Introduction
Fibrotic skin disease is an umbrella term for a
group of diseases, which is driven by inflammation, autoimmune
mechanisms or environmental factors (1). Examples include all forms of scleroderma,
graft-versus-host disease (GvHD), nephrogenic fibrosing dermopathy,
mixed connective tissue disease, scleromyxedema, scleredema,
eosinophilic fasciitis, keloids and hypertrophic scars. Fibrotic
skin diseases have a significant negative effect on quality of
life, specifically including issues with self-esteem.
A hallmark of fibrotic skin diseases is the
involvement of the extracellular matrix (ECM), which occurs as a
result of pathological and/or abnormal wound healing (1). Progression of fibrosis is a complex
process and consists of immune, autoimmune and inflammatory
mechanisms, as well as certain cytokines and chemical or physical
factors (1,2). Fibrotic skin diseases may be accompanied
by psychological issues, as a result of reduced self-esteem. A
small number of treatment options are useful; however, further
therapeutic strategies are required for the management of skin
fibrosis (3,4). Accumulating clinical and experimental
evidence indicate that the distribution of the balance of ECM
remodelling is a key component (5).
Generally, tissue fibrosis occurs following injury
and presents an abnormal wound healing process (5–7). Skin
fibrosis progression may be followed by chronic inflammation,
inflammation and intrinsic profibrotic changes in fibroblasts, and
involves cytokines, growth factors and different cell types
(2,8).
Endothelial cells, when damaged, release certain cytokines that
activate immune cells. As a result, activated immune cells secrete
fibrotic growth factors. Different cytokines and growth factors,
such as transforming growth factor (TGF)-β, interleukin-4 (IL-4),
interferon-γ and tumour necrosis factor (TNF)-α, connective tissue
growth factor (CTGF) and platelet-derived growth factor (PDGF),
participate in skin fibrosis. Growth factor actions lead to
proliferation of fibroblasts and they differentiate into
myofibroblasts during the fibrosis process (9,10).
Following the discovery of microRNAs (miRNAs or
miRs) in the early 2000s, studies have begun to discuss their roles
in the physiological and pathological processes of diseases,
including fibrosis (11). miRNAs are
small non-coding RNAs (18–25 nucleotides) that block the
translation or induce the degradation of target messenger RNA
(mRNA) (12). miRNAs have different
expression patterns and regulate various biological (such as
cellular differentiation and cellular proliferation) and
pathological (cancer and neurodegenerative disease) processes
(11,13–16).
Increasing evidence indicates that miRNAs participate in fibrotic
disorders, including those of the skin, heart, kidney, liver and
lung (3,6,8). Previous
studies have evaluated the possible roles of miRNAs in skin
diseases (3,4,8,17,18).
The miR-29 family includes miR-29a, miR-29b-1,
miR-29b-2 and miR-29c. This family demonstrates anti-fibrotic
activity and regulates a number of ECM proteins, including
collagens, fibronectin, laminin, matrix metalloproteinase-2
(MMP-2), secreted protein acidic and rich in cysteine (19–25).
Accumulating data suggest that the miR-29 family may be important
in the homeostasis of the ECM.
In the present review, the role and function of the
miR-29 family is summarized, with an emphasis on its role in the
pathogenesis of fibrotic skin diseases, and its potential as a
therapeutic molecule.
miRNAs
miRNAs are a class of non-coding RNAs that regulate
mRNA processing at the post-transcriptional level (26). In 1993, Lee et al (27) discovered the first miRNA, lin-4 in
Caenorhabditis elegans. In the early 2000s, Reinhart et
al (28) identified the second
miRNA, let-7 and, during the 2000s, more than a dozen miRNAs were
identified in plant and animal species (11,12,29). The chromosomal locations of miRNAs
affect their expression and function. While the majority of
mammalian miRNA genes are located in intronic regions, certain
miRNA genes are located in exons, and ~30% are located in
intergenic regions (13,30).
miR-29 family
The miR-29 family consists of four miRNAs: miR-29a,
miR-29b-1, miR-29b-2 and miR-29c. miR-29a and miR-29b-1 are encoded
by genes located on chromosome 7 (7q32.3), whereas miR-29b-2 and
miR-29c are encoded on chromosome 1 (1q32.2). All three miRNAs
share an identical 5-nucleotide sequence in their seed region,
which results in an overlap in the genes they target (22). Expression levels of miR-29 family
members are regulated by multiple transcription factors, including
Myc, nuclear factor (NF)-κB and Gli (20,31).
