Introduction
Cancer is an international public health problem,
has been considered as a hyper-proliferative disorder. Cancer
contains of irreversible cell change, dysregulation of apoptosis,
proliferation, invasion, angiogenesis and metastasis (1). More than 7 million deaths per year have
been reported worldwide and it is predicted that the new cancer
cases will reach 15 million every year by 2020. Prostate cancer
(PCa) as the most commonly diagnosed urologic malignancy is the
second most common cause of cancer death in men in developed
countries (2). Androgen ablation is
the most common and successful treatment for progressive PCa.
However, presence of androgen-independent cells increase problems
with PCa treatment (3). Hence, studies
have attempted to replace the new targets for treatment of PCa
including telomerase activity (4,5).
Telomerase is one of the most important targets of
therapeutic intervention in various cancers (6). Telomeres are conserved, repetitive and
large non-coding sequences located at the ends of eukaryotic
chromosomes these sequences responsible for the protection of the
genomic DNA integrity (7). During each
round of cell division, DNA polymerase is not able to replicate the
5′ ends of chromosomes in normal somatic cells; consequently, the
telomeres are progressively shortened. When the telomeres reach a
critically short length, cells leave the cell cycle and DNA damage
responses such as apoptosis and replicative senescence are
initiated. Hence, a limited number of passages could be seen in
normal somatic cells due to their telomere length (7,8).
In most tumor cells, telomerase is responsible for
telomere maintenance. Telomerase is a RNA dependent DNA polymerase,
synthesize TTAGGG tandem repeats to the 3′ end of the telomere on
the lagging strand and confers cellular immortality. In this
mechanism, telomerase is preventing telomere shortening and
permitting continued proliferation (9). Telomerase contains two main subunits: The
catalytic subunit called human telomerase reverse transcriptase
(hTERT), which synthesizes the addition of TTAGGG tandem repeats to
the 3′ end; and the telomerase RNA template subunit, the second
subunit is an RNA template complementary to the 3′ end which used
as primer for telomere synthesize (10).
Although continuing improvement in treatment of
cancer is observed, more patients suffer from cancer
treatment-related symptoms including fatigue, paresthesias and
dysesthesias, chronic pain, anorexia, insomnia, limbs edema and
constipation (11,12).
In traditional medicine, natural and herbal products
have been considered as a main way for treatment of different
diseases including infections and malignant diseases for a long
time (13). In addition, several
studies indicated that various herbal plants might have the
anti-cancer effect by cell differentiation induction, inhibition of
telomerase activity, induction of apoptosis in cancer cells and
improving the immune system (13–15).
Although it is believed that natural and herbal medicine have no
side effects and lower dependency, different herbs could be toxic
mainly in higher doses and long usage (16).
The Achillea genus (Asteraceae) with
>130 species has been spread throughout the world, including
Asia and Europe (17). In Iran,
nineteen species of this genus have been recognized and approved
for their essential oil (18).
Achillea genus is widely used in traditional medicine
(17,18). A previous study demonstrated that the
Achillea species extracts exhibit antimicrobial, antioxidant
and cytotoxic activities (19).
Achillea wilhelmsii (AW) is a member of the Achillea
genus, which is widely found in different regions of Iran. The
effects of AW have been the topic of several studies, these effects
may be attributed to high flavonoids and sesquiterpene lactones
content (20,21). Evidences have reported that the extract
and oil of Achillea species present cytotoxic effects
against several cancer cell lines (19,22) and a
recent study revealed cytotoxic activities of the hydroalcoholic
extract of AW. on Breast cancer cell lines (22).
Although several research papers have evaluated the
effects of AW extract on some cancers (19,22), there
is no published report on the effects of AW extract in PCa cell
lines. Therefore, in the current study, the effect of AW extract on
apoptosis and expression of mRNA-hTERT gene was investigated in the
PCa PC3 cell line.
Materials and methods
Plant material
AW C. Koch was collected from the Taftan area in
Sistan and Baluchistan Province, Iran in spring 2015. The specimen
was identified by one of the members of Research Institute of the
University of Sistan and Baluchistan (Dr Ali Shahraki). The stem
and leaf of AW were air dried in dark room and then powdered. Then,
a solution (70% alcohol) of the AW was extracted using a Soxhlet
extractor.
Chemicals and regents
RPMI-1640, fetal bovine serum (FBS),
phosphate-buffered saline (PBS), streptomycin and Penicillin,
trypsin, EDTA, trypan blue, were obtained from Gibco; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). MTT and dimethyl sulfoxide
(DMSO) were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). An Annexin V/fluorescein isothiocyanate (FITC) apoptosis
detection kit was purchased from BioVision, Inc. (San Francisco,
CA, USA) and a hTERT colorimetric assay kit was procured from
ZellBio GmbH (Ulm, Germany). All the other materials were of
analytical grade and purchased locally.
Cell culture
PCa cell line PC3 was purchased from National Cell
Bank of Iran (Pasteur Institute, Tehran, Iran). PC3 cells were
cultured at a density between 0.5–1.0×106 cells/ml in
RPMI-1640 medium, containing 10% heat-inactivated FBS, 100 U/ml
penicillin, 100 µg/ml streptomycin in a CO2 incubator at
37°C in a moistened atmosphere of 5% CO2 and 95% air.
When the PC3 cells reached logarithmic growth phase, the cells were
sub-cultured and the experiments were performed immediately on the
sub-cultured cells.
Cell treatments
For treatment, the powder of AW was dissolved in
DMSO (HPLC grade) and kept frozen in the −20°C. The cells were
seeded into sterile 96-well microplates at a density of
5×103 cells/well. The cells were incubated overnight
(37°C, 5% CO2 and 95% air) for adhering the cells to
button plates. Then the medium was removed, and different
concentrations of extract were added.
