Introduction
Glioblastoma multiforme (GBM) is among the most
common malignant cancers of the brain (1). Although the main cause of this disease
remains unknown, possible risk factors include genetic disorder
(2), viral infection (3), alcohol consumption (4) and ionizing radiation exposure (5). Standard treatments for GBM include
surgicael resection, chemotherapy (e.g., temozolomide therapy) and
radiotherapy (6,7). As GBM spreads rapidly and has a high
potential for relapse even the most aggressive treatment rarely
achieves survival >3-years and the median survival time is
approximately 1-year (7,8). Complementary and alternative therapies
commonly used in GBM patients include vitamin and nutrient
supplements as well as herbal extracts (9). Some clinical evidence shows that
treatment with herbal extracts can reduce mortality in GBM patients
(10).
Pluchea indica (L.) Less. (Asteraceae) is a
perennial shrub with medicinal properties that are widely
recognized in many Asian countries (11). P. indica is traditionally used
to heal minor wounds because it has astringent, antipyretic and
anti-inflammatory activities (11,12). The
leaves of P. indica contain α-glucosidase inhibitors that
could help treat diabetes mellitus by suppressing carbohydrate
digestion (13). Methanol extracts of
the P. indica root are known to neutralize snake venoms
(14) and contain anti-amoebic
activities (15). The methanol
fraction of P. indica root and leaf extracts has revealed
potent anti-ulcer (16) and
anti-tuberculosis effects (17). The
aqueous extract of P. indica has antiviral activity against
human immunodeficiency virus type 1 (18). Quinic acid esters from the leaves of
P. indica showed inhibitory activities towards collagenase,
MMP-2 and −9 (19). In vitro
studies also revealed that crude aqueous extracts and ethanol
extracts of P. indica root effectively suppress human
malignant glioma cancer cells and human cervical cancer cells
(20,21).
Therefore, the aim of this study was to investigate
the underlying mechanisms of the anticancer effects of the hexane
fraction of P. indica root extract (H-PIRE) in human U87 GBM
cells and primary GBM cells.
Materials and methods
Plant material and extraction
The P. indica plants used in experiments in
this study were collected and verified as described in a previous
study (20). The roots of P.
indica were prepared as a dried powder and deposited at the
National Sun Yat-sen University (Kaohsiung, Taiwan, R.O.C.) and the
H-PIRE was extracted using the method described by Kao et al
(21). Briefly, the P. indica
extract was prepared by immersing the root powder in 95% (v/v)
ethanol at room temperature overnight, filtering with 0.45 µm
filters, then partitioning the water and ethyl acetate (v/v, 1:1).
The ethyl acetate fraction was further partitioned with a 1:1 (v/v)
mixture of 75% ethanol and hexane. The hexane layer was collected
and concentrated to obtain powdered H-PIRE. The powdered H-PIRE was
then dissolved in dimethyl sulfoxide (DMSO) to desired
concentrations in subsequent experiments.
Preliminary phytochemical
analysis
Total phenolic compound content was determined with
Folin-Ciocalteu reagent and presented as gallic acid equivalents in
mg/g of extract. Total flavonoid content was determined by aluminum
chloride colorimetric method and expressed as catechin equivalents
in mg/g of extract (22). Condensed
tannin (proanthocyanidin) content was analyzed by vanillin assay
method as described by Butler et al (23) and presented as catechin equivalents in
mg/g of extract. All assays were performed in triplicate.
Cell culture
Primary GBM cells were enzymatically dissociated
from fresh glioblastoma samples as described by Pavon et al
(24), according to a protocol
approved by the Kaohsiung Medical University Institutional Review
Board (Kaohsiung, Taiwan, R.O.C.). The U87 and primary GBM cell
lines were cultured in Dulbecco's modified Eagle's medium with 10%
fetal bovine serum (all from HyClone; GE Healthcare Life Sciences,
Logan, UT, USA), 200 mM l-glutamine (Invitrogen Life Technologies;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 1% penicillin
(10,000 U/ml)/streptomycin (10,000 U/ml) (HyClone; GE Healthcare
Life Sciences Hyclone), 1X non-essential amino acids and 1 mM
sodium pyruvate (both from Invitrogen Life Technologies; Thermo
Fisher Scientific, Inc.). The cells were cultured in 5%
CO2 at 37°C in a humidified incubator. Institutional
board approval was obtained before experimental use of primary GBM
cells derived from patients.
Cell viability assays
Cell viability was determined using the WST-1
colorimetric assay method. The cells were cultured overnight in
96-well plates (2×103 cells/well) and treated with
0–1,000 µg/ml H-PIRE or DMSO (negative control) for 24 to 72 h. The
medium was then carefully removed and replaced with fresh medium
containing the cell proliferation reagent WST-1 (Roche Diagnostics
GmbH, Mannheim, Germany) and the cells were incubated for an
additional 3 h at 37°C. Absorbance was measured at 450 nm with an
ELISA plate reader (Micro Quant; BioTek Instruments, Inc.,
Winooski, VT, USA). The IC50 values were determined as
previously described (20) and
presented as mean ± standard deviation (SD) (n=3).
Flow cytometric analysis
Apoptosis was detected with an fluorescein
isothiocyanate (FITC) Annexin V apoptosis detection kit I (BD
Pharmingen, BD Biosciences, Franklin Lakes, NJ, USA). After 24 and
48 h of incubation with H-PIRE or DMSO, 1×105 cells were
collected and suspended in 100 µl staining buffer containing 5 µl
of FITC Annexin V and 5 µl propidium iodide (PI) at room
temperature in the dark. After 15 min, 400 µl 1X binding buffer was
added and flow cytometric analysis was performed as previously
described (20).