The miR-29 family appears to exert a range of
regulatory functions, controlling apoptosis (32,33),
proliferation (34,35) and differentiation (36,37). The
antifibrotic activity of the miR-29 family is primarily based on
its ability to target ECM genes. Various studies have independently
demonstrated that the miR-29 family targets >20 ECM-associated
genes, including collagen, elastin and integrin B1; therefore, the
miR-29 family is a major target of this miRNA family (22,24). miR-29
family members are actively involved in the fibrotic processes of
various types of tissue, such as liver (25,36,37), pulmonary (19,38,39), cardiac (40,41) and
renal (24,42), as well as in systemic sclerosis
(23,43–45),
trabecular meshwork (9) and bone
remodelling (20,21,46).
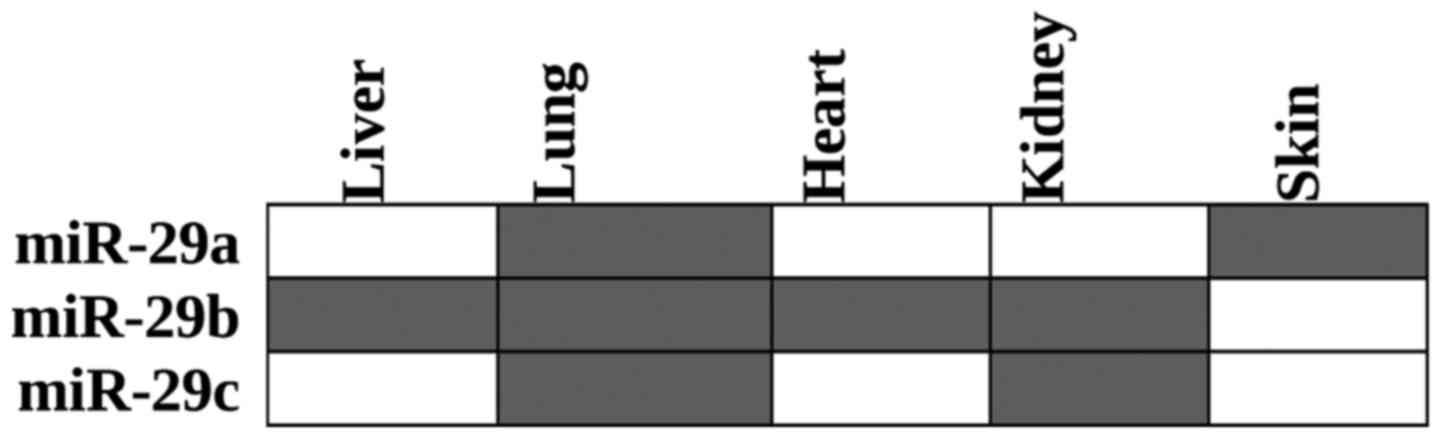
Downregulation of different miR-29 family members
has been reported in the liver (25,37,47,48), lungs
(19,49), heart (25,41), kidneys
(50,51)
and skin (23) (Fig. 1).
miR-29 family in the pathogenesis of
fibrotic skin diseases
The association between downregulated miR-29
expression levels and tissue fibrosis has been reported in
different tissues, and it is not surprising that the miR-29 family
is described as the ‘master fibromiRNA’ due to its pivotal role
during the process of fibrosis (44).
The roles of miR-29 individually in fibrotic skin diseases are
discussed further in the following sections.
Systemic sclerosis (SSc)
SSc is an autoimmune disease that is closely
associated with inflammation and fibrosis (7). The pathogenesis of this disease remains
unclear and there is no effective treatment. The miR-29 family is
predominantly evaluated in scleroderma, with regard to other
fibrotic skin diseases. Maurer et al (23) demonstrated that miR-29a post
transcriptionally regulates collagens in vitro and in
vivo. The authors investigated primary skin fibroblasts and
bleomycin-induced skin fibrosis in mice. It was determined that
miR-29a was significantly downregulated in SSc fibroblasts and skin
sections compared with healthy control samples. Furthermore,
miR-29a expression was demonstrated to be decreased by major
profibrotic mediators, TGF-β, PDGF subunit B and IL-4. Inhibition
of TGF-β signalling reregulates the miR-29 levels in SSc
fibroblasts and bleomycin-induced mice (23). Kawashita et al (43) evaluated patients diagnosed with
scleroderma and grouped all patients according to the subtypes of
the disorder. It was determined that serum miR-29a was
downregulated only in the scleroderma spectrum disorders.
