Introduction
Acute myocardial infarction (AMI) remains a leading
cause of mortality worldwide (1), and
reperfusion of the ischemic myocardium is a valuable approach for
limiting infarct size (IS). However, reperfusion alone leads to
reversible and irreversible injuries in the ischemic myocardium,
which is called ischemia-reperfusion (I/R) injury (2,3).
Prostaglandin E2 (PGE2) has been demonstrated to be
beneficial during cardiac I/R (4,5). Previous
studies indicate that endogenous PGE2 protects the heart
from I/R injury in vivo and in vitro by promoting
collateral vessel growth (4). However,
the underlying mechanism of PGE2 in cardiac I/R injury
remains unknown.
In AMI, expression levels of vascular endothelial
growth factor (VEGF) have been reported to be upregulated, which
diminished I/R injury (6). Endothelial
nitric oxide synthase (eNOS), a rate-limiting enzyme for the
synthesis of prostaglandins (PGs), has been reported to be induced
in the heart during I/R (7). This
result is consistent with the fact that production of
PGE2 in the heart increases significantly during
ischemia (8), suggesting that it is
significant in cardiac I/R injury. The aim of the present study was
to investigate whether PGE2 affected expression levels
of VEGF and eNOS in a catheter-based porcine model of AMI.
Materials and methods
Animal experiment protocol
The Animal Research Committee of China-Japan
Friendship Hospital (Beijing, China) provided ethical approval for
the experiments. The investigations conformed to the Guide for the
Care and Use of Laboratory Animals published by the US National
Institutes of Health (NIH publication, 8th edition) (9).
Twenty-two male Chinese miniature pigs (weight,
25±3.2 kg; age, 6 months) procured from China Agricultural
University (Beijing, China) were selected for the experiment. The
pigs were housed separately in the Animal Lab Center of China-Japan
Friendship Hospital at a temperature of 20°C and humidity of 50%
under a 12-h light/dark cycle. They had free access to food of
normal cholesterol content. The porcine model of AMI was created on
the basis of a previous study by Suzuki et al (10) with modifications. The distal segment of
the left anterior descending (LAD) coronary artery was completely
occluded by a dilated balloon (2.0×10 mm) for 2 h. Successful
construction of the AMI model was confirmed by findings of coronal
artery angiography (CAG) and electrocardiogram (ECG). The LAD
coronary artery was then reperfused for 3 h, followed by repeat CAG
to ensure the presence of thrombolysis in MI (TIMI) grade 3 blood
flow in the LAD coronary artery.
Twenty-two Chinese miniature pigs were randomized
into 3 groups as follows: Sham-surgery (n=6), control (n=8) and
PGE2 (n=8) groups. The distal segment of the LAD
coronary artery of the control and PGE2 groups were
occluded by dilated balloon for 2 h followed by a 3-h reperfusion.
PGE2 (1 µg/kg; Beijing Tide Pharmaceutical Co., Ltd.,
Beijing, China) was injected from 10 min before LAD occlusion to 1
h after reperfusion in the PGE2 group. Saline was used
instead of PGE2 in the control group. In the
sham-surgery group animals, a balloon was placed in the LAD
coronary artery, but was not dilated. There was no AMI reperfusion
and no-reflow in the sham-surgery group.
Hemodynamic assessment
Left ventricular systolic pressure (LVSP), LV
end-diastolic pressure (LVEDP) and heart rate (HR) were obtained
via a 6F pigtail catheter method prior to AMI, 2 h after occlusion,
and 1, 2 and 3 h after reperfusion for serial monitoring of cardiac
function. The baseline hemodynamic parameters were measured prior
to AMI.
Measurement of necrosis and no-reflow
area (NRA)
Double-staining with 0.01 g/ml Evans blue dye and
0.04 g/ml Thioflavin-S was performed to delineate the reperfusion
area (RA) and no-reflow area (NRA). Three hours after reperfusion,
1 ml/kg of 4% Thioflavin-S in saline was injected as a bolus. The
reperfused area was stained, but the NRA was not stained. After
another complete occlusion of the LAD coronary artery, 0.01 g/ml
Evans Blue dye was infused into the left ventricle and the normal
myocardium was stained to negatively mark the territory of the
occluded artery (i.e., the risk area).
The deeply anesthetized swine were sacrificed by
injection of 15% KCl (1 ml/kg). Then the heart was excised and
rinsed in ice-cold saline solution to remove the blood and excess
dye. The atria and right ventricular free wall were removed, and
the remaining LV tissue was sectioned perpendicular to its long
axis into six to seven sections and photographed. The risk area
(the area unstained by Evans Blue) was traced and visualized under
natural light. The NRA (the area not perfused by Thioflavin-S) was
photographed using ultraviolet light (wavelength, 365 nm) and a
yellow filter. The area between the risk area and NRA was the area
of reflow. The normal area was defined as the area not including
the RA, NRA and risk area. The RA, NRA and LV wall area (LVWA) were
measured using image processing software IPP 6.0. Outcomes were
calculated as follows: RA (%) = (RA/area of left ventricle) × 100;
NRA (%) = (NRA/risk area) × 100%. In addition, RA/LVWA and NRA/LVWA
were calculated.
