleton to guide vesicle transport (30). The Rab protein interacts with adhesion
factors during the formation of vesicles (i.e., in the absorption
of SNARE to transport vesicles). Rab protein interactions occur
instantaneously by the same adhesion factor, in any order, during
the two stages (formation and adhesion) of vesicular transport and
have an important role (9). In the
target membrane vesicles are anchored by pairing of v- and
t-SNAREs, and this pairing provides specificity for the membrane
fusion reactions. The Rab protein interacts with adhesion factors
and other effectors to promote the pairing of v- and t-SNAREs, and
subsequently promotes vesicle fusion with the target membrane
(27).
Regulation of insulin secretory
granule exocytosis
Peptide and protein hormones are stored in and
released by the secretory vesicles. Large secretory vesicles are
generated by the Golgi to form a network structure. Subsequently, a
series of steps result in exocytosis as follows: Recruitment (the
release of secretory vesicles to the cell membrane), anchoring,
priming (the maturation of the vesicles), and the final vesicle
membrane and cell membrane fusion. The inclusions are then released
into the extracellular fluid through the fusion pore (Fig. 3).
There are two types of factors regulating insulin
secretion: A class of nutrients, such as glucose, and the
neurotransmitters and hormones. Islet β-cells integrate these two
kinds of signal functions of regulating factors, and adjust their
insulin levels in steady state. At the molecular level, the insulin
secretory vesicle exocytotic mechanism and its regulation include
two parts: One is the proximal step, which involves adjusting the
level of intracellular second messenger substances. The second is
the terminal adjustment steps. In the proximal step, metabolism of
glucose and other nutrients, via alterations of the cytoplasm
ATP/ADP ratio, closes the ATP-sensitive potassium (K+)
channel resulting in membrane depolarization-induced calcium
(Ca2+) channel opening. Increased intracellular
Ca2+ ion levels stimulate the release of insulin.
Elevated intracellular Ca2+ leads to a rise of ATP/ADP
in the cytoplasm, and the K+ channels close causing
membrane depolarization, Ca2+ influx and insulin
granular exocytosis (31).
Glucose-dependent insulinotropic polypeptide (32) and glucagon-like peptide 1 (GLP-1)
(33) act on membrane receptors to
generate cytoplasmic cAMP and regulate insulin secretion. The
insulin pathway is initiated by the binding of insulin to the
receptor on the target tissue. Under physiological conditions,
insulin action on the insulin receptor (INSR) of the target cell
membrane combined with the α subunit of the receptor, results in
multiple β subunit tyrosine phosphorylation of the INSR substrate,
and subsequently combines with the INSR substrates (IRS-1 and
IRS-2). The tyrosine protein substrates are phosphorylated by
tyrosine kinases of the INSR (34).
Phosphorylated IRS-1 and IRS-2 interact with the p85 subunit of
phosphoinositide 3-kinase (PI3K), to bring it closer to the plasma
membrane, leading to the generation of phosphatidylinositol
(3,4,5)-trisphosphate (35). Through a series of signal transduction
pathways, inhibition of glycogen synthase kinase 3 (GSK-3) promotes
glycogen synthase (GS) phosphorylation, improves its activity,
promotes tissue glycogen synthesis and decreases the peripheral
blood glucose concentration (34).
Conclusion and future perspectives
The current review introduces the proteins that are
involved in insulin secretory vesicle exocytosis. It is these
proteins that pull the vesicle membrane and the cell membrane
together, resulting in the exocytosis process. Membrane fusion
failure may be associated with the occurrence of diabetes;
therefore, the specific mechanisms of membrane fusion are
considered to be significant in the prevention of diabetes.
Currently, the understanding of the various aspects of membrane
fusion remains hypothetical. Understanding the process of membrane
fusion and the underlying molecular mechanisms will markedly
promote the development of biology, as well as lay a solid
foundation for disease treatment. Furthermore, the pathogenesis of
DM, particularly the early mechanisms, is unclear. Inhibition of
insulin secretory granule exocytosis is the important pathological
manifestation in the early stage of DM development. Therefore,
further in-depth investigation of DM and disordered early insulin
secretion, and timely clarification of pathophysiologic mechanisms
are considered to be particularly urgent. In biology and
morphology, secretory vesicles and lysosomes possess a high degree
of homology and similarity. In addition, lysosome membrane
proteins, and the association between insulin secretory vesicles
and their role in exocytosis have not yet been investigated in
detail. Due to the biological homology of lysosomes and vesicles,
further investigations are required to establish whether lysosomal
membrane proteins have an important role in the formation of
insulin secretion vesicles and insulin secretion. Thus, their
involvement in the early development of DM and insulin secretion
disorder requires further investigation.