Furthermore, the authors demonstrated that collagen levels were
upregulated in the SSc patients. Notably, a correlation between
reduced miR-29a levels and increased right ventricular systolic
pressure was determined in patients with SSc (43–45).
Ciechomska et al (52)
demonstrated that TGF-β-activated kinase-binding protein is a novel
target of miR-29a, and it regulates TIMP metallopeptidase inhibitor
1, which is a key molecule for ECM deposition. Jafarinejad-Farsangi
et al (53) determined that
miR-29a possesses pro-apoptotic properties in addition to its
anti-fibrotic effects. The protein expression of B cell lymphoma-2
(Bcl-2) family was altered by miR-29a in dermal fibroblasts from
SSc patients and miR-29a induced apoptosis in this type of cell.
Mcl-1, an anti-apoptotic BCL2 family member, is a target of miR-29b
and is a member of the Bcl-2 family; therefore, the miR-29 family
directly or indirectly affects Mcl-1 (53,54).
According to Takemoto et al (55) hair miR-29a levels in patients with
scleroderma were markedly reduced when compared with the
controls.
miR-29 is not the only miRNA that is involved in the
pathogenesis of SSc. As discussed by Zhu et al (56), at least four other miRNAs are involved
in SSc. Among these, miR-21 and miR-92a promote fibrosis, while
miR-150 and miR-196a exert antifibrotic effects.
Graft-versus-host diseases
A common complication associated with allogeneic
hematopoietic stem cell transplantation is the development of GvHD,
which is observed in 50% of recipient patients (57). GvHD is an immune-mediated disease,
which leads to morbidity and mortality. The major problem regarding
GvHD prognosis and treatment depends only on clinical progression
and, the unique method for establishing the GvHD prognosis is to
determine any validated laboratory tests and results (58). Given the involvement of the skin and
immune system in GvHD, the role of miRNAs in the pathogenesis of
GvHD has been hypothesized. Xiao et al (59) provided supporting evidence for this
hypothesis, and demonstrated that the plasma levels of miR-423,
miR-199a-3p, miR-93* and miR-377 were significantly upregulated in
patients with acute GvHD. In another study, Atarod and Dickinson
(6) used Ingenuity Pathway Analysis to
further investigate the interactions between the GvHD signalling
pathway and miRNAs. The authors speculated that the miR-29 family
targets IL-6 and TNF-α, and acts as downstream regulators of tissue
damage (60). Notably, a large number
of molecules have a role in GvHD pathogenesis, and various miRNAs
are likely to exert regulatory roles in this process. In
vivo models of GvHD, as well as clinical samples from GvHD
patients may be utilized to identify the precise roles of the
miR-29 family in this disease.
Keloids
Keloids are defined as benign dermal tumours.
Keloids generally occur following an abnormal wound healing;
however, the pathogenesis remains unclear (61). Zhang et al (62) determined that all miR-29 family members
are expressed in keloid fibroblasts (KFs) and fresh skin biopsy
specimens (obtained 24–48 h after surgery) and, in particular,
miR-29a is highly expressed in KFs. The authors also evaluated the
interaction of miR-29a and collagen type III α1 chain (COL3α1) and
demonstrated that miR-29a directly regulates enhancement of
collagen production. Furthermore, in normal fibroblasts, miR-29a
directly regulates collagen production via affecting TGF-β
expression (62). In another study,
Liu et al (63) used microarray
analysis for comparative miRNA profiling between keloid tissue and
normal skin tissue samples. The authors identified 32
differentially expressed miRNAs, 23 of which were upregulated in
keloid tissue samples. Notably, miR-21 was the most upregulated
miRNA, whereas miR-203 was the most downregulated. Notably, none of
the miR-29 family members were identified to be differentially
expressed in the study. The most likely explanation for this
observation is the stringent expression analysis, which excludes
any miRNA that is not differentially expressed in all paired
keloid-normal tissues (63).
