Introduction
Multiple sclerosis (MS) is a chronic, inflammatory,
demyelinating and neurodegenerative disease that predominantly
affects people aged between 20 and 40 years (1). The damage appears to be due to
inflammatory processes in which lymphocytes become activated at the
periphery, disrupt the intracellular matrix of the blood-brain
barrier and invade the central nervous system. Environmental
(including toxic substances, metabolic stress and possible pathogen
infection) and genetic factors may facilitate the movement of
auto-reactive T cells and demyelinating antibodies into the central
nervous system (1–4).
To date, MS has no cure and only partially efficient
therapeutic strategies are available [for example beta interferons,
glatiramer acetate (Copaxone), Tysabri, fingolimod and laquinimod].
None of these therapeutic agents reduce the disability experienced
by patients suffering from MS. Thus, novel therapeutic strategies
and agents are required for the treatment of MS (5). One such therapeutic strategy/agent could
be Endotherapia (GEMSP) (6,7). Endotherapia is a novel therapeutic
approach against chronic conditions (for example neurodegenerative
and auto-immune diseases) that accounts for genetic predisposition,
and environmental, bacterial and immunological factors. This
includes the identification of specific circulating antibodies in
the serum of patients and the use of therapeutic tools, such as
small compounds linked to the carrier poly-L-lysine (PLL) and the
physiological actions of such compounds being known (e.g., GEMSP)
(6,7).
PLL is edible, water-soluble and non-toxic for humans (8). Depending on the circulating antibodies
directed against the antigens found during the course of MS, the
chemical composition and doses of the therapeutic agent (e.g.,
GEMSP) can be established for each patient (9).
GEMSP was originally conceived for the treatment of
the secondary progressive form of MS and it is a combination of
amino acids, fatty acids, free radical scavengers and antioxidants
linked to PLL (6,7,9). The
beneficial effects of GEMSP have been demonstrated in acute and
chronic experimental autoimmune encephalomyelitis (EAE) models
(10,11). Subsequently, an open clinical trial
stated that following treatment with GEMSP for 6 months, the
Expanded Disability Status Scale (EDSS) improved in 18% of patients
and remained unchanged in 55% (1). Due
to the promising results found in preclinical and clinical studies,
another study with a larger number of MS patients (n=102) was
conducted (6) and again, treatment
with GEMSP was beneficial for 72% of patients. The present study
follows up the evolution of the disease in a larger population of
MS patients (n=193) that received GEMSP treatment.
Materials and methods
Population description
The present study was performed in 193 volunteer
patients [152 women (78.8%) and 41 men (21.2%)]. The patients
provided written informed consent and treatment subsequently
commenced. The study was national, multicentre, non-blinded and
non-randomized, and the experimental unit was the patient. The EDSS
score (9) was evaluated, and treatment
with GEMSP (M) and worldwide reference (R) scores were established
and compared. The M score was evaluated in each patient following
clinical evaluation, whereas the R score was the estimated
theoretical international score evolution (0.25
points/year/patient). The period of treatment with GEMSP was from
150 to 7,709 days (21.1 years) and the median was 1,289 days (3.5
years). The following data for each patient affected by MS and
treated with GEMSP were collected: Age, gender, date of diagnosis,
start/termination of GEMSP treatment and the MS Assessment
Questionnaire follow-up for each patient over time. In addition,
the evolution of the EDSS score was tracked during the study. A
plot of the evolution of the EDSS score (R) over time was
conducted. The slope (P=0.25) corresponds to the mean R of the
speed of EDSS evolution (0.25 point/year), allowing the evolution
of individual scores to be compared with the worldwide mean trend
of progression. Finally, the duration of the GEMSP treatment, the
number of medical consultations and the interval between these
consultations were also taken into consideration.
Score
The individual EDSS introduced fluctuations along
time. In order to assess the total effect of treatment with GEMSP
for each patient, the individual mean of EDSS evolution was
determined over time as follows: i) When the individual mean rate
of EDSS evolution with GEMSP was greater or equal to the mean speed
of the worldwide EDSS reference, this indicated disease progression
(a worsening of the state of the patient); ii) when the individual
mean rate of EDSS evolution with GEMSP was recorded to be between
the mean speed of the worldwide EDSS reference and 0, this
indicated a decrease in the progression of the disease; iii) when
the individual mean rate of EDSS evolution with GEMSP was equal to
0, this indicated a stabilization of the disease; and iv) when the
individual mean rate of EDSS evolution with GEMSP was below 0, the
evolution of the disease was reversed (an improvement of the
disease and a recovery from lesions occurred).
Statistical analysis
In order to overcome the high variability of the MS
cohort included in the current study, a method of adapted valuation
was applied as previously described (6). Thus, the Mann-Whitney U test was used to
compare the current population with the population of reference
[where the score evolution of each patient was compared with the
worldwide reference score (R)], allowing the comparison of patients
with each other and to assess the efficacy of treatment with GEMSP.