Acknowledgements
The study was supported by the National Natural
Science Foundation of China (grant nos. 81200632 and 81471002), the
Natural Science Foundation of Anhui, China (grant no.
1308085QH134), and the Introduction of Talents Foundation of
Yijishan Hospital (grant no. YR201104).
References
|
1
|
Liu T, Li H, Gounko NV, Zhou Z, Xu A, Hong
W and Han W: Detection of insulin granule exocytosis by an
electrophysiology method with high temporal resolution reveals
enlarged insulin granule pool in BIG3-knockout mice. Am J Physiol
Endocrinol Metab. 307:E611–E618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Cantley J, Burchfield JG, Meoli CC,
Stöckli J, Whitworth PT, Pant H, Chaudhuri R, Groffen AJ, Verhage
M, et al: DOC2 isoforms play dual roles in insulin secretion and
insulin-stimulated glucose uptake. Diabetologia. 57:2173–2182.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jewell JL, Oh E and Thurmond DC:
Exocytosis mechanisms underlying insulin release and glucose
uptake: Conserved roles for Munc18c and syntaxin 4. Am J Physiol
Regul Integr Comp Physiol. 298:R517–R531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Südhof TC and Rothman JE: Membrane fusion:
Grappling with SNARE and SM proteins. Science. 323:474–477. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hong W: SNAREs and traffic. Biochim
Biophys Acta. 1744:493–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jahn R: Sec1/Munc18 proteins: Mediators of
membrane fusion moving to center stage. Neuron. 27:201–204. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ulloa F, Gonzàlez-Juncà A, Meffre D,
Barrecheguren PJ, Martínez-Mármol R, Pazos I, Olivé N, Cotrufo T,
Seoane J and Soriano E: Blockade of the SNARE protein syntaxin 1
inhibits glioblastoma tumor growth. PLoS One. 10:e01197072015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alonso-Curbelo D, Riveiro-Falkenbach E,
Pérez-Guijarro E, Cifdaloz M, Karras P, Osterloh L, Megías D, Cañón
E, Calvo TG, Olmeda D, et al: RAB7 controls melanoma progression by
exploiting a lineage-specific wiring of the endolysosomal pathway.
Cancer Cell. 26:61–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Torii S, Takeuchi T, Nagamatsu S and Izumi
T: Rab27 effector granuphilin promotes the plasma membrane
targeting of insulin granules via interaction with syntaxin 1a. J
Biol Chem. 279:22532–22538. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin RC and Scheller RH: Mechanisms of
synaptic vesicle exocytosis. Annu Rev Cell Dev Biol. 16:19–49.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borisovska M, Zhao Y, Tsytsyura Y, Glyvuk
N, Takamori S, Matti U, Rettig J, Südhof T and Bruns D: v-SNAREs
control exocytosis of vesicles from priming to fusion. EMBO J.
24:2114–2126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Müller HK, Kragballe M, Fjorback AW and
Wiborg O: Differential regulation of the serotonin transporter by
vesicle-associated membrane protein 2 in cells of neuronal versus
non-neuronal origin. PLoS One. 9:e975402014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagamatsu S, Nakamichi Y, Watanabe T,
Matsushima S, Yamaguchi S, Ni J, Itagaki E and Ishida H:
Localization of cellubrevin-related peptide, endobrevin, in the
early endosome in pancreatic beta cells and its physiological
function in exo-endocytosis of secretory granules. J Cell Sci.
114:219–227. 2001.PubMed/NCBI
|
|
14
|
Antonin W, Holroyd C, Tikkanen R, Höning S
and Jahn R: The R-SNARE endobrevin/VAMP-8 mediates homotypic fusion
of early endosomes and late endosomes. Mol Biol Cell. 11:3289–3298.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Forgac M: Vacuolar ATPases: Rotary proton
pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol.
8:917–929. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toei M, Saum R and Forgac M: Regulation
and isoform function of the V-ATPases. Biochemistry. 49:4715–4723.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morel N: Neurotransmitter release: The
dark side of the vacuolar-H+ATPase. Biol Cell.
