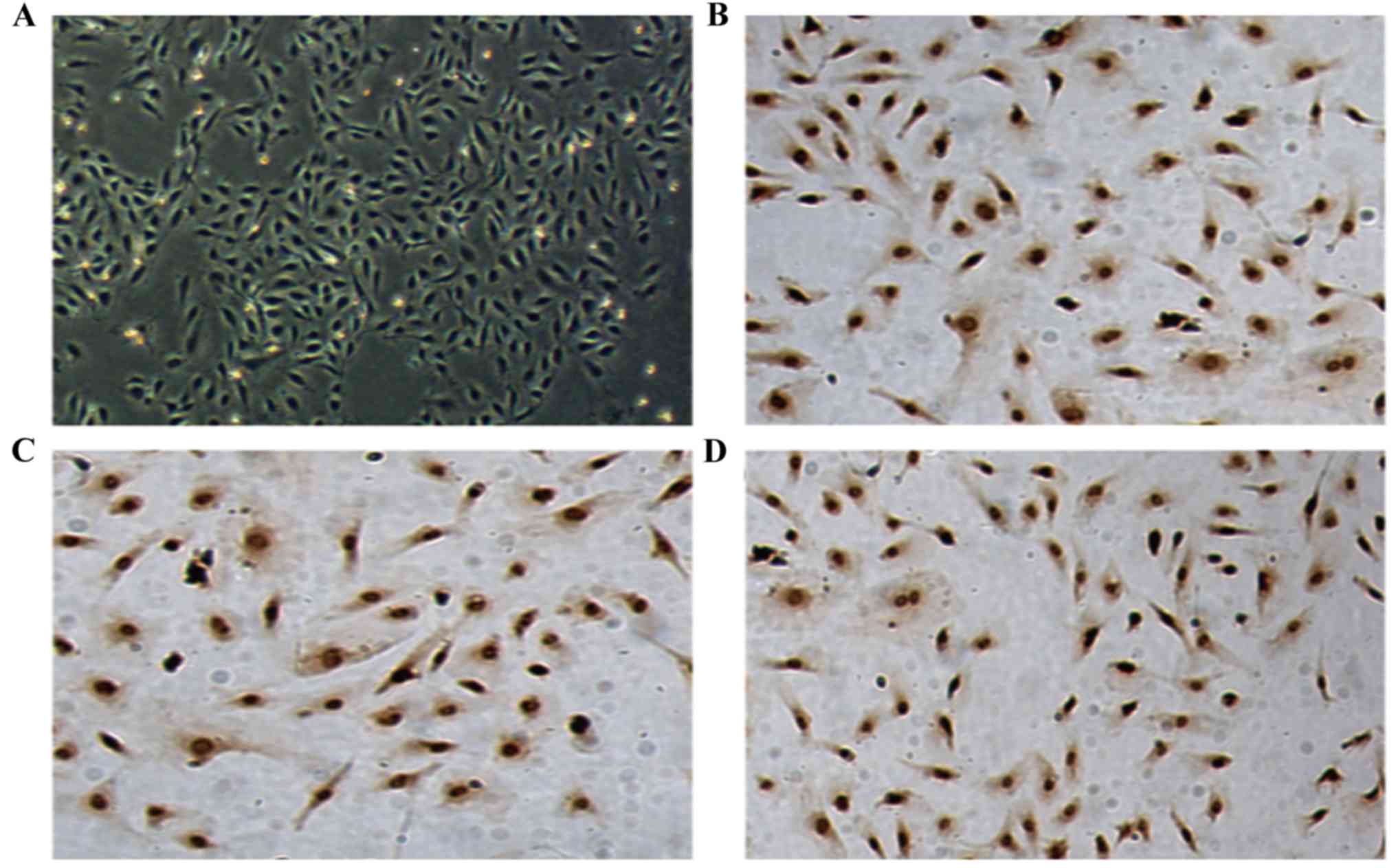
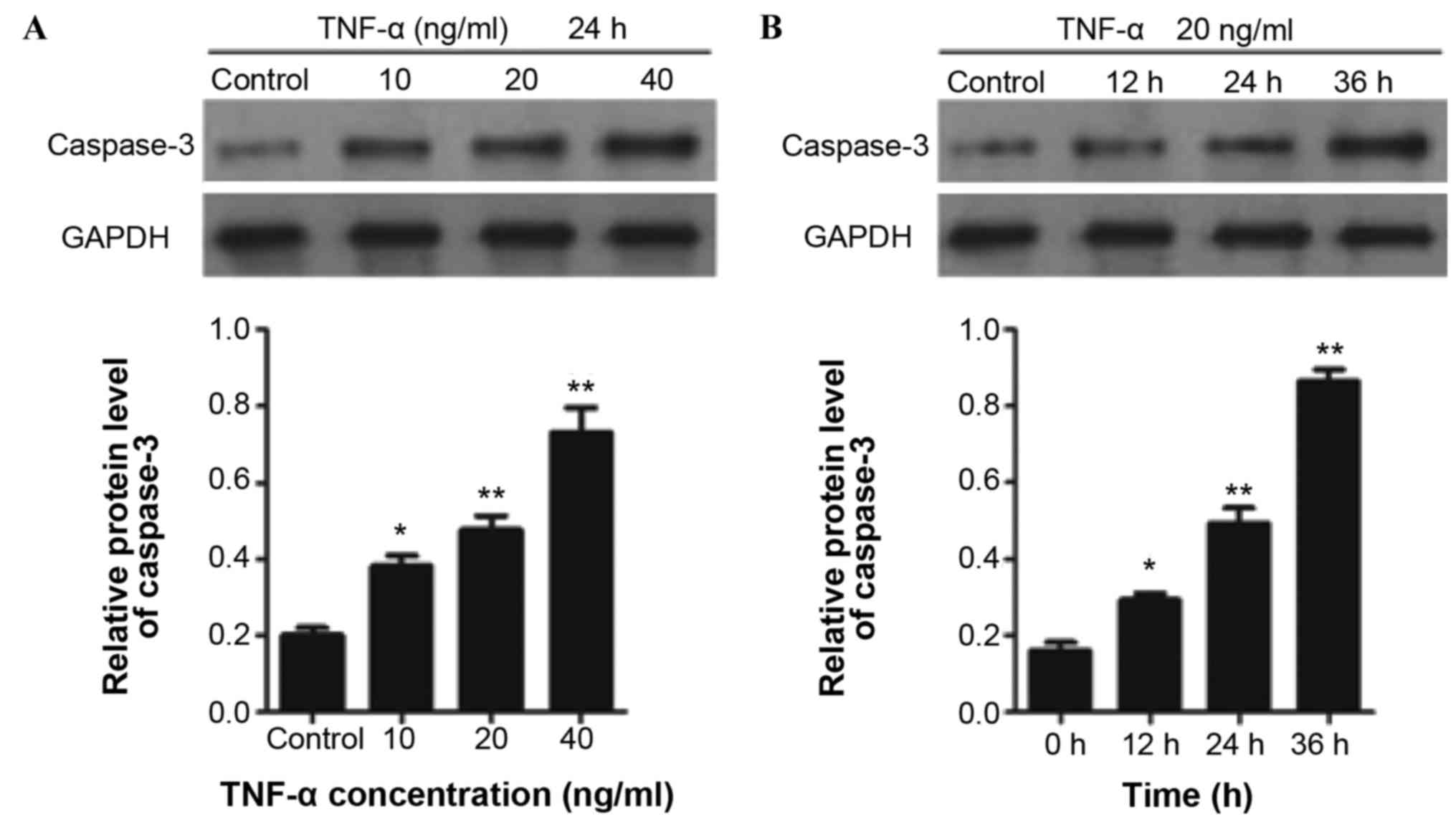
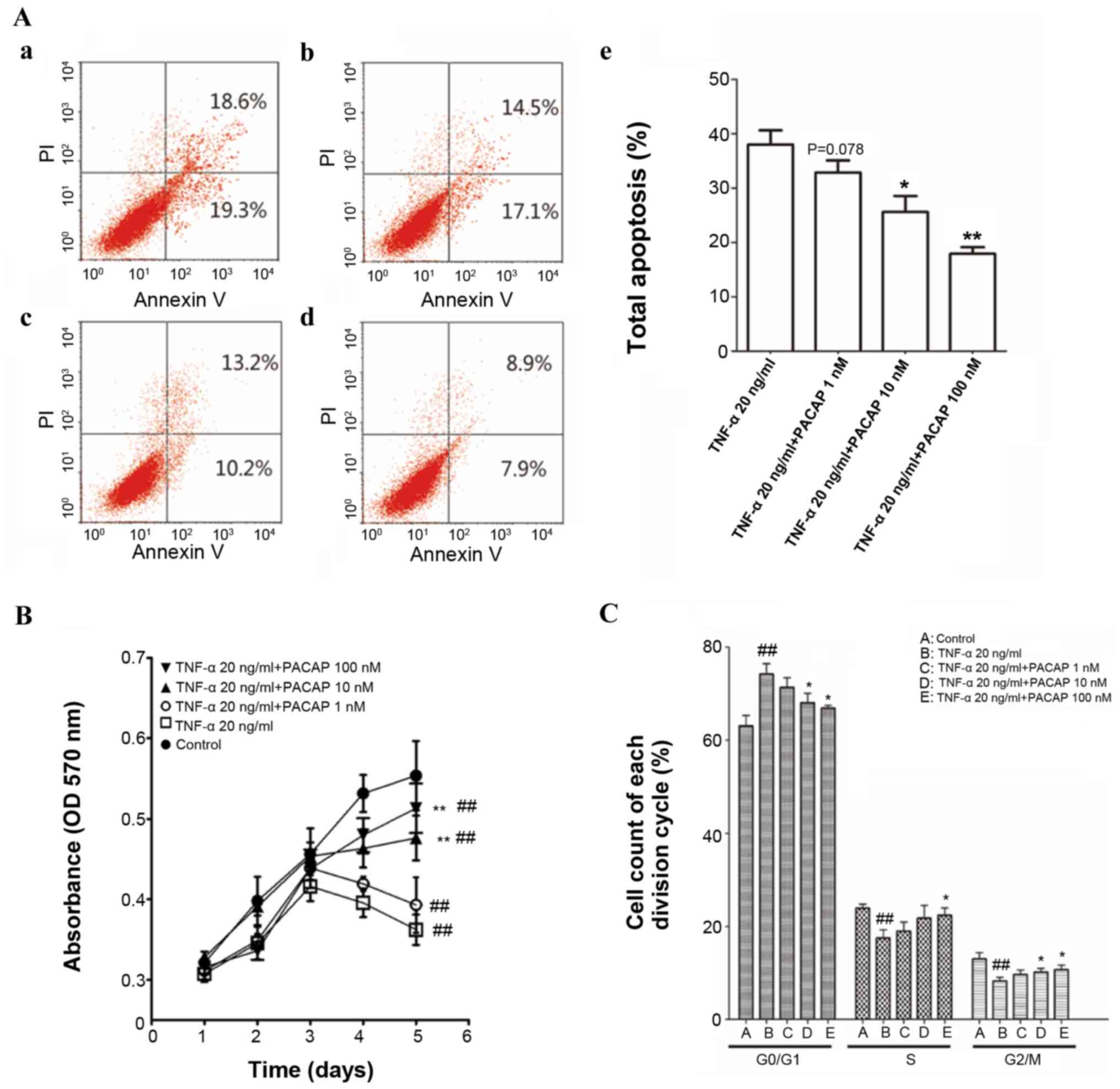
|
1
|
Asahara T: Cell therapy and gene therapy
using endothelial progenitor cells for vascular regeneration. Handb
Exp Pharmacol. 180:181–194. 2007. View Article : Google Scholar
|
|
2
|
Ingram DA, Mead LE, Tanaka H, Meade V,
Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D and Yoder
MC: Identification of a novel hierarchy of endothelial progenitor
cells using human peripheral and umbilical cord blood. Blood.