P<0.05 was considered to indicate a statistically significant
difference and analyses were performed using the version 9 software
(SAS Institute Inc., Cary, NC, USA); the conditions of the test
application were verified in order to confirm that the statistics
were correctly conducted. A comprehensive study of the EDSS score
and the EDSS evolution was conducted. In the descriptive analysis,
the following parameters were investigated: Strength, percentage,
distribution, minimum, 1st quartile, median,
3rd quartile, maximum (Max), average, standard deviation
and 95% confidence interval. In the comparative analysis, a t-test
between the M group and R group final scores was performed.
Equality of the variance was verified using the Folded F test. In
the descriptive analysis the following parameters were evaluated:
Strength, percentage and distribution. Regarding the analysis of
the evolution of the data by a linear regression model, the
following parameters were analyzed for the M and R scores:
Coefficient of correlation, R2 and the associated
P-value, intercept and slope (ax + b). For comparison of the
success/failure rates, the χ2 test or Fisher's exact
test were applied according to the theoretical strength obtained by
unilateral assumption (% success > % failure).
Furthermore, a qualitative study of the EDSS score
(global study) was conducted. In the descriptive analysis the
following parameters were evaluated: Strength, percentage and
distribution. For comparison of the success/failures rates: The
χ2 test or Fisher's exact test were performed according
to the theoretical strength obtained by unilateral assumption (%
success > % failure).
Dose and synthesis of GEMSP
GEMSP (15 mg per day) was administered via the
sublingual route. GEMSP was synthesized according to patent numbers
792167 (EU) and 6114388 (USA) (6,9). GEMSP is a
‘tailor-made’ mixture of functional polypeptides: Fatty acids
linked to PLL (e.g., thioctic acid, oleic acid and linoleic acid),
antioxidants linked to PLL (e.g., ascorbic acid), free radical
scavengers and amino acids linked to PLL (e.g., taurine, cysteine
and methionine).
Results
General considerations