Hypertrophic scars
Hypertrophic scars occur as a result of trauma,
injury, surgery or burns. This type of scar is characterized by
excessive deposition of the ECM. The incidence is more frequent
when compared with keloids (64). To
the best of our knowledge, the first analysis of differential miRNA
expression in hypertrophic scars was performed by Ning et al
(65), who demonstrated that miR-29b-1
expression was downregulated in hypertrophic scar tissue samples
when compared with healthy control tissue samples. In another
study, Guo et al (66)
demonstrated that miR-29b treatment inhibited the TGF-β1/Smad/CTGF
signalling pathway in scalded model in mice. Zhou et al
(67) reported that miR-21 and
miR-200b are overexpressed in profibrotic hypertrophic scars, and
speculated that these miRNAs are involved in the pathogenesis of
hypertrophic scars through TGF-β/Smad7/zinc finger E-box-binding
homeobox 1 signalling. In a follow-up study, Li et al
(68) reported that miR-21 inhibitor
suppresses hypertrophic scar growth in a mouse model. Furthermore,
downregulation of miR-143-3p is associated with COLI/III
accumulation, and upregulation of CTGF (also termed CCN2)
expression hypertrophic scars. Mu et al (69) demonstrated that forced overexpression
of miR-143-3p inhibits proliferation of hypertrophic scar
fibroblasts, and induces apoptosis via the Akt/mechanistic target
of rapamycin (mTOR) signalling pathway. Taken together, these
results indicate that multiple miRNAs are involved in the
pathogenesis of hypertrophic scars, and their effects are performed
predominantly via TGF-β-mediated signalling.
Notably, the interaction between the miR-29 family
and TGF-β has been revealed in cardiac and lung fibrosis (19,41).
Following treatment of TGF-β1 on miR-29 knockdown IMR-90 cells,
Cushing et al (19)
demonstrated that certain genes were upregulated by TGF-β and the
authors observed that these genes predominantly consisted of
miR-29-predicted targets. Decreased miR-29 expression levels lead
to upregulation of Col3A1 and Col4A1. Furthermore, the stimulation
of TGF-β1 and knockdown of miR-29 exhibit the same behaviour in
lung cells, and lead to upregulation of specific genes, including
Col1A1, Col3A1 and Col1A2. In certain cases, TGF-β1 stimulation may
be insufficient compared with miR-29 inhibition, and this suggests
that downregulation of miR-29 may facilitate TGF-β-mediated
upregulation. Conversely, certain laminins, integrins, MMPs and
ADAMs family are upregulated with downregulation of miR-29, but not
with TGF-β1 stimulation (19). Thus,
these observations indicate that the miR-29 family regulates gene
expression via TGF-β-dependent and -independent signalling
pathways.
miR-29 family as therapeutic target for
fibrotic skin diseases
The effective treatment options have not been
established in fibrotic skin diseases. Current treatment options
are far from adequate; they only provide relief and assist patients
by easing the side effects associated with main fibrotic disease
(3,8).
Therefore, it is necessary to develop novel treatment options.
Recent evidence indicates that miRNAs may serve as
therapeutic targets for certain fibrotic skin diseases. In
particular, the miR-21 and miR-29 family have become prominent;
however, considering that deregulated miR-21 expression is also
associated with other pathological conditions, it is possible that
negative effects associated with miR-21 deregulation may not be
specific to fibrotic skin diseases (4,6).
Gay et al (70)
developed an miR-29 upregulator for scleroderma treatment and have
submitted a patent application (http://www.google.ch/patents/US20110218233).
Considering the anti-fibrotic properties of the miR-29 family, this
approach is considered to be a suitable treatment option. In
addition, synthetic short double-stranded oligonucleotide mimics
and viral (lentiviral, adenoviral and adeno-associated viral)
vectors may be used for treatment of fibrotic skin diseases in
order to increase miR-29 expression (3,4,6).