The normal area, RA and NRA were then fixed using
10% formalin and embedded in paraffin for histopathological
examination by hematoxylin and eosin (H&E) staining. Neutrophil
infiltration in the area of reflow was semi-quantified by light
microscopy (Nikon Eclipse E400; Nikon Corporation, Tokyo, Japan) at
a magnification of ×400, in a blinded manner by a cardiac
pathologist.
Immunohistochemical staining for VEGF
and eNOS
In immunohistochemical analysis, cross-sectional
myocardial slices at the level of LV papillary muscles were
selected. Normal area, RA and NRA were fixed by 10% formalin and
embedded in paraffin. Tissue sections (5 µm) were deparaffinized
and re-hydrated. Samples were then subjected to 0.1% Triton X-100
for permeability. The endogenous peroxidase activity was subdued by
treating with 3% hydrogen peroxide for 10 min. The
paraffin-embedded sections were incubated with anti-VEGF antibody
(Abcam, Cambridge, USA; cat. no. ab69479) and anti-eNOS antibody
(Abcam; cat. no. ab66127) at a dilution of 1:200, or with negative
control (normal serum) at 4°C overnight. The sections were stained
with horseradish peroxidase conjugated secondary antibody
(Histofine Simple Stain Mouse MAX PO (R), Nichirei Biosciences Inc.
Tokyo, Japan; cat. no. 414341F) and 3,3′-diaminobenzidine
tetrahydrochloride and counterstained with hematoxylin. The
percentage of positive staining for VEGF and eNOS were calculated
and six fields selected in a clockwise direction were observed at a
magnification of ×200 under a stereo microscope. The expression
levels of VEGF and eNOS were observed as brown particles in the
myocardial and perivascular tissue samples, and the sum of
integrated optical density (IOD) and total positive areas of each
group were measured using an image processing software IPP 6.0. IOD
to area ratios were calculated as the sum of IOD divided by the
total positive area. These measurements were performed three times
and were analyzed by two independent observers who were blinded to
the treatment allocation.
Western blot analysis for protein
expression of VEGF and eNOS
Total protein was extracted from the left ventricle
using cell lysis buffer (Cell Signaling Technology, Inc., Danvers,
MA, USA) with protease inhibitor cocktail (BD Biosciences, San
Jose, CA, USA). Protein samples (20 g) were denatured in SDS sample
buffer [125 mmol/l Tris-HCl (pH 6.8), 50% glycerol, 2% SDS, 5%
mercaptoethanol and 0.01% bromophenol blue] were subjected to
SDS-PAGE and blotted onto Immobilon-FL transfer membranes (EMD
Millipore, Billerica, MA, USA). The blotted membranes were blocked
with 5% skimmed milk in Tris-buffered saline containing 0.1%
Tween-20 for 2 h and subsequently incubated with the primary
antibodies against VEGF, eNOS and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH; Quanshijin Biotechnology Inc., Beijing,
China; cat. no. HC301-02) overnight at 4°C. After three washes in
Tris-buffered saline containing 0.1% Tween-20, the membranes were
incubated with mouse anti-human IgM monoclonal secondary antibody
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA; cat.
no. MA5-14712) diluted in phosphate-buffered saline for 50 min at
room temperature. Immunoreactivity was quantified by using the
Odyssey dual color infrared fluorescence imaging system (LI-COR,
Lincoln, NE, USA) and normalized to GAPDH, which served as an
internal control.
The signals from immunoreactive bands were
visualized using an Amersham ECL system (GE Healthcare Life
Sciences, Chalfont, UK) and quantified using densitometric
analysis. The ratio for the protein examined was normalized against
GAPDH.
Statistical analysis
All data were expressed as means ± standard
deviation. Comparisons between two groups were performed using an
unpaired Student's t-test. Differences among groups were evaluated
by one-way ANOVA and P<0.05 was considered to indicate a
statistically significant difference.
Results
Procedure success rate
Twenty-six male Chinese mini swines were used in the
current study; however, four succumbed due to laryngeal edema as a
result of intubation failure, sensitivity to anesthesia, thrombosis
in the left main coronary artery due to balloon inflation and
recurrent ventricular tachycardia following reperfusion. Complete
occlusion of the LAD coronary artery by dilated balloon was
confirmed by CAG in the control (n=8) and PGE2 (n=8)
groups. CAG was performed again to ensure reperfusion following
AMI, which was presented as TIMI grade 3 blood flow. The procedure
success rate was 84.6%.