Patients included in the current study were born
between the 1st September 1937 and the 1st
June 1994 (59% of patients were born between 1951 and 1970). The
diagnosis of MS was performed between the 1st October
1962 and the 28th April 2011 (44% of cases were
diagnosed between 1996 and 2005). The diagnosis of the disease was
performed when patients were aged between 12 and 62 years (median,
36 years). The beginning of the treatment with GEMSP was between
the 1st September 1994 and the 1st August
2011 (median, 1st November 2006). The majority of
patients began the treatment after the year 2005. The delay between
the date of diagnosis and the beginning of the treatment with GEMSP
was between 0 and 500 months (median, 106 months). Upon inclusion
in the study, global EDSS scores were between 0 and 8.5 (median,
5); the mean score was 4.4. The median of the delay between the
date of the EDSS score evaluation and the date of the treatment
with GEMSP was 31 days (mean delay, 301 days). The follow-up period
was between 150 and 7,709 days [21.1 years; median, 1,289 days (3.5
years)]. The number of visits was between 1 and 29 (median, 7).
Finally, the median interval between visits was 121 days.
EDSS global study
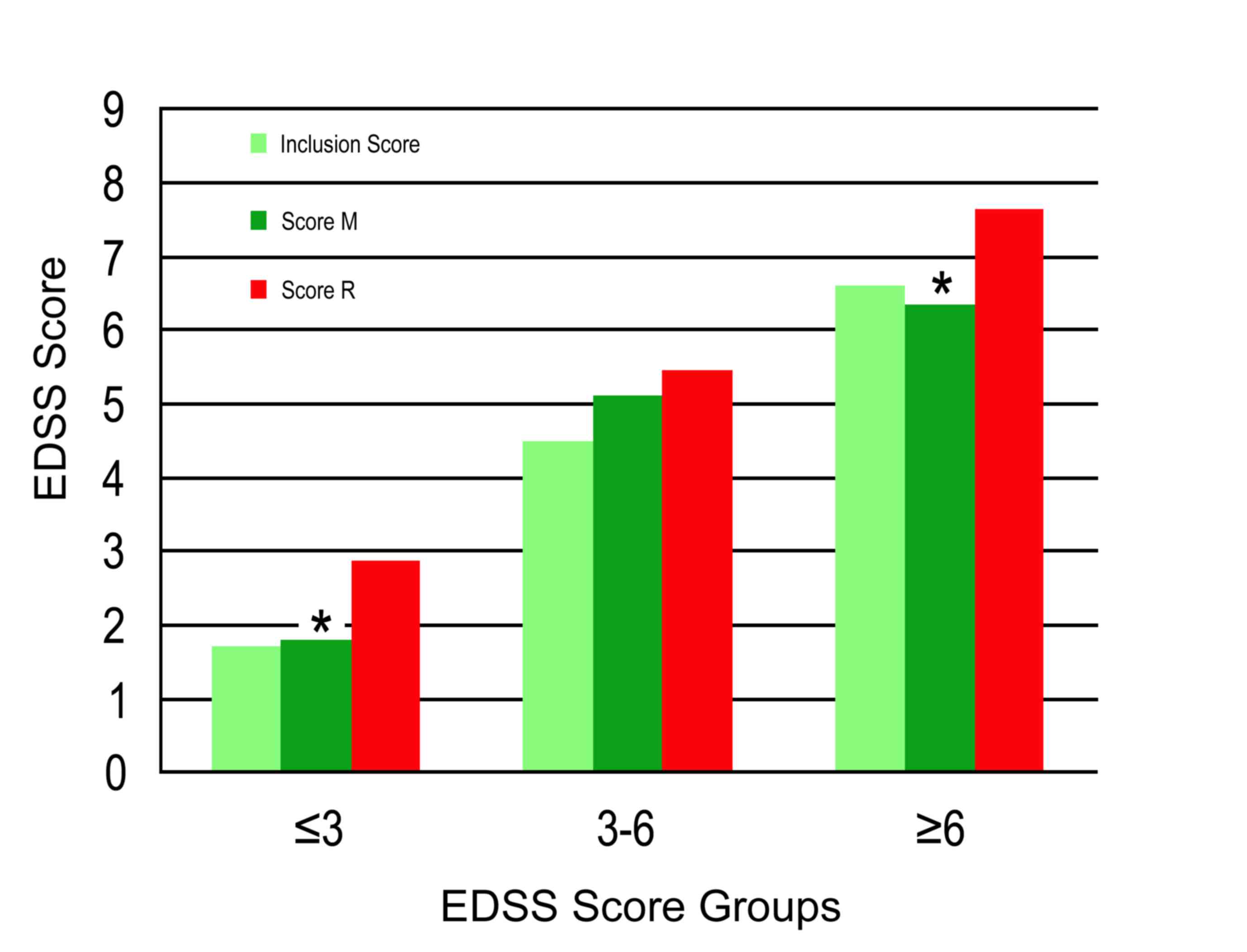
A statistically significant difference (P<0.0001)
was observed between the EDSS mean scores of the M and R groups
(Fig. 1 and Table I). The EDSS mean score of the M group
was 4.44 and that of the R group was 5.43. Regarding the EDSS mean
score upon inclusion in the study, an increase of 1.8% was observed
in the M group, whereas in the R group the increase was 24.5%. In
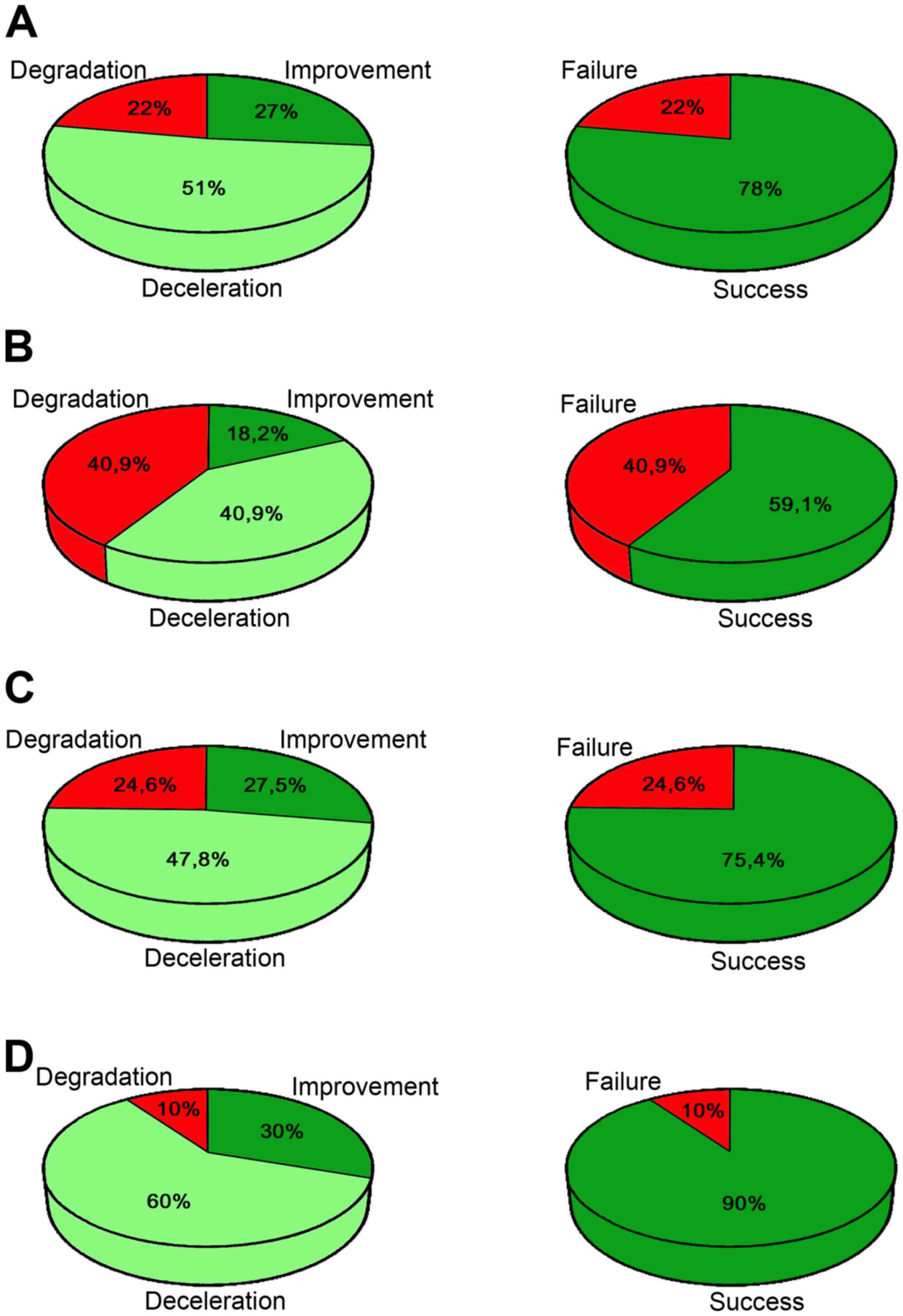
193 patients treated with GEMSP, 22% showed a worsening of their
state (degradation); 51% showed a decrease in the progression of
the disease (deceleration) and 27% showed a reversal of the disease
evolution, which is considered to be an improvement in their status
(Fig. 2A; Table II). Thus, 78% of patients showed an
improvement or deceleration of the disease (Fig. 2A). The difference between the
percentages of success and failure was identified to be
significantly different (P≤0.0001).
