Introduction
Leber's hereditary optic neuropathy (LHON; OMIM
535000) is a classic mitochondrial disease, associated with a
rapid, painless, acute or sub-acute bilateral visual loss in young
adults, predominantly caused by the primary and secondary mutations
in mitochondrial DNA (mtDNA). It has been reported that 1:8,500
individuals harbor a primary LHON-causing mutation and 1:31,000
experience visual loss as a result of LHON in the North East of
England (1). Few significant
improvements in visual acuity are reported following atrophy of the
optic discs. LHON typically affects males more frequently than
females, with the incomplete and variable penetrance estimated at
~50% in males and 10% in females (2–4).
Additionally, certain LHON cases have additional clinical symptoms,
such as movement disorders, dystonia, and multiple-sclerosis-like
illness, which complicate the diagnosis in the clinical setting
(5–7).
Although the majority ofcases of LHON transmitted by maternal
inheritance have a history of visual loss in families, up to 40% of
cases are sporadic (5).
The genetic cause of LHON is mutations in the
mitochondrial genome, which is a double-stranded 16,569-nucleotide
pair, circular molecule, consisting of one D-Loop region and 37
genes. The three most causative mutations, m.11778G>A
(MT-ND4), m.14484T>C (MT-ND6) and m.3460G>A
(MT-ND1), have been reported to account for 90% of LHON
patients in a Caucasian population, but for only 38.3 and 46.5% of
cases in two large cohorts of Chinese Han subjects with LHON
(7–10). Our previous studies have shown the
spectrum of genes, MT-ND1, MT-ND4 and MT-ND6, and the
frequency of the three primary mutations in a Chinese LHON
population (8–10) using Sanger sequencing. In addition,
secondary mutations that contributed to the high penetrance,
including m.3394T>C (MT-ND1), m.11696G>A
(MT-ND4), m.12338T>C (MT-ND5) and m.15951A>G
(MT-TT) areusually synergized with m.11778G>A or
m.14484T>C or m.3460G>A (11).
According to Mitomap (http://www.mitomap.org/), >40 point mutations in
mtDNA are associated with LHON, of which the incidence varies
between different ethnic backgrounds.
To further understand the spectrum of mutations
associated with LHON in a Chinese population, 46 LHON-associated
mutations distributed among 13 mitochondrial genes were selected
from Mitomap, and multi-gene target sequencing was performed in 275
cases of LHON as well as in 281 Chinese control subjects to
distinguish the most frequent mtDNA mutations associated with LHON
in the Han population.
Materials and methods
DNA samples, extraction,
quantification and quality control
A total of 275 unrelated LHON samples and 281
Chinese control samples were enrolled from the ophthalmology
clinics at Zhejiang University School of Medicine (Hangzhou, China)
and Wenzhou Medical College (Wenzhou, China) between 2004 and 2015,
as described previously (8–10,12), under
protocols approved by Zhejiang University and Wenzhou Medical
University Ethics Committees. DNA was extracted from 1 ml
peripheral blood using a QIAamp DNA Blood Minikit (51106; Qiagen
China Co., Ltd., Shanghai, China). The quality and quantity of DNA
were assessed using Qubit 3.0 fluorometers (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). DNA samples with concentration
>1.0 ng/µl were employed in the sequencing experiments.
Multi-genepanel design
Multi-gene target sequencing was performed using the
VariantPro™ Capture Technology by LC Sciences (Hangzhou, China) as
described previously (13). The 46
LHON-associated mutations were selected from Mitomap and previous
studies (8–10). As presented in Table I, they were distributed in the
following 13 genes: MT-ND1, MT-ND2, MT-ATP6, MT-CO3, MT-ND3,
MT-ND4L, MT-ND4, MT-ND5, MT-ND6, MT-CYB, MT-TM, MT-TT and
MT-TE. Twenty-seven amplicons that covered all 46 mutations
were designed by LC Sciences, as described previously (13). All amplicons were pooled into two
polymerase chain reaction (PCR) tubes (tube 1 and tube 2) as
VariantPro™ PCR mastermix with an average length of 184 nt (range,
167–203 nt).
 | Table I.Mutations in the multi-gene panel
(n=46). |
Table I.
