Introduction
Endothelial progenitor cells (EPCs) have been
demonstrated as important in neovascularisation and contribute to
vascular repair (1). Endothelial
colony-forming cells (ECFCs) are a subset of EPCs, also referred to
as late-outgrowth EPCs, which exhibit essential progenitor
characteristics, including high proliferative potential and the
capacity for self-renewal. ECFCs have received widespread attention
as the primary progenitor population contributing to
neovasculogenesis, in particular due to their reparative capacity
to ameliorate vascular injury (2,3). Reduced
numbers and altered function of ECFCs are associated with
endothelial dysfunction under disease conditions, such as diabetic
hyperglycemia and arterial hypertension (4–6).
Furthermore, endothelial dysfunction is an early abnormality in the
process that leads to atherosclerosis and its associated
complications (7). Currently, the
transfer of ECFCs is considered to be a therapeutic modality that
is potentially effective in ischemia-associated conditions, such as
myocardial infarction (8) and in
non-ischemic inflammatory models, such as dilated cardiomyopathy
(9). ECFC levels have been shown to be
negatively correlated with risk factors for atherosclerosis
(10), suggesting that decreased
repair of vascular plaques may be the result of ECFC depletion or
insufficient production (11). Repair
of a damaged endothelium by ECFCs is likely critical in preventing
or limiting blood vessel injury (12–14).
Evidence from in vitro and clinical studies
indicates that inflammation and oxidative stress triggers ECFC
apoptosis. Atherosclerosis develops through an inflammatory
pathology, which results in plaque formation and acute coronary
events. Tumor necrosis factor (TNF)-α is a proinflammatory cytokine
that is central in the pathogenesis of atherosclerosis (15,16) and a
contributing risk factor in metabolic disorders, such as insulin
resistance and dyslipidemia. Primarily produced by activated
macrophages (17), TNF-α regulates
numerous immune cell functions, including rolling, adhesion,
proliferation and apoptosis (18).
Additionally, TNF-α is key as an inflammatory mediator and as an
inducer of apoptosis in endothelial cells and ECFCs.
Pituitary adenylate cyclase-activating polypeptide
(PACAP), encoded by the ADCYAP1 gene, was originally isolated from
an ovine hypothalamus extract (19),
and has been identified in gastrointestinal, respiratory,
cardiovascular and urogenital systems (20,21). PACAP
is one of the members of the vasoactive intestinal
peptide/secretin/growth hormone-releasing hormone/glucagon
superfamily and exists in two biological active forms, PACAP 38 and
PACAP 27, with three currently identified receptors: PAC1, VPAC1
and VPAC2. PACAP has been reported to promote survival in various
types of cell, including lymphocytes, chondrocytes, endothelial
cells and Schwann cells, as well as in liver, lung, and ovarian
tissue samples (22,23). Adcyap1-deficient mice exhibited a
markedly higher degree of, as well as more wide spread,
inflammation (24), increased levels
of proinflammatory cytokines, such as interleukin (IL)-6, TNF-α,
interferon (IFN)-γ and decreased levels of IL-4 (25). Due to its broad distribution in tissues
and its cytoprotective effects on many cell types, PACAP is
considered to be an attractive therapeutic candidate possessing
anti-inflammatory and anti-atherosclerotic potential.
Materials and methods
Materials
TNF-α was obtained from PeproTech, Inc. (Rocky Hill,
NJ, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5 diphenyltetrazolium
bromide (MTT) was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Synthetic PACAP38 was obtained from GL Biochem
(Shanghai) Ltd. (Shanghai, China). Cluster of differentiation CD34
(dilution, 1:200; cat. no. 561440), CD31 (dilution, 1:200; cat. no.
562861) and KDR (dilution, 1:400; cat. no. 560494) antibodies were
obtained from BD Biosciences (San Jose, CA, USA). The secondary
antibody (goat anti-rabbit HRP-IgG; cat. no. sc-2048) was purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The FITC
Annexin V Apoptosis Detection kit was purchased from BD Pharmingen
(San Diego, CA, USA) and endothelial differentiation medium
(EGM-2MV) was obtained from Clonetics Corp. (San Diego, CA, USA).
Lymphoprep™ was obtained from Axis-shield (Oslo, Norway). The human
umbilical cord blood (UCB) was obtained from six patients of the
First Affiliated Hospital of Jinan University (Guangzhou, China)
between May 2014 to January 2015. Patients with known genetic
diseases, cancer, a history of anemia, or medical conditions that
involved cardiopulmonary insufficiency, were excluded. All subjects
provided written informed consent and the present study was
approved by the Regional Ethics Committee of Jinan University.