Hemodynamic effect of
PEG2
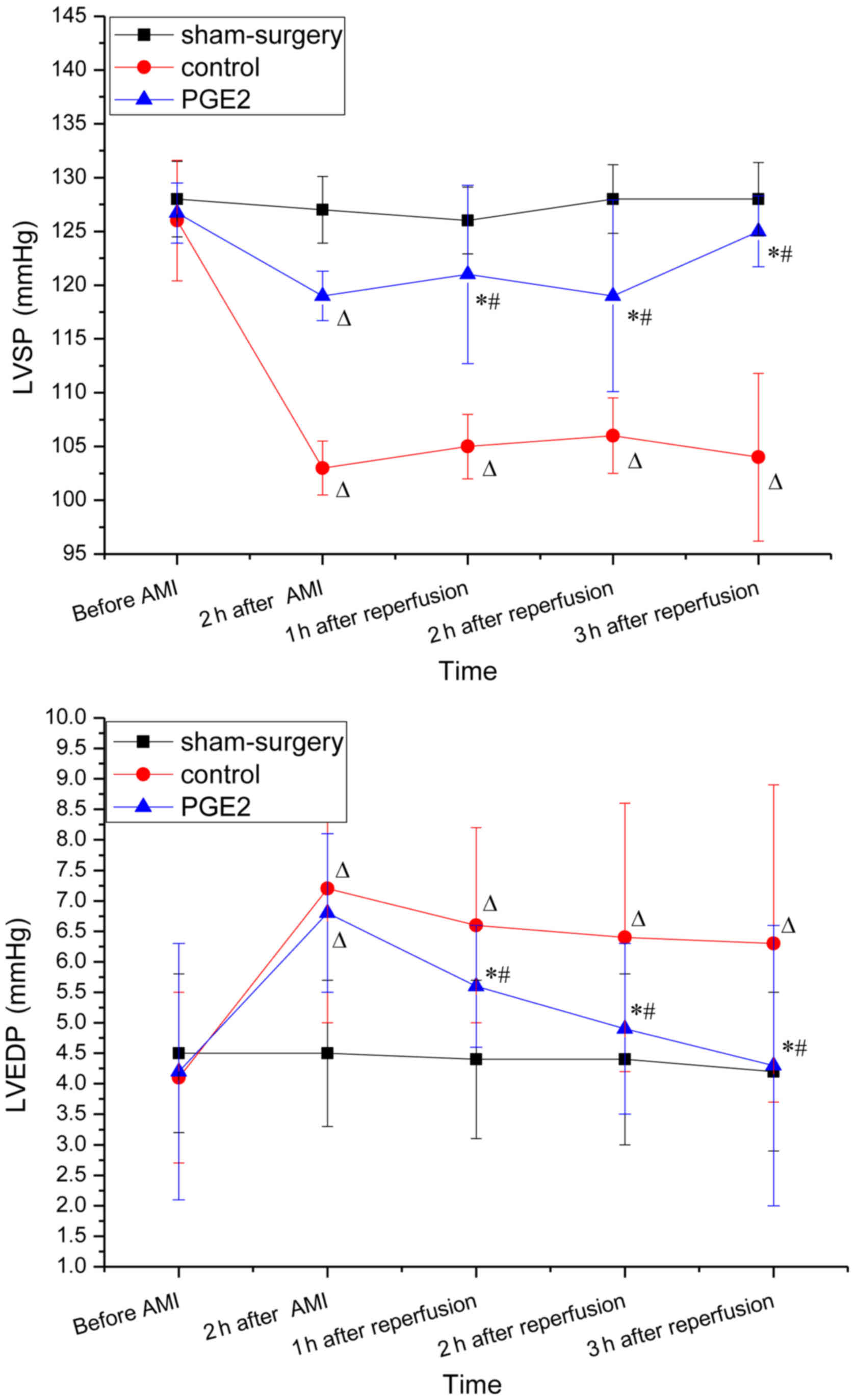
The baseline hemodynamic parameters (obtained prior
to surgery) were similar in each of the three groups (Fig. 1). Two hours after occlusion and 3 h
after reperfusion, LVEDP in the control group increased
significantly when compared with the baseline (prior to AMI;
P<0.05 for 2 and 3 h), whereas LVSP decreased significantly
(P<0.05 for for 2 and 3 h). No changes in LVSP and LVEDP were
observed 2 h after occlusion between the control and
PGE2 groups, while 1, 2 and 3 h after reperfusion,
increased LVSP and decreased LVEDP were observed in the
PGE2 group when compared with the control group
(P<0.05). For the PGE2 group, LVSP increased
significantly after reperfusion compared with 2 h after occlusion,
while the change of LVEDP exhibited the opposite trend (Fig. 1).
Effect of PEG2 on
pathological changes
Pathological changes were analyzed by
double-staining and H&E staining. For double-staining, the
normal myocardium, RA and NRA appeared dark blue, yellow and dark
red, respectively under natural light, whereas their color changed
to black, bright yellow, and deep red, respectively under UV light.
Infarct size was expressed as a percentage of the myocardium at
risk. Following reperfusion, no significant difference in RA/LVWA
was identified between the PGE2 and the control groups
(54.37±8.72 vs. 50.73±3.93%; P>0.05). NRA/LVWA of the
PGE2 group was found to be significantly lower than that
of the control group (14.83±5.51 vs. 25.39±3.49%; P<0.01). The
modified reperfusion area of LV [(RA-NRA)/LVWA] of the
PGE2 group increased significantly when compared with
the control group (39.54±7.55 vs. 25.34±2.68%; P<0.01) (Table I and Fig.
2).
 | Figure 2.Double staining of myocarium in the
different groups (A and B) Sham-surgery (C and D) control and (E
and F) PGE2 groups under natural (left column) and UV
(right column) light. Normal myocardium, RA and NRA appeared dark
blue, yellow, and dark red under natural light, respectively. Under
UV light, the colors changed to black, bright yellow, and deep red,
respectively. RAs of the PGE2 and control groups were
not significantly different, whereas NRA of the PGE2
group was markedly smaller than of the control group.