Mutations in the multi-gene panel
(n=46).
| Index | Gene name | Var start | Var end | Ref allele | Var allele | Amino acid
change |
|---|
| 1 | MT-ND1 | 3316 | 3316 | G | A | A-T |
| 2 | MT-ND1 | 3376 | 3376 | G | A | E-K |
| 3 | MT-ND1 | 3394 | 3394 | T | C | Y-H |
| 4 | MT-ND1 | 3460 | 3460 | G | A | A-T |
| 5 | MT-ND1 | 3497 | 3497 | C | T | A-V |
| 6 | MT-ND1 | 3571 | 3571 | C | T | L-F |
| 7 | MT-ND1 | 3635 | 3635 | G | A | S-N |
| 8 | MT-ND1 | 3700 | 3700 | G | A | A-T |
| 9 | MT-ND1 | 3733 | 3733 | G | A | E-K |
| 10 | MT-ND1 | 3866 | 3866 | T | C | I-T |
| 11 | MT-ND1 | 4025 | 4025 | C | T | T-M |
| 12 | MT-ND1 | 4171 | 4171 | C | A | L-M |
| 13 | MT-ND1 | 4216 | 4216 | T | C | Y-H |
| 14 | MT-ND2 | 4640 | 4640 | C | A | I-M |
| 15 | MT-ND2 | 5244 | 5244 | G | A | G-S |
| 16 | MT-ATP6 | 9101 | 9101 | T | C | I-T |
| 17 | MT-CO3 | 9804 | 9804 | G | A | A-T |
| 18 | MT-ND3 | 10237 | 10237 | T | C | I-T |
| 19 | MT-ND4L | 10663 | 10663 | T | C | V-A |
| 20 | MT-ND4L | 10680 | 10680 | G | A | A-T |
| 21 | MT-ND4 | 11253 | 11253 | T | C | I-T |
| 22 | MT-ND4 | 11696 | 11696 | G | A | V–I |
| 23 | MT-ND4 | 11778 | 11778 | G | A | R-H |
| 24 | MT-ND5 | 12338 | 12338 | T | C | M-T |
| 25 | MT-ND5 | 12811 | 12811 | T | C | Y-H |
| 26 | MT-ND5 | 12848 | 12848 | C | T | A-V |
| 27 | MT-ND5 | 13051 | 13051 | G | A | G-S |
| 28 | MT-ND5 | 13528 | 13528 | A | G | T-A |
| 29 | MT-ND5 | 13637 | 13637 | A | G | Q-R |
| 30 | MT-ND5 | 13730 | 13730 | G | A | G-E |
| 31 | MT-ND6 | 14279 | 14279 | G | A | S-L |
| 32 | MT-ND6 | 14325 | 14325 | T | C | N-D |
| 33 | MT-ND6 | 14482 | 14482 | C | A | M-I |
| 34 | MT-ND6 | 14482 | 14482 | C | G | M-I |
| 35 | MT-ND6 | 14484 | 14484 | T | C | M-V |
| 36 | MT-ND6 | 14495 | 14495 | A | G | L-S |
| 37 | MT-ND6 | 14498 | 14498 | T | C | Y-C |
| 38 | MT-ND6 | 14502 | 14502 | T | C | I–V |
| 39 | MT-ND6 | 14568 | 14568 | C | T | G-S |
| 40 | MT-ND6 | 14596 | 14596 | A | T | I-M |
| 41 | MT-CYB | 14831 | 14831 | G | A | A-T |
| 42 | MT-CYB | 15812 | 15812 | G | A | V-M |
| 43 | MT-TM | 4435 | 4435 | A | G | tRNAMet |
| 44 | MT-TT | 15951 | 15951 | A | G | tRNAThr |
| 45 | MT-TE | 14693 | 14693 | A | G | tRNAGlu |
| 46 | MT-TE | 14727 | 14727 | T | C | tRNAGlu |
Library preparation and
sequencing
Library generation was performed according to the
manufacturer's protocol (LC Sciences). Briefly, 5 ng DNA per pool
was amplified in 25 cycles of PCR using probe sequences of 27
library amplicons (Table II) and a
VariantPro™ PCR mastermix as described previously (13). PCR runs of 18, 20, 24 and 26 cycles
were performed to evaluate the influence of the PCR cycles on the
experiment. The correlation coefficient, R2, ranged from
0.90–1.00 (average, 0.95), implicating that cycles between 18 and
26 had no influence on the experiment outcome. The amplified
products were purified using Agencourt AMPure XP beads [Beckman
Coulter (UK) Ltd., High Wycombe, UK]. Each library was diluted to
20 pM and sequenced on an Illumina Miseq with a minimum of 2X
150-bp paired-end reads.
 | Table II.PCR amplicons. |
Table II.