MTT assay
The anti-proliferative activity of the AW was
assessed using MTT assay as previously described. Cells were plated
onto 96-well plates at 5×103 cells/well in 0.1 ml RPMI
medium. Following 24 h of incubation, cells were treated with 0,
12.5, 25, 50, 100, 150 and 200 µg/ml AW extract for 24, 48 and 72
h. Then, 20 µl MTT were added to each well and incubated at 37°C
for 4 h. Then the culture medium was discarded and finally, 200 µl
DMSO was mixed with the cells for solubilization of Formazan
crystals and incubated in a dark place for 2 h at room
temperature.
Absorbance of cells in each well was measured at 570
nm by a microplate reader (Anthos 2020; Biochrom Ltd., Cambridge,
UK). All experiments were conducted independently three times. The
OD treated/OD control was considered as the cell viability
percentage.
Cell apoptosis
Cell apoptosis was measured using Annexin V-Cy5 and
propidium iodide (PI) staining and analyzed by flow cytometry
(18). 1×105 cells per well
were seeded in six well plates and incubated overnight, and then
treated with 0, 50, 100, 150 and 200 µg/ml of HAWE for 48 h.
All cells were washed two times with cold PBS.
Subsequently, the washed PC3 cells were stained in a 250 µl 1X
binding buffer, 2.5 µl Annexin V/FITC, and 2.5 µl PI. Then, the
stained cells were inculcated in a dark place for 15 min at room
temperature.
Cell cycle analysis was conducted using a flow
cytometer (Partec CyFlow Space, Sysmex Partec GmbH, Görlitz,
Germany) with FloMax version 2.7 software. The distribution of