The possibility of restoring downregulated miR-29
expression via small molecules or synthetic oligonucleotides (miRNA
mimics) has been investigated in different studies. In one example,
Zhu et al (71) indicated that
carvedilol, a non-selective β-adrenoreceptor antagonist, attenuates
myocardial fibrosis in rats. The authors determined that carvedilol
treatment exerts its effect via inhibition of Smad3 signalling and
activation of miR-29b expression. Forced overexpression by miRNA
mimics also exerted similar effects, which was demonstrated by a
significant decrease in Col1A1, Col3α1, and α-smooth muscle actin
expression levels in the two experimental conditions (71). In another example, van Rooij et
al (41) suggested that restoring
miRNA expression with synthetic oligonucleotides (miRNA mimics) or
pharmacological inhibitors may be a useful approach to counteract
the adverse effects of miR-29 downregulation in myocardial
fibrosis. Thus, these suggest that restoring miR-29 expression may
facilitate with reversing fibrosis.
Conclusion
Fibrosis is a complex process that involves
excessive deposition and reorganization of the ECM, leading to
epithelial-mesenchymal transition and TGF-β activity. The activity
of miRNAs is significant for fibrosis. Over the past decade, a
numerous preclinical studies have revealed the role of miRNAs,
particularly the miR-29 family, in fibrotic skin diseases. Briefly,
downregulation of miR-29 expression levels leads to increased ECM
protein expression levels. In addition, TGF-β signalling is
responsible for decreased miR-29 expression levels during the
course of fibrosis. Therefore, targeting the miR-29 family and
TGF-β may be an effective strategy for the treatment of fibrosis. A
substantial level experimental evidence has indicated that
restoring downregulated miRNA expression with synthetic miRNA
mimics may be a useful strategy to overcome fibrosis. However, such
a strategy has certain technical (such as route of delivery and
stability in circulation) and biological (targeted delivery, uptake
rate and effective dose) limitations, which require further
investigation using animal models and clinical trials.
Acknowledgements
The authors would like to thank Dr Dimitris Kletsas,
Research Director of the Laboratory of Cell Proliferation &
Ageing (the Institute of Biology, National Centre for Scientific
Research ‘Demokritos’, Athens, Greece) for his valuable
contribution and comments.
References
|
1
|
Gardet A, Zheng TS and Viney JL: Genetic
architecture of human fibrotic diseases: Disease risk and disease
progression. Front Pharmacol. 4:1592013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vassiliadis E, Veidal SS, Barascuk N,
Mullick JB, Clausen RE, Larsen L, Simonsen H, Larsen DV, Bay-Jensen
AC, Segovia-Silvestre T, et al: Measurement of matrix
metalloproteinase 9-mediated collagen type III degradation fragment
as a marker of skin fibrosis. BMC Dermatol. 11:62011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Babalola O, Mamalis A, Lev-Tov H and
Jagdeo J: The role of microRNAs in skin fibrosis. Arch Dermatol
Res. 305:763–776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jinnin M: Various applications of
microRNAs in skin diseases. J Dermatol Sci. 74:3–8. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karsdal MA, Manon-Jensen T, Genovese F,
Kristensen JH, Nielsen MJ, Sand JM, Hansen NU, Bay-Jensen AC, Bager
CL, Krag A, et al: Novel insights into the function and dynamics of
extracellular matrix in liver fibrosis. Am J Physiol Gastrointest
Liver Physiol. 308:G807–G830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang X, Tsitsiou E, Herrick SE and
Lindsay MA: MicroRNAs and the regulation of fibrosis. FEBS J.