Acridine orange staining
Cells were cultured in 60-mm dishes until 60%
confluent and then treated with 300 µg/ml H-PIRE for 24 h. The
cells were then stained with 1 mg/ml acridine orange (AO) for 15
min and washed with phosphate buffer saline (PBS) to detect acidic
vesicular organelles (AVOs). A fluorescence microscope (Olympus
IX70; Tokyo, Japan) with an excitation filter set to 490 nm and a
515 nm long-pass filter was used for cell analysis and digital
imaging.
Western blot analysis
The cells were cultured in 60-mm dishes until 60%
confluent and then treated with 300 µg/ml H-PIRE for 0 to 24 h.
After incubation, the cells were washed with PBS and lysed in T-PER
protein extraction reagent (Pierce). Cell lysates containing 20 µg
total protein were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
polyvinylidene difluoride membranes. Antibodies against p-p38
(1:1,000, rabbit monoclonal, Cell Signaling Technology cat. no.
4511), p-JNK (1:1,000, rabbit monoclonal, Cell Signaling
Technology, cat. no. 4668), LC3B (1:1,000, rabbit monoclonal, Cell
Signaling Technology cat. no. 3868) and β-actin (1:10,000, mouse
monoclonal, Sigma-Aldrich,) were used for immunoblotting. The
immunoblots were visualized using enhanced chemiluminescence
detection reagent (Amersham; GE Healthcare, Chicago, IL, USA). A
LAS-3000 CCD system was used for imaging and a Multi Gauge V2.02
both from (Fujifilm Holdings Corporation, Tokyo, Japan) was used
for analysis.
Statistical analysis
Data were expressed as the mean ± SD of three
independent experiments. Statistical significance was determined by
performing independent Student's t-test with SPSS 12.0 software
(SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Phytochemical screening
Table I shows that the
preliminary phytochemical analysis of H-PIRE revealed a high
content of phenols, flavonoids and proanthocyanidins, which are
biologically active phytochemical compounds found in many medicinal
herbal extracts. Secondary metabolites such as polyphenols and
flavonoids interact with proteins and are useful for inhibiting
multidrug resistance (25),
suppressing tumor angiogenesis and preventing inflammation
(26). Thus, the phytochemical
composition of H-PIRE was consistent with the reported medicinal
properties of P. indica.
 | Table I.Phytochemical content of H-PIRE. |
Table I.
Phytochemical content of H-PIRE.
| Phytochemical
content | Mean ± SD |
|---|
| Total phenols |
196.7±0.7
mg GAE/g DW |
| Total
flavonoids |
113.6±0.6
mg CE/g DW |
|
Proanthocyanidins |
28.947±0.0062 mg CE/g DW |
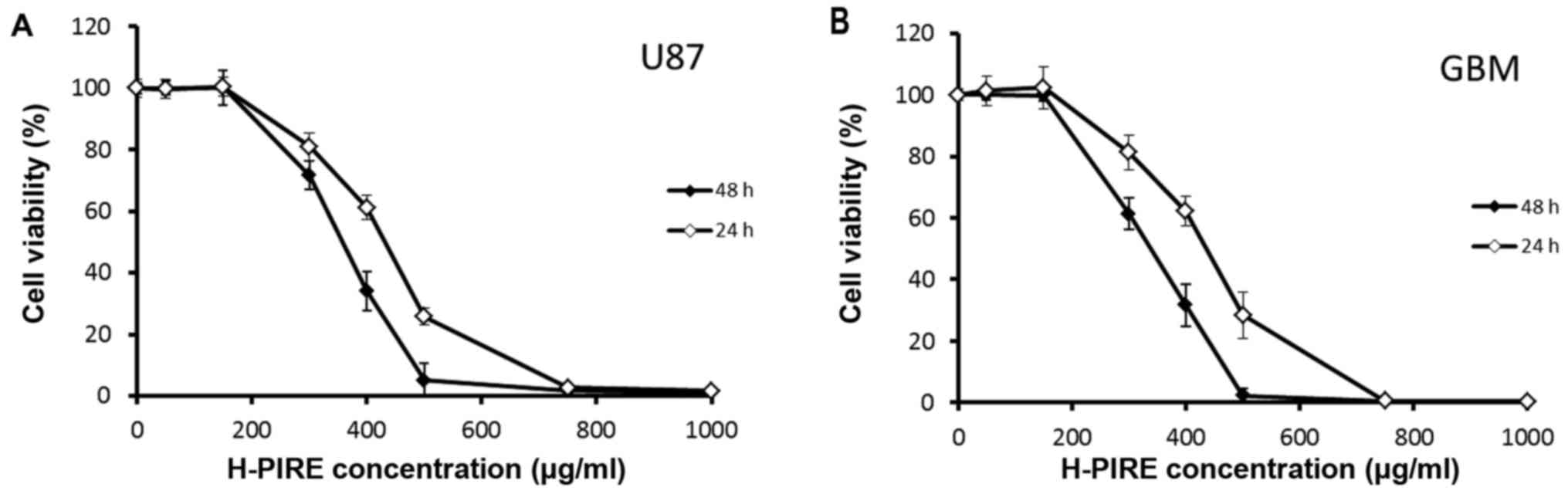
Suppression of GBM cell proliferation
by H-PIRE
The cytotoxic effects of H-PIRE on GBM cells were
examined by assays of WST-1 cell proliferation. Two GBM cell lines
were treated with varying concentrations of H-PIRE (range, 0–1,000
µg/ml) at 24 and 48 h. Fig. 1 shows
the experimental results. In both cell lines, H-PIRE reduced cell
viability in a dose-dependent manner. At 24 and 48 h, the
IC50 values (mean ± SD, µg/ml) for H-PIRE were
422.6±38.3 and 353.3±18.7 in U87 cells and 416.4±35.5 and
334.1±29.6 in GBM cells, respectively. That is, the IC50
values indicated that H-PIRE suppressed proliferation in both human
GBM cell lines.