cells in different cell cycle phases including living, early
apoptosis, late apoptosis and necrosis phases was assessed.
mRNA expression analysis by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
PC3 cells (2×105 cells/well) were
cultured in a six-well plates and incubated at 37°C in a moistened
air of 5% CO2 overnight. Cells were then treated for 2,
4, 8, 12, 24, 48 and 72 h with a 150 µg/ml concentration of AW
extract. Total RNA was isolated using RNx (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol
(17). Isolated RNA was treated with
RNase-free DNase I (Promega Corporation, Madison, WI, USA), and the
concentration and purity of the isolated RNA were determined.
First strand cDNA was synthesized from the cells'
extracted RNA by using the RevertAid First Strand cDNA Synthesis
kit (cat. no. K1621; Thermo Fisher Scientific, Inc.) based on the
manufacturer's protocol.
The expression levels of hTERT were detected using
the SYBR ExScript RT-qPCR kit (Takara Bio, Inc., Otsu, Japan). PCR
amplification was performed with Master PCR kit (CinnaGen, Tehran,
Iran). PCR amplification for hTERT and β-actin mRNA consisted of 35
cycles (95°C for 45 sec, 56°C for 50 sec and 72°C for 50 sec). To
identify hTERT mRNA the following primers were used: forward
primer, 5′-CGGAAGAGTGTCTGGAGCAA-3′, reverse primer,
5′-GGATGAAGCGGAGTCTGGA-3′ (23). To
identify β-actin mRNA following primers were used: forward primer:
5′-AGAAAATCTGGCACCACACC-3′, reverse primer:
5′-GGAAGGAAGGCTGGAAGAGT-3′.
hTERT concentration assay
The hTERT concentration was measured by ELISA assay