104:2752–2760. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steinmetz M, Nickenig G and Werner N:
Endothelial-regenerating cells: An expanding universe.
Hypertension. 55:593–599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blue EK, DiGiuseppe R, Derr-Yellin E,
Acosta JC, Pay SL, Hanenberg H, Schellinger MM, Quinney SK, Mund
JA, Case J, et al: Gestational diabetes induces alterations in the
function of neonatal endothelial colony-forming cells. Pediatr Res.
75:266–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ingram DA, Lien IZ, Mead LE, Estes M,
Prater DN, Derr-Yellin E, DiMeglio LA and Haneline LS: In vitro
hyperglycemia or a diabetic intrauterine environment reduces
neonatal endothelial colony-forming cell numbers and function.
Diabetes. 57:724–731. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ikutomi M, Sahara M, Nakajima T, Minami Y,
Morita T, Hirata Y, Komuro I, Nakamura F and Sata M: Diverse
contribution of bone marrow-derived late-outgrowth endothelial
progenitor cells to vascular repair under pulmonary arterial
hypertension and arterial neointimal formation. J Mol Cell Cardiol.
86:121–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vanhoutte PM: Endothelial dysfunction: The
first step toward coronary arteriosclerosis. Circ J. 73:595–601.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi
JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, et al:
Therapeutic potential of ex vivo expanded endothelial progenitor
cells for myocardial ischemia. Circulation. 103:634–637. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Werner L, Deutsch V, Barshack I, Miller H,
Keren G and George J: Transfer of endothelial progenitor cells
improves myocardial performance in rats with dilated cardiomyopathy
induced following experimental myocarditis. J Mol Cell Cardiol.
39:691–697. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hill JM, Zalos G, Halcox JP, Schenke WH,
Waclawiw MA, Quyyumi AA and Finkel T: Circulating endothelial
progenitor cells, vascular function, and cardiovascular risk. N
Engl J Med. 348:593–600. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scalone G, De Caterina A, Leone AM,
Tritarelli A, Mollo R, Pinnacchio G, D'Amario D, Lanza GA and Crea
F: Effect of exercise on circulating endothelial progenitor cells
in microvascular angina. Circ J. 77:1777–1782. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujita Y and Asahara T: Evaluation of
circulating endothelial progenitor cells in cardiovascular risk.
Circ J. 75:2541–2542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Etemadifar M, Dehghani L, Ganji H,
Soleimani R, Talebi M, Eskandari N, Samani FS and Meamar R:
Evaluation of the circulating CD34(+), CD309(+), and endothelial
progenitor cells in patients with first attack of optic neuritis.
Adv Biomed Res. 4:1512015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duckers HJ, Silber S, De Winter R, den
Heijer P, Rensing B, Rau M, Mudra H, Benit E, Verheye S, Wijns W,
et al: Circulating endothelial progenitor cells predict
angiographic and intravascular ultrasound outcome following
percutaneous coronary interventions in the HEALING-II trial:
Evaluation of an endothelial progenitor cell capturing stent.
EuroIntervention. 3:67–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaehler J, Osterholz S, Patten M, Koester
R and Meinertz T: Cytokines in the pathogenesis of atherosclerosis.