Cell culture
ECFCs derived from human UCB were isolated by
Lymphoprep™ density gradient centrifugation at 800 × g for 20 min
at 4°C. Following centrifugation, the mononuclear cell (MNC) layer
was harvested and washed twice with 0.9% saline. The MNCs were
cultured at 37°C in 5% CO2 in endothelial
differentiation medium (EGM-2MV) containing 5% fetal bovine serum,
vascular endothelial growth factor, fibroblast growth factor-2,
epidermal growth factor and insulin-like growth factor-1.
Approximately two days after the initial plating, non-adherent
cells were discarded and fresh medium was applied; thereafter, the
medium was replaced on alternating days. Subsequent to 1–2 weeks of
culture, the cells exhibited a paving stone-like morphology and
were identified as ECFCs by immunohistochemical staining. In order
to evaluate the effect of TNF-α on ECFCs, ECFCs were treated with
TNF-α (10, 20 and 40 ng/ml) for 24 h and the cleaved caspase-3
expression level was determined by western blot analysis.
Subsequently, flow cytometric apoptosis assays were performed on
the control group (cells were cultured in EGM-2MV), the TNF-α group
(cells were cultured in EGM-2MV, pulsed with 20 ng/ml TNF-α) and 3
PACAP groups [the ECFCs were treated with 20 ng/ml TNF-α for 5 min
and the medium was replaced with fresh medium, which contained
PACAP (1, 10 and 100 nM)]. The MTT assay and flow cytometry were
performed to evaluate cell proliferation and cell cycle.
ECFC immunohistochemistry
ECFCs were fixed with 4% paraformaldehyde for 15 min
and permeabilized with 0.5% Triton X-100 in phosphate-buffered
saline (PBS) buffer (0.1 mmol/l) for 20 min. After blocking with
10% goat serum for 20 min, cells were incubated with anti-CD31
(1:200), -CD34 (1:200), or -KDR (1:400) antibodies overnight at
4°C. Cells were washed three times with PBS, which was followed by
application of the goat anti-rabbit IgG secondary antibody (1:500)
with an avidin-peroxidase conjugate. Visualization was performed
using a DAB chromogen kit (cat. no. ST033; Beyotime Institute of
Biotechnology, Shanghai, China) and images were obtained by
bright-field microscopy (IX71; Olympus Corporation, Tokyo,
Japan).
Western blot analysis
ECFCs were homogenized in PBS containing a protease
inhibitor cocktail (50 mM Tris, pH 7.6, 150 mM NaCl, 1 mM EDTA, 1%
Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS and 10% glycerol).
Samples were separated by 10% SDS-PAGE and then transferred to
nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) at 30 V for 20 min. The membranes were blocked with 5% bovine
serum albumin (w/v) at room temperature for 1 h, and incubated with
primary anti-cleaved caspase 3 antibody (1:1,000; Abcam, Cambridge,
MA, USA; cat. no. ab-2302) at 4°C overnight, and then incubated
with secondary antibody (1:3,000; Santa Cruz Biotechnology, Inc;
cat. no. sc-2048) at room temperature for 1 h, developed with
chemiluminescence ECL reagent (LumiGold; SignaGen Laboratories,
Rockville, MD, USA) and exposed to Hyperfilm MP (Beyotime Institute
of Biotechnology). The results of each time point in each group
were normalized to GAPDH. The relative band intensities of the
blots were determined using Adobe Photoshop software (Adobe
Photoshop 6.0; Adobe, San Jose, CA, USA).
Apoptosis analysis
ECFCs were harvested, washed with ice-cold PBS,
resuspended in binding buffer (500 µl), and incubated with
propidium iodide (PI; 5 µl) and Annexin V-fluorescein
isothiocyanate (5 µl) at 4°C in the dark for 15 min. The cells were
then washed and resuspended in PBS (500 µl) and analyzed by flow
cytometry (BD Biosciences, San Jose, CA, USA) according to the
manufacturer's instructions.
MTT assay
ECFCs were seeded in 96-well plates (4,000 cells per
well). At a series of time points (24, 48, 72 and 96 h) following
treatment with TNF-α and/or PACAP, depending on the group, the
cells were incubated with MTT (5 mg/ml) in PBS for 3 h, and
dissolved with 50% N,N dimethylformamide (Beyotime Institute of
Biotechnology) and 10% SDS for 3 h at 37°C. The optical density was
measured at 570 nm. Data are representative of three biological
replicates.
Cell cycle analysis
The cell cycle was determined using a Cell Cycle
Assay kit from GenMed Scientifics Inc. (Shanghai, China). Briefly,
cells were washed twice with PBS and fixed in 80% ethanol. The
fixed cells were washed with PBS, incubated in PI (50 µg/ml) at
room temperature for 20 min and immediately analyzed by flow
cytometry according to the manufacturer's instructions.
Statistical analysis
An unpaired Student's t-test was used to evaluate
statistical differences between the groups. Data are expressed as
the mean ± standard deviation and P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using SPSS version 19 software (IBM SPSS,
Armonk, NY, USA).