kit (cat. no. ZB-10934B-H9648; ZellBio GmbH) according to
manufacturer's protocol.
Statistical analysis
The SPSS software package (version 23.0; IBM SPSS,
Armonk, NY, USA) was used for statistical analysis. Each experiment
was performed at least three times for all data. All the data were
represented as mean ± standard deviation, and the nonparametric
analysis of variance test, with post hoc Tukey test, was used for
statistical analysis between the groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
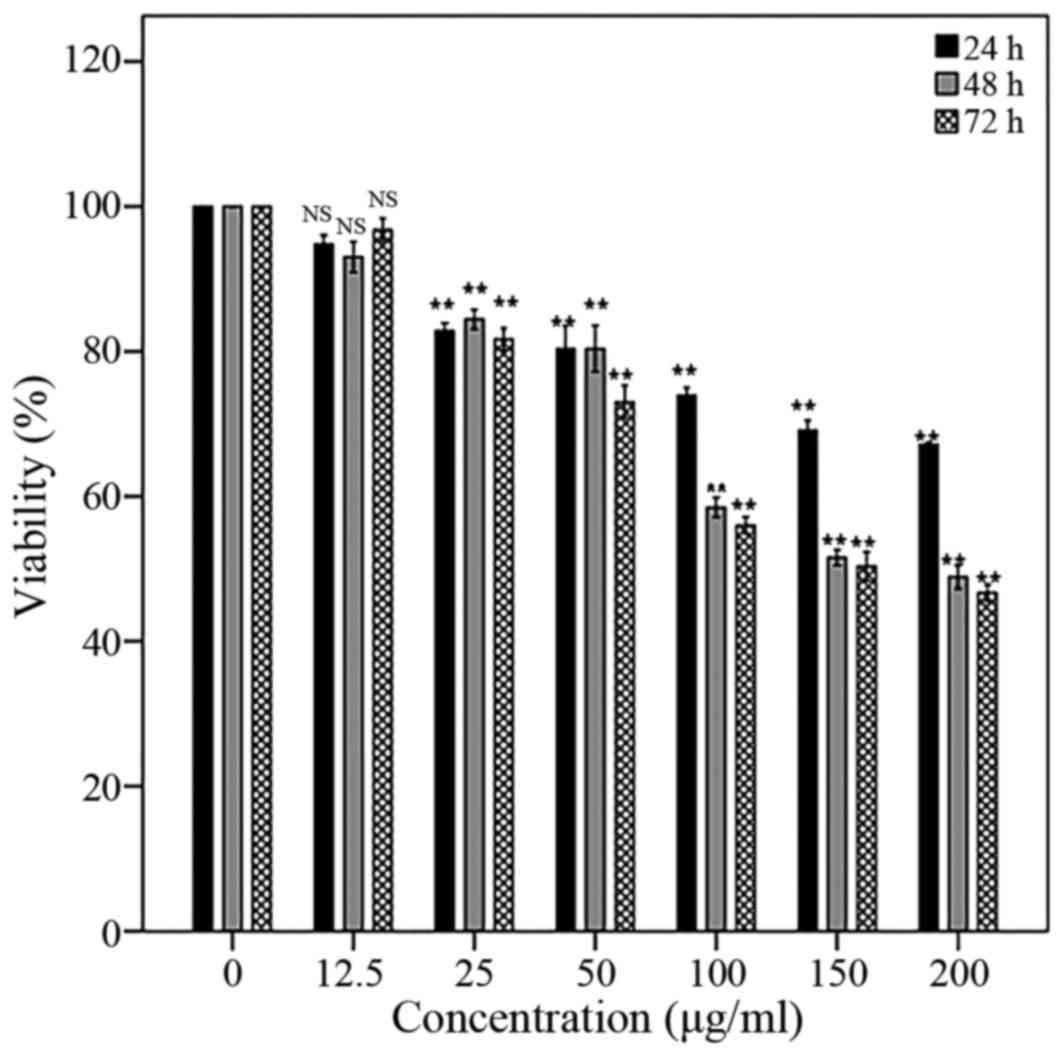
Cytotoxicity activity
Significant inhibitory effect of AW extract has been
seen in 150 µg/ml concentration (IC50) on PC3 cell line
after treatment for 48 h. In addition, the inhibitory effect of AW
extract was in dose- and time-dependent manner (Fig. 1). The half maximal inhibitory
concentration (IC50) was defined as the AW concentration
value which inhibits the viability of PC3 cells in culture by 50%
compared to the untreated cells (control).
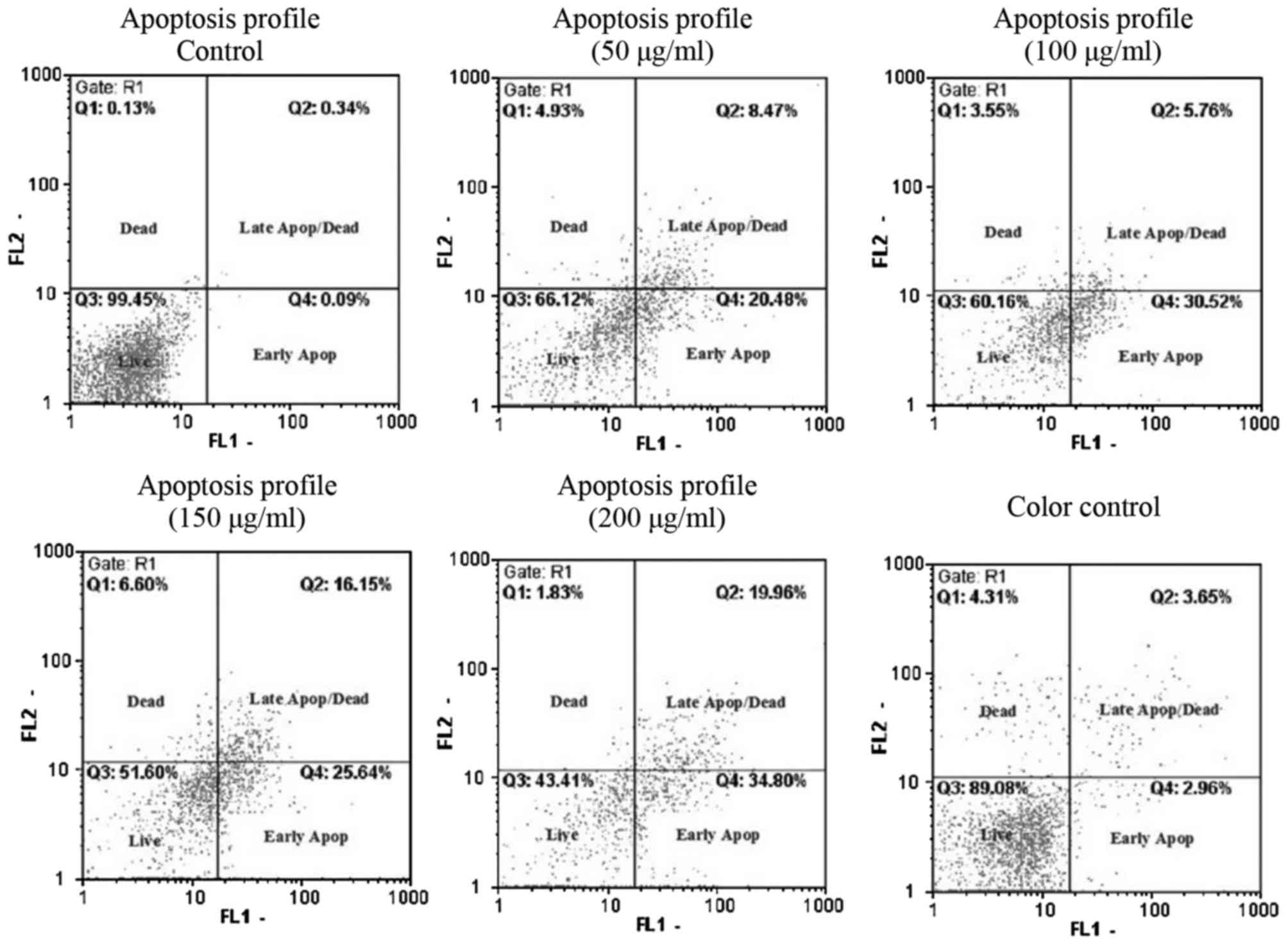
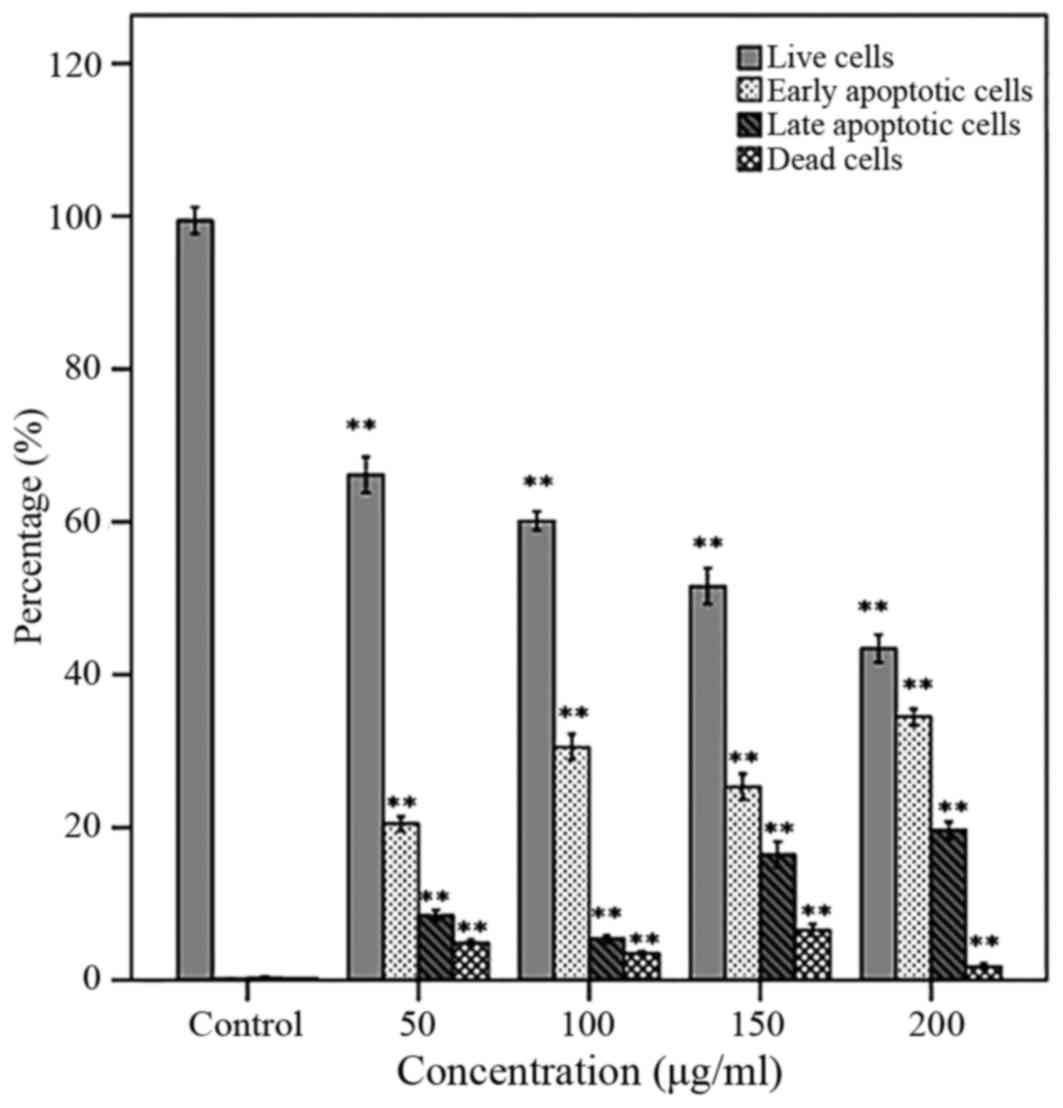
Cell apoptosis
The percentage of cells in Live (Annexin V-/PI-),
early apoptosis (Annexin V+/PI-), late apoptosis and dead (Annexin
V+/PI+) phases are presented in Fig.
2. Following a 48 h treatment of the PC3 cells with AW extract,
a significant increase in early and late apoptotic cells and a
decrease in live cells was observed in a dose-dependent manner.
Moreover, the early apoptotic cells were significantly higher than
late apoptotic cells (Fig. 3).