Dtsch Med Wochenschr. 127:94–99. 2002.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takahashi M: Inflammatory cytokines in the
pathogenesis of atherosclerosis. Nihon Rinsho. 69:30–33. 2011.(In
Japanese). PubMed/NCBI
|
|
17
|
Prisco AR, Prisco MR, Carlson BE and
Greene AS: TNF-α increases endothelial progenitor cell adhesion to
the endothelium by increasing bond expression and affinity. Am J
Physiol Heart Circ Physiol. 308:H1368–H1381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walsh LJ, Trinchieri G, Waldorf HA,
Whitaker D and Murphy GF: Human dermal mast cells contain and
release tumor necrosis factor alpha, which induces endothelial
leukocyte adhesion molecule 1. Proc Natl Acad Sci USA. 88:pp.
4220–4224. 1991; View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyata A, Arimura A, Dahl RR, Minamino N,
Uehara A, Jiang L, Culler MD and Coy DH: Isolation of a novel 38
residue-hypothalamic polypeptide which stimulates adenylate cyclase
in pituitary cells. Biochem Biophys Res Commun. 164:567–574. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heimesaat MM, Dunay IR, Schulze S, Fischer
A, Grundmann U, Alutis M, Kühl AA, Tamas A, Toth G, Dunay MP, et
al: Pituitary adenylate cyclase-activating polypeptide ameliorates
experimental acute ileitis and extra-intestinal sequelae. PLoS One.
9:e1083892014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vaudry D, Falluel-Morel A, Bourgault S,
Basille M, Burel D, Wurtz O, Fournier A, Chow BKC, Hashimoto H,
Galas L, et al: Pituitary adenylate cyclase-activating polypeptide
and its receptors: 20 years after the discovery. Pharmacol Rev.
61:283–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giunta S, Castorina A, Adorno A, Mazzone
V, Carnazza ML and D'Agata V: PACAP and VIP affect NF1 expression
in rat malignant peripheral nerve sheath tumor (MPNST) cells.
Neuropeptides. 44:45–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Castorina A, Waschek JA, Marzagalli R,
Cardile V and Drago F: PACAP interacts with PAC1 receptors to
induce tissue plasminogen activator (tPA) expression and activity
in schwann cell-like cultures. PLoS One. 10:e01177992015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan YV, Abad C, Lopez R, Dong H, Liu S,
Lee A, Gomariz RP, Leceta J and Waschek JA: Pituitary adenylyl
cyclase-activating polypeptide is an intrinsic regulator of Treg
abundance and protects against experimental autoimmune
encephalomyelitis. Proc Natl Acad Sci USA. 106:pp. 2012–2017. 2009;
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Armstrong BD, Abad C, Chhith S, Cheung-Lau
G, Hajji OE, Nobuta H and Waschek JA: Impaired nerve regeneration
and enhanced neuroinflammatory response in mice lacking pituitary
adenylyl cyclase activating peptide. Neuroscience. 151:63–73. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du G, Song Y, Zhang T, Ma L, Bian N, Chen
X, Feng J, Chang Q and Li Z: Simvastatin attenuates TNF-α induced
apoptosis in endothelial progenitor cells via the upregulation of
SIRT1. Int J Mol Med. 34:177–182. 2014.PubMed/NCBI
|
|
27
|
Sasi SP, Song J, Enderling H, Yan X and
Goukassian DA: TNF-α and IL-1α but not MCP 1 and Rantes increase
significantly the formation of p-H2AX foci in naive BM-derived
TNFR1/p55KO EPCs. J Radiat Res. 55 Suppl 1:i122–i123. 2014.