PGE2, prostaglandin E2; UV, ultraviolet; RA,
reperfusion area; NRA, non-reflow area. |
 | Table I.RA, NRA of the three groups following
double staining. |
Table I.
RA, NRA of the three groups following
double staining.
| Group | n | RA/LVWA (%) | NRA/RA (%) | NRA/LVWA (%) | (RA-NRA)/LVWA
(%) |
|---|
| Control | 8 | 50.73±3.93 | 49.84±5.04 | 25.39±3.49 | 25.34±2.68 |
| PGE2 | 8 | 54.37±8.72 |
27.13±8.71a |
14.83±5.51a |
39.54±7.55a |
For H&E staining, no necrosis or neutrophil
infiltration was observed in the myocardium of the normal area in
the three groups, whereas the myocardium in the RA and NRA of the
control group exhibited myocardial necrosis, local tissue swelling,
fibrosis, large quantities of neutrophil infiltration and a greater
leucocyte count when compared with the normal area of the control
group (P<0.01 and P<0.05, respectively). In RA and NRA of the
PGE2 group, myocardial cells with normal structure and
shape were apparent, and the myocardial cells were mildly swollen
or partially ruptured with fewer leucocytes compared with the RA
and NRA of the control group (P<0.01 and P<0.05,
respectively; Table II and Fig. 3).
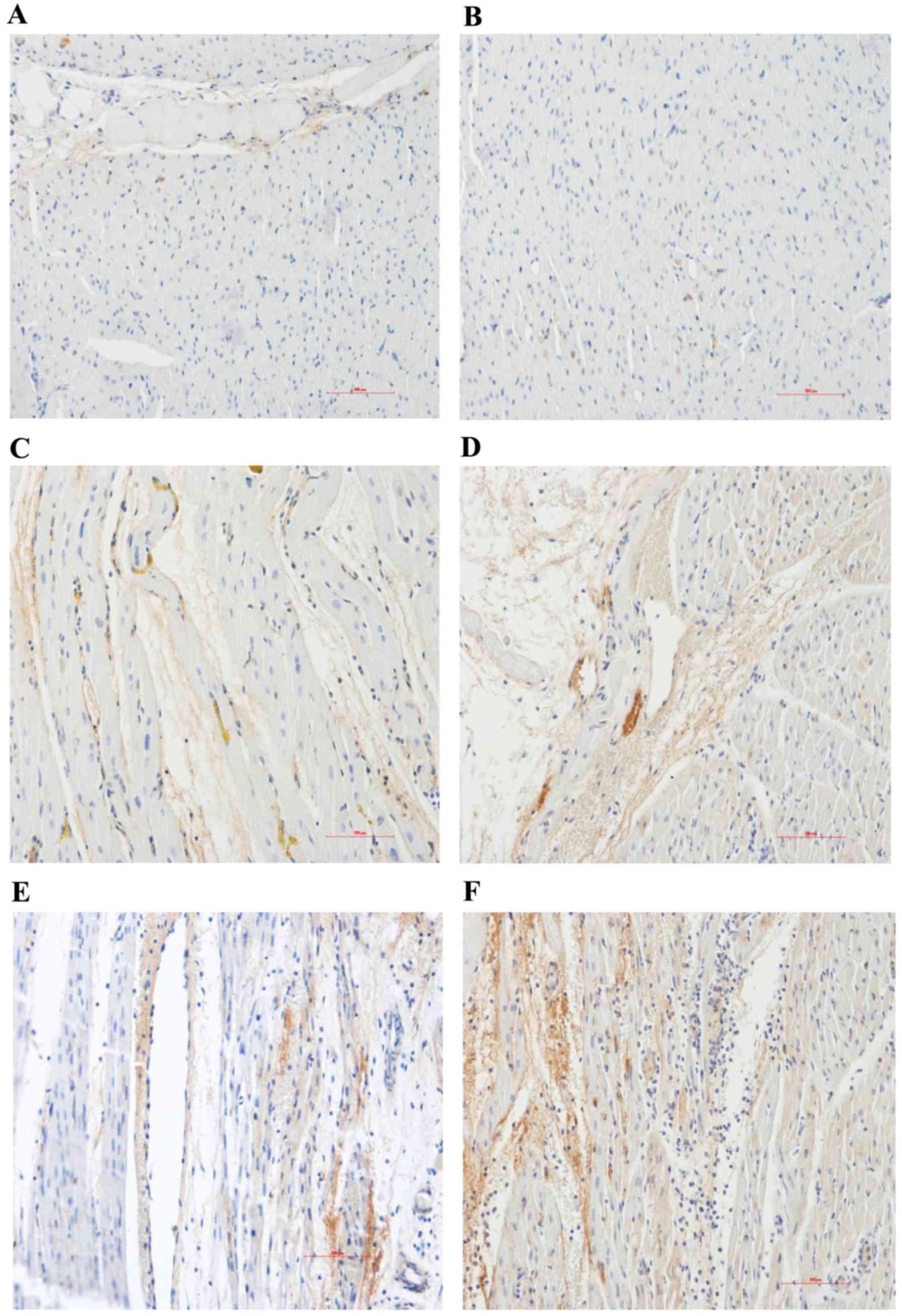