95:453–457. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Poëa-Guyon S, Amar M, Fossier P and Morel
N: Alternative splicing controls neuronal expression of v-ATPase
subunit a1 and sorting to nerve terminals. J Biol Chem.
281:17164–17172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Camacho M, Machado JD, Montesinos MS,
Criado M and Borges R: Intragranular pH rapidly modulates
exocytosis in adrenal chromaffin cells. J Neurochem. 96:324–334.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Giovanni J, Boudkkazi S, Mochida S,
Bialowas A, Samari N, Lévêque C, Youssouf F, Brechet A, Iborra C,
Maulet Y, et al: V-ATPase membrane sector associates with
synaptobrevin to modulate neurotransmitter release. Neuron.
67:268–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahmad A, Khundmiri SJ, Pribble F, Merchant
ML, Ameen M, Klein JB, Levi M and Lederer ED: Role of vacuolar
ATPase in the trafficking of renal type IIa sodium-phosphate
cotransporter. Cell Physiol Biochem. 27:703–714. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kwan EP and Gaisano HY: Rescuing the
subprime meltdown in insulin exocytosis in diabetes. Ann N Y Acad
Sci. 1152:154–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kwan EP, Xie L, Sheu L, Nolan CJ, Prentki
M, Betz A, Brose N and Gaisano HY: Munc13-1 deficiency reduces
insulin secretion and causes abnormal glucose tolerance. Diabetes.
55:1421–1429. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu D, Zhang Y, Lam PP, Dolai S, Liu Y,
Cai EP, Choi D, Schroer SA, Kang Y, Allister EM, et al: Dual role
of VAMP8 in regulating insulin exocytosis and islet β cell growth.
Cell Metab. 16:238–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mandic SA, Skelin M, Johansson JU, Rupnik
MS, Berggren PO and Bark C: Munc18-1 and Munc18-2 proteins modulate
beta-cell Ca2+ sensitivity and kinetics of insulin
exocytosis differently. J Biol Chem. 286:28026–28040. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parsaud L, Li L, Jung CH, Park S, Saw NM,
Park S, Kim MY and Sugita S: Calcium-dependent activator protein
for secretion 1 (CAPS1) binds to syntaxin-1 in a distinct mode from
Munc13-1. J Biol Chem. 288:23050–23063. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brunner Y, Couté Y, Iezzi M, Foti M,
Fukuda M, Hochstrasser DF, Wollheim CB and Sanchez JC: Proteomics
analysis of insulin secretory granules. Mol Cell Proteomics.
6:1007–1017. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jordens I, Marsman M, Kuijl C and Neefjes
J: Rab proteins, connecting transport and vesicle fusion. Traffic.
6:1070–1077. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iezzi M, Escher G, Meda P, Charollais A,
Baldini G, Darchen F, Wollheim CB and Regazzi R: Subcellular
distribution and function of Rab3A, B, C, and D isoforms in
insulin-secreting cells. Mol Endocrinol. 13:202–212. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Waselle L, Coppola T, Fukuda M, Iezzi M,
El-Amraoui A, Petit C and Regazzi R: Involvement of the Rab27
binding protein Slac2c/MyRIP in insulin exocytosis. Mol Biol Cell.
14:4103–4113. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang SN and Berggren PO: Beta-cell CaV
channel regulation in physiology and pathophysiology. Am J Physiol
Endocrinol Metab. 288:E16–E28. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gremlich S, Porret A, Hani EH, Cherif D,
Vionnet N, Froguel P and Thorens B: Cloning, functional expression,
and chromosomal localization of the human pancreatic islet
glucose-dependent insulinotropic polypeptide receptor. Diabetes.
44:1202–1208. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thorens B: Expression cloning of the
pancreatic beta cell receptor for the gluco-incretin hormone
glucagon-like peptide 1. Proc Natl Acad Sci USA. 89:8641–8645.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dominguez V, Raimondi C, Somanath S,
Bugliani M, Loder MK, Edling CE, Divecha N, da Silva-Xavier G,
Marselli L, Persaud SJ, et al: Class II phosphoinositide 3-kinase
regulates exocytosis of insulin granules in pancreatic beta cells.
J Biol Chem. 286:4216–4225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo L, Li Q, Wang W, Yu P, Pan H, Li P,
Sun Y and Zhang J: Apelin inhibits insulin secretion in pancreatic
beta-cells by activation of PI3-kinase-phosphodiesterase 3B. Endocr
Res. 34:142–154. 2009. View Article : Google Scholar : PubMed/NCBI
|