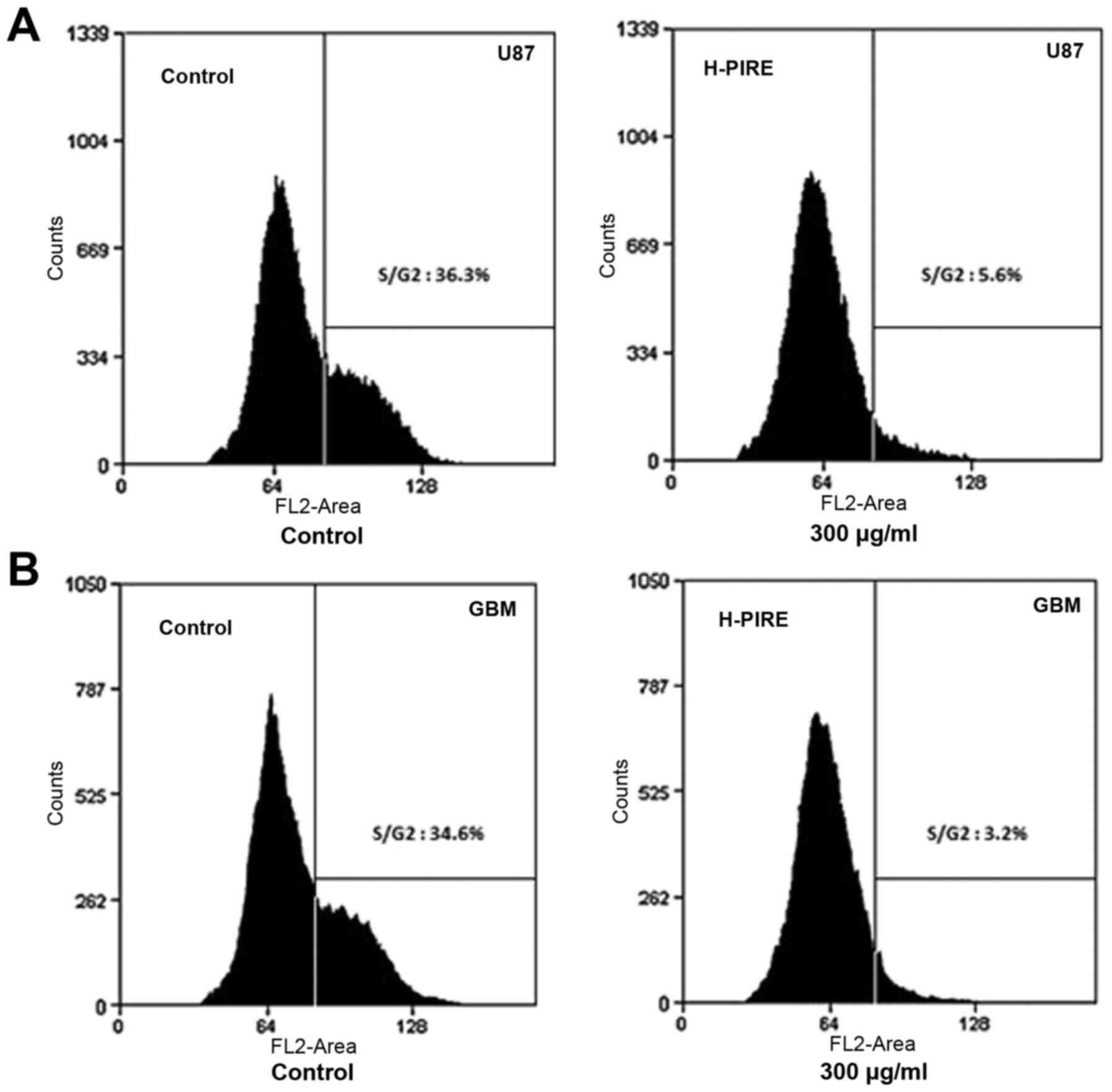
Suppression of cell cycle progression
by H-PIRE
The effect of H-PIRE on the cell cycle was
investigated by flow cytometry. The U87 and GBM cells were
incubated with 300 µg/ml H-PIRE for 24 h. The incubated cells were
then collected, fixed and stained with PI for flow cytometric
analysis of DNA content. Fig. 2 shows
that the H-PIRE treatment significantly decreased the cell
populations in the S and G2/M phases. However, the sub-G1
population showed no increase, which indicated that the treatment
did not induce apoptosis. Thus, H-PIRE suppressed progression of
the human GBM cell cycle through the G0/G1 phase.