Results
Culture and identification of
ECFCs
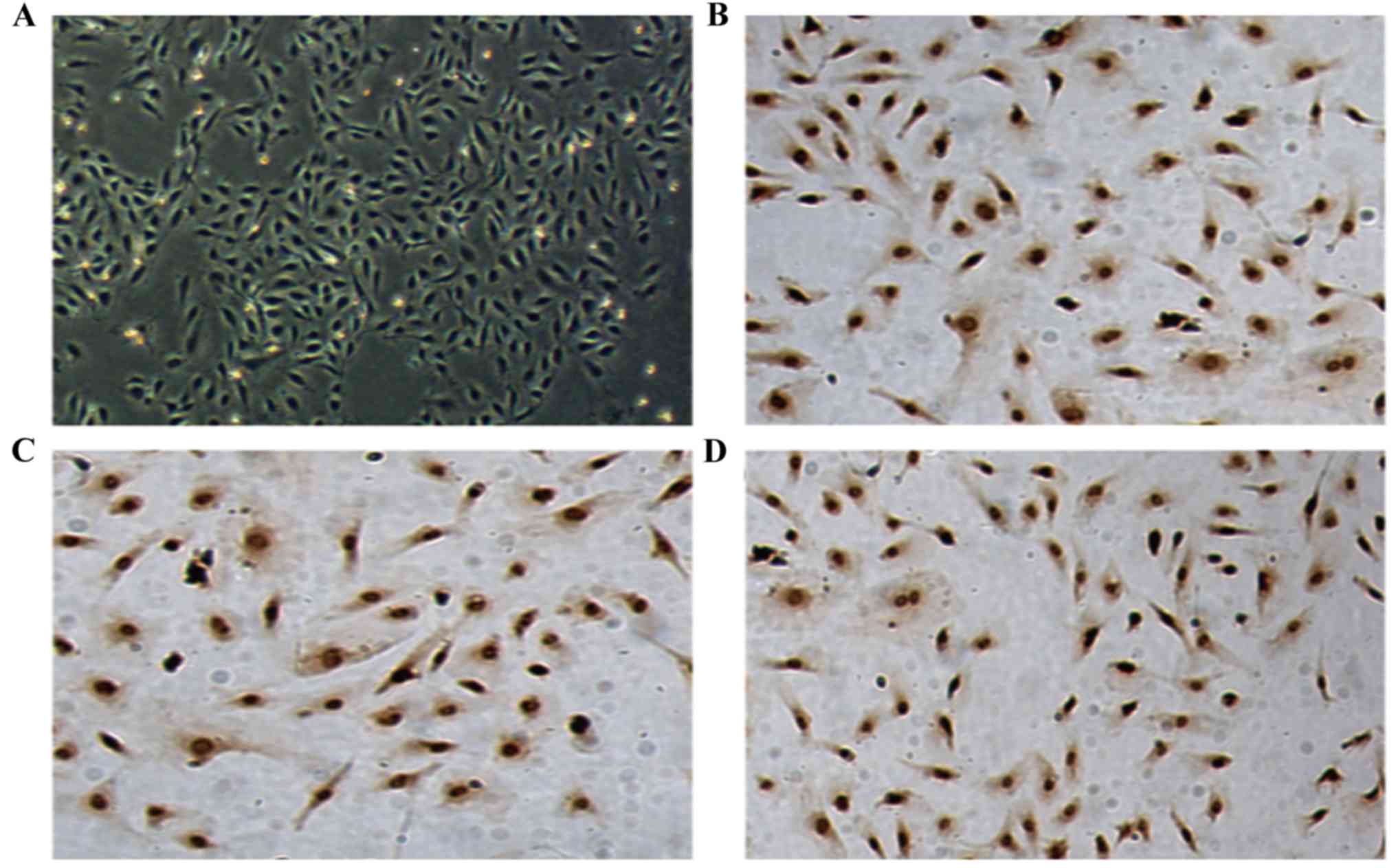
MNCs were isolated from human UCB and seeded in
collagen-coated tissue culture plates in EGM-2MV media. Following
~1 week of culture, the cells exhibited a paving stone-like
morphology (Fig. 1A).
Immunocytochemical staining was positive for CD34 (dilution,
1:200), CD31 (dilution, 1:200) and KDR (dilution, 1:400; Fig. 1B-D), identifying the cells as
ECFCs.