277:2015–2021. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jinnin M: Mechanisms of skin fibrosis in
systemic sclerosis. J Dermatol. 37:11–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vettori S, Gay S and Distler O: Role of
microRNAs in fibrosis. Open Rheumatol J. 6:130–139. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Villarreal G Jr, Oh DJ, Kang MH and Rhee
DJ: Coordinated regulation of extracellular matrix synthesis by the
microRNA-29 family in the trabecular meshwork. Invest Ophthalmol
Vis Sci. 52:3391–3397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wynn TA: Cellular and molecular mechanisms
of fibrosis. J Pathol. 214:199–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Almeida MI, Reis RM and Calin GA: MicroRNA
history: Discovery, recent applications, and next frontiers. Mutat
Res. 717:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bhaskaran M and Mohan M: MicroRNAs:
History, biogenesis, and their evolving role in animal development
and disease. Vet Pathol. 51:759–774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bushati N and Cohen SM: MicroRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Erkan EP, Breakefield XO and Saydam O:
miRNA signature of schwannomas: Possible role(s) of ‘tumor
suppressor’ miRNAs in benign tumors. Oncotarget. 2:265–270. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Senol O, Schaaij-Visser TB, Erkan EP,
Dorfer C, Lewandrowski G, Pham TV, Piersma SR, Peerdeman SM,
Ströbel T, Tannous B, et al: miR-200a-mediated suppression of
non-muscle heavy chain IIb inhibits meningioma cell migration and
tumor growth in vivo. Oncogene. 34:1790–1798. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Huang J, Guo M and Zuo X: MicroRNAs
Regulating Signaling Pathways: Potential Biomarkers in Systemic
Sclerosis. Genomics Proteomics Bioinformatics. 13:234–241. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Noetel A, Kwiecinski M, Elfimova N, Huang
J and Odenthal M: microRNA are Central Players in Anti- and
Profibrotic Gene Regulation during Liver Fibrosis. Front Physiol.
3:492012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cushing L, Kuang PP, Qian J, Shao F, Wu J,
Little F, Thannickal VJ, Cardoso WV and Lü J: miR-29 is a major
regulator of genes associated with pulmonary fibrosis. Am J Respir
Cell Mol Biol. 45:287–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kapinas K, Kessler C, Ricks T, Gronowicz G
and Delany AM: miR-29 modulates Wnt signaling in human osteoblasts
through a positive feedback loop. J Biol Chem. 285:25221–25231.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kapinas K, Kessler CB and Delany AM:
miR-29 suppression of osteonectin in osteoblasts: Regulation during
differentiation and by canonical Wnt signaling. J Cell Biochem.
108:216–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kriegel AJ, Liu Y, Fang Y, Ding X and
Liang M: The miR-29 family: Genomics, cell biology, and relevance
to renal and cardiovascular injury. Physiol Genomics. 44:237–244.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maurer B, Stanczyk J, Jüngel A,
Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE,
Michel BA, Distler JH, et al: MicroRNA-29, a key regulator of
collagen expression in systemic sclerosis. Arthritis Rheum.
62:1733–1743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Zhang X, Li H, Yu J and Ren X: The
role of miRNA-29 family in cancer. Eur J Cell Biol. 92:123–128.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Ghazwani M, Li J, Sun M, Stolz
DB, He F, Fan J, Xie W and Li S: miR-29b inhibits collagen
maturation in hepatic stellate cells through down-regulating the
expression of HSP47 and lysyl oxidase. Biochem Biophys Res Commun.
446:940–944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruvkun G: Molecular biology. Glimpses of a
tiny RNA world. Science. 294:797–799. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mott JL, Kurita S, Cazanave SC, Bronk SF,
Werneburg NW and Fernandez-Zapico ME: Transcriptional suppression
of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J
Cell Biochem. 110:1155–1164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kole AJ, Swahari V, Hammond SM and
Deshmukh M: miR-29b is activated during neuronal maturation and
targets BH3-only genes to restrict apoptosis. Genes Dev.
25:125–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mott JL, Kobayashi S, Bronk SF and Gores
GJ: mir-29 regulates Mcl-1 protein expression and apoptosis.
Oncogene. 26:6133–6140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei W, He HB, Zhang WY, Zhang HX, Bai JB,
Liu HZ, Cao JH, Chang KC, Li XY and Zhao SH: miR-29 targets Akt3 to
reduce proliferation and facilitate differentiation of myoblasts in
skeletal muscle development. Cell Death Dis. 4:e6682013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu K, Liu L, Zhang J, Wang Y, Liang H,
Fan G, Jiang Z, Zhang CY, Chen X and Zhou G: MiR-29b suppresses the
proliferation and migration of osteosarcoma cells by targeting
CDK6. Protein Cell. 7:434–444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roderburg C and Luedde T: Circulating
microRNAs as markers of liver inflammation, fibrosis and cancer. J
Hepatol. 61:1434–1437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Roderburg C, Urban GW, Bettermann K, Vucur
M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi
M, et al: Micro-RNA profiling reveals a role for miR-29 in human
and murine liver fibrosis. Hepatology. 53:209–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cushing L, Kuang P and Lü J: The role of
miR-29 in pulmonary fibrosis. Biochem Cell Biol. 93:109–118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang T, Liang Y, Lin Q, Liu J, Luo F, Li
X, Zhou H, Zhuang S and Zhang H: miR-29 mediates TGFβ1-induced
extracellular matrix synthesis through activation of PI3K-AKT
pathway in human lung fibroblasts. J Cell Biochem. 114:1336–1342.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maegdefessel L, Azuma J and Tsao PS:
MicroRNA-29b regulation of abdominal aortic aneurysm development.
