Introduction
Atrial fibrillation (AF) is the most commonly
encountered cardiac arrhythmia in clinical practice and is
associated with significantly increased morbidity and mortality
(1,2).
Catheter-based AF ablation with the primary aim of pulmonary vein
(PV) electrical isolation has been established as a treatment
option for patients with symptomatic, drug-refractory AF (3,4). AF ablation
is a relatively complex procedure, and is associated with the
potential risk of periprocedural thromboembolic complications. The
endothelial lesion caused by the ablation energy may serve a
significant role in activating the clotting cascade. In addition,
the cardioversion or the restored contractility post-ablation may
dislodge left atrial microthrombi (5,6). However,
minimizing thromboembolic complications with periprocedural
anticoagulation could potentially increase the risk of bleeding
events. As such, maintaining the balance between bleeding and
thrombosis is critical to the safety of the ablation procedure.
Warfarin administration has been the mainstay for AF
ablation anticoagulation, with a target international normalized
ratio (INR) level of 2.0 to 3.0. Bridging anticoagulation refers to
the temporary interruption of oral anticoagulation and introduction
of a short-acting anticoagulant such as low-molecular-weight
heparin (LMWH). Recently, uninterrupted therapeutic warfarin has
been demonstrated to be associated with less bleeding events, and
it has the benefit of preventing stroke in patients undergoing
catheter ablation of AF compared to use of warfarin with bridging
LMWH (7). In patients with AF who need
to cease warfarin treatment in preparation for an invasive
procedure, forgoing bridging therapy was non-inferior to
perioperative bridging with LMWH for reducing the risk of stroke
and major bleeding (8). The current
international guidelines suggest that bridging therapy should be
considered for patients at high risk of thrombosis; low-risk
patients do not require bridging (9–11). The
CHADS2 score can be calculated to assess the risk of stroke.
Low-risk patients include those with a CHADS2 score of 0–2,
moderate-risk with a CHADS2 score of 3–4, and high-risk with a
CHADS2 score of 5 or 6. Previous studies have demonstrated that
Asian patients are more sensitive to the anticoagulant effects of
warfarin (12) and warfarin has a long
half-life, uninterrupted warfarin without bridging therapy remains
controversial and has not been widely accepted in China.
Conversely, the direct thrombin inhibitor dabigatran has more
predictable pharmacokinetics and the unique property of a rapid
onset and short half-life. Therefore, it may not necessitate
bridging therapy, and uninterrupted administration appears to be
easier to achieve. Although the availability of these new oral
anticoagulants (OACs) would obviate the need for bridging, the use
of new OACs is limited due to their high medical cost.
Materials and methods
Inclusion and exclusion criteria
A prospective, observational study was performed
using patients undergoing AF ablation at an electrophysiology
center (at Qilu Hospital, Shandong University, Jinan, Shandong) in
China between July 2013 and June 2015. The research protocol was
approved by the institutional review board of Qilu Hospital (Jinan,
China) and the signed informed consent was provided by each
patient. The inclusion criteria were: i) Age 18–75 years, ii)
presence of AF as evidenced by a 12-lead electrocardiogram or 24 h
Holter monitoring, iii) absence of thrombus in atrium/atrial
appendage by transesophageal echocardiography (TEE) and iv)
hemodynamic stable non-valvular atrial fibrillation. The exclusion
criteria were: i) Hypersensitivity to the active ingredient or any
excipients, ii) patients with severe renal impairment (creatinine
clearance <30 ml/min), iii) clinically active bleeding, iv)
significant risk factors for major bleeding, v) concomitant
treatment with any other anticoagulants except switching therapy to
or from dabigatran, or when unfractionated heparin is given to
maintain an open central venous or arterial catheter, vi) severe
hepatic impairment or liver disease, vii) concomitant treatment
with systemic ketoconazole, cyclosporine, itraconazole, tacrolimus
and dronedarone, and viii) a prosthetic heart valve or
hemodynamically significant valvular disease. The study included
240 patients with drug-refractory, paroxysmal or persistent AF who
underwent AF catheter ablation. Among them, 137 patients received
110 mg dabigatran twice a day (group D) and the remaining 103
received dose-adjusted warfarin (group W). The design flow chart is
presented in Fig. 1.