TNF-α induces ECFC apoptosis
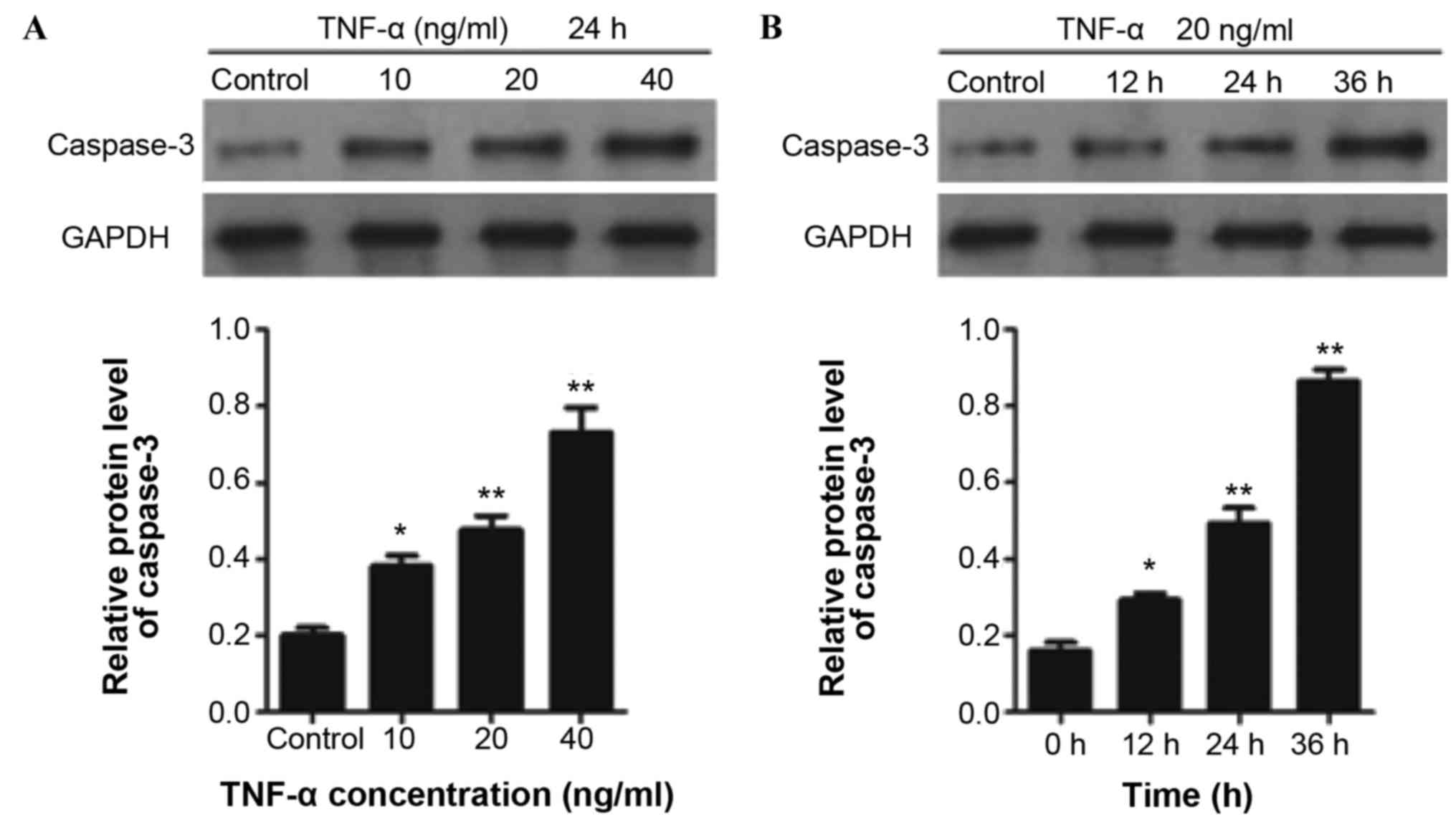
TNF-α significantly increased the cleaved caspase-3
expression level in ECFCs in a concentration-dependent manner
(Fig. 2A). In addition, a time course
test of caspase-3 expression in ECFCs was performed by treating
ECFCs with TNF-α (20 ng/ml) for 12, 24 and 36 h. The results
indicated that TNF-α treatment significantly increased the cleaved
caspase-3 expression level in a time-dependent manner (Fig. 2B).