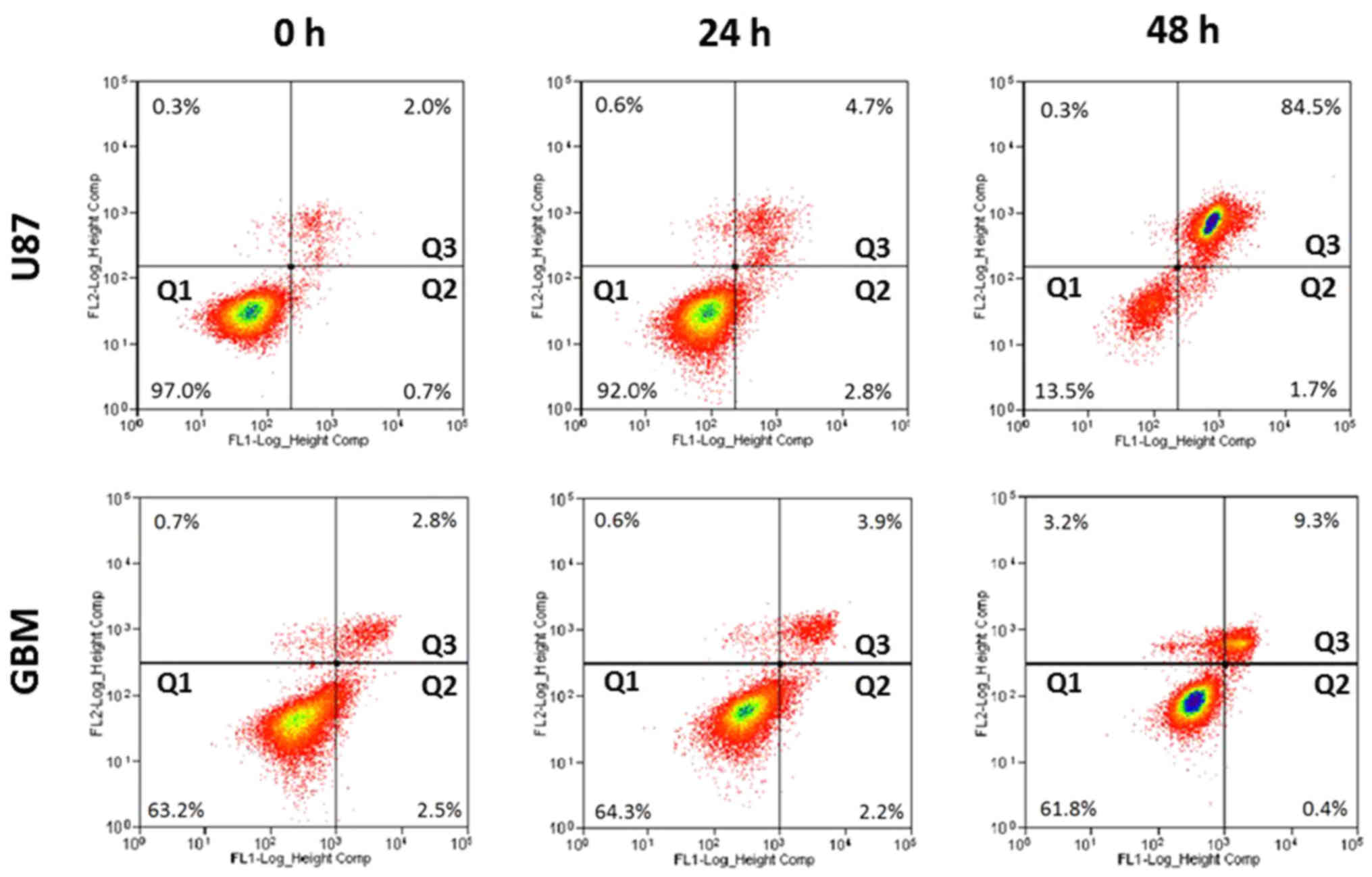
Induction of apoptosis by H-PIRE
Apoptotic effects of H-PIRE were investigated by
flow cytometry of unfixed cells stained with Annexin V and PI.
Fig. 3 shows that, at 24 and 48 h
after treatment with 300 µg/ml H-PIRE, the U87 and GBM cells showed
significantly increased percentages of dead cells in quadrant 3 but
no increase in early apoptosis in quadrant 2. These experimental
results suggest that cell death induced by H-PIRE in glioblastoma
cells is probably not associated with apoptosis.