Trends Cardiovasc Med. 24:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van Rooij E, Sutherland LB, Thatcher JE,
DiMaio JM, Naseem RH, Marshall WS, Hill JA and Olson EN:
Dysregulation of microRNAs after myocardial infarction reveals a
role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 105:pp.
13027–13032. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang G, Kwan BC, Lai FM, Chow KM, Li PK
and Szeto CC: Urinary miR-21, miR-29, and miR-93: Novel biomarkers
of fibrosis. Am J Nephrol. 36:412–418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kawashita Y, Jinnin M, Makino T, Kajihara
I, Makino K, Honda N, Masuguchi S, Fukushima S, Inoue Y and Ihn H:
Circulating miR-29a levels in patients with scleroderma spectrum
disorder. J Dermatol Sci. 61:67–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
O'Reilly S: miRNA-29a in systemic
sclerosis: A valid target. Autoimmunity. 48:511–512. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Peng WJ, Tao JH, Mei B, Chen B, Li BZ,
Yang GJ, Zhang Q, Yao H, Wang BX, He Q, et al: MicroRNA-29: A
potential therapeutic target for systemic sclerosis. Expert Opin
Ther Targets. 16:875–879. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kapinas K and Delany AM: MicroRNA
biogenesis and regulation of bone remodeling. Arthritis Res Ther.
13:2202011. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Knabel MK, Ramachandran K, Karhadkar S,
Hwang HW, Creamer TJ, Chivukula RR, Sheikh F, Clark KR, Torbenson
M, Montgomery RA, et al: Systemic Delivery of scAAV8-Encoded
MiR-29a Ameliorates Hepatic Fibrosis in Carbon
Tetrachloride-Treated Mice. PLoS One. 10:e01244112015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang J, Chu ES, Chen HY, Man K, Go MY,
Huang XR, Lan HY, Sung JJ and Yu J: microRNA-29b prevents liver
fibrosis by attenuating hepatic stellate cell activation and
inducing apoptosis through targeting PI3K/AKT pathway. Oncotarget.
6:7325–7338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xiao J, Meng XM, Huang XR, Chung AC, Feng
YL, Hui DS, Yu CM, Sung JJ and Lan HY: miR-29 inhibits
bleomycin-induced pulmonary fibrosis in mice. Mol Ther.
20:1251–1260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fang Y, Yu X, Liu Y, Kriegel AJ, Heng Y,
Xu X, Liang M and Ding X: miR-29c is downregulated in renal
interstitial fibrosis in humans and rats and restored by HIF-α
activation. Am J Physiol Renal Physiol. 304:F1274–F1282. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Qin W, Chung AC, Huang XR, Meng XM, Hui
DS, Yu CM, Sung JJ and Lan HY: TGF-β/Smad3 signaling promotes renal
fibrosis by inhibiting miR-29. J Am Soc Nephrol. 22:1462–1474.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ciechomska M, O'Reilly S, Suwara M,
Bogunia-Kubik K and van Laar JM: miR-29a reduces TIMP-1 production
by dermal fibroblasts via targeting TGF-β activated kinase 1
binding protein 1, implications for systemic sclerosis. PLoS One.
9:e1155962014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jafarinejad-Farsangi S, Farazmand A,
Mahmoudi M, Gharibdoost F, Karimizadeh E, Noorbakhsh F, Faridani H
and Jamshidi AR: MicroRNA-29a induces apoptosis via increasing the
Bax: Bcl-2 ratio in dermal fibroblasts of patients with systemic
sclerosis. Autoimmunity. 48:369–378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Steele R, Mott JL and Ray RB: MBP-1
upregulates miR-29b that represses Mcl-1, collagens, and
matrix-metalloproteinase-2 in prostate cancer cells. Genes Cancer.