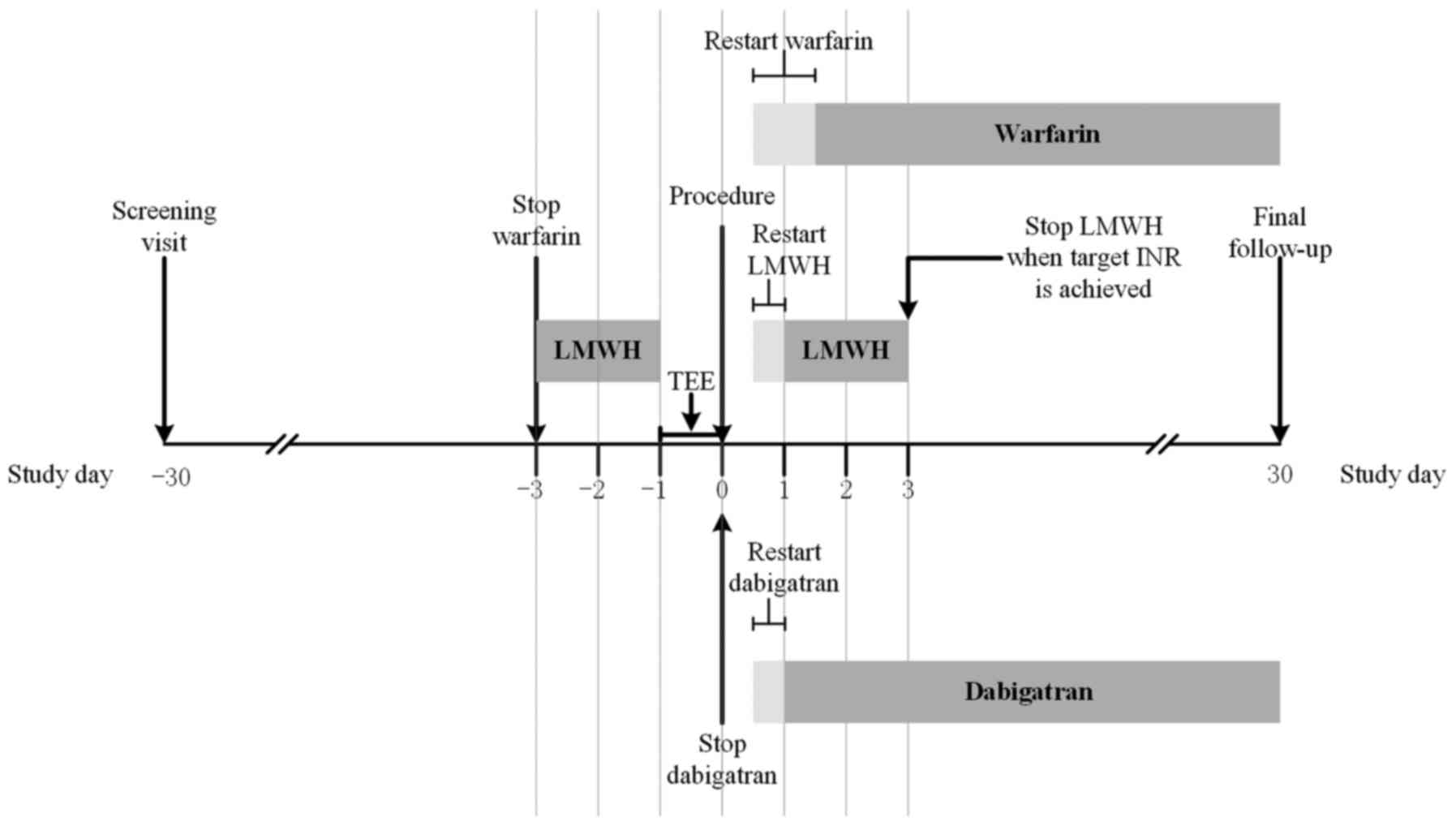
Periprocedural anticoagulation
In group D, dabigatran was not discontinued until
the morning of the procedure; it was restarted 4 h following
hemostasis at the same dosage as was given previously that morning.