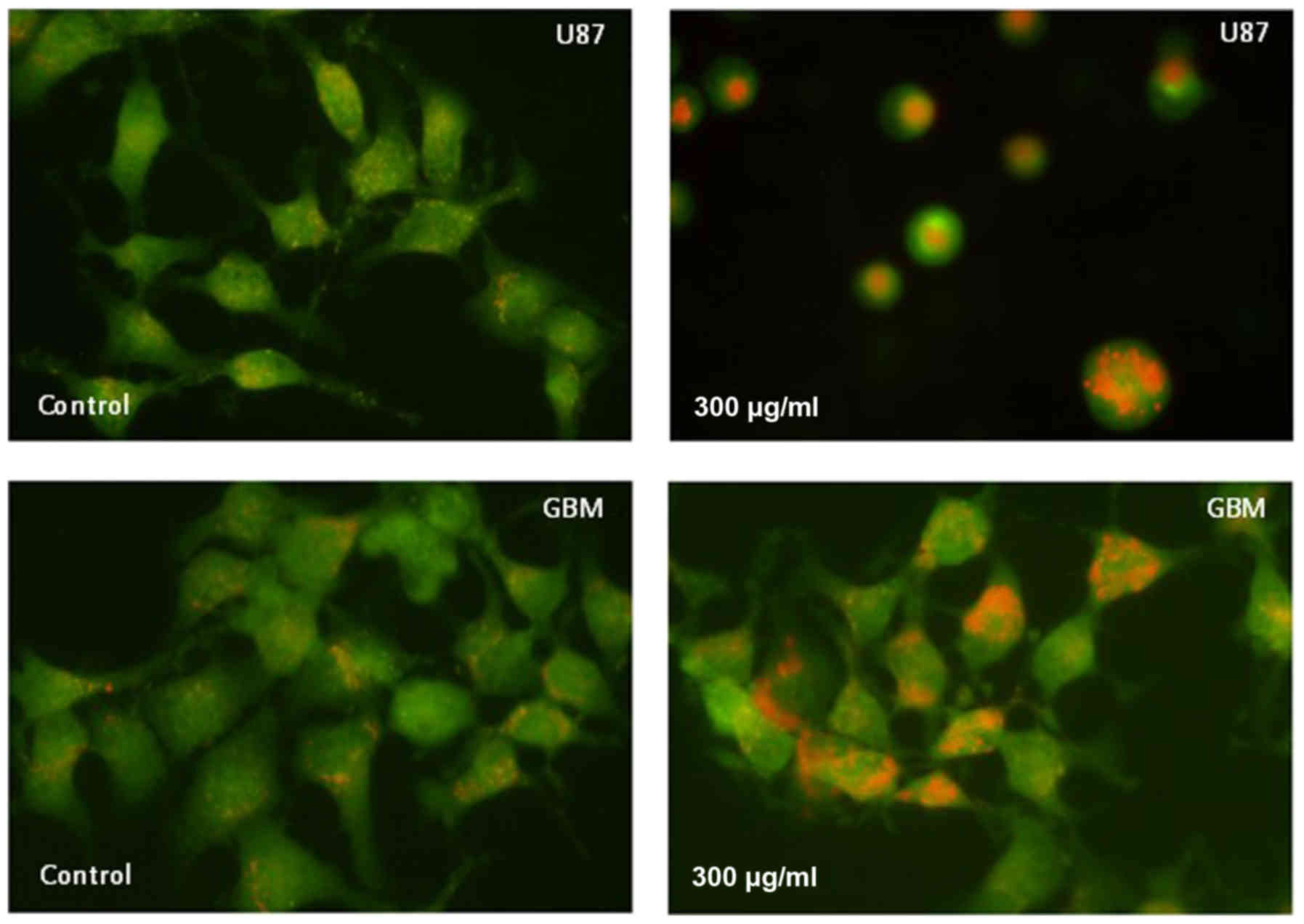
Detection of AVOs
Formation of AVOs, which is a morphological
characteristic of cells undergoing autophagy, can be detected by
fluorescence microscopy of living cells stained with AO, which
emits red fluorescence at low pH. The staining results in Fig. 4 show that AVO expression was increased
in U87 and GBM cells at 24 h after treatment with 300 µg/ml H-PIRE.
These results clearly indicate that H-PIRE induces autophagy in
human glioblastoma cells.
Expression of autophagy-related
proteins
The mechanism of cell death induced by H-PIRE was
investigated by examining the expression of autophagy-related
proteins in U87 and GBM cells. The cells were treated with 300
µg/ml of H-PIRE for 0 to 24 h. Western blot analysis was then used
to examine the expression of microtubule-associated protein light
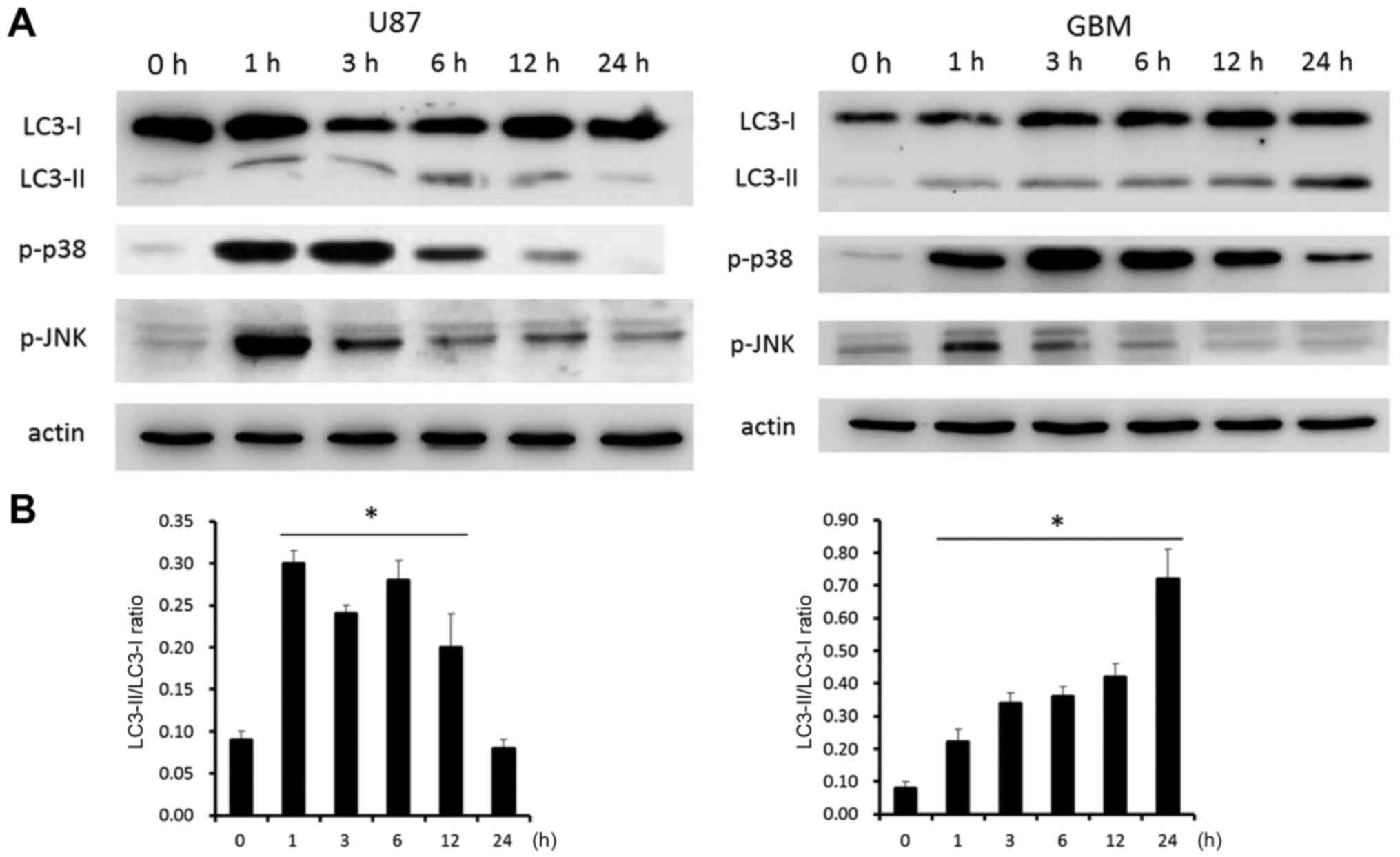
chain 3 (LC3)-I, LC3-II, phosphorylated-p38 and phosphorylated-JNK.
Fig. 5A shows that, at 1 h after the
H-PIRE treatment, LC3-II was significantly upregulated relative to
LC3-I, which significantly increased autophagosome formation
(27). In both cell lines, expression
of phosphorylated-p38 protein was significantly increased at 1 h
after H-PIRE treatment and peaked at 3 h after treatment.
Phosphorylated-p38 protein expression remained at the peak level
for >12 h in U87 cells and for 24 h in GBM cells before dropping
back to the base level. Expression of phosphorylated-JNK protein
was substantially increased at 1 h after H-PIRE treatment and then
gradually decreased to the base level in the two cell lines. The
results indicated that, in both cell lines, the stress induced by
H-PIRE activated the p38 and JNK signaling pathways, which are
known to play key roles in the induction of autophagy (27).
Fig. 5B shows that the
LC3-II/LC3-I ratio increased in a time-dependent manner in both
cell lines. The ratio peaked at 0.30 at 1 h and started to decline
after 12 h in U87 cells. By contrast, the ratio increased steadily
and peaked at 0.73 at 24 h in GBM cells. In addition, H-PIRE
treatment resulted in different FITC-AV and PI staining pattern at
48 h (Fig. 3) and cell morphology at