 | Table I.Expanded Disability Status Scale
global study (n=193). |
Table I.
Expanded Disability Status Scale
global study (n=193).
| Parameter | Score at
inclusion | Final M score | Final R score |
|---|
| Minimum | 0.00 | 0.00 | 0.25 |
| Quartile, 25% | 2.50 | 2.00 | 3.50 |
| Median | 5.00 | 5.50 | 5.82 |
| Quartile, 75% | 6.50 | 6.50 | 7.23 |
| Maximum | 8.50 | 8.50 | 10.00 |
| Mean ± SD | 4.36±2.30 |
4.44±2.48a | 5.43±2.43 |
| 95% CI | 4.03–4.68 | 4.08, 4.79 | 5.08, 5.77 |
 | Table II.Success/failure global evolution
(n=193). |
Table II.
Success/failure global evolution
(n=193).
| Evolution | Distribution, n
(%) | Success, n (%) |
|---|
| Improvement | 51 (26.4) | 150/193
(77.7)a |
| Deceleration | 99 (51.3) |
|
| Degradation | 43 (22.3) |
|
EDSS score depending on the score upon
inclusion in the study
A statistically significant difference was
identified between the EDSS mean scores of the M and R groups for
the EDSS score ≤3 (P=0.0001) and ≥6 (P<0.0001), although this
difference was not significant for the EDSS score 3–6 (Fig. 1 and Table
III; P=0.20). According to the final EDSS scores and in
comparison with the EDSS inclusion scores, the percentages of
improvement for the M group were as follows: 62% (score ≤3), 7.8%
(score 3–6) and 19.6% (score ≥6). Regarding the qualitative
evolution of the EDSS scores, a statistically significant
difference was identified when comparing the M and R group mean
evolutions (for the scores of ≤3 and ≥6), but not in the case of
score 3–6. The percentages of improvement for the M group were as
follows: 49.3% (score ≤3), 79.1% (score 3–6) and 19.3% (score
≥6).
 | Table III.Expanded Disability Status Scale
score depending on the score at inclusion. |
Table III.
Expanded Disability Status Scale
score depending on the score at inclusion.
| A, Score ≤3 |
|---|
|
|---|
| Parameter | Inclusion
score | Final M score | Final R score |
|---|
| Patients, n | 69 | 69 | 69 |
| Minimum | 0 | 0 | 0.25 |
| Quartile, 25% | 1.00 | 1.00 | 1.75 |
| Median | 2.00 | 1.50 | 2.96 |
| Quartile, 75% | 3.00 | 2.50 | 3.81 |
| Maximum | 3.00 | 7.00 | 7.53 |
| Mean ± SD | 1.71±1.13 |
1.80±1.47a | 2.86±1.63 |
| 95% CI | 1.44, 1.98 | 1.45, 2.16 | 2.47, 3.25 |
|
| B, Score 3–6 |
|
| Parameter | Inclusion
score | Final M score | Final R score |
|
| Patients, n | 44 | 44 | 44 |
| Minimum | 3.50 | 1.00 | 3.66 |
| Quartile, 25% | 4.00 | 4.50 | 4.84 |
| Median | 4.50 | 5.50 | 5.60 |
| Quartile, 75% | 5.00 | 6.00 | 5.90 |
| Maximum | 5.50 | 7.00 | 8.52 |
| Mean ± SD | 4.48±0.68 |
5.11±1.49b | 5.46±0.92 |
| 95% CI | 4.27, 4.69 | 4.66, 5.57 | 5.18, 5.74 |
|
| C, Score ≥6 |
|
| Parameter | Inclusion
score | Final M score | Final R score |
|
| Patients, n | 80 | 80 | 80 |
| Minimum | 6.00 | 1.00 | 6.24 |
| Quartile, 25% | 6.00 | 6.00 | 6.83 |
| Median | 6.50 | 6.50 | 7.50 |
| Quartile, 75% | 7.00 | 7.00 | 8.20 |
| Maximum | 8.50 | 8.50 | 10.0 |
| Mean ± SD | 6.58±0.59 | 6.33±1.38 | 7.62±0.99 |
| 95% CI | 6.44, 6.71 | 6.02, 6.64 | 7.40, 7.84 |
| P-value |
| <0.0001 |
Evolution depending on the score upon
inclusion
A statistically significant difference was observed
between the percentages of success and failure for the EDSS scores
3–6 (P<0.0001) and ≥6 (P<0.0001). However, the EDSS score ≤3
was at the limit of significance (P=0.088; Table IV). In the case of those patients with
an EDSS score ≤3, the following results were observed (Fig. 2B and Table
IV): Improvement (18.2%), deceleration (40.9%) and degradation
(40.9%); that is, success (59.1%) and failure (40.9%). For patients
with an EDSS score 3–6, the following results were observed
(Fig. 2C and Table IV): Improvement (27.5%), deceleration