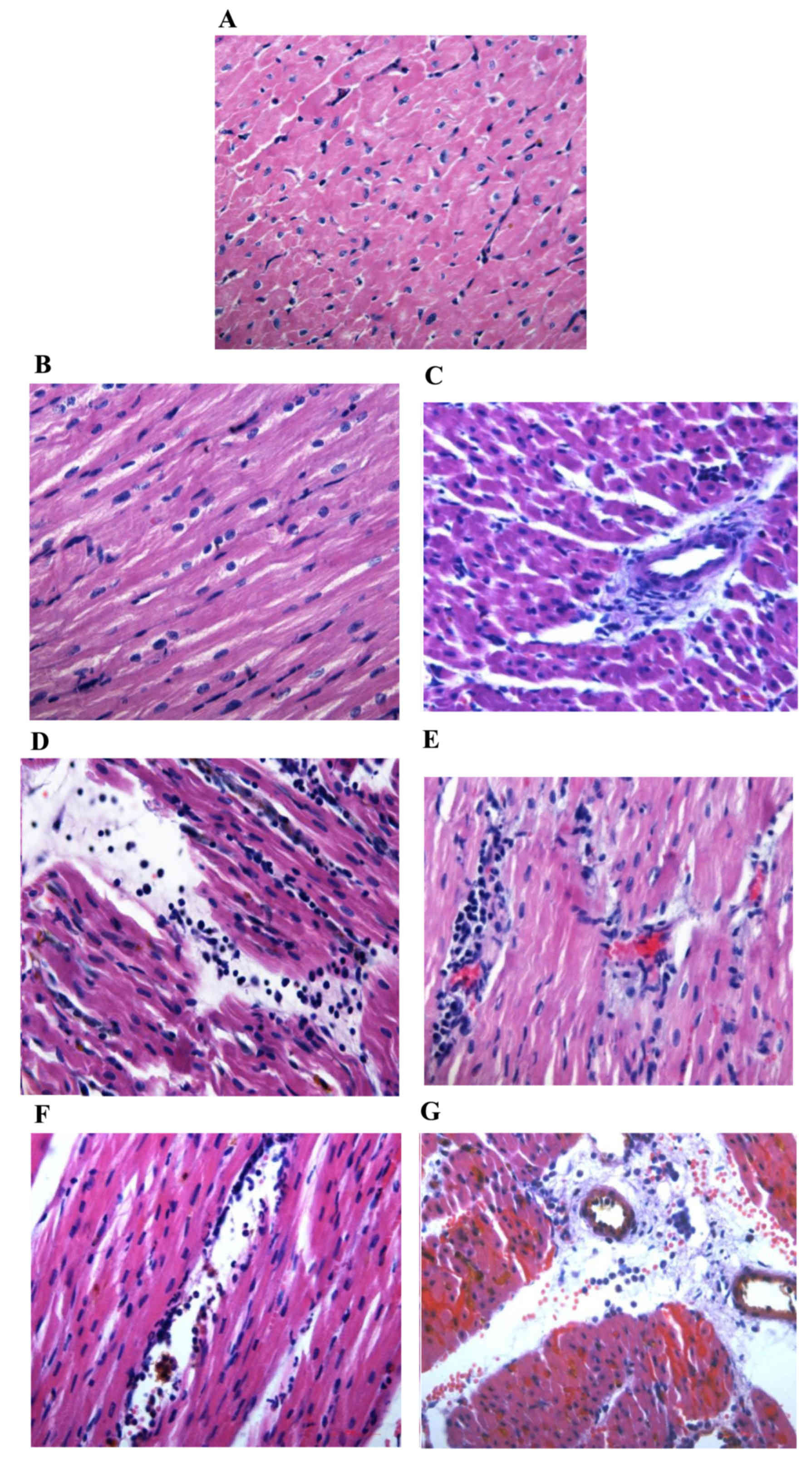 | Figure 3.Hematoxylin and eosin staining of
myocarium in the different groups (magnification, ×400). Normal
area of the three groups exhibited normal size cardiomyocytes, no
hemorrhaging or neutrophil granulocyte infiltration. (A)
Sham-surgery group, and normal areas of the (B) control and (C)
PGE2 groups. The RA and NRA in the control group
exhibited cardiomyocyte degeneration, hemorrhaging, edema, and
significant interstitial neutrophil granulocyte infiltration
compared with the normal area of the same group. (D) Reperfusion
area and (E) NRA of the control group. No significant cardiomyocyte
degeneration was identified in the RA and NRA in the
PGE2 group, however, slight edema between the myocardial
fibers, and mild neutrophil granulocyte infiltration was observed
compared with the RA and NRA of the control group. (F) Reperfusion
area and (G) NRA of the PGE2 group. The intravascular
yellow staining is Thioflavin-S stain. PGE2,
prostaglandin E2; RA, reperfusion area; NRA, no-reflow
area. |
 | Table II.Leucocyte count per single field of
view from the three groups following hematoxylin and eosin
staining). |
Table II.
