Introduction
Gastric metastases are rare, with a reported
incidence of 0.2–0.7% based on clinical and autopsy findings,
whereas primary gastric cancer is the most commonly diagnosed
cancer worldwide and is the leading cause of cancer-related deaths
(1–3). It
may be very difficult to distinguish metastatic gastric tumors from
primary gastric cancers based on clinical, endoscopic,
radiological, and histopathological features. With gradual
improvements in prognosis for cancer patients, it seems that
metastatic tumors in the stomach are being encountered more
frequently (1). Therefore, it is
important to distinguish metastatic gastric tumor from primary
gastric cancer for accurate diagnosis and optimal treatment.
Although metastatic gastric tumors are not that
common, recognizing the range of possible presentations is
important for the early and accurate diagnosis and treatment. The
aim of the present study was to analyze the clinicopathologic
features and treatment outcomes of gastric metastasis from other
malignancies of solid organs.
Materials and methods
Patients and methods
Patients with metastatic tumors in the stomach from
other malignancies of solid organs detected endoscopically at the
Department of Surgery, Kochi Medical School, between January 1991
and December 2015 were reviewed. Diagnoses of metastatic gastric
cancer were made by esophagogastroduodenoscopy (EGD), analysis of
biopsy specimens, computed tomography (CT), magnetic resonance
imaging, ultrasonography of the abdomen, and positron emission
tomography. Patients with malignant lymphoma involving the stomach
or with direct invasion from neighboring organs were excluded from
the study. All the tumors were subjected to detailed examinations,