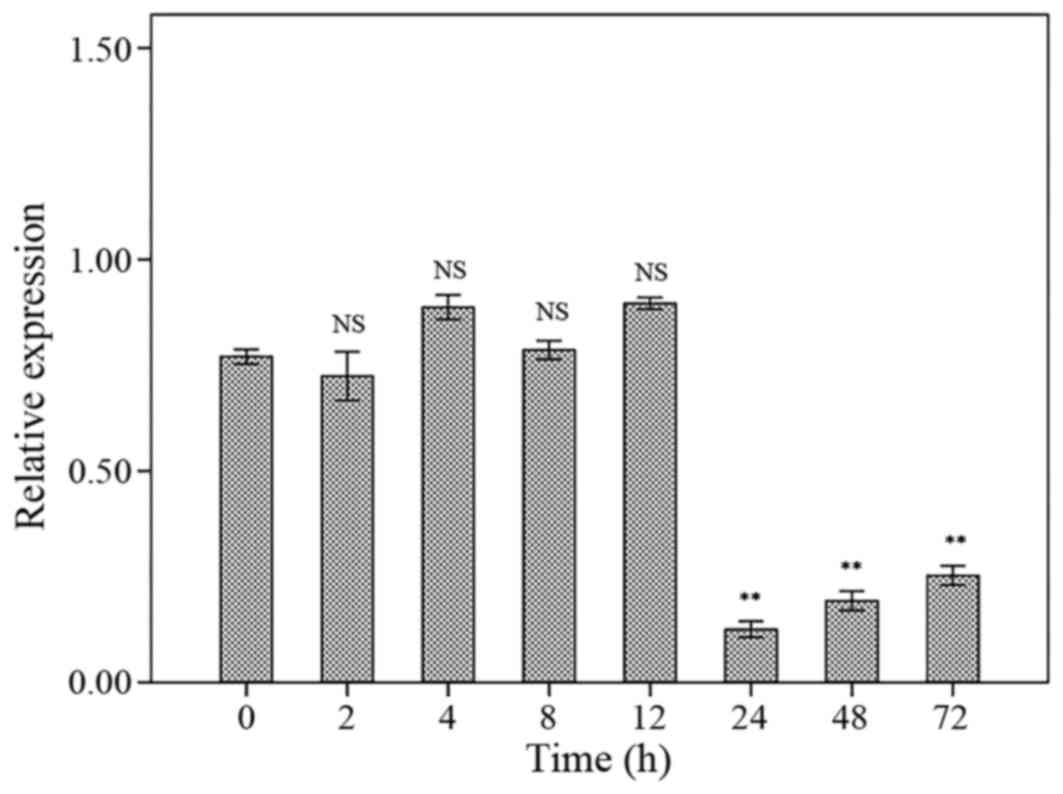
hTERT expression
The hTERT mRNA expression levels were not different
compared to control group after 2, 4, 8 and 12 h. However, the
hTERT mRNA expression levels were significantly decreased after 24,
48 and 72 h treatment with 150 μg/ml concentration of AW extract
compared to control. There were no significant differences
regarding to hTERT mRNA expression levels between 24, 48 and 72 h
of treatment (Fig. 4).
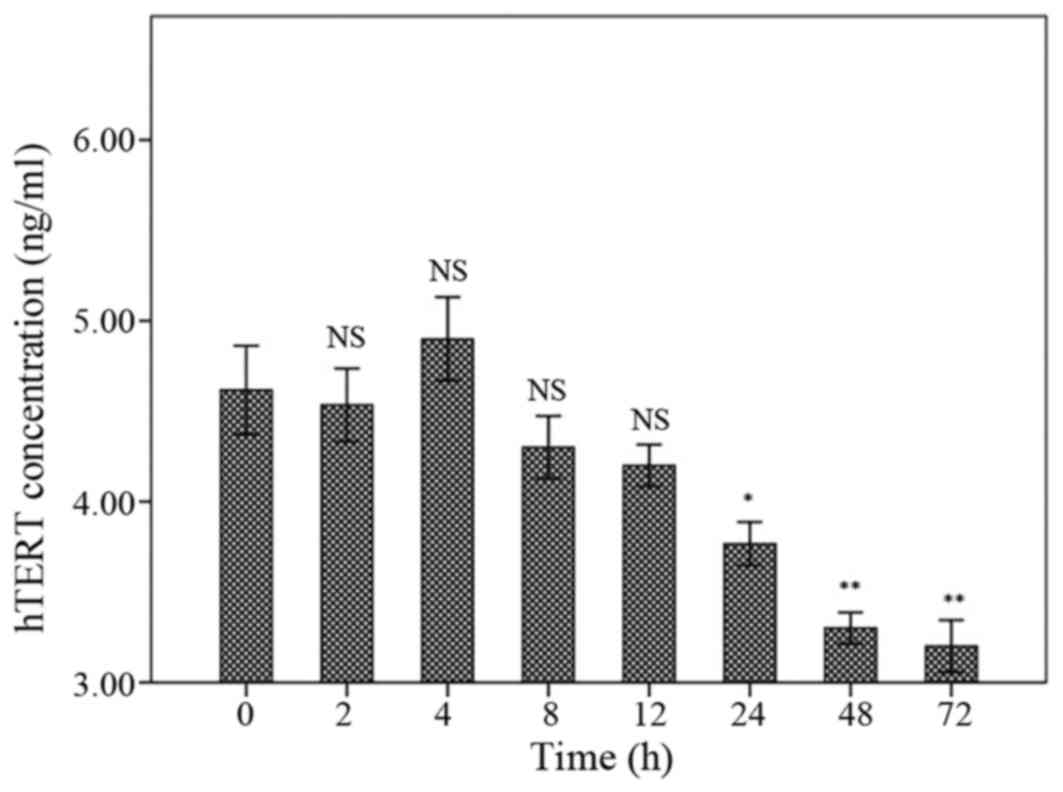
hTERT level
The hTERT levels have been presented in Fig. 5. Following treatment for 2, 4, 8 or 12
h, the hTERT levels were not different compared to control. The
hTERT level was significantly decreased following 24 h treatment
with marginal P-value (P=0.043). However, the hTERT levels were
significantly declined in 48 and 72 h following treatment
(P<0.001). There were no significant differences between 24, 48
and 72 h treatments (Fig. 5).