1:381–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Takemoto R, Jinnin M, Wang Z, Kudo H,
Inoue K, Nakayama W, Ichihara A, Igata T, Kajihara I, Fukushima S,
et al: Hair miR-29a levels are decreased in patients with
scleroderma. Exp Dermatol. 22:832–833. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhu H, Luo H and Zuo X: MicroRNAs: Their
involvement in fibrosis pathogenesis and use as diagnostic
biomarkers in scleroderma. Exp Mol Med. 45:e412013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pavletic SZ and Fowler DH: Are we making
progress in GVHD prophylaxis and treatment? Hematology Am Soc
Hematol Educ Program. 2012:251–264. 2012.PubMed/NCBI
|
|
58
|
Paczesny S, Raiker N, Brooks S and Mumaw
C: Graft-versus-host disease biomarkers: Omics and personalized
medicine. Int J Hematol. 98:275–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xiao B, Wang Y, Li W, Baker M, Guo J,
Corbet K, Tsalik EL, Li QJ, Palmer SM, Woods CW, et al: Plasma
microRNA signature as a noninvasive biomarker for acute
graft-versus-host disease. Blood. 122:3365–3375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Atarod S and Dickinson AM: MicroRNAs: The
Missing Link in the Biology of Graft-Versus-Host Disease? Front
Immunol. 4:4202013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hahn JM, McFarland KL, Combs KA and Supp
DM: Partial epithelial-mesenchymal transition in keloid scars:
Regulation of keloid keratinocyte gene expression by transforming
growth factor-β1. Burns Trauma. 4:302016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang GY, Wu LC, Liao T, Chen GC, Chen YH,
Zhao YX, Chen SY, Wang AY, Lin K, Lin DM, et al: A novel regulatory
function for miR-29a in keloid fibrogenesis. Clin Exp Dermatol.
41:341–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu Y, Yang D, Xiao Z and Zhang M: miRNA
expression profiles in keloid tissue and corresponding normal skin
tissue. Aesthetic Plast Surg. 36:193–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lian N and Li T: Growth factor pathways in
hypertrophic scars: Molecular pathogenesis and therapeutic
implications. Biomed Pharmacother. 84:42–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ning P, Liu DW, Mao YG, Peng Y, Lin ZW and
Liu DM: Differential expression profile of microRNA between
hyperplastic scar and normal skin]. Zhonghua Yi Xue Za Zhi.
92:692–694. 2012.(In Chinese). PubMed/NCBI
|
|
66
|
Guo J, Lin Q, Shao Y, Rong L and Zhang D:
miR-29b promotes skin wound healing and reduces excessive scar
formation by inhibition of TGF-β1/Smad/CTGF signaling pathway. Can
J Physiol Pharmacol. 95:437–442. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhou R, Zhang Q, Zhang Y, Fu S and Wang C:
Aberrant miR-21 and miR-200b expression and its pro-fibrotic
potential in hypertrophic scars. Exp Cell Res. 339:360–366. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li G, Zhou R, Zhang Q, Jiang B, Wu Q and
Wang C: Fibroproliferative effect of microRNA-21 in hypertrophic
scar derived fibroblasts. Exp Cell Res. 345:93–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mu S, Kang B, Zeng W, Sun Y and Yang F:
MicroRNA-143-3p inhibits hyperplastic scar formation by targeting
connective tissue growth factor CTGF/CCN2 via the Akt/mTOR pathway.
Mol Cell Biochem. 416:99–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gay S, Distler O and Maurer B: Treatment
of scleroderma US Patent US20110218233 A1. Filed September 4, 2009;
issued September 8. 2011
|
|
71
|
Zhu JN, Chen R, Fu YH, Lin QX, Huang S,
Guo LL, Zhang MZ, Deng CY, Zou X, Zhong SL, et al: Smad3
inactivation and miR-29b upregulation mediate the effect of
carvedilol on attenuating the acute myocardium infarction-induced
myocardial fibrosis in rat. PLoS One. 8:e755572013. View Article : Google Scholar : PubMed/NCBI
|