24 h (Fig. 4) in U87 and GBM cells.
These data may reflect the intrinsic difference in the manner the
two types of cells responded to autophagic stress. The overall
results indicate that H-PIRE activates p38 and JNK and induces
autophagy in human GBM cells.
Additive cytotoxic effects of combined
treatment with H-PIRE with PI3K inhibitor
Rapamycin and LY294002 (a pan-PI3K inhibitor)
reportedly exert synergistic effects on the induction of autophagy
and reduction of cell survival in malignant glioma cell lines,
including U87 (28). Specifically,
rapamycin induces autophagy by deactivating mTOR (29). To determine whether inhibition of the
PI3K pathway and H-PIRE treatment synergistically affect cell
viability, U87 cells were treated with H-PIRE (0–1,000 µg/ml) with
or without 10 µM LY274002. Fig. 6
shows the cell viability assay results, which indicated that the
addition of LY294002 enhanced the reduction of cell viability
induced by H-PIRE treatment. The addition of LY294002 reduced
IC50 of H-PIRE from 353.3±18.7 to 298.6±20.3 µg/ml.
These results indicate that the combined treatment of H-PIRE and
LY294002 have additive effects on the reduction of cell survival in
human GBM cells.
Discussion
Previous findings have demonstrated the anticancer
properties of crude aqueous fraction and ethanol fraction of the
P. indica root extract (20).
The present study further investigated the suppressive effects of
H-PIRE on GBM cells. The hydrophobic nature of H-PIRE enables it to
penetrate the blood-brain barrier and effectively target GBM cells
in the brain.
Our experimental results indicate that H-PIRE has a
potent suppressive effect on human GBM cell growth, apparently by
inducing cell cycle arrest in the G0/G1 phase but without inducing
apoptosis (the sub-G1 fraction). However, due to the complex nature
of H-PIRE, we cannot exclude that apoptosis may be induced by
H-PIRE at much higher concentrations. Another possible mechanism of
the suppressive effects of H-PIRE is the induction of autophagy
through the increased formation of AVOs, increased expression of
LC3-II, and increased phosphorylation of JNK and p38 proteins.