Discussion
PCa is the second reason of cancer-related deaths
after lung cancer in men. Androgen ablation is the prominent and
the most successful treatment of progressive PCa, however, after
treatment the tumor cells become insensitive to androgen hormone.
Therefore, new targets required for therapeutic intervention of
androgen-independent PCa. Therefore, telomerase could be a suitable
and main target for therapeutic intervention in this cancer
(2,3).
Most normal cells typically lack telomerase activity, but this
enzyme expressed in >85% of all human cancer cells (23,24). Higher
telomerase levels are identified in the most of PCas, but, not in
normal or benign prostatic hyperplasia tissues (6). Existing data suggest that hTERT
expression and telomerase activity are positively regulated by
androgenic stimuli in androgen-dependent PC (ADPC) cells. Evidence
demonstrated that AR regulates the EGR1 expression that in turn
controls positively the hTERT expression in CRPC cells. Thus, AR
exerts an inhibitory effect on hTERT expression and telomerase
activity by modulating EGR1 levels in CRPC cells (25).
Expression and reactivation of telomerase has also
been described as an important feature of PCa. Telomerase activity
was found in up to 100% of analyzed PCa cases (26). Interestingly, high expression of
telomerase components does not always result in mandatory
telomerase activity (27). In
addition, hTERT expression was significantly associated with the
aggressive behavior of prostate tumors (28). Previously, promising in vitro
data were published identifying telomerase as a main target of an
anti-androgen therapy in PCa, and the effectiveness of boron
derivatives as a telomerase inhibitors (29,30). These
data suggested telomerase inhibition as a reasonable therapeutic
approach for the treatment of PCa, though, the molecular and
cellular pathways involved in telomerase reactivation in PCa are
still not clear. Expression of TERT and the telomerase activity
were regulated by androgen receptor signaling, whereas exogenous
expression of AR surprisingly led to inhibition of TERT
transcription in PCa cells (31,32).
According to this fact that some PCa cells become
insensitive to AR treatment, in the current study, the authors
investigated the effects of the herbal extract of AW on apoptosis
and hTERT expression in an insensitive prostatic cancer cell line
to AR treatment.
PC3 is a PCa cell line that is established from bone
metastasis of grade IV of PCa. This cell line has potential
metastatic activity and do not respond to androgens,
glucocorticoids or fibroblast growth factors. This cell line is
useful in investigating the therapeutic interventions in
progressive prostatic cancer cells (33).
In the current study, the apoptotic effect of AW
extract on the PC3 cell line was assessed and the effects of this
plant on hTERT expression and concentration were determined.
The findings demonstrated the appropriate inhibitory
effect of AW extract in 150 µg/ml concentration (IC50)
on PC3 cell line following AW treatment for 48 h. Proliferation of
the PC3 cells was significantly inhibited in a dose- and
time-dependent manner. A significant increase in early and late
apoptotic cells and a decrease in live cells in a dose-dependent
manner was observed following treatment with AW extract for 48 h.
Moreover, the early apoptotic cells were significantly higher than
late apoptotic cells. The relative mRNA expression was decreased
following 24 h treatment of AW extract compared to control, however
it was not different between 2, 4, 8 and 12 h treatments or 24, 48
and 72 h treatments. In addition, the hTERT concentration was
significantly decreased after 24 h treatment of AW extract with
marginal P-value. There was no significant difference regarding to
hTERT concentration between 2, 4, 8 and 12 h treatments or 24, 48
and 72 h treatments.
The apoptotic effects of AW species extracts have
been investigated in various cancer cell lines. The flavonoid
casticin derived from Achilleamillefolium, demonstrated an
important effect in cancer therapy (34). The anticancer effects of the various
species of Achillea have been reported in different cancer
cell lines (35,36). The cytotoxic and pro- apoptotic effects
of methanol and water extracts of Achillea teretifolia on
DU145 and PC3 PCa cell lines were considered in a recent study
(37).
However, studies on the apoptotic effects of AW
extract are rare. In a study, the cytotoxic effects of essential
oils extracted from leaves of AW C. Koch have been described in
several cancer cell lines including PC3 (38). In a similar study, the
anti-proliferative and apoptosis-inducing potential of
hydroalcoholic AW C. Koch extract were investigated on MCF-7 and
MDA-Mb-468 human breast carcinoma cell lines. Consistent to results
of current study, the apoptosis-inducing potential of the
hydroalcoholic AW extract was indicated, however they observed this
effect with lower concentration of AW extract compared with the
present authors' results on the PC3 cell line (25 vs. 150 µg/ml)
(22).
In addition, there was no published report on the
effects of not only AW C. Koch extract but also other species of
Achillea on the hTERT mRNA expression or concentration in
different cancer cell lines. However, several studies investigated
the effect of other herbal extracts on telomerase expression and
concentration in PCa cell lines. Fruit extract of Gleditsia
sinensis exhibited anti-cancer and telomerase inhibition
effects on ESCC (esophageal squamous cell carcinoma) cell line
(39). In human lung carcinoma cell
line A549, aqueous extract of the Platycodon grandiflorum
root represents the apoptotic events with the reduced telomerase
activity and downregulation of Bcl2 expression (40).
The effect of hydroalcoholic extract of Melissa
officinalis on human lung, breast and PCa cell lines
demonstrated the potent anti-proliferative activity of this extract
via parallel downregulation of VEGF-A and hTERT (41).
The investigation on the pristimerin effects on PCa
LNCaP and PC3 cell lines exhibited that this quinonemethide
triterpenoid inhibited the hTERT mRNA expression and suppressed the
native and phosphorylated hTERT protein and hTERT telomerase
activity (42).