(47.8%) and degradation (24.6%); that is, success (75.4%) and
failure (24.6%). For patients with an EDSS score ≥6, the following
results were observed (Fig. 2D and
Table IV): Improvement (30%),
deceleration (60%) and degradation (10%); that is, success (90%)
and failure (10%). Thus, the three groups exhibited a percentage of
success between 59.1 and 90.0% (Fig.
2B-D).
 | Table IV.Evolution depending on the score at
inclusion. |
Table IV.
Evolution depending on the score at
inclusion.
| Score | Evolution | Distribution, n
(%) | Success, n (%) |
|---|
| ≤3 | Improvement | 8
(18.2) | 26/44 (59.1) |
|
| Deceleration | 18 (40.9) |
|
|
| Degradation | 18 (40.9) |
|
| 3–6 | Improvement | 19 (27.5) | 52/69
(75.4)a |
|
| Deceleration | 33 (47.8) |
|
|
| Degradation | 17 (24.6) |
|
| ≥6 | Improvement | 24 (30.0) | 72/80
(90.0)a |
|
| Deceleration | 48 (60.0) |
|
|
| Degradation | 8
(10.0) |
|
Discussion
Approved MS treatments are focused towards the
relapsing-remitting phases, but not the progressive phases.
Inflammation is important during the relapsing-remitting phase of
MS, but not during the secondary progressive phase (6). The majority of the therapeutic agents
currently tested for the treatment of MS act against inflammatory
processes. However, existing data demonstrate that the inflammatory
processes are not exclusive in the pathogenesis of MS, as there are
other mechanisms involved (e.g., oxidative stress, axonal injury
and neuronal loss) (5,12). A global treatment for MS, including the
progressive phases, is required (5).
Endotherapia takes into consideration environmental, immunological
and bacterial factors, and is proposed as a treatment for chronic
incurable diseases exhibiting a multifactorial etiology (e.g., MS
and amyotrophic lateral sclerosis). Therefore, GEMSP was developed
as an anti-inflammatory therapeutic strategy (the therapeutic agent
contains fatty acids), as well as for myelin/neuron protection
(1,12).
GEMSP contains compounds (e.g., vitamins), which are effective
against nitrosative and oxidative stress (1).
The linkage of heterogeneous molecules (e.g., amino
acids, vitamins and fatty acids) to PLL offers various advantages
as follows (6): Molecule stablility;
prevention of metabolic degradation of the linked molecules;
improvement of the kinetics, and increased half-life and
permeability of membranes. Furthermore, in GEMSP, the different
molecules are linked to PLL by reduced glutaraldehyde linkages
resulting in particularly flexible bonds (free conformation in
space improves interactions and facilitates access to the lesion
site) (13). The beneficial effects of
GEMSP only occur when the molecules are linked to PLL, since free
constituents (not linked to PLL) are less active against MS
symptoms and it seems that these free molecules either degrade or
are rapidly incorporated into the metabolism (1).
In preclinical studies (acute model of EAE)
(10), it has been reported that GEMSP
(0.75 mg/day) inhibited brain leukocyte infiltration (10). Later, in a chronic model of EAE, it was
demonstrated that GEMSP (7.5 mg/day) abolished EAE episodes, the
clinical score and brain leukocyte infiltration. In addition, one
compound of the GEMSP (methionine) was located in the motoneurons
of the spinal cord (11). This finding
indicates that GEMSP may be involved in the neutralization of free
radicals, exerting a neuroprotective action by inhibiting apoptotic
mechanisms (11). Furthermore, in a
chronic EAE model, it was demonstrated that GEMSP protected and
enhanced formation of the myelin sheath (14). In a phase IIa trial, 22 patients were
treated with GEMSP (0.75 mg/day, sublingual route) for six months.