The activation of p38 and JNK is usually associated
with antiproliferative activity (30)
and induction of autophagy (31,32). Recent
studies suggest that p38 and JNK have important roles in the
crosstalk between apoptosis and autophagy under cellular stress
conditions (33). Although autophagy
may be associated with cell survival or death (34), significantly higher than normal
autophagy is usually associated with cell death (35). Although inducing autophagy is
considered an effective way to induce GBM cell death (36,37), our
experiments showed that GBM cells are insensitive to most apoptotic
stimuli.
The PI3K, the protein kinase B (Akt), and the
mammalian target of rapamycin (mTOR) form a cell survival pathway
important for cell growth and proliferation. This pathway is also
critical for tumorigenesis (38) and
is now recognized as an important target in cancer treatment
(39). Our experiments showed that
pre-treating U87 cells with LY294002 inhibited PI3K activity, which
then increased their sensitivity to H-PIRE treatment. Thus, since
H-PIRE treatment substantially reduces GBM cell survival through an
autophagy-related pathway, combined treatments with H-PIRE and
other growth inhibitors may have synergistic effects in
conventional cancer treatment.
In conclusion, findings of this study show that
H-PIRE suppresses proliferation and reduces viability in GBM cells.
As it activates the p38 and JNK pathways, H-PIRE can also be used
to induce autophagy in GBM cells. However, further studies are
needed to elucidate the molecular pathway of anticancer activity
induced by H-PIRE.
Acknowledgements
This study was supported by grants from the ‘Aim for
the Top University Plan’ of the National Sun Yat-sen University and
from the NSYSU-KMU Joint Research Project (no. NSYSUKMU 105-P012)
and MOST 104–2320-B-037-030-MY3 from the Ministry of Science and
Technology (Taiwan, R.O.C.). We would like to thank Dr L-J Liao
(National Kaohsiung Normal University, Kaohsiung, Taiwan, R.O.C.)
for providing and authenticating the P. indica plants used
in experiments in the present study.
References
|
1
|
Bleeker FE, Molenaar RJ and Leenstra S:
Recent advances in the molecular understanding of glioblastoma. J
Neurooncol. 108:11–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bleeker FE, Lamba S, Zanon C, Molenaar RJ,
Hulsebos TJM, Troost D, van Tilborg AA, Vandertop WP, Leenstra S,
van Noorden CJF, et al: Mutational profiling of kinases in
glioblastoma. BMC Cancer. 14:7182014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crawford JR, Santi MR, Thorarinsdottir HK,
Cornelison R, Rushing EJ, Zhang H, Yao K, Jacobson S and Macdonald
TJ: Detection of human herpesvirus-6 variants in pediatric brain
tumors: Association of viral antigen in low grade gliomas. J Clin
Virol. 46:37–42. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baglietto L, Giles GG, English DR,
Karahalios A, Hopper JL and Severi G: Alcohol consumption and risk
of glioblastoma; evidence from the Melbourne Collaborative Cohort
Study. Int J Cancer. 128:1929–1934. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Braganza MZ, Kitahara CM, de González
Berrington A, Inskip PD, Johnson KJ and Rajaraman P: Ionizing
radiation and the risk of brain and central nervous system tumors:
A systematic review. Neuro-oncol. 14:1316–1324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ellis HP, Greenslade M, Powell B, Spiteri
I, Sottoriva A and Kurian KM: Current challenges in glioblastoma:
Intratumour heterogeneity, residual disease and models to predict
disease recurrence. Front Oncol. 5:2512015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnson DR and O'Neill BP: Glioblastoma
survival in the United States before and during the temozolomide
era. J Neurooncol. 107:359–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wrensch M, Minn Y, Chew T, Bondy M and
Berger MS: Epidemiology of primary brain tumors: Current concepts
and review of the literature. Neuro Oncol. 4:278–299. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Emsen B, Aslan A, Togar B and Turkez H: In
vitro antitumor activities of the lichen compounds olivetoric,
physodic and psoromic acid in rat neuron and glioblastoma cells.
Pharm Biol. 54:1748–1762. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mulpur BH, Nabors LB, Thompson RC, Olson
JJ, LaRocca RV, Thompson Z and Egan KM: Complementary therapy and
survival in glioblastoma. Neurooncol Pract. 2:122–126.
2015.PubMed/NCBI
|
|
11
|
Sen T, Dhara AK, Bhattacharjee S, Pal S
and Chaudhuri Nag AK: Antioxidant activity of the methanol fraction
of Pluchea indica root extract. Phytother Res. 16:331–335.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rahman Ab MR, Razak Abdul F and Bakri Mohd
M: Evaluation of wound closure activity of Nigella sativa,
Melastoma malabathricum, Pluchea indica and Piper
sarmentosum extracts on scratched monolayer of human gingival
fibroblasts. Evid Based Complement Alternat Med. 2014:1903422014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arsiningtyas IS, Gunawan-Puteri MD, Kato E
and Kawabata J: Identification of α-glucosidase inhibitors from the
leaves of Pluchea indica (L.) Less., a traditional
Indonesian herb: Promotion of natural product use. Nat Prod Res.
28:1350–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gomes A, Saha A, Chatterjee I and
Chakravarty AK: Viper and cobra venom neutralization by
β-sitosterol and stigmasterol isolated from the root extract of
Pluchea indica Less. (Asteraceae). Phytomedicine.