View Article : Google Scholar
|
|
28
|
Botz B, Bölcskei K, Kereskai L, Kovács M,
Németh T, Szigeti K, Horváth I, Máthé D, Kovács N, Hashimoto H, et
al: Differential regulatory role of pituitary adenylate
cyclase-activating polypeptide in the serum-transfer arthritis
model. Arthritis Rheumatol. 66:2739–2750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Juhász T, Helgadottir SL, Tamás A, Reglődi
D and Zákány R: PACAP and VIP signaling in chondrogenesis and
osteogenesis. Peptides. 66:51–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu R, Xie S, Chen J, Zhang L and Dai Y:
The effects of PACAP and related peptides on leptin, soluble leptin
receptor and resistin in normal condition and LPS-induced
inflammation. Peptides. 30:1456–1459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsumuraya T, Ohtaki H, Song D, Sato A,
Watanabe J, Hiraizumi Y, Nakamachi T, Xu Z, Dohi K, Hashimoto H, et
al: Human mesenchymal stem/stromal cells suppress spinal
inflammation in mice with contribution of pituitary adenylate
cyclase-activating polypeptide (PACAP). J Neuroinflammation.
12:352015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Waschek JA: VIP and PACAP: Neuropeptide
modulators of CNS inflammation, injury, and repair. Br J Pharmacol.
169:512–523. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang Q, Li ZC and Deng Y: The
distribution of pacap receptor on the cardiovascular tissues and
cells. J Clin Cardiol. 18:329–331. 2002.
|
|
34
|
Chang Q, Zhang L, Tang HL, Huang HM and Zi
Cheng LI: Effects of pituitary adenylate cyclase activating
polypeptide on cardiac remodeling in coronary atherosclerotic
rabbits. Chin J Pathophysiology. 23:529–532. 2007.
|
|
35
|
Cheng ZL: The changes of collagen and mmp
2 in atherosclerotic rabbit aorta and effect of pacap. Guangdong
Med. 29:1107–1109. 2008.
|
|
36
|
Qiao J, Qi K, Chu P, Mi H, Yang N, Yao H,
Xia Y, Li Z, Xu K and Zeng L: Infusion of endothelial progenitor
cells ameliorates liver injury in mice after haematopoietic stem
cell transplantation. Liver Int. 35:2611–2620. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martini G, Biscaro F, Boscaro E, Calabrese
F, Lunardi F, Facco M, Agostini C, Zulian F and Fadini GP: Reduced
levels of circulating progenitor cells in juvenile idiopathic
arthritis are counteracted by anti TNF-α therapy. BMC Musculoskelet
Disord. 16:1032015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang XX, Yang JX, Pan YY and Zhang YF:
Protective effects of tanshinone IIA on endothelial progenitor
cells injured by tumor necrosis factor-α. Mol Med Rep.
12:4055–4062. 2015.PubMed/NCBI
|
|
39
|
Cao Q, Wang Y, Huang L, Wang F and Chen S:
TNF receptor-associated factor 6 (TRAF6) mediates the
angiotensin-induced non-canonical TGF-β pathway activation of
c-kit(+) cardiac stem cells. Am J Transl Res. 7:2233–2243.
2015.PubMed/NCBI
|
|
40
|
Liao L, Su X, Yang X, Hu C, Li B, Lv Y,
Shuai Y, Jing H, Deng Z and Jin Y: TNF-α Inhibits FoxO1 by
Upregulating miR-705 to Aggravate Oxidative Damage in Bone
Marrow-Derived Mesenchymal Stem Cells during Osteoporosis. Stem
Cells. 34:1054–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Salvesen GS: Caspases: Opening the boxes
and interpreting the arrows. Cell Death Differ. 9:3–5. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shioda S, Ohtaki H, Nakamachi T, Dohi K,
Watanabe J, Nakajo S, Arata S, Kitamura S, Okuda H, Takenoya F, et
al: Pleiotropic functions of PACAP in the CNS: Neuroprotection and
neurodevelopment. Ann N Y Acad Sci. 1070:550–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
May V, Lutz E, MacKenzie C, Schutz KC,
Dozark K and Braas KM: Pituitary adenylate cyclase-activating
polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates
multiple neurotrophic signaling pathways: Akt activation through
phosphatidylinositol 3-kinase gamma and vesicle endocytosis for
neuronal survival. J Biol Chem. 285:9749–9761. 2010. View Article : Google Scholar : PubMed/NCBI
|