PACAP inhibits TNF-α-induced apoptosis
of ECFCs
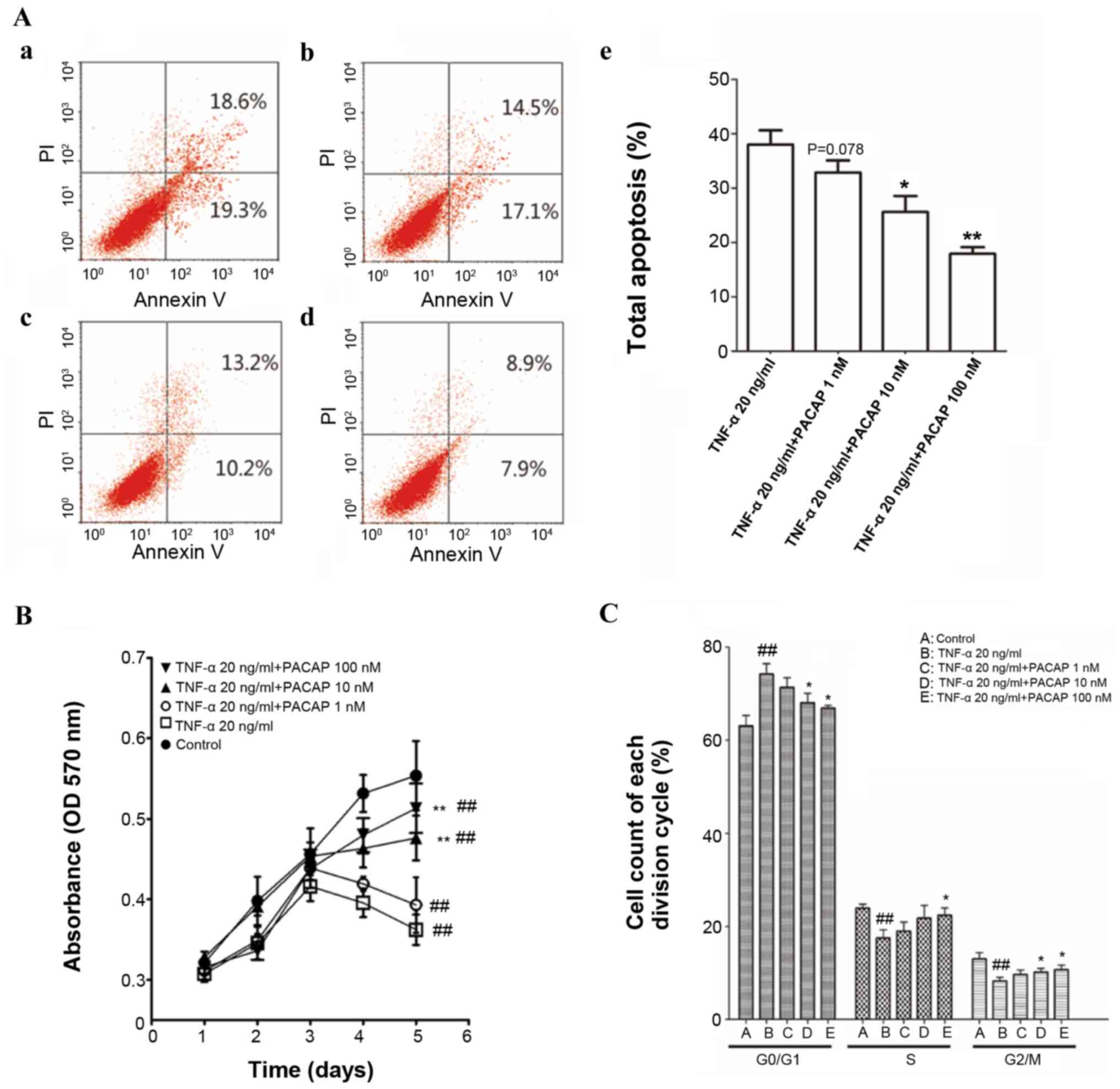
Flow cytometric apoptosis assays indicated that
PACAP significantly decreased the number of apoptotic cells in a
concentration-dependent manner compared with the TNF-α (20 ng/ml)
group. The higher concentrations of PACAP (10 and 100 nM) induced a
statistically significant reduction in apoptotic cell number
compared with the TNF-α (20 ng/ml) group (P<0.05 and P<0.01).
The lower concentration of PACAP (1 nM) reduced the apoptotic cell
number, but this was not statistically significant when compared
with the TNF-α (20 ng/ml) group (P=0.078; Fig. 3A).
PACAP restored cell proliferation,
which was reduced by TNF-α
The viability of ECFCs was evaluated by MTT assay.
The proliferation of TNF-α (20 ng/ml) group was markedly inhibited
compared with the control group (P<0.01). High concentrations of
PACAP (10 and 100 nM) rescued cell proliferation when compared with
the TNF-α (20 ng/ml) group (P<0.01). No significant differences
were observed in cell proliferation between the TNF-α (20 ng/ml)
group and the low concentration PACAP (1 nM) group (Fig. 3B).
Effect of PACAP on cell cycle
progression
A dose-dependent effect of PACAP on the cell cycle
was observed in the present study. The effect of PACAP on cells
appears to be dose-dependent, as a higher dosage of PACAP resulted
in a greater number of cells in the G2/M phase. After 48 h of 100
nM PACAP treatment, cells in the G2/M population increased from
8.27 to 10.75% compared with the TNF-α group, whereas the group of
cells treated with 10 nM PACAP, the G2/M population increased to
10.17%. The increase of cell population at the G2/M phase was
accompanied by a decrease of cell population in the G1 phase of the
cell cycle (Fig. 3C).
Discussion
Increased expression levels of inflammation
mediators have been implicated in numerous types of vascular
disease and these mediators are known to negatively impact
endothelial cell function in atherosclerosis. Cytoprotective agents
present as a promising preventative therapeutic strategy for these
conditions, as they negate the apoptotic effects of inflammation.