In 55% of patients, the EDSS was stable and in 18% the EDSS
decreased (instead of a normal progression of 0.25 points on the
mean EDSS scale) (1). Furthermore, 28%
of patients did not react to the GEMSP treatment. No side effects
(biological, hematological or hepatic) were observed and hence
GEMSP was demonstrated to be safe. In addition, no toxicity was
observed in experimental animals (10 mg/kg, single intravenous
injection) or in humans (11). Another
study was conducted where the MS patients (n=102) received 15
mg/day GEMSP (sublingual route) for ranging from 3 months to 15
years and the results were as follows: 28% of patients showed a
worsening of their state; 20% showed a decrease in the progression
of the disease; 35% showed an improvement of their state; and 17%
showed stabilization. That is, 72% of patients showed a positive
evolution of the disease (6). The
present study (n=193) validates the favorable previous clinical
study results, with 22 and 102 patients, that were previously
published (1,6). However, it is important to remark that
the positive effect of treatment with GEMSP differs depending on
the initial EDSS score of the patients. Thus, with a higher score,
a greater positive effect of the treatment is observed: 59.1% of
patients with initial EDSS scores ≤3 showed a positive evolution;
when the initial EDSS scores were between 3–6, the positive effect
was found in 75.3% of patients, increasing to 90.0% when the
initial EDSS scores were ≥6. In the previous study performed in 102
patients (6), the percentages for the
three groups (scores ≤3, 3–6 and ≥6) were as follows: 74, 62 and
73%, respectively. In order to compare the results found in the
current study (n=193) with those published in the previous work
(n=102) (6), it is important to remark
that the MS patients of the latter study are included in the
present work with an extended follow-up period. Regarding the group
in which the initial EDSS scores at inclusion were ≤3 it was found
that, in the present study, the absence of efficacy of GEMSP
treatment increased from 26 to 40.9% and that the improvement of
the treatment decreased from 33 to 18.2% when compared with the
previous study (n=102). The deceleration in the progression of the
disability was similar in the two studies (40.9 and 41%). Regarding
the group in which the initial EDSS scores at the inclusion were
3–6, the absence of effectiveness of GEMSP treatment decreased from
38 to 24.6%; deceleration in disability progression increased from
19 to 47.8% and the improvement decreased from 43 to 27.5%.
Regarding the group in which the initial EDSS scores at inclusion
were ≥6, the absence of efficacy of GEMSP treatment decreased from
26 to 10%; deceleration in disability progression increased from 40
to 60% and the improvement was similar in the two studies (33 and
30%).
Compared with other therapeutic agents, treatment
with beta-interferon was demonstrated to be less efficient than
treatment with GEMSP in the present study, as the former
decelerates the progression of disability, whereas GEMSP improves
the disability score. This finding is consistent with the results
published in a previous study (n=102) (6). Approved therapeutic agents for the
treatment of MS exert marked side effects. Alemtuzumab is more
effective than GEMSP, but the undesirable effects of alemtuzumab
are important (e.g., infection, immune thrombocytopenic purpura and
thyroid disorder) as are those that are caused by the use of
beta-interferon (e.g., elevated liver enzymes, leukopenia,
influenza-like syndrome, formation of neutralizing antibodies).
However, no side effects were experienced when GEMSP was
administered (1,6,9). GEMSP
contains compounds that are not foreign, but are endogenous; these
compounds are known by the organism and, for this reason, the
clinical trials performed to date have shown a good safety profile
for GEMSP (neither adverse effects nor toxicity have been
reported). Furthermore, other therapeutic agents, such as linomide,
mitoxantrone and natalizumab are associated with considerable side
effects (1). Conventional therapeutic
strategies (e.g., β-interferon and glatiramer acetate) are based on
immunomodulatory drugs modifying the number of immunological cells
(1,15),
whereas GEMSP counteracts the inflammatory mechanisms, as well as
other pathogenic mechanisms (e.g., oxidative stress, demyelination
and neurotoxicity) (12).
In the pathogenesis of MS, oxidative stress is
important (16). In GEMSP, the
presence of vitamins and certain amino acids and their derivatives
(alpha-tocopherol-succinate, ascorbic acid, taurine and
5-methoxy-tryptamine) are important neuroprotective components and
radical scavengers, exerting a crucial role against nitrosative and
oxidative stress (1,11). Cysteine and methionine also act as
antioxidants and scavengers contributing to reduced apoptosis and
neuronal death induced by reactive oxygen species (17). Alpha-tocopherol and neurotransmitters,
such as histamine and 5-methoxy-tryptamine and amino acids, such as
histidine act as neuroprotective agents (1,18).
Polyunsaturated fatty acids exert a neuroprotective effect and act
as free radical scavengers preventing oxidation of cell membrane
unsaturated fatty acids (19,20). GEMSP protects and enhances the
formation of myelin sheath (11,14).
Deficiencies in essential fatty acids impair myelin synthesis
(21) and hence it is possible that
the fatty acids present in GEMSP are involved in the process of
remyelinization, as observed in a chronic EAE model (14). Additionally, it is known that the oleic
acid, present in GEMSP, acts as a neurotrophic factor in neurons
(22) and that fatty acids exert
anti-inflammatory activity (7).