In another study, the effects of PC-SPES, herbal
formulation for PCa, on LNCaP, PC-3 and DU 145 PCa cell lines have
been studied and the finding presented unchanged levels of
telomerase in each of the lines (43).
In conclusion, the current findings demonstrated
that the herbal extract of AW has an apoptotic effect on PC3 PCa
cell line. In addition, the herbal extract of AW can inhibit hTERT
mRNA expression. Further studies with each component of AW are
required in various cancer cell lines to identify the active
substance of this plant on telomerase expression and activity.
Acknowledgements
This study was extracted from the MS thesis
(registered no. 7534) at Zahedan University of Medical Sciences
(Zahedan, Iran). The authors would like to thank the Deputy of
Research Affairs at the University for funding the current
project.
References
|
1
|
The Lancet: Moving cancer up the global
health agenda. Lancet. 375:20512010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rove KO and Crawford ED: Traditional
androgen ablation approaches to advanced prostate cancer: New
insights. Can J Urol. 21:14–21. 2014.PubMed/NCBI
|
|
4
|
Yu EM, Jain M and Aragon-Ching JB:
Angiogenesis inhibitors in prostate cancer therapy. Discov Med.
10:521–530. 2010.PubMed/NCBI
|
|
5
|
Garrison JB and Kyprianou N: Novel
targeting of apoptosis pathways for prostate cancer therapy. Curr
Cancer Drug Targets. 4:85–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Biroccio A and Leonetti C: Telomerase as a
new target for the treatment of hormone-refractory prostate cancer.
Endocr Relat Cancer. 11:407–421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wright WE, Tesmer VM, Huffman KE, Levene
SD and Shay JW: Normal human chromosomes have long G-rich telomeric
overhangs at one end. Genes Dev. 11:2801–2809. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McElligott R and Wellinger RJ: The
terminal DNA structure of mammalian chromosomes. EMBO J.
16:3705–3714. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Lange T: Protection of mammalian
telomeres. Oncogene. 21:532–540. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Venteicher AS, Abreu EB, Meng Z, McCann
KE, Terns RM, Veenstra TD, Terns MP and Artandi SE: A human
telomerase holoenzyme protein required for Cajal body localization
and telomere synthesis. Science. 323:644–648. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pachman DR, Barton DL, Swetz KM and
Loprinzi CL: Troublesome symptoms in cancer survivors: Fatigue,
insomnia, neuropathy, and pain. J Clin Oncol. 30:3687–3696. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stark L, Tofthagen C, Visovsky C and
McMillan SC: The Symptom Experience of Patients with Cancer. J Hosp
Palliat Nurs. 14:61–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tavakoli J, Miar S, Zadehzare M Majid and
Akbari H: Evaluation of effectiveness of herbal medication in
cancer care: A review study. Iran J Cancer Prev. 5:144–156.
2012.PubMed/NCBI
|
|
14
|
Baum M, Ernst E, Lejeune S and Horneber M:
Role of complementary and alternative medicine in the care of
patients with breast cancer: Report of the European Society of
Mastology (EUSOMA) Workshop, Florence, Italy, December 2004. Eur J
Cancer. 42:1702–1710. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lian Z, Niwa K, Gao J, Tagami K, Mori H
and Tamaya T: Association of cellular apoptosis with anti-tumor
effects of the Chinese herbal complex in endocrine-resistant cancer
cell line. Cancer Detect Prev. 27:147–154. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fong HH: Integration of herbal medicine
into modern medical practices: Issues and prospects. Integr Cancer
Ther. 1:287–293; discussion 293. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saeidnia S, Gohari A, Mokhber-Dezfuli N
and Kiuchi F: A review on phytochemistry and medicinal properties
of the genus Achillea. Daru. 19:173–186. 2011.PubMed/NCBI
|
|
18
|
Zargari A: Medicinal Plants. 2. 6th
edition. Tehran University Publication; Tehran: 1996
|
|
19
|
Zengin G, Aktumsek A, Ceylan R, Uysal S,
Mocan A, Guler GO, Mahomoodally MF, Glamočlija J, Ćirić A and
Soković M: Shedding light on the biological and chemical
fingerprints of three Achillea species (A. biebersteinii,
A. millefolium and A. teretifolia). Food Funct. 8:1152–1165.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khazneh E, Hribova P, Hosek J, Pavel
Suchý, Peter Kollár, Gabriela Pražanová, Jan Muselík, Zuzana
Hanaková and Jiří Václavík: The chemical composition of Achillea
wilhelmsii C. Koch and its desirable effects on hyperglycemia,
inflammatory mediators and hypercholesterolemia as risk factors for
cardiometabolic disease. Molecules. 21:4042016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niazmand S, Harandizadeh F, Mahmoudabady
M, Hosseini M, Hasanzadeh M and Fereidouni E: Mechanism of
vasorelaxation induced by Achillea wilhelmsii in rat
isolated thoracic aorta. Adv Biomed Res. 3:912014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Galavi HR, Saravani R, Shahraki A and
Ashtiani M: Anti-proliferative and apoptosis inducing potential of
hydroalcoholic Achillea wilhelmsii C. Koch extract on human
breast adenocarcinoma cell lines MCF-7 and MDA-Mb-468. Pak J Pharm
Sci. 29:(Suppl 6). 2397–2403. 2016.
|
|
23
|
Krams M, Hero B, Berthold F, Parwaresch R,
Harms D and Rudolph P: Full-length telomerase reverse transcriptase
messenger RNA is an independent prognostic factor in neuroblastoma.
Am J Pathol. 162:1019–1026. 2003. View Article : Google Scholar
|
|
24
|
Broccoli D, Young JW and de Lange T:
Telomerase activity in normal and malignant hematopoietic cells.