During the pathological progression of atherosclerosis, monocytes
and T-lymphocytes are recruited to blood vessel walls and release
chemokines, such as monocyte chemoattractant protein-1, TNF-α and
IL-6, which are involved in maintaining the inflammatory process.
ECFCs are recruited from the bone marrow to these sites of
inflammatory signals. Previous studies indicated that acute
exposure to low concentrations of TNF-α increases the adhesive
properties of ECFCs to vascular endothelial cells (19), although chronic inflammatory
stimulation induces ECFC apoptosis (26,27).
PACAP has been shown to limit the effects of chronic
inflammation in rheumatoid arthritis and osteoarthritis (28,29).
Furthermore, there is strong evidence that PACAP is involved in
repair of nerve injury and damage induced by inflammation and
oxidative stress (30–32). In addition, our previous studies
demonstrated a role for PACAP in atherosclerosis in rabbits and
identified receptors for PACAP in cardiovascular tissue (33–35). As a
powerful stimulator of inflammation, TNF-α has been reported to
induce apoptosis and senescence of ECFCs or other stem cells in
vitro and in vivo (36–40);
however, to the best of our knowledge, no previous studies have
linked PACAP to attenuation of TNF-α-induced apoptosis in ECFCs.
Caspase-3 is activated in the apoptotic cell by extrinsic and
intrinsic signaling pathways (41). A
positive correlation was identified between TNF-α and cleaved
caspase-3 expression levels in ECFCs in the present study,
indicating that TNF-α-mediated inflammation induced ECFC apoptosis.
Furthermore, PACAP was shown to exert a direct anti-apoptotic
effect on ECFCs treated with TNF-α and enhance proliferation of
ECFCs that are inhibited by TNF-α. It has been reported that low
concentrations of PACAP cross the blood-brain barrier and may
stimulate neurons directly, as well as stimulate astrocytes and
microglia to secrete neuroprotective factors (42). During the process of cytoprotection,
PACAP may regulate the dynamic balance between the growth
factor-activated extracellular signal-regulated kinases and
stress-activated c-Jun N-terminal kinases-p38 signaling pathways in
neuronal systems (43). However, the
anti-apoptosis mechanism of PACAP has not yet been elucidated in
ECFCs. Further studies are required to identify the molecular
pathways mediating the effects of PACAP and identify the PACAP
receptors in ECFCs.
In conclusion, the present results indicate that
TNF-α induced apoptosis in ECFCs in a concentration- and
time-dependent manner; however, PACAP partially blocks the negative
effects of prolonged TNF-α exposure, including ECFC apoptosis,
growth inhibition and cell cycle distribution, indicating its
therapeutic potential for the treatment of circulatory
diseases.
Acknowledgements
The current study was supported by the Key Subject
Construction of the First Affiliated Hospital of Jinan University
(Guangzhou, China; grant no. 2010-4).
References
|
1
|
Asahara T: Cell therapy and gene therapy
using endothelial progenitor cells for vascular regeneration. Handb
Exp Pharmacol. 180:181–194. 2007. View Article : Google Scholar
|
|
2
|
Ingram DA, Mead LE, Tanaka H, Meade V,
Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D and Yoder
MC: Identification of a novel hierarchy of endothelial progenitor
cells using human peripheral and umbilical cord blood. Blood.
104:2752–2760. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steinmetz M, Nickenig G and Werner N:
Endothelial-regenerating cells: An expanding universe.
Hypertension. 55:593–599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blue EK, DiGiuseppe R, Derr-Yellin E,
Acosta JC, Pay SL, Hanenberg H, Schellinger MM, Quinney SK, Mund
JA, Case J, et al: Gestational diabetes induces alterations in the
function of neonatal endothelial colony-forming cells. Pediatr Res.
75:266–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ingram DA, Lien IZ, Mead LE, Estes M,
Prater DN, Derr-Yellin E, DiMeglio LA and Haneline LS: In vitro
hyperglycemia or a diabetic intrauterine environment reduces
neonatal endothelial colony-forming cell numbers and function.
Diabetes. 57:724–731. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ikutomi M, Sahara M, Nakajima T, Minami Y,
Morita T, Hirata Y, Komuro I, Nakamura F and Sata M: Diverse
contribution of bone marrow-derived late-outgrowth endothelial
progenitor cells to vascular repair under pulmonary arterial
hypertension and arterial neointimal formation. J Mol Cell Cardiol.
86:121–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vanhoutte PM: Endothelial dysfunction: The
first step toward coronary arteriosclerosis. Circ J. 73:595–601.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi
JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, et al:
Therapeutic potential of ex vivo expanded endothelial progenitor
cells for myocardial ischemia. Circulation. 103:634–637. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Werner L, Deutsch V, Barshack I, Miller H,
Keren G and George J: Transfer of endothelial progenitor cells
improves myocardial performance in rats with dilated cardiomyopathy
induced following experimental myocarditis. J Mol Cell Cardiol.