including CT, ultrasonography, EGD, and pathological confirmation
using biopsy or surgically resected specimens.
Statistical analysis
Correlations among continuous variables in each
group were evaluated using the Mann-Whitney U-test, whereas
categorical variables were evaluated using Pearson's Chi-square
test. P<0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
SPSS for Windows v13.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Seven patients who had been treated for metastatic
gastric tumors arising from other malignancies of solid organs were
included in the present study. The clinical features of these seven
patients are listed in Table I. Four
patients (57.1%) were men and three (42.9%) were women, with
patient age ranging from 42 to 71 years (median, 65 years). Four
patients had lesions in the upper third of the stomach, one had
lesions in the middle third of the stomach, and two had lesions in
the entire stomach. Median tumor size was 7.3 cm (range, 2.5–12.0
cm). The primary malignancy leading to metastatic tumors in the
stomach was esophageal cancer in three patients, breast cancer in
two patients, renal cell carcinoma in one patient, and ovarian
cancer in one patient. The pathology of both tumors arising from
breast cancer showed invasive lobular carcinoma, and the pathology
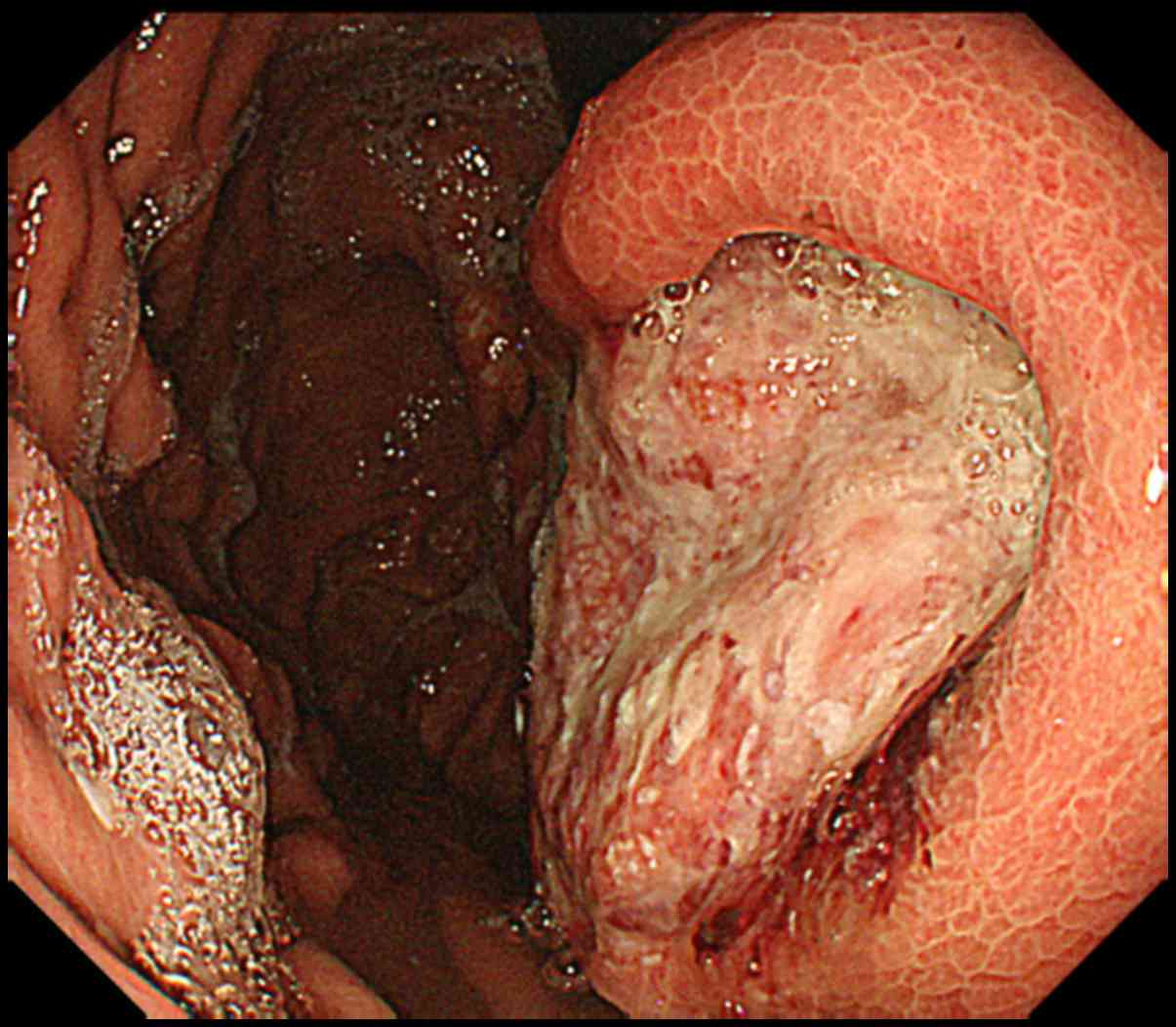
of the esophageal cancers showed squamous cell carcinoma. Fig. 1 shows the EGD results in a 71-year-old
patient who was diagnosed with metastatic gastric cancer arising
from esophageal cancer.
 | Table I.Clinical data for patients with
metastatic tumors in the stomach from other malignancies of solid
organs. |
Table I.
Clinical data for patients with
metastatic tumors in the stomach from other malignancies of solid
organs.