Previous studies have proposed the involvement of
bacteria in the pathogenesis of MS (23–25).
Commensal bacteria usually protect the organism, however, may cause
autoimmune processes when bacteria pass through the mucosal
epithelium to the submucosal tissues. Certain bacterial components
act as super-antigens, and induce the proliferation of autoreactive
lymphocyte clones and the raising of autoantibodies. In response to
lipopolysaccharides (LPS), immune cells produce reactive oxygen
species and pro-inflammatory mediators, which are involved in
oxidative stress, the demyelination processes and axonal damage
(26). Systemic injection of LPS leads
to the invasion of the brain by granulocytes and a breakdown of the
blood-brain barrier (27). This
breakdown may allow the toxins produced by the bacteria to enter
the central nervous system. Inside the central nervous system, LPS
may exert direct neurotoxic action on microglial cells and LPS is
associated with extensive oligodendrocyte death due to LPS-induced
neurotoxicity (28). LPS may bind to
the blood-brain barrier through their lipid A (gram-negative
bacteria) and cross the barrier. Lipid A provides the hydrophobic
anchor that secures the molecule within the membrane, while the
polysaccharide component interacts with the external environment,
including the defences of the host species (29). Thus, an important goal would be to
inhibit this induction process by preventing the interaction of
bacteria with target cells. In this sense, an amide linkage to
fatty acid is preferable to an unstable ester linkage to achieve
the same fatty acid presentation as the lipid A component of the
LPS of gram-negative bacteria. The similar structure of lipid A and
fatty acid, linked to PLL, leads to the neutralization of LPS
through the onset of lipid bilayer formation. This approach may
prevent LPS from linking to target cells and may allow the
inhibition of chronicity factors (6).
In conclusion, preclinical and clinical data suggest
that GEMSP presents a novel therapeutic agent/approach for the
treatment of MS. GEMSP targets the different aspects of MS rather
than the inflammatory aspect alone. GEMSP decreases inflammatory
mechanisms, controls oxidative stress, counteracts demyelination,
acts as a neuroprotector and fights chronic factors. The present
data, using a larger population of MS patients than previous
clinical studies (1,6), confirms the beneficial effects of GEMSP
that were previously reported.
Acknowledgements
The present study was supported by the Institut pour
le Développement de la Recherche en Pathologie Humaine et
Thérapeutique (Talence, France). The authors would like to thank
Mr. B. Combes for the statistical analysis (Stalphamis; Le Bourg,
France).
References
|
1
|
Mangas A, Coveñas R, Bodet D, Duleu S and
Geffard M: A new drug candidate (GEMSP) for multiple sclerosis.
Curr Med Chem. 16:3203–3214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sawcer S, Hellenthal G, Pirinen M, Spencer
CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt
SE, et al: International Multiple Sclerosis Genetics Consortium;
Wellcome Trust Case Control Consortium 2: Genetic risk and a
primary role for cell-mediated immune mechanisms in multiple
sclerosis. Nature. 476:214–219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kutzelnigg A, Lucchinetti CF, Stadelmann
C, Brück W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE and
Lassmann H: Cortical demyelination and diffuse white matter injury
in multiple sclerosis. Brain. 128:2705–2712. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Villar LM, Sádaba MC, Roldán E, Masjuan J,
González-Porqué P, Villarrubia N, Espiño M, García-Trujillo JA,
Bootello A and Alvarez-Cermeño JC: Intrathecal synthesis of
oligoclonal IgM against myelin lipids predicts an aggressive
disease course in MS. J Clin Invest. 115:187–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mangas A, Coveñas R and Geffard M: New
drug therapies for multiple sclerosis. Curr Opin Neurol.
23:287–292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geffard M, de Bisschop L, Duleu S, Pouns
O, Ferran G, Bassede A, Hassaine N, Autran JL, Bodet D, Mangas A
and Coveñas R: Endotherapia. Antiinflamm Antiallergy Agents Med
Chem. 9:197–211. 2010. View Article : Google Scholar
|
|
7
|
Geffard M, Duleu S, Bessede A, Vigier V,
Bodet D, Mangas A and Coveñas R: GEMSP: A new therapeutic approach
to multiple sclerosis. Cent Nerv Syst Agents Med Chem. 12:173–181.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shih IL, Van YT and Shen MH: Biomedical
applications of chemically and microbiologically synthesized
poly(glutamic acid) and poly(lysine). Mini Rev Med Chem. 4:179–188.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Geffard M, de Bisschop L, Duleu S,
Hassaine N, Mangas A and Coveñas R: Endotherapia: A new frontier in
the treatment of multiple sclerosis and other chronic diseases.
Discov Med. 10:443–451. 2010.PubMed/NCBI
|
|
10
|
Mangas A, Coveñas R, Bodet D, Dabadie MP,
Glaize G and Geffard M: Evaluation of the effects of a new drug on
brain leukocyte infiltration in an experimental model of autoimmune
encephalomyelitis. Lett Drug Des Discov. 3:138–148. 2006.