39:691–697. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hill JM, Zalos G, Halcox JP, Schenke WH,
Waclawiw MA, Quyyumi AA and Finkel T: Circulating endothelial
progenitor cells, vascular function, and cardiovascular risk. N
Engl J Med. 348:593–600. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scalone G, De Caterina A, Leone AM,
Tritarelli A, Mollo R, Pinnacchio G, D'Amario D, Lanza GA and Crea
F: Effect of exercise on circulating endothelial progenitor cells
in microvascular angina. Circ J. 77:1777–1782. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujita Y and Asahara T: Evaluation of
circulating endothelial progenitor cells in cardiovascular risk.
Circ J. 75:2541–2542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Etemadifar M, Dehghani L, Ganji H,
Soleimani R, Talebi M, Eskandari N, Samani FS and Meamar R:
Evaluation of the circulating CD34(+), CD309(+), and endothelial
progenitor cells in patients with first attack of optic neuritis.
Adv Biomed Res. 4:1512015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duckers HJ, Silber S, De Winter R, den
Heijer P, Rensing B, Rau M, Mudra H, Benit E, Verheye S, Wijns W,
et al: Circulating endothelial progenitor cells predict
angiographic and intravascular ultrasound outcome following
percutaneous coronary interventions in the HEALING-II trial:
Evaluation of an endothelial progenitor cell capturing stent.
EuroIntervention. 3:67–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaehler J, Osterholz S, Patten M, Koester
R and Meinertz T: Cytokines in the pathogenesis of atherosclerosis.
Dtsch Med Wochenschr. 127:94–99. 2002.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takahashi M: Inflammatory cytokines in the
pathogenesis of atherosclerosis. Nihon Rinsho. 69:30–33. 2011.(In
Japanese). PubMed/NCBI
|
|
17
|
Prisco AR, Prisco MR, Carlson BE and
Greene AS: TNF-α increases endothelial progenitor cell adhesion to
the endothelium by increasing bond expression and affinity. Am J
Physiol Heart Circ Physiol. 308:H1368–H1381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walsh LJ, Trinchieri G, Waldorf HA,
Whitaker D and Murphy GF: Human dermal mast cells contain and
release tumor necrosis factor alpha, which induces endothelial
leukocyte adhesion molecule 1. Proc Natl Acad Sci USA. 88:pp.
4220–4224. 1991; View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyata A, Arimura A, Dahl RR, Minamino N,
Uehara A, Jiang L, Culler MD and Coy DH: Isolation of a novel 38
residue-hypothalamic polypeptide which stimulates adenylate cyclase
in pituitary cells. Biochem Biophys Res Commun. 164:567–574. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heimesaat MM, Dunay IR, Schulze S, Fischer
A, Grundmann U, Alutis M, Kühl AA, Tamas A, Toth G, Dunay MP, et
al: Pituitary adenylate cyclase-activating polypeptide ameliorates
experimental acute ileitis and extra-intestinal sequelae. PLoS One.
9:e1083892014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vaudry D, Falluel-Morel A, Bourgault S,
Basille M, Burel D, Wurtz O, Fournier A, Chow BKC, Hashimoto H,
Galas L, et al: Pituitary adenylate cyclase-activating polypeptide
and its receptors: 20 years after the discovery. Pharmacol Rev.
61:283–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giunta S, Castorina A, Adorno A, Mazzone
V, Carnazza ML and D'Agata V: PACAP and VIP affect NF1 expression
in rat malignant peripheral nerve sheath tumor (MPNST) cells.
Neuropeptides. 44:45–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Castorina A, Waschek JA, Marzagalli R,
Cardile V and Drago F: PACAP interacts with PAC1 receptors to
induce tissue plasminogen activator (tPA) expression and activity
in schwann cell-like cultures. PLoS One. 10:e01177992015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan YV, Abad C, Lopez R, Dong H, Liu S,
Lee A, Gomariz RP, Leceta J and Waschek JA: Pituitary adenylyl
cyclase-activating polypeptide is an intrinsic regulator of Treg
abundance and protects against experimental autoimmune
encephalomyelitis. Proc Natl Acad Sci USA. 106:pp. 2012–2017. 2009;
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Armstrong BD, Abad C, Chhith S, Cheung-Lau
G, Hajji OE, Nobuta H and Waschek JA: Impaired nerve regeneration
and enhanced neuroinflammatory response in mice lacking pituitary
adenylyl cyclase activating peptide. Neuroscience. 151:63–73. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du G, Song Y, Zhang T, Ma L, Bian N, Chen
X, Feng J, Chang Q and Li Z: Simvastatin attenuates TNF-α induced
apoptosis in endothelial progenitor cells via the upregulation of
SIRT1. Int J Mol Med. 34:177–182. 2014.PubMed/NCBI
|
|
27
|
Sasi SP, Song J, Enderling H, Yan X and
Goukassian DA: TNF-α and IL-1α but not MCP 1 and Rantes increase
significantly the formation of p-H2AX foci in naive BM-derived
TNFR1/p55KO EPCs. J Radiat Res. 55 Suppl 1:i122–i123. 2014.