In group W, all patients received dose-adjusted warfarin with a
target INR of 2.0–3.0. Warfarin was discontinued 3 days prior to
the procedure and patients were bridged with subcutaneous LMWH
until the evening before the ablation procedure. The administration
of LMWH was restarted on the evening of the procedure and continued
until an INR of 2.0–3.0 was achieved. Warfarin treatment was
restarted on the evening of or the day after the procedure. For all
patients in the two groups, TEE was performed prior to the
procedure to exclude the presence of an intracardiac thrombus.
Ablation procedure
Before the transseptal puncture, intraprocedural
anticoagulation was conducted with a weight-based (80–100 U/kg)
heparin bolus administered intravenously. Based on the activated
clotting time (ACT), infusion of heparin continuously at 1,000 U/h
was given and adjusted, which was monitored every 30 min and
targeted at 300 to 350 sec. Meanwhile, the transseptal sheaths were
continuously infused with heparinized saline. Vascular access was
achieved through the right femoral vein and left subclavian vein. A
circular mapping catheter was performed to guide pulmonary vein
antrum isolation. Electrical activity recorded by placing a
circular mapping catheter (Lasso, Biosense Webster, Inc. Diamond
Bar, CA, USA) in the ostium of each pulmonary vein (PV), and
pulmonary venography was performed for each PV. Following
successful isolation of all the PVs, burst atrial pacing was
performed to confirm that an atrial arrhythmia was induced.
Additional procedures including the complex fractionated atrial
electrograms ablation, linear ablation of the LA, and the superior
vena cava isolation were performed, if AF/atrial tachyarrhythmia
was induced and sustained. Either the elimination or dissociation
of the PV potentials was the end-point of the PV isolation,
recorded through the circular catheters placed within the PVs. If
termination was unsuccessful, cardioversion was performed to
restore the sinus rhythm. During the procedure, the blood pressure
was noninvasively monitored.
Follow-up
Following the ablation, the patients leave hospital
in 3 to 5 days, and were checked at the outpatient clinic 1 week
and 1 month after the ablation for any postprocedural
complications. Thromboembolic complications defined as ischemic
stroke, transient ischemic attack (TIA), or systemic
thromboembolism were the primary end-points (efficacy end-points).
Major (requiring a transfusion or surgical intervention) and minor
bleeding complications were the secondary end-points (safety
end-points).
Statistical analysis
Statistical analyses were performed using SPSS
software (version, 17.0; SPSS Inc., Chicago, IL, USA). Continuous
variables are presented as the as mean ± standard deviation and
compared using a Student's t-test or Mann-Whitney U test.
Categorical variables were presented as counts and percentages then
analyzed using either a Chi-squared test or Fisher's exact test.
Bleeding complications (minor and major), thromboembolic
complications and total complications were compared between groups.
P<0.05 (two-sided) was considered to indicate a statistically
significant difference.
Results
Patient characteristics
A total of 240 patients presenting with AF between
July 2013 and June 2015 were enrolled in the present study.
Patients were treated according to their preference to undergo the
procedure with an anticoagulation strategy of dabigatran (group D,
n=139) or warfarin with bridging therapy (group W, n=101). The
baseline characteristics of the two groups were similar with no
significant differences in age, sex, body mass index, blood
pressure, left atrial size, left ventricular ejection fraction,
CHADS2 score, CHA2DS2VASc score, alcohol consumption, proportion of
patients with persistent AF or the number of patients with a
history of previous stroke/TIA (Table
I). In group D, 8 patients (6%) had a HAS-BLED ≥3 compared with
15 (15%) in group W (P<0.05). Those receiving dabigatran were
more likely to be smokers and had a shorter duration of atrial
fibrillation, when compared with the warfarin patients. During the
procedure, 28 patients (20%) in group D and 15 patients (15%) in
group W (P>0.05) received cryoablation. Intraprocedural
cardioversions were conducted in 22 patients receiving dabigatran
(16%), compared with 18 patients receiving warfarin (18%,
P>0.05).
 | Table I.Patient baseline characteristics. |
Table I.