View Article : Google Scholar
|
|
11
|
Mangas A, Coveñas R, Bodet D, de León M,
Duleu S and Geffard M: Evaluation of the effects of a new drug
candidate (GEMSP) in a chronic EAE model. Int J Biol Sci.
4:150–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Villoslada P: Neuroprotective therapies
for multiple sclerosis and other demyelinating diseases Mult Scler
Demyel Dis. 1:12016. View Article : Google Scholar
|
|
13
|
Mangas A, Coveñas R, Bodet D and Geffard
M: Antisera and immunocytochemical techniquesBrain molecules: From
vitamins to molecules for axonal guidance. Mangas A, Coveñas R and
Geffard M: Transworld Research Network; Trivandrum: pp. 1–25.
2008
|
|
14
|
Mangas A, Vecino E, Rodríguez F David,
Geffard M and Coveñas R: GEMSP exerts a myelin-protecting role in
the rat optic nerve. Neurol Res. 35:903–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Noseworthy JH, Lucchinetti C, Rodríguez M
and Weinshenker BG: Multiple sclerosis. N Engl J Med. 343:938–952.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Meeteren ME, Teunissen CE, Dijkstra CD
and van Tol EA: Antioxidants and polyunsaturated fatty acids in
multiple sclerosis. Eur J Clin Nutr. 59:1347–1361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Agar J and Durham H: Relevance of
oxidative injury in the pathogenesis of motor neuron diseases.
Amyotroph Lateral Scler Other Motor Neuron Disord. 4:232–242. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crouzin N, de Jesus Ferreira MC,
Cohen-Solal C, Aimar RF, Vignes M and Guiramand J:
Alpha-tocopherol-mediated long-lasting protection against oxidative
damage involves an attenuation of calcium entry through TRP-like
channels in cultured hippocampal neurons. Free Radic Biol Med.
42:1326–1337. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blondeau N, Widmann C, Lazdunski M and
Heurteaux C: Polyunsaturated fatty acids induce ischemic and
epileptic tolerance. Neuroscience. 109:231–241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshida H, Yanai H, Namiki Y,
Fukatsu-Sasaki K, Furutani N and Tada N: Neuroprotective effects of
edaravone: A novel free radical scavenger in cerebrovascular
injury. CNS Drug Rev. 12:9–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Di Biase A and Salvati S: Exogenous lipids
in myelination and myelination. Kaohsiung J Med Sci. 13:19–29.
1997.PubMed/NCBI
|
|
22
|
Medina JM and Tabernero A:
Astrocyte-synthesized oleic acid behaves as a neurotrophic factor
for neurons. J Physiol Paris. 96:265–271. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ebringer A, Hughes L, Rashid T and Wilson
C: Acinetobacter immune responses in multiple sclerosis:
Etiopathogenetic role and its possible use as a diagnostic marker.
Arch Neurol. 62:33–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hughes LE, Bonell S, Natt RS, Wilson C,
Tiwana H, Ebringer A, Cunningham P, Chamoun V, Thompson EJ, Croker
J, et al: Antibody responses to Acinetobacter spp. and Pseudomonas
aeruginosa in multiple sclerosis: Prospects for diagnosis using the
myelin-acinetobacter-neurofilament antibody index. Clin Diagn Lab
Immunol. 8:1181–1188. 2001.PubMed/NCBI
|
|
25
|
Furrows SJ, Hartley JC, Bell J, Silver N,
Losseff N, Stevenson S, Chapman M, Thompson EJ, Ridgway GL and
Giovannoni G: Chlamydophila pneumoniae infection of the central
nervous system in patients with multiple sclerosis. J Neurol
Neurosurg Psychiatry. 75:152–154. 2004.PubMed/NCBI
|
|
26
|
Victor VM and De La Fuente M: Changes in
the superoxide production and other macrophage functions could be
related to the mortality of mice with endotoxin-induced oxidative
stress. Physiol Res. 52:101–110. 2003.PubMed/NCBI
|
|
27
|
Bohatschek M, Werner A and Raivich G:
Systemic LPS injection leads to granulocyte influx into normal and
injured brain: Effects of ICAM-1 deficiency. Exp Neurol.
172:137–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lehnardt S, Lachance C, Patrizi S,
Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ and
Vartanian T: The toll-like receptor TLR4 is necessary for
lipopolysaccharide-induced oligodendrocyte injury in the CNS. J
Neurosci. 22:2478–2486. 2002.PubMed/NCBI
|
|
29
|
Johnson KP, Brooks BR, Cohen JA, Ford CC,
Goldstein J, Lisak RP, Myers LW, Panitch HS, Rose JW, Schiffer RB,
et al: Extended use of glatiramer acetate (Copaxone) is well
tolerated and maintains its clinical effect on multiple sclerosis
relapse rate and degree of disability. Copolymer 1 Multiple
Sclerosis Study Group. Neurology. 50:701–708. 1998. View Article : Google Scholar : PubMed/NCBI
|