View Article : Google Scholar
|
|
28
|
Botz B, Bölcskei K, Kereskai L, Kovács M,
Németh T, Szigeti K, Horváth I, Máthé D, Kovács N, Hashimoto H, et
al: Differential regulatory role of pituitary adenylate
cyclase-activating polypeptide in the serum-transfer arthritis
model. Arthritis Rheumatol. 66:2739–2750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Juhász T, Helgadottir SL, Tamás A, Reglődi
D and Zákány R: PACAP and VIP signaling in chondrogenesis and
osteogenesis. Peptides. 66:51–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu R, Xie S, Chen J, Zhang L and Dai Y:
The effects of PACAP and related peptides on leptin, soluble leptin
receptor and resistin in normal condition and LPS-induced
inflammation. Peptides. 30:1456–1459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsumuraya T, Ohtaki H, Song D, Sato A,
Watanabe J, Hiraizumi Y, Nakamachi T, Xu Z, Dohi K, Hashimoto H, et
al: Human mesenchymal stem/stromal cells suppress spinal
inflammation in mice with contribution of pituitary adenylate
cyclase-activating polypeptide (PACAP). J Neuroinflammation.
12:352015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Waschek JA: VIP and PACAP: Neuropeptide
modulators of CNS inflammation, injury, and repair. Br J Pharmacol.
169:512–523. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang Q, Li ZC and Deng Y: The
distribution of pacap receptor on the cardiovascular tissues and
cells. J Clin Cardiol. 18:329–331. 2002.
|
|
34
|
Chang Q, Zhang L, Tang HL, Huang HM and Zi
Cheng LI: Effects of pituitary adenylate cyclase activating
polypeptide on cardiac remodeling in coronary atherosclerotic
rabbits. Chin J Pathophysiology. 23:529–532. 2007.
|
|
35
|
Cheng ZL: The changes of collagen and mmp
2 in atherosclerotic rabbit aorta and effect of pacap. Guangdong
Med. 29:1107–1109. 2008.
|
|
36
|
Qiao J, Qi K, Chu P, Mi H, Yang N, Yao H,
Xia Y, Li Z, Xu K and Zeng L: Infusion of endothelial progenitor
cells ameliorates liver injury in mice after haematopoietic stem
cell transplantation. Liver Int. 35:2611–2620. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martini G, Biscaro F, Boscaro E, Calabrese
F, Lunardi F, Facco M, Agostini C, Zulian F and Fadini GP: Reduced
levels of circulating progenitor cells in juvenile idiopathic
arthritis are counteracted by anti TNF-α therapy. BMC Musculoskelet
Disord. 16:1032015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang XX, Yang JX, Pan YY and Zhang YF:
Protective effects of tanshinone IIA on endothelial progenitor
cells injured by tumor necrosis factor-α. Mol Med Rep.
12:4055–4062. 2015.PubMed/NCBI
|
|
39
|
Cao Q, Wang Y, Huang L, Wang F and Chen S:
TNF receptor-associated factor 6 (TRAF6) mediates the
angiotensin-induced non-canonical TGF-β pathway activation of
c-kit(+) cardiac stem cells. Am J Transl Res. 7:2233–2243.
2015.PubMed/NCBI
|
|
40
|
Liao L, Su X, Yang X, Hu C, Li B, Lv Y,
Shuai Y, Jing H, Deng Z and Jin Y: TNF-α Inhibits FoxO1 by
Upregulating miR-705 to Aggravate Oxidative Damage in Bone
Marrow-Derived Mesenchymal Stem Cells during Osteoporosis. Stem
Cells. 34:1054–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Salvesen GS: Caspases: Opening the boxes
and interpreting the arrows. Cell Death Differ. 9:3–5. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shioda S, Ohtaki H, Nakamachi T, Dohi K,
Watanabe J, Nakajo S, Arata S, Kitamura S, Okuda H, Takenoya F, et
al: Pleiotropic functions of PACAP in the CNS: Neuroprotection and
neurodevelopment. Ann N Y Acad Sci. 1070:550–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
May V, Lutz E, MacKenzie C, Schutz KC,
Dozark K and Braas KM: Pituitary adenylate cyclase-activating
polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates
multiple neurotrophic signaling pathways: Akt activation through
phosphatidylinositol 3-kinase gamma and vesicle endocytosis for
neuronal survival. J Biol Chem. 285:9749–9761. 2010. View Article : Google Scholar : PubMed/NCBI
|