Patient baseline characteristics.
|
| Dabigatran group | Warfarin group |
|
|---|
|
| (n=139) | (n=101) | P-value |
|---|
| Age (years) | 55.12±11.10 | 55.96±10.40 | 0.554 |
| Male (%) | 101 (73) | 72 (71) | 0.815 |
| Body mass index
(kg/m2) | 27.25±3.78 | 26.32±3.49 | 0.055 |
| Blood pressure
(mmHg) |
|
|
|
|
Systolic | 132.87±18.33 | 130.70±17.55 | 0.358 |
|
Diastolic | 82.14±13.37 | 80.47±15.21 | 0.368 |
| Persistent atrial
fibrillation (%) | 33 (24) | 29 (29) | 0.385 |
| Duration of atrial
fibrillation (months) | 37.08±51.69 | 45.34±41.74 | 0.037 |
| Hypertension (%) | 64 (46) | 43 (43) | 0.594 |
| Diabetes (%) | 18 (13) | 16 (16) | 0.526 |
| Coronary artery
disease (%) | 9 (7) | 13 (13) | 0.090 |
| Heart failure
(%) | 2 (1) | 1 (1) | P>0.99 |
| Transient ischemic
attacks or stroke (%) | 6 (4) | 8 (8) | 0.240 |
| Smoking (%) | 60 (43) | 30 (30) | 0.033 |
| Alcohol drinking
(%) | 37 (27) | 24 (24) | 0.616 |
| Alanine
aminotransferase (U/l) | 22.05±11.87 | 22.36±11.12 | 0.840 |
| Serum creatinine
(µmol/l) | 71.63±14.06 | 69.74±11.59 | 0.272 |
| Fasting blood glucose
(mmol/l) | 5.03±1.00 | 5.26±1.74 | 0.202 |
| Platelet count
(x109/l) | 218.24±45.20 | 208.76±47.59 | 0.118 |
| INR | 1.04±0.13 | 1.07±0.23 | 0.900 |
| APTT-S | 31.22±8.80 | 32.21±4.23 | 0.649 |
| CHADS2
scorea | 0.68±0.80 | 0.77±0.89 | 0.598 |
| CHADS2 ≥2 (n, %) | 17 (12) | 19 (19) | 0.159 |
| CHA2DS2VASc
scoreb | 1.14±1.17 | 1.32±1.21 | 0.241 |
| CHA2DS2VASc ≥2 (n,
%) | 42 (30) | 35 (35) | 0.467 |
| HAS-BLED
scorec | 1.04±0.96 | 1.11±1.10 | 0.900 |
| HAS-BLED ≥ 3 (n,
%) | 8 (6) | 15 (15) | 0.018 |
| Left atrial size
(mm) | 39.75±5.83 | 39.51±5.63 | 0.746 |
| Left ventricular
ejection fraction (%) | 61.49±5.03 | 60.56±6.28 | 0.207 |
| AF ablation
procedure |
|
Cryoablation | 28 (20) | 15
(15) | 0.291 |
|
Intraprocedural
cardioversion | 22 (16) | 18
(18) | 0.682 |
|
Anticoagulation-experienced (n, %) | 19 (14) | 13
(13) | 0.858 |
|
Warfarin (n, %) | 3
(2) | 9
(9) | 0.038 |
|
Dabigatran (n, %) | 16 (12) | 4
(4) | 0.242 |
Study outcomes
Complications associated with the procedures are
presented in Table II. A
periprocedural stroke event occurred in one patient in the
dabigatran group and no patients in the warfarin group. No major
bleeding complications were observed in any patients within the
study population. The incidence of minor bleeding complications was
23 in group D (17%) and 11 in group W (11%, P>0.05). In
addition, 11 patients receiving dabigatran treatment developed a
groin hematoma compared with five patients receiving warfarin. All
of these patients underwent an ultrasound scan that excluded the
presence of a pseudo-aneurysm or an arteriovenous (AV) fistula. As
a result, one patient in each of the groups was indicated to have
an AV fistula. A hemothorax was observed in one patient in the
dabigatran group and one patient from the warfarin group.