| Patient | Age (years) | Sex | Primary cancer | Tumor location | Tumor size (cm) | Gross appearance | Additional
metastases | Pathology | Treatment | Outcome |
|---|
| 1 | 43 | Female | Breast | Entire stomach | 12 | Diffuse | Lung, bone, liver,
kidney, skin | Invasive lobular
carcinoma | Chemotherapy | Died 5.5 months after
therapy |
| 2 | 65 | Male | RCC | U | 2.5 | Elevated | Solitary | Clear cell-type
RCC | Partial resection of
the stomach | 58 months
survival |
| 3 | 63 | Female | Ovarian | M | 2.5 | Ulcerated | Solitary | Serous ovarian
adenocarcinoma | Distal gastrectomy
treatment | Died 9 months after
therapy |
| 4 | 70 | Female | Breast | Entire stomach | 12 | Diffuse | Peritoneum | Invasive lobular
carcinoma | Chemotherapy +
hormone therapy | Survived 11
months |
| 5 | 42 | Male | Esophageal | U | 6.6 | Ulcerated | Solitary | SCC | Chemotherapy | Survived 10
months |
| 6 | 71 | Male | Esophageal | U | 7.3 | Ulcerated | Solitary | SCC | Total
gastrectomy | Survived 6
months |
| 7 | 69 | Male | Esophageal | U | 7.5 | Elevated | Liver | SCC | Total
gastrectomy | Survived 6
months |
Gastric metastasis presented as solitary lesions in
four patients and as multiple lesions in three patients. The median
tumor size was significantly smaller in patients with solitary
rather than multiple metastases (4.6 vs. 12.0 cm, respectively;
P=0.038). Six patients had a single lesion in the stomach and one
patient had multiple metastatic tumors in the stomach, as well as
metastases to the lung, bone, liver, kidney, and skin. Examination
of the tumors revealed ulcerated tumors in three patients, linitis
plastica lesion in two, and a protruding tumor in another two
patients. In one patient, the condition was diagnosed from biopsy
specimens using endoscopic ultrasound-guided fine needle aspiration
(EUS-FNA) (5).
The interval between treatment of the primary tumor
and diagnosis of the metastatic tumor in the stomach was 23 years
in the case of solitary gastric metastasis from renal cell
carcinoma and 6 years in the case of solitary gastric metastasis
from ovarian cancer. In all other cases (i.e., gastric metastases
from breast cancer and esophageal cancer), presentation was
synchronous.
Treatment of gastric metastases
One patient with multiple metastases arising from
breast cancer was treated with chemotherapy using fluorouracil;
however, this patient died 5.5 months after therapy. In the case of