Leucocyte count per single field of
view from the three groups following hematoxylin and eosin
staining).
| Group | Normal area (per
field of view) | RA (per field of
view) | NRA (per field of
view) |
|---|
| Sham-surgery | 3±1 | – | – |
| Control | 4±2 | 70±5a | 57±8b |
| PGE2 | 3±1 | 30±3c | 45±5d |
Effect of PEG2 on
expression levels of VEGF and eNOS
Content and distribution of VEGF and eNOS
proteins: Immunohistochemical analysis
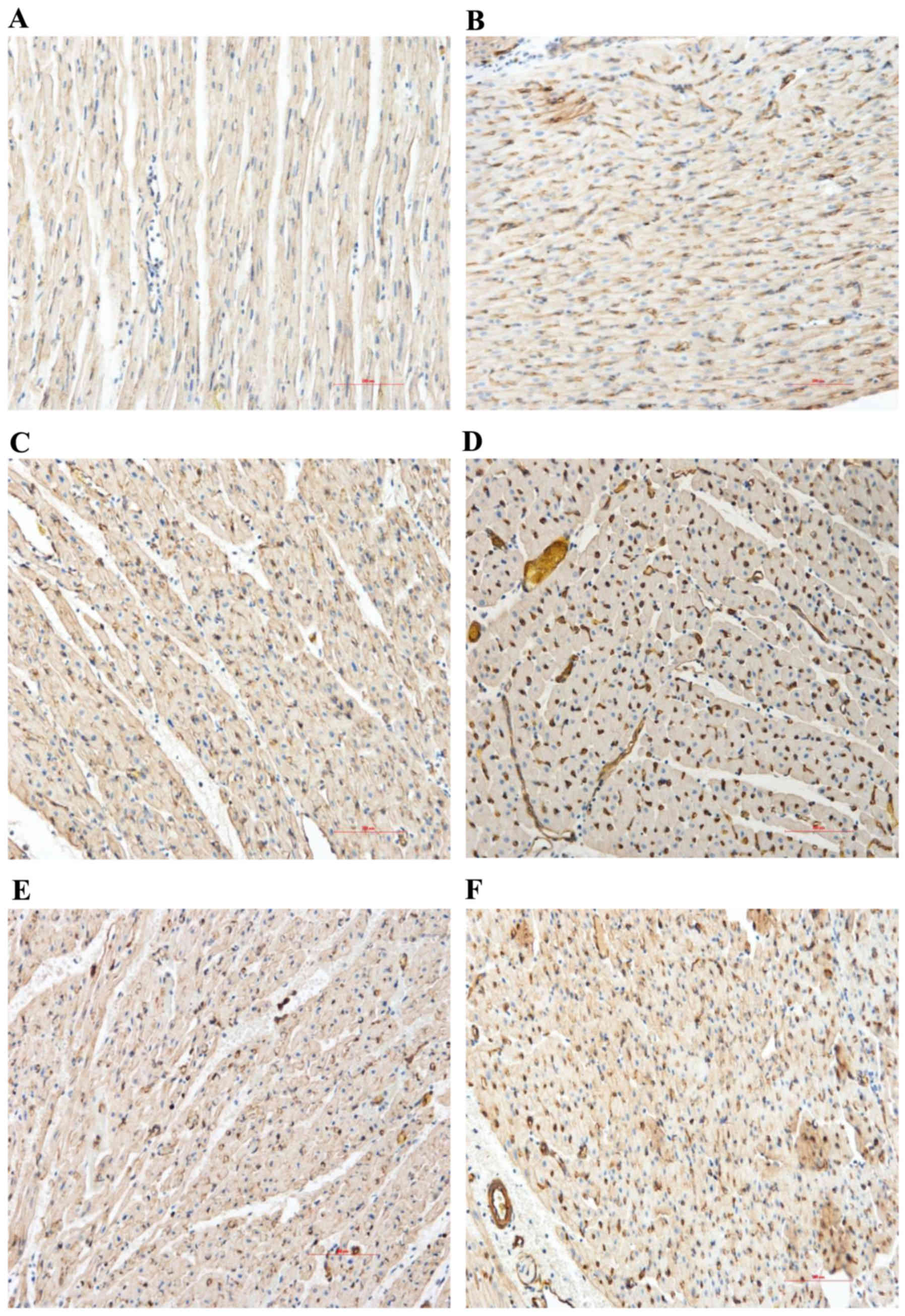
The expression levels of VEGF and eNOS in the
myocardial sections were evaluated by immunohistochemical analysis.
Compared with the normal area, the myocardial VEGF and eNOS protein
expression levels of the control group significantly increased in
the RA and NRA. These expression levels were significantly
upregulated in the PGE2 group compared with the control
group, particularly in the NRA (Table
III and Figs. 4 and 5; P<0.05).
 | Table III.Immunohistochemical analysis of the
content and distribution of eNOS and VEGF proteins (integrated
optical density/area ratio). |
Table III.