Hemoptysis occurred in one patient who received dabigatran
treatment, and the patient developed a large groin hematoma two
days following the procedure and a high fever of 38.6°C. Dabigatran
was discontinued imminently. Two days later, massive hemoptysis
occurred and pulmonary computed tomography (CT) presented bilateral
pulmonary frosted glass. At three days later, no hemoptysis
occurred and a lung CT presented markedly reduced frosted glass.
Overall, there were no significant differences in complication
rates between the two groups (P>0.05; Table II).
 | Table II.Comparison of complications between
patients on dabigatran and warfarin. |
Table II.
Comparison of complications between
patients on dabigatran and warfarin.
|
| Dabigatran
(n=139) | Warfarin
(n=101) | Total (n=240) | P-value |
|---|
| Major bleeding
complications (n, %) | 0 | 0 | 0 |
| Cardiac
tamponade | 0 | 0 | 0 |
|
Intracranial bleeding | 0 | 0 | 0 |
|
Extracranial | 0 | 0 | 0 |
| Minor bleeding
complications (n, %) | 23 (17) | 11 (11) | 34 (14) | 0.215 |
| Groin
hematoma | 11 (8) | 5 (5) | 16 (7) | 0.364 |
|
Hemothorax | 1 (1) | 1 (1) | 2 (1) | >0.050 |
|
Hemoptysis | 1 (1) | 0 | 1 (1) | >0.050 |
|
Urogenital bleeding | 7 (5) | 3 (3) | 10 (4) | 0.643 |
|
Gastrointestinal bleeding | 3 (2) | 2 (2) | 5 (2) | >0.050 |
| Total bleeding
complications (n, %) | 23 (17) | 11 (11) | 34 (14) | 0.215 |
| Embolic
complications (stroke/TIA) (n, %) | 1 (1) | 0 | 1 (1) | >0.050 |
| Composite of
bleeding and embolic complications | 24 (17) | 11 (11) | 35 (15) | 0.167 |
| Other complications
(n, %) | 5 (4)a | 0 | 5 (2) | 0.076 |
Discussion
Stroke is the most serious complication in patients
with AF. Therefore, in AF patients with a CHADS2 score ≥2,
conventional OAC is recommended to reduce the risk of stroke
(13,14). During the period of anticoagulation
therapy, the risk of both stroke and bleeding complications are
highest when OAC is initiated. During the initiation of OAC, there
is a theoretical transient hypercoagulable state, because the
vitamin K-dependent anticoagulant proteins C and S are decreased,
while the vitamin K-dependent procoagulant factors II and X remain
elevated due to their longer half-lives (15). Bridging anticoagulation therapy is
designed to minimize the risk of thromboembolism in high-risk
patients when anticoagulation therapy is suspended and to minimize
the risk of bleeding following procedures, and it is usually used
in patients receiving warfarin treatment when warfarin has been
discontinued and the INR falls below the therapeutic range
(16). During the interruption of
warfarin treatment, typically LMWH bridging anticoagulation therapy
can be given to minimize the interval that patients do not in a
state of anticoagulation, with the purpose of decreasing the risk
of perioperative stroke (8,16). In patients who require temporary
interruption of warfarin therapy for invasive procedures, a
standardized periprocedural bridging anticoagulant therapy with
subcutaneous LMWH is feasible and associated with a low risk of
thromboembolic and major bleeding complications (17,18). Some
studies have assessed perioperative bridging with LMWH, however,
the practice guidelines have provided weak and inconsistent
recommendations regarding whether bridging anticoagulation is
necessary during perioperative warfarin interruption (19–21).
Previous research has indicated that use of bridging
anticoagulation is associated with an increased risk of bleeding
and adverse events after interruption (22,23) and in
patients who interrupted dabigatran or warfarin in the RE-LY trial,
bridging anticoagulation appeared to increase the risk for major
bleeding irrespective of dabigatran or warfarin interruption
(24). The use of interrupted warfarin
bridging with LMWH has been widely used, although studies have
suggested that continuous warfarin treatment protects against the
risk of periprocedural stroke with no increased bleeding risk
(8,22–24).