the patient with gastric metastasis of renal cell carcinoma
presenting 23 years after radical nephrectomy, 58 months have
elapsed since the surgical resection of the tumor and the patient
is alive without the occurrence of any new lesions. One patient
with solitary gastric metastasis of ovarian cancer underwent distal
gastrectomy; however, this patient developed peritoneal metastasis
and died 9 months after surgery. One patient with gastric
metastasis of breast cancer is currently undergoing chemotherapy
with anti-estrogen therapy, and one patient with solitary gastric
metastasis of esophageal cancer is undergoing chemotherapy with
fluorouracil and cisplatin. One patient with solitary gastric
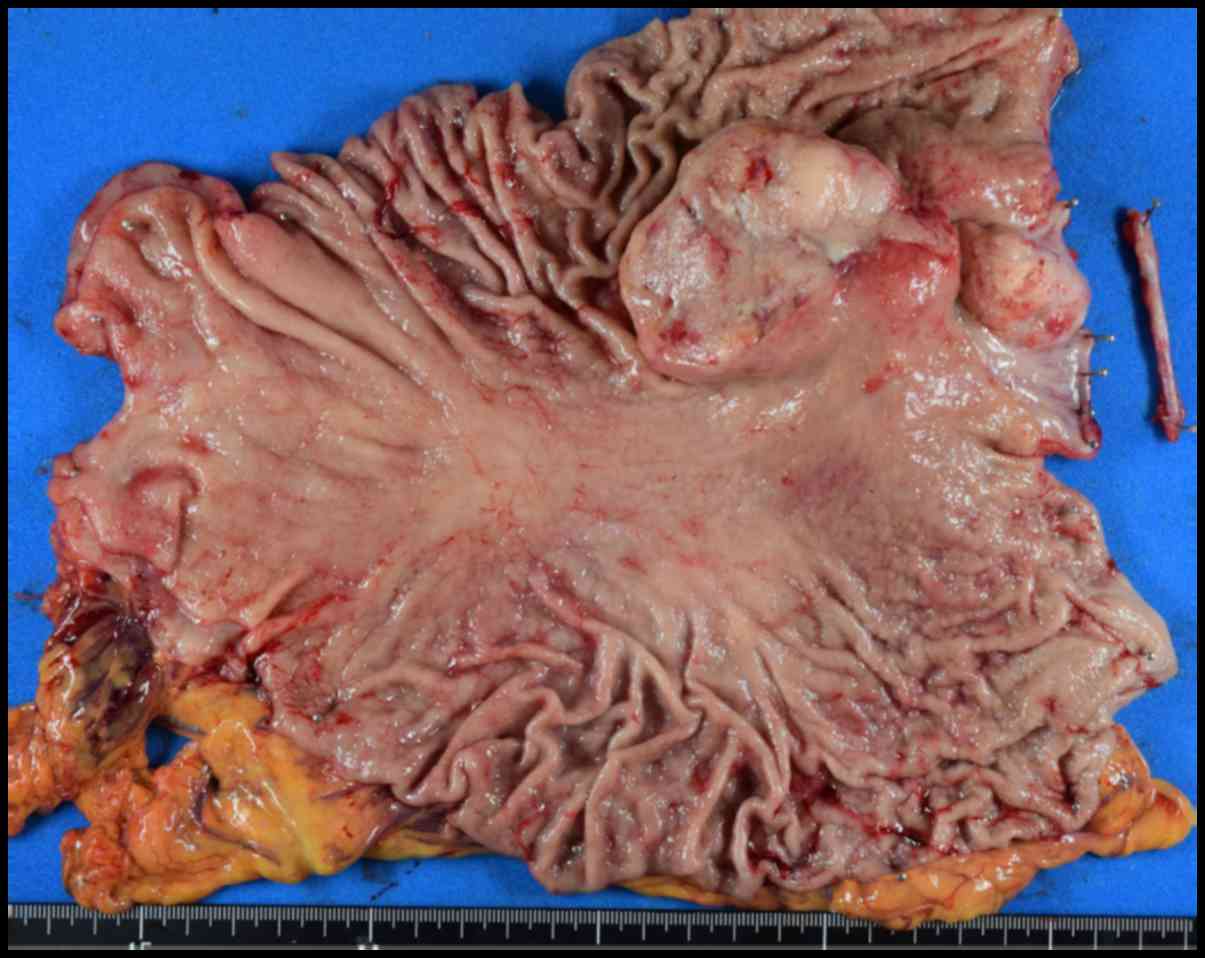
metastasis of esophageal cancer (which was an ulcerated tumor
measuring 7.3×7.2 cm) underwent total gastrectomy and is undergoing
chemotherapy with docetaxel, cisplatin, and fluorouracil
post-surgery (Fig. 2). One patient
with gastric and liver metastases arising from esophageal cancer is
undergoing chemotherapy with docetaxel, cisplatin, and
fluorouracil.
Discussion
In the present study, the incidence of esophageal
and breast cancer as the primary malignancies leading to metastatic
tumors in the stomach was high, which is similar to findings of
previous studies reporting that the most common primary sites of
metastases to the stomach are the breast, melanoma, lung, and
esophagus (1,4,5). Although
the mechanisms underlying gastric metastasis have not been clearly
elucidated, potential pathways may include peritoneal
dissemination, hematogenous dissemination, lymphatic spread, and
direct tumor invasion (1).
In the case of hematogenous dissemination, tumor
cells may become trapped in vessels in areas of the stomach wall
with a rich blood supply, such as the submucosal or subserosal
layers (6). Investigations into the
mechanisms responsible for gastric metastasis depending on the
primary malignancy are expected in the near future. At the same
time, risk factors for the development of metastatic gastric tumors
need to be elucidated to enable early diagnosis and to establish
the best therapeutic strategy. In addition, when planning treatment
for a gastric neoplasm, it is important to differentiate a primary
gastrointestinal tract tumor from a metastatic tumor, especially in
patients who have previously been treated for lobular breast
carcinoma or esophageal cancer.