Immunohistochemical analysis of the
content and distribution of eNOS and VEGF proteins (integrated
optical density/area ratio).
| Protein | Group | Normal area | Reperfusion
area | No-reflow area |
|---|
| eNOS | Sham-surgery | 0.13±0.05 | – | – |
|
| Control | 0.12±0.01 |
0.34±0.08a |
0.41±0.04a |
|
|
PGE2 | 0.13±0.01 |
0.48±0.05a,b |
0.62±0.04a,b |
| VEGF | Sham-surgery | 0.13±0.03 | – | – |
|
| Control | 0.12±0.05 |
0.24±0.03a |
0.26±0.06a |
|
|
PGE2 | 0.13±0.02 |
0.46±0.03a,b |
0.66±0.05a,b |
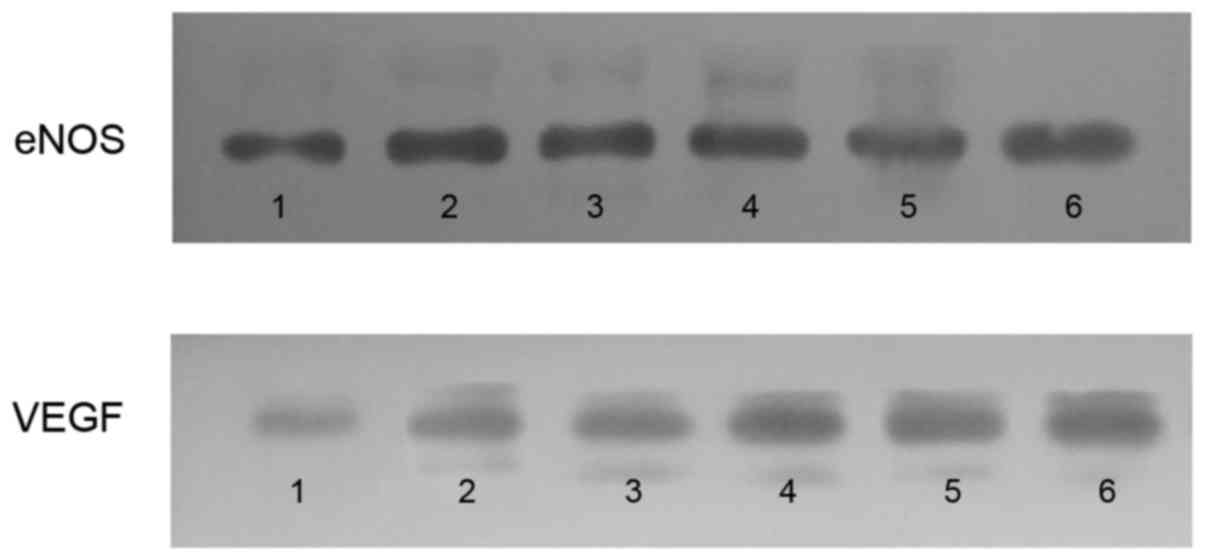
VEGF and eNOS protein expression levels: Western
blot analysis. Western blot analysis was performed to detect the
protein expression levels of VEGF and eNOS. The myocardial VEGF and
eNOS expression levels of the control group significantly increased
in the RA and NRA compared with the normal area. PGE2
significantly upregulated the expression of VEGF and eNOS in the RA
and NRA when compared with the control group (Fig. 6).
Discussion
AMI is currently the leading cause of morbidity and
mortality worldwide (1). Increasing
numbers of AMI patients receive early restoration of coronary flow
from the emergency medical services, such as percutaneous coronary
intervention and thrombolysis, which significantly improves the
prognosis (11). However, I/R injury
of the heart, which is caused by reperfusion itself, affects the
cardiac function and prognosis of patients. Micro-vascular spasms,
neutrophil infiltration and micro-thrombus, amongst others, have
been identified as mechanisms of I/R injury in a recent study
(12).
PGE2 is an endogenous lipid mediator,
which is important in the control of vascular tone and platelet
aggregation (13). A recent study has
shown that PGE2 is protective against MI (14). In the present porcine model of AMI,
significant reductions in myocardial injury were observed following
PGE2 therapy, which improved LVSP (by alleviating
myocardial ischemia and improving impaired ventricular systolic
function), and reduced LVEDP and NRA following reperfusion in AMI.
In the pathological analysis, myocardial cells were mildly swollen
with fewer leucocytes in the RA and NRA of the PGE2
group when compared with the control group. These findings provide
evidence of the potential benefits of PGE2 in reducing
NRA 2 h after AMI and 3 h after reperfusion. It is hypothesized
that this occurs by alleviating neutrophil infiltration and
myocardial cell edema.
To date, studies examining the role of VEGF in
ischemia over extended periods of time suggest promising efficacy
(15). VEGF has been shown to cause NO
release, resulting in vasodilation and increased blood flow
(16). Recent studies have revealed
that trans-coronary arterial delivery of VEGF to an isolated heart
immediately prior to ischemia improves myocardial functional
recovery (17). In the current study,
exogenous PGE2 increased the expression level of VEGF 2
h after AMI and 3 h after reperfusion when compared with the
control group, and simultaneously reduced the NRA of the
myocardium. VEGF may be an important intermediate factor of
PGE2 in attenuating the deleterious effects of I/R.
Protection of PGE2 against myocardial I/R injury may be
achieved by enhancement of VEGF formation. The underlying mechanism
of this protection may be via vasodilation, and subsequently the
improvement of collateral blood flow and alleviation of
micro-vascular spasms in the coronary artery.
eNOS is one of the key enzymes in the synthesis and
release of NO. Previous studies have shown that activation of eNOS
is important for mediating the cardioprotective effect of NO
against I/R injury (18). NO
generation elicits vasodilation, which exerts protective effects
during I/R by influencing platelet aggregation, leukocyte adhesion
and neutrophil infiltration (19). In
the present study, PGE2 enhanced protein production of
eNOS and VEGF following myocardial reperfusion. The myocardial
protection of PGE2 may be achieved by diminishing
platelet aggregation, leukocyte adhesion and neutrophil
infiltration, which are performed by VEGF and eNOS. This is
hypothesized to be an important cardio-protective mechanism of
PGE2. In conclusion, PGE2 induces myocardial
protection against myocardial I/R injury via enhancement of VEGF
and eNOS expression levels.