Given the growing concern regarding hemorrhagic
complications associated with bridging therapies, some experts
suggest that bridging should only be considered in those at highest
risk for thrombosis (11). A recent
study indicated that performing AF catheter ablation with
uninterrupted warfarin in patients at high risk for stroke and with
nonparoxysmal AF reduced the periprocedural stroke and bleeding
complications compared to bridging therapy (7). The results of the present study suggested
that interrupted warfarin bridged with LMWH is safe and effective
among the low-risk population compared to anticoagulation with
dabigatran. In the current study, there was no difference in
thromboembolic events in the two anticoagulant groups. The
incidence and outcomes of bleeding complications were also similar
between the two groups. Of note, some of the complications were
likely the result of technical or mechanical complications, as
opposed to the choice of anticoagulant.
In the present study, the mean CHADS2 score was
0.68±0.80 vs. 0.77±0.89 in dabigatran and warfarin groups,
respectively (P>0.05). There were 17 patients with CHADS2 ≥ 2
(12%) in the dabigatran group and 19 patients in the warfarin group
(19%, P>0.05). This also applied to the CHA2DS2VASc score and
HAS-BLED score. As such, the entire population tended to have a low
risk of both thromboembolic and bleeding events.
Anticoagulation-experienced is defined as the total lifetime use of
the anticoagulant for more than two months. There was no difference
between the two groups in ratio of the anticoagulation-experienced
(P>0.05) and only >20% of the patients were treated with OAC
at baseline. Asian patients are more sensitive to the anticoagulant
effects of warfarin and have higher rates of bleeding when they are
in the therapeutic range (12).
Therefore, frequent therapeutic monitoring and concern about
bleeding events remains an important barrier to the appropriate use
of anticoagulant therapy in the Chinese population (25,26). The
cost of routine dabigatran or rivaroxaban anticoagulation is too
high to be affordable for ordinary patients. Therefore, it is
convenient and likely safe to leave low-risk patients on aspirin or
no OAC pre-ablation. In addition, the interrupted anticoagulation
strategy with LMWH bridging was safe and effective for the AF
patients who had a low risk score.
Despite a recent trend towards uninterrupted OAC for
AF ablation (7,27,28),
interrupted OAC provides several advantages. Some patients on
uninterrupted warfarin will not have a stable INR pre-ablation and
may arrive with a subtherapeutic or supratherapeutic INR (29). With the NOACs, there is no test to
verify patient compliance pre-ablation, and the lack of proven
reversal agents may add additional risk if they are not
interrupted. In addition, bleeding complications are easier to
handle with interrupted OAC. The use of uninterrupted warfarin was
followed by an era of using interrupted warfarin. Thus, published
retrospective comparisons have been conducted by examining these
two different time periods of AF ablation. It was demonstrated that
some of the apparent advantages of uninterrupted warfarin may
instead be due to improvements in ablation safety over time. Arshad
et al (30) recently compared
peri-ablation anticoagulation at four experienced AF ablation
centers and indicated that major complications (stroke/TIA,
pericardial tamponade, major bleeding and surgical intervention)
occur more frequently in the uninterrupted warfarin group (4.3%)
vs. both the dabigatran group (0.8%) and the bridged warfarin group
(2.6%) (P<0.01).
In conclusion, the administration of dabigatran
during AF ablation procedures did not cause any significantly
different effects on the safety and efficiency as compared to those
of the conventional anticoagulation with warfarin bridged with LMWH
for patients with a low CHADS2 score. Low-risk patients may remain
on aspirin or no OAC pre-ablation and do not need to receive
warfarin or dabigatran before ablation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81270238) and the
Scientific Research Foundation for the Doctoral Degree, State
Education Ministry of China (grant no. 20130131110065).
References
|
1
|
Ball J, Carrington MJ, McMurray JJ and
Stewart S: Atrial fibrillation: Profile and burden of an evolving
epidemic in the 21st century. Int J Cardiol. 167:1807–1824. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alonso A and Bengtson LG: A rising tide:
The global epidemic of atrial fibrillation. Circulation.