PCR amplicons.
| Index | Gene name | Tgt start | Tgt end | Prb strand | Prb start | Prb end | Var start | Var end | Prb length
(bp) | Amp length
(bp) |
|---|
| 1 | MT-ND1 | 3,316 | 3,866 | − | 3,269 | 3,439 | 3,288 | 3,425 | 171 | 303 |
| 2 | MT-ND1 | 3,316 | 3,866 | + | 3,402 | 3,585 | 3,415 | 3,571 | 184 | 316 |
| 3 | MT-ND1 | 3,316 | 3,866 | − | 3,526 | 3,699 | 3,543 | 3,684 | 174 | 306 |
| 4 | MT-ND1 | 3,316 | 3,866 | + | 3,643 | 3,812 | 3,661 | 3,794 | 170 | 302 |
| 5 | MT-ND1 | 3,316 | 3,866 | − | 3,745 | 3,936 | 3,767 | 3,918 | 192 | 324 |
| 6 | MT-ND1 | 4,025 | 4,025 | − | 3,952 | 4,140 | 3,962 | 4,126 | 189 | 321 |
| 7 | MT-ND1 | 4,171 | 4,216 | + | 4,132 | 4,334 | 4,145 | 4,315 | 203 | 335 |
| 8 | MT-TM | 4,435 | 4,435 | − | 4,363 | 4,529 | 4,380 | 4,514 | 167 | 299 |
| 9 | MT-ND2 | 4,640 | 4,640 | − | 4,482 | 4,667 | 4,494 | 4,655 | 186 | 318 |
| 10 | MT-ND2 | 5,244 | 5,244 | + | 5,140 | 5,317 | 5,154 | 5,302 | 178 | 310 |
| 11 | MT-ATP6 | 9,101 | 9,101 | − | 9,026 | 9,223 | 9,041 | 9,208 | 198 | 330 |
| 12 | MT-CO3 | 9,804 | 9,804 | − | 9,639 | 9,839 | 9,653 | 9,824 | 201 | 333 |
| 13 | MT-ND3 | 10,237 | 10,237 | − | 10,161 | 10,331 | 10,178 | 10,310 | 171 | 303 |
| 14 | MT-ND4L | 10,663 | 10,680 | − | 10,541 | 10,710 | 10,560 | 10,692 | 170 | 302 |
| 15 | MT-ND4 | 11,253 | 11,253 | + | 11,226 | 11,404 | 11,239 | 11,386 | 179 | 311 |
| 16 | MT-ND4 | 11,696 | 11,778 | + | 11,642 | 11,833 | 11,660 | 11,813 | 192 | 324 |
| 17 | MT-ND5 | 12,338 | 12,338 | − | 12,213 | 12,399 | 12,230 | 12,385 | 187 | 319 |
| 18 | MT-ND5 | 12,811 | 12,848 | + | 12,764 | 12,941 | 12,776 | 12,924 | 178 | 310 |
| 19 | MT-ND5 | 13,051 | 13,051 | − | 12,967 | 13,146 | 12,984 | 13,128 | 180 | 312 |
| 20 | MT-ND5 | 13,528 | 13,730 | + | 13,488 | 13,682 | 13,503 | 13,665 | 195 | 327 |
| 21 | MT-ND5 | 13,528 | 13,730 | + | 13,577 | 13,774 | 13,590 | 13,760 | 198 | 330 |
| 22 | MT-ND6 | 14,279 | 14,325 | − | 14,251 | 14,421 | 14,267 | 14,408 | 171 | 303 |
| 23 | MT-TE;
MT-ND6; MT-CYB | 14,482 | 14,831 | + | 14,444 | 14,645 | 14,460 | 14,628 | 202 | 334 |
| 24 | MT-TE;
MT-ND6; MT-CYB | 14,482 | 14,831 | − | 14,587 | 14,762 | 14,610 | 14,748 | 176 | 308 |
| 25 | MT-TE;
MT-ND6; MT-CYB | 14,482 | 14,831 | + | 14,699 | 14,866 | 14,720 | 14,857 | 168 | 300 |
| 26 | MT-CYB;
MT-TT | 15,812 | 15,951 | + | 15,780 | 15,966 | 15,801 | 15,945 | 187 | 319 |
| 27 | MT-CYB;
MT-TT | 15,812 | 15,951 | − | 15,792 | 15,989 | 15,809 | 15,974 | 198 | 330 |
Data analysis
Low quality reads (reads containing sequencing
adaptors or nucleotides with quality scores <20) were removed
before alignment. Cleaned, paired-end sequence reads in paired
FASTQ files were aligned using Burrows-Wheeler Alignment version
0.1.19 (14). Variant calling was
generated using the Genome Analysis Toolkit version 3.3.0 and its
Unified Genotyper module (https://www.broadinstitute.org/gatk/guide/tagged?tag=unifiedgenotyper).
A Gaussian mixture model was used to evaluate the confidence score
for each putative mutation call and novel potential variants.
Sequence reads were aligned to the human mtDNA sequence data
relative to the revised Cambridge Reference Sequence (GenBank
accession no. NC_012920) (15).