Proc Natl Acad Sci USA. 92:9082–9086. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jacob S, Nayak S, Kakar R, Chaudhari UK,
Joshi D, Vundinti BR, Fernandes G, Barai RS, Kholkute SD and
Sachdeva G: A triad of telomerase, androgen receptor and early
growth response 1 in prostate cancer cells. Cancer Biol Ther.
17:439–448. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meeker AK: Telomeres and telomerase in
prostatic intraepithelial neoplasia and prostate cancer biology.
Urol Oncol. 24:122–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kamradt J, Drosse C, Kalkbrenner S, Rohde
V, Lensch R, Lehmann J, Fixemer T, Bonkhoff H, Stoeckle M and
Wullich B: Telomerase activity and telomerase subunit gene
expression levels are not related in prostate cancer: A real-time
quantification and in situ hybridization study. Lab Invest.
83:623–633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Kok JB, Verhaegh GW, Roelofs RW,
Hessels D, Kiemeney LA, Aalders TW, Swinkels DW and Schalken JA:
DD3(PCA3), a very sensitive and specific marker to detect prostate
tumors. Cancer Res. 62:2695–2698. 2002.PubMed/NCBI
|
|
29
|
Korkmaz M, Avcı CB, Gunduz C, Aygunes D
and Erbaykent-Tepedelen B: Disodium pentaborate decahydrate (DPD)
induced apoptosis by decreasing hTERT enzyme activity and
disrupting F-actin organization of prostate cancer cells. Tumour
Biol. 35:1531–1538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu S, Qi Y, Ge Y, Duplessis T, Rowan BG,
Ip C, Cheng H, Rennie PS, Horikawa I, Lustig AJ, et al: Telomerase
as an important target of androgen signaling blockade for prostate
cancer treatment. Mol Cancer Ther. 9:2016–2025. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo C, Armbruster BN, Price DT and Counter
CM: In vivo regulation of hTERT expression and telomerase activity
by androgen. J Urol. 170:615–618. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moehren U, Papaioannou M, Reeb CA,
Grasselli A, Nanni S, Asim M, Roell D, Prade I, Farsetti A and
Baniahmad A: Wild-type but not mutant androgen receptor inhibits
expression of the hTERT telomerase subunit: A novel role of AR
mutation for prostate cancer development. FASEB J. 22:1258–1267.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaighn ME, Narayan KS, Ohnuki Y, Lechner
JF and Jones LW: Establishment and characterization of a human
prostatic carcinoma cell line (PC-3). Invest Urol. 17:16–23.
1979.PubMed/NCBI
|
|
34
|
Haïdara K, Zamir L, Shi QW and Batist G:
The flavonoid Casticin has multiple mechanisms of tumor
cytotoxicity action. Cancer Lett. 242:180–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grossini E, Marotta P, Farruggio S,
Sigaudo L, Qoqaiche F, Raina G, de Giuli V, Mary D, Vacca G and
Pollastro F: Effects of artemetin on nitric oxide release and
protection against peroxidative injuries in porcine coronary artery
endothelial cells. Phytother Res. May 29–2015.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baharara J, Namvar F, Ramezani T, Mousavi
M and Mohamad R: Silver nanoparticles biosynthesized using
Achillea biebersteinii flower extract: Apoptosis induction
in MCF-7 cells via caspase activation and regulation of Bax and
Bcl-2 gene expression. Molecules. 20:2693–2706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bali EB, Açık L, Elçi P, Sarper M, Avcu F
and Vural M: In vitro anti-oxidant, cytotoxic and pro-apoptotic
effects of Achillea teretifolia Willd extracts on human
prostate cancer cell lines. Pharmacogn Mag. 11:(Suppl 2).
S308–S315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ahmadi-Jouibaria T, Nikbakht MR, Mansouri
K and Majnooni MB: Cytotoxic effects of the essential oil from
Achillea wilhelmsii C. Koch. J Rep Pharm Sci. 2:98–102.
2013.
|
|
39
|
Tang WK, Chui CH, Fatima S, Kok SH, Pak
KC, Ou TM, Hui KS, Wong MM, Wong J, Law S, et al: Inhibitory
effects of Gleditsia sinensis fruit extract on telomerase
activity and oncogenic expression in human esophageal squamous cell
carcinoma. Int J Mol Med. 19:953–960. 2007.PubMed/NCBI
|
|
40
|
Park DI, Lee JH, Moon SK, Kim CH, Lee YT,
Cheong J, Choi BT and Choi YH: Induction of apoptosis and
inhibition of telomerase activity by aqueous extract from
Platycodon grandiflorum in human lung carcinoma cells.
Pharmacol Res. 51:437–443. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jahanban-Esfahlan R, Seidi K, Monfaredan
A, Shafie-Irannejad V, Abbasi MM, Karimian A and Yousefi B: The
herbal medicine Melissa officinalis extract effects on gene
expression of p53, Bcl-2, Her2, VEGF-A and hTERT in human lung,
breast and prostate cancer cell lines. Gene. 613:14–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu YB, Gao X, Deeb D, Pindolia K and
Gautam SC: Role of telomerase in anticancer activity of pristimerin
in prostate cancer cells. J Exp Ther Oncol. 11:41–49.
2015.PubMed/NCBI
|
|
43
|
Kubota T, Hisatake J, Hisatake Y, Said JW,
Chen SS, Holden S, Taguchi H and Koeffler HP: PC-SPES: A unique
inhibitor of proliferation of prostate cancer cells in vitro and in
vivo. Prostate. 42:163–171. 2000. View Article : Google Scholar : PubMed/NCBI
|