129:829–830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cappato R, Calkins H, Chen SA, Davies W,
Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, et al:
Updated worldwide survey on the methods, efficacy, and safety of
catheter ablation for human atrial fibrillation. Circ Arrhythm
Electrophysiol. 3:32–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morillo CA, Verma A, Connolly SJ, Kuck KH,
Nair GM, Champagne J, Sterns LD, Beresh H, Healey JS and Natale A:
RAAFT-2 Investigators: Radiofrequency ablation vs antiarrhythmic
drugs as first-line treatment of paroxysmal atrial fibrillation
(RAAFT-2): A randomized trial. JAMA. 311:692–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gaita F, Caponi D, Pianelli M, Scaglione
M, Toso E, Cesarani F, Boffano C, Gandini G, Valentini MC, De Ponti
R, et al: Radiofrequency catheter ablation of atrial fibrillation:
A cause of silent thromboembolism? Magnetic resonance imaging
assessment of cerebral thromboembolism in patients undergoing
ablation of atrial fibrillation. Circulation. 122:1667–1673. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Phan K, Wang N, Pison L, Kumar N, Hitos K
and Thomas SP: Meta-analysis of dabigatran vs warfarin in patients
undergoing catheter ablation for atrial fibrillation. Int J
Cardiol. 189:199–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Biase L, Burkhardt JD, Santangeli P,
Mohanty P, Sanchez JE, Horton R, Gallinghouse GJ, Themistoclakis S,
Rossillo A, Lakkireddy D, et al: Periprocedural stroke and bleeding
complications in patients undergoing catheter ablation of atrial
fibrillation with different anticoagulation management: Results
from the role of coumadin in preventing thromboembolism in Atrial
Fibrillation (AF) patients undergoing catheter ablation (COMPARE)
randomized trial. Circulation. 129:2638–2644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Douketis JD, Spyropoulos AC, Kaatz S,
Becker RC, Caprini JA, Dunn AS, Garcia DA, Jacobson A, Jaffer AK,
Kong DF, et al: Perioperative bridging anticoagulation in patients
with atrial fibrillation. N Engl J Med. 373:823–833. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
You JJ, Singer DE, Howard PA, Lane DA,
Eckman MH, Fang MC, Hylek EM, Schulman S, Go AS, Hughes M, et al:
Antithrombotic therapy for atrial fibrillation: antithrombotic
therapy and prevention of thrombosis, 9th ed: American college of
chest physicians evidence-based clinical practice guidelines.
Chest. 141:(Suppl 2). e531S–e575S. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Veen JJ and Makris M: Management of
peri-operative anti-thrombotic therapy. Anaesthesia. 70:(Suppl 1).
S58–S67. 2015. View Article : Google Scholar
|
|
11
|
Kim TH, Kim JY, Mun HS, Lee HY, Roh YH,
Uhm JS, Pak HN, Lee MH and Joung B: Heparin bridging in warfarin
anticoagulation therapy initiation could increase bleeding in
non-valvular atrial fibrillation patients: A multicenter
propensity-matched analysis. J Thromb Haemost. 13:182–190. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hori M, Connolly SJ, Zhu J, Liu LS, Lau
CP, Pais P, Xavier D, Kim SS, Omar R, Dans AL, et al: Dabigatran
versus warfarin: Effects on ischemic and hemorrhagic strokes and
bleeding in Asians and non-Asians with atrial fibrillation. Stroke.
44:1891–1896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Crandall MA, Bradley DJ, Packer DL and
Asirvatham SJ: Contemporary management of atrial fibrillation:
Update on anticoagulation and invasive management strategies. Mayo
Clin Proc. 84:643–662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gage BF, Waterman AD, Shannon W, Boechler
M, Rich MW and Radford MJ: Validation of clinical classification
schemes for predicting stroke: Results from the National Registry
of Atrial Fibrillation. JAMA. 285:2864–2870. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeuthen EL, Lassen JF and Husted SE: Is
there a hypercoagulable phase during initiation of antithrombotic
therapy with oral anticoagulants in patients with atrial
fibrillation? Thromb Res. 109:241–246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baron TH, Kamath PS and McBane RD:
Management of antithrombotic therapy in patients undergoing
invasive procedures. N Engl J Med. 368:2113–2124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Douketis JD, Johnson JA and Turpie AG:
Low-molecular-weight heparin as bridging anticoagulation during
interruption of warfarin: Assessment of a standardized
periprocedural anticoagulation regimen. Arch Intern Med.