Acknowledgements
The present study was supported by the Beijing
Natural Science Foundation (grant no. 7152128).
References
|
1
|
Bulluck H, Yellon DM and Hausenloy DJ:
Reducing myocardial infarct size: Challenges and future
opportunities. Heart. 102:341–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heusch G, Musiolik J, Gedik N and
Skyschally A: Mitochondrial STAT3 activation and cardioprotection
by ischemic postconditioning in pigs with regional myocardial
ischemia/reperfusion. Circ Res. 109:1302–1308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei M, Xin P, Li S, Tao J, Li Y, Li J, Liu
M, Li J, Zhu W and Redington AN: Repeated remote ischemic
postconditioning protects against adverse left ventricular
remodeling and improves survival in a rat model of myocardial
infarction. Circ Res. 108:1220–1225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hishikari K, Suzuki J, Ogawa M, Isobe K,
Takahashi T, Onishi M, Takayama K and Isobe M: Pharmacological
activation of the prostaglandin E2 receptor EP4 improves cardiac
function after myocardial ischaemia/reperfusion injury. Cardiovasc
Res. 81:123–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pang L, Cai Y, Tang EH, Irwin MG, Ma H and
Xia Z: Prostaglandin E receptor subtype 4 signaling in the heart:
role in ischemia/reperfusion injury and cardiac hypertrophy. J
Diabetes Res. 2016:13243472016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Infanger M, Faramarzi S, Grosse J, Kurth
E, Ulbrich C, Bauer J, Wehland M, Kreutz R, Kossmehl P, Paul M, et
al: Expression of vascular endothelial growth factor and receptor
tyrosine kinases in cardiac ischemia/reperfusion injury. Cardiovasc
Pathol. 16:291–299. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao J, Xie H, Sun Y, Zhu J, Ying M, Qiao
S, Shao Q, Wu H and Wang C: Sevoflurane post-conditioning reduces
rat myocardial ischemia reperfusion injury through an increase in
NOS and a decrease in phopshorylated NHE1 levels. Int J Mol Med.
36:1529–1537. 2015.PubMed/NCBI
|
|
8
|
Siu KL, Lotz C, Ping P and Cai H: Netrin-1
abrogates ischemia/reperfusion-induced cardiac mitochondrial
dysfunction via nitric oxide-dependent attenuation of NOX4
activation and recoupling of NOS. J Mol Cell Cardiol. 78:174–185.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press (US); Washington, DC: 2011, PubMed/NCBI
|
|
10
|
Suzuki Y, Lyons JK, Yeung AC and Ikeno F:
In vivo porcine model of reperfused myocardial infarction: in situ
double staining to measure precise infarct area/area at risk.
Catheter Cardiovasc Interv. 71:100–107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fordyce CB, Gersh BJ, Stone GW and Granger
CB: Novel therapeutics in myocardial infarction: Targeting
microvascular dysfunction and reperfusion injury. Trends Pharmacol
Sci. 36:605–616. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang G and Chen L: An update of microsomal
prostaglandin E synthase-1 and PGE2 receptors in cardiovascular
health and diseases. Oxid Med Cell Longev. 2016:52490862016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kezeli T, Rukhadze T, Gongadze N, Sukoyan
G, Dolidze N, Chipashvili M and Mirziashvili M: Effect of
calcitonin gene-related peptide antagonist on the cardiovascular
events, mortality, and prostaglandin E2 production by
nitrate-induced tolerant rats with acute myocardial infarction.
EPMA J. 7:62016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Zhang X, Pang N, Xiao L, Li Y,
Chen N, Ren M, Deng X and Wu J: Glycation of vitronectin inhibits
VEGF-induced angiogenesis by uncoupling VEGF receptor-2-αvβ3
integrin cross-talk. Cell Death Dis. 6:e17962015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park YS, Jeon YJ, Kim HS, Chae KY, Oh SH,
Han IB, Kim HS, Kim WC, Kim OJ, Kim TG, et al: The role of VEGF and
KDR polymorphisms in moyamoya disease and collateral
revascularization. PLoS One. 7:e471582012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie J, Wang H, Wang Y, Ren F, Yi W, Zhao
K, Li Z, Zhao Q, Liu Z, Wu H, et al: Induction of angiogenesis by
controlled delivery of vascular endothelial growth factor using
nanoparticles. Cardiovasc Ther. 31:e12–e18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Simon JN, Duglan D, Casadei B and Carnicer
R: Nitric oxide synthase regulation of cardiac
excitation-contraction coupling in health and disease. J Mol Cell
Cardiol. 73:80–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li XD, Yang YJ, Geng YJ, Zhao JL, Zhang
HT, Cheng YT and Wu YL: Phosphorylation of endothelial NOS
contributes to simvastatin protection against myocardial no-reflow
and infarction in reperfused swine hearts: partially via the PKA
signaling pathway. Acta Pharmacol Sin. 33:879–887. 2012. View Article : Google Scholar : PubMed/NCBI
|