14:637–643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Biswas R, Dutta PK, Achari B,
Bandyopadhyay D, Mishra M, Pramanik KC and Chatterjee TK: Isolation
of pure compound R/J/3 from Pluchea indica (L.) Less. and
its anti-amoebic activities against Entamoeba histolytica.
Phytomedicine. 14:534–537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sen T, Ghosh TK and Chaudhuri AK: Studies
on the mechanism of anti-inflammatory and anti-ulcer activity of
Pluchea indica-probable involvement of 5-lipooxygenase
pathway. Life Sci. 52:737–743. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mohamad S, Zin NM, Wahab HA, Ibrahim P,
Sulaiman SF, Zahariluddin ASM and Noor SSM: Antituberculosis
potential of some ethnobotanically selected Malaysian plants. J
Ethnopharmacol. 133:1021–1026. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Locher CP, Witvrouw M, De Béthune MP,
Burch MT, Mower HF, Davis H, Lasure A, Pauwels R, De Clercq E and
Vlietinck AJ: Antiviral activity of Hawaiian medicinal plants
against human immunodeficiency Virus Type-1 (HIV-1). Phytomedicine.
2:259–264. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohtsuki T, Yokosawa E, Koyano T, Preeprame
S, Kowithayakorn T, Sakai S, Toida T and Ishibashi M: Quinic acid
esters from Pluchea indica with collagenase, MMP-2 and −9
inhibitory activities. Phytother Res. 22:264–266. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cho JJ, Cho CL, Kao CL, Chen CM, Tseng CN,
Lee YZ, Liao LJ and Hong YR: Crude aqueous extracts of Pluchea
indica (L.) Less. inhibit proliferation and migration of cancer
cells through induction of p53-dependent cell death. BMC Complement
Altern Med. 12:2652012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kao CL, Cho J, Lee YZ, Cheng YB, Chien CY,
Hwang CF, Hong YR, Tseng CN and Cho CL: Ethanolic extracts of
Pluchea indica induce apoptosis and antiproliferation
effects in human nasopharyngeal carcinoma cells. Molecules.
20:11508–11523. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harborne JB: Phytochemical methods: a
guide to modern techniques of plant analysis. 3rd. Chapman and
Hall; London, UK: 1998, View Article : Google Scholar
|
|
23
|
Butler LG, Price ML and Brotherton JE:
Vanillin assay for proanthocyanidins (condensed tannins):
Modification of the solvent for estimation of the degree of
polymerization. J Agric Food Chem. 30:1087–1089. 1982. View Article : Google Scholar
|
|
24
|
Pavon LF, Marti LC, Sibov TT, Malheiros
SM, Brandt RA, Cavalheiro S and Gamarra LF: In vitro analysis of
neurospheres derived from gioblastoma primary culture: A novel
methodology paradigm. Front Neurol. 4:2142014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wink M, Ashour ML and El-Readi MZ:
Secondary metabolites from plants inhibiting ABC transporters and
reversing resistance of cancer cells and microbes to cytotoxic and
antimicrobial agents. Front Microbiol. 3:1302012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tabrez S, Priyadarshini M, Urooj M, Shakil
S, Ashraf GM, Khan MS, Kamal MA, Alam Q, Jabir NR, Abuzenadah AM,
et al: Cancer chemoprevention by polyphenols and their potential
application as nanomedicine. J Environ Sci Health C Environ
Carcinog Ecotoxicol Rev. 31:67–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hansen TE and Johansen T: Following
autophagy step by step. BMC Biol. 9:392011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takeuchi H, Kondo Y, Fujiwara K, Kanzawa
T, Aoki H, Mills GB and Kondo S: Synergistic augmentation of
rapamycin-induced autophagy in malignant glioma cells by
phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer
Res. 65:3336–3346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Berger Z, Ravikumar B, Menzies FM, Oroz
LG, Underwood BR, Pangalos MN, Schmitt I, Wullner U, Evert BO,
O'Kane CJ, et al: Rapamycin alleviates toxicity of different
aggregate-prone proteins. Hum Mol Genet. 15:433–442. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signalling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun T, Li D, Wang L, Xia L, Ma J, Guan Z,
Feng G and Zhu X: c-Jun NH2-terminal kinase activation is essential
for up-regulation of LC3 during ceramide-induced autophagy in human
nasopharyngeal carcinoma cells. J Transl Med. 9:1612011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou C, Zhou J, Sheng F, Zhu H, Deng X,
Xia B and Lin J: The heme oxygenase-1 inhibitor ZnPPIX induces
non-canonical, Beclin 1-independent, autophagy through p38 MAPK
pathway. Acta Biochim Biophys Sin (Shanghai). 44:815–822. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tanida I: Autophagosome formation and
molecular mechanism of autophagy. Antioxid Redox Signal.
14:2201–2214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shimizu S, Yoshida T, Tsujioka M and
Arakawa S: Autophagic cell death and cancer. Int J Mol Sci.
15:3145–3153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koukourakis MI, Mitrakas AG and
Giatromanolaki A: Therapeutic interactions of autophagy with
radiation and temozolomide in glioblastoma: Evidence and issues to
resolve. Br J Cancer. 114:485–496. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yan Y, Xu Z, Dai S, Qian L, Sun L and Gong
Z: Targeting autophagy to sensitive glioma to temozolomide
treatment. J Exp Clin Cancer Res. 35:232016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Morgensztern D and McLeod HL:
PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer
Drugs. 16:797–803. 2005. View Article : Google Scholar : PubMed/NCBI
|