Breast cancer is the most commonly diagnosed cancer
worldwide and the second leading cause of cancer-related deaths
(7). Despite breast cancer metastases
to the gastrointestinal tract being less common than to other
sites, because breast cancer is extremely common, it may be
responsible for a high proportion of metastatic gastric tumors. In
the present study, the pathological results for both breast cancer
patients showed invasive lobular carcinoma, which agrees with
findings reported previously (4,8). As tumor
histology is one of the predictors of metastatic spread, lobular
carcinoma is more likely to metastasize to the gastrointestinal
tract, although metastatic gastric tumors are less common than
ductal carcinoma and the mechanisms involved are not clear
(8–10).
These results emphasize the importance of considering metastatic
gastric cancer, especially in patients with a previous history of
breast cancer. In such cases, clinicians should undertake
additional immunohistochemical examinations, such as staining for
estrogen or progesterone receptors (9).
In the present study, the metastatic gastric tumors
in the two breast cancer patients exhibited a diffuse-type
appearance, such as linitis plastica. One of the characteristic
endoscopic features in these two cases was diffuse infiltration of
the gastric wall. This finding is similar to linitis plastica,
which is a diffuse-type gastric cancer that presents in the area of
the fundic gland and is characterized by thickening of the stomach
wall and deformation of the stomach resulting in a leather
bottle-like appearance of the stomach (1,4,8,11). In a
previous study, the median interval to metastatic tumors in the
stomach from primary breast cancer and renal cell carcinoma was
50–78 and 75.6 months, respectively (1), which highlights the fact that metastatic
spread to the stomach may occur many years after initial treatment
for the primary tumor.
The incidence of metastases in the stomach from
esophageal carcinoma is in the range of 0–15% in autopsy cases, and
1.7% in clinical cases before autopsy (12–14). The
microlymphatic system in the esophageal submucosa is thought to be
continuous with that in the gastric submucosa, and this may be
associated with the mechanism underlying gastric metastasis
(6,14).
In the present study, in all three patients with gastric metastases
from esophageal cancer, the metastatic tumors were located in the
upper gastric body close to the esophagocardial junction, which
could be explained by metastases occurring via the lymphatic
system.
Gastric metastasis arising from ovarian cancer can
be diagnosed using EUS-FNA as lesions exhibiting fold convergence
with a central depression. Hassan et al (15) reported that EUS-FNA significantly
changed patient management in 15% of patients fit for surgery when
lymph nodes or lesions were considered to be distant metastases of
primary gastric cancer. In the diagnosis of metastatic gastric
carcinoma, endoscopy with biopsies is the most common modality:
However, results from endoscopic biopsies may be negative for tumor
cells because the infiltration of tumor cells is localized to the
deeper layers, which are often inaccessible to biopsy forceps. In
these cases, accurate diagnosis may be supported by EUS-FNA with
other radiological techniques, such as CT and positron emission
tomography (16).
In the present study, the median tumor size was
significantly smaller in patients with solitary rather than
multiple metastases. Furthermore, in the case of solitary gastric
metastasis from a renal cell carcinoma, the patient achieved
long-term survival without the occurrence of any new lesions.
Previous studies have also reported that patients with solitary
gastric metastasis arising from renal cell carcinoma have good
outcomes following treatment compared with patients with multiple
metastases (5,17).
One of the limitations of the present study is the
small number of patients from a single institution. Thus, further
studies with a larger number of patients are required to gain a
better understanding of the various presentations of metastatic
gastric cancer from other malignancies of solid organs.
In conclusion, clinicians should be aware of the
possible existence of metastatic gastric cancer from other
malignancies of solid organs, especially in breast lobular
carcinoma and esophageal cancer. Although appropriate systemic
treatment including chemotherapy or hormonal therapy for metastatic
tumors in the stomach is the preferred treatment, surgical
resection of metastatic gastric tumors may be recommended to
improve patients' quality of life, when there is a risk of
bleeding, tumor perforation, and/or a solitary metastasis.