164:1319–1326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kovacs MJ, Kearon C, Rodger M, Anderson
DR, Turpie AG, Bates SM, Desjardins L, Douketis J, Kahn SR,
Solymoss S and Wells PS: Single-arm study of bridging therapy with
low-molecular-weight heparin for patients at risk of arterial
embolism who require temporary interruption of warfarin.
Circulation. 110:1658–1663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
BRIDGE Study Investigators: Bridging
anticoagulation: Is it needed when warfarin is interrupted around
the time of a surgery or procedure? Circulation. 125:e496–e498.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Patel JP and Arya R: The current status of
bridging anticoagulation. Br J Haematol. 164:619–629. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Douketis JD, Spyropoulos AC, Spencer FA,
Mayr M, Jaffer AK, Eckman MH, Dunn AS and Kunz R: American College
of Chest Physicians: Perioperative management of antithrombotic
therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th
ed: American College of Chest Physicians Evidence-Based Clinical
Practice Guidelines. Chest. 141:(2 Suppl). e326S–e350S. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Steinberg BA, Peterson ED, Kim S, Thomas
L, Gersh BJ, Fonarow GC, Kowey PR, Mahaffey KW, Sherwood MW, Chang
P, et al: Use and outcomes associated with bridging during
anticoagulation interruptions in patients with atrial fibrillation:
Findings from the Outcomes Registry for Better Informed Treatment
of Atrial Fibrillation (ORBIT-AF). Circulation. 131:488–494. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Siegal D, Yudin J, Kaatz S, Douketis JD,
Lim W and Spyropoulos AC: Periprocedural heparin bridging in
patients receiving vitamin K antagonists: Systematic review and
meta-analysis of bleeding and thromboembolic rates. Circulation.
126:1630–1639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Douketis JD, Healey JS, Brueckmann M,
Eikelboom JW, Ezekowitz MD, Fraessdorf M, Noack H, Oldgren J,
Reilly P, Spyropoulos AC, et al: Perioperative bridging
anticoagulation during dabigatran or warfarin interruption among
patients who had an elective surgery or procedure. Substudy of the
RE-LY trial. Thromb Haemost. 113:625–632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sabir I, Khavandi K, Brownrigg J and Camm
AJ: Oral anticoagulants for Asian patients with atrial
fibrillation. Nat Rev Cardiol. 11:290–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eikelboom JW and Hart RG: Intensity and
quality of warfarin anticoagulation in chinese patients: Setting
the record straight. Stroke. 46:5–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hussein AA, Martin DO, Saliba W, Patel D,
Karim S, Batal O, Banna M, Williams-Andrews M, Sherman M, Kanj M,
et al: Radiofrequency ablation of atrial fibrillation under
therapeutic international normalized ratio: A safe and efficacious
periprocedural anticoagulation strategy. Heart Rhythm. 6:1425–1429.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wazni OM, Heheiry S, Fahmy T, Barrett C,
Hoa S, Patel D, Di Biase L, Martin DO, Kanj M, Arruda M, et al:
Atrial fibrillation ablation in patients with therapeutic
international normalized ratio: Comparison of strategies of
anticoagulation management in the periprocedural period.
Circulation. 116:2531–2534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nam D, Sadhu A, Hirsh J, Keeney JA, Nunley
RM and Barrack RL: The use of warfarin for DVT prophylaxis
following hip and knee arthroplasty: How often are patients within
their target INR range? J Arthroplasty. 30:315–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arshad A, Johnson CK, Mittal S, Buch E,
Hamam I, Tran T, Shaw RE, Musat D, Preminger M, Sichrovsky T, et
al: Comparative safety of periablation anticoagulation strategies
for atrial fibrillation: Data from a large multicenter study.
Pacing Clin Electrophysiol. 37:665–673. 2014. View Article : Google Scholar : PubMed/NCBI
|