References
|
1
|
Namikawa T and Hanazaki K:
Clinicopathological features and treatment outcomes of metastatic
tumors in the stomach. Surg Today. 44:1392–1399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oda I, Kondo H, Yamao T, Saito D, Ono H,
Gotoda T, Yamaguchi H, Yoshida S and Shimoda T: Metastatic tumors
to the stomach: Analysis of 54 patients diagnosed at endoscopy and
347 autopsy cases. Endoscopy. 33:507–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kobayashi O, Murakami H, Yoshida T, Cho H,
Yoshikawa T, Tsuburaya A, Sairenji M, Motohashi H, Sugiyama Y and
Kameda Y: Clinical diagnosis of metastatic gastric tumors:
Clinicopathologic findings and prognosis of nine patients in a
single cancer center. World J Surg. 28:548–551. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taal BG, Peterse H and Boot H: Clinical
presentation, endoscopic features, and treatment of gastric
metastases from breast carcinoma. Cancer. 89:2214–2221. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Namikawa T, Munekage M, Kitagawa H,
Okabayashi T, Kobayashi M and Hanazaki K: Metastatic gastric tumors
arising from renal cell carcinoma: Clinical characteristics and
outcomes of this uncommon disease. Oncol Lett. 4:631–636.
2012.PubMed/NCBI
|
|
6
|
Hashimoto T, Arai K, Yamashita Y, Iwasaki
Y and Hishima T: Characteristics of intramural metastasis in
gastric cancer. Gastric Cancer. 16:537–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nazareno J, Taves D and Preiksaitis HG:
Metastatic breast cancer to the gastrointestinal tract: A case
series and review of the literature. World J Gastroenterol.
12:6219–6224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Namikawa T, Kobayashi M and Hanazaki K:
Unusual thickened gastric folds in a patient with breast cancer.
Gastroenterology. 152:e8–e9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borst MJ and Ingold JA: Metastatic
patterns of invasive lobular versus invasive ductal carcinoma of
the breast. Surgery. 114:637–641, discussion 641–642.
1993.PubMed/NCBI
|
|
11
|
Pectasides D, Psyrri A, Pliarchopoulou K,
Floros T, Papaxoinis G, Skondra M, Papatsibas G, Macheras A,
Athanasas G, Arapantoni-Datioti P, et al: Gastric metastases
originating from breast cancer: Report of 8 cases and review of the
literature. Anticancer Res. 29:4759–4763. 2009.PubMed/NCBI
|
|
12
|
Anderson LL and Lad TE: Autopsy findings
in squamous-cell carcinoma of the esophagus. Cancer. 50:1587–1590.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mandard AM, Chasle J, Marnay J, Villedieu
B, Bianco C, Roussel A, Elie H and Vernhes JC: Autopsy findings in
111 cases of esophageal cancer. Cancer. 48:329–335. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saito T, Iizuka T, Kato H and Watanabe H:
Esophageal carcinoma metastatic to the stomach. A clinicopathologic
study of 35 cases. Cancer. 56:2235–2241. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hassan H, Vilmann P and Sharma V: Impact
of EUS-guided FNA on management of gastric carcinoma. Gastrointest
Endosc. 71:500–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Namikawa T, Kobayashi M and Hanazaki K:
Metastatic gastric tumor arising from ovarian cancer. Gastrointest
Endosc. 79:332–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Namikawa T, Iwabu J, Kitagawa H,
Okabayashi T, Kobayashi M and Hanazaki K: Solitary gastric
metastasis from a renal cell carcinoma, presenting 23 years after
radical nephrectomy. Endoscopy. 44 Suppl 2 UCTN:E177–E178. 2012.
View Article : Google Scholar : PubMed/NCBI
|