Sanger validation
Thirteen LHON cases associated with m.11778G>A
(11) or m.14484T>C (2) mutations and four healthy control samples
(available upon request) were selected as the positive and negative
controls, respectively, for runs of the panel following validation
by Sanger sequencing. Furthermore, 100% correlation was derived
from the panel assay and the Sanger sequencing for the positive and
negative controls.
Results
Summary of sequencing data
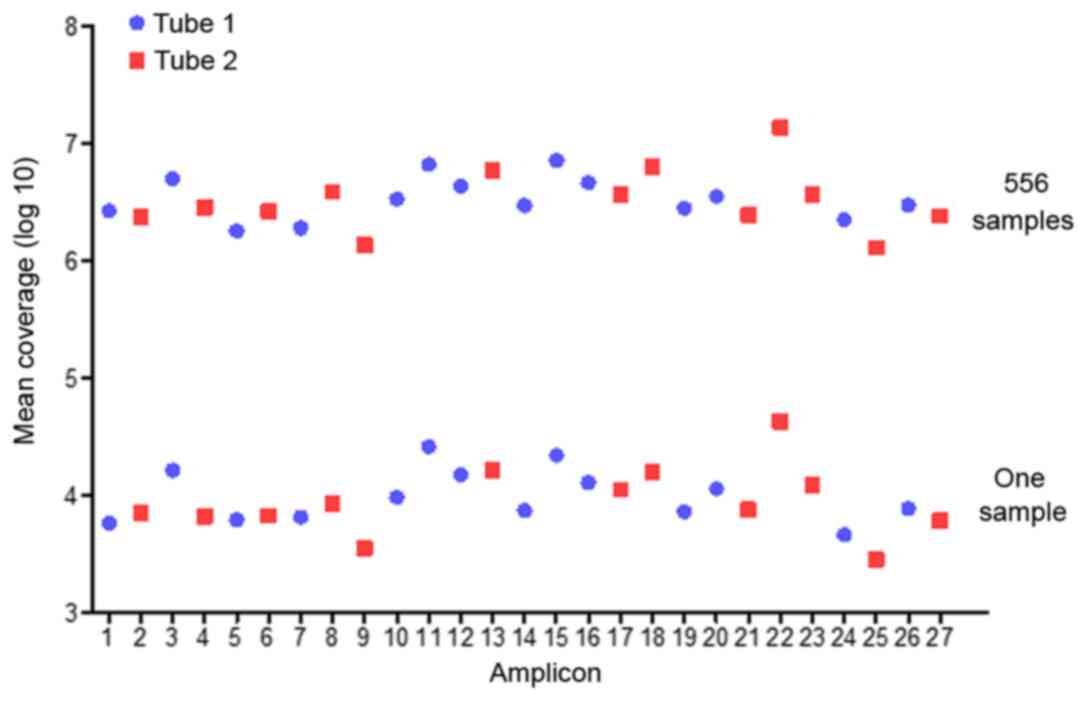
A total of 118 milion reads were obtained, on
average 89% of which were mapped to the amplicon targets and
resultedin a mean of 7,001 reads to each sample per amplicon
(Table III). The mean reads over
the 27 amplicons were distrubed with an average uniformity of
coverage of 98.0% (amplicon mean coverage, 20%) and an average
read-depth of 1,000 X (Fig. 1). A
total of 363 variants were distributed in 46 LHON-associated
mutations of all samples.
 | Table III.Summary of sequencing data in the
panel for 556 samples. |
Table III.
Summary of sequencing data in the
panel for 556 samples.
| Variable | Outcome |
|---|
| Total no. of
reads | 118,156,518 |
| Reads mapped to the
amplicons (forward primer) | 110,440,487 |
| Reads mapped to the
amplicons (reverse primer) | 108,812,285 |
| Reads mapped to the
amplicon targets | 105,107,193 |
| Reads mapped to
each amplicon (average) | 3,892,859 |
| Reads mapped to
each sample per amplicon (mean) | 7,001.5 |
| Reads enrichment to
the targets,% (average) | 89 |
| Uniformity of
coverage, % (20% mean) | 98 |
| Total no. of
variants among 46 point mutations | 363 |
Mutations analysis
A total of 363 variants were identified in the
cohort of all 556 samples; 285 variants were detected in LHON cases
as an average incidence of 104%, whereas only 78 variants were
identified in 281 controls with a mean incidence of 28% (Table IV).
 | Table IV.Summary information of 46-point
mutations in the cohort. |
Table IV.
Summary information of 46-point
mutations in the cohort.
|
|
|
|
|
| Primer
mutations |
| Incidence (%) |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Indexa | Gene | SNP | Total variants | Patients
(n=275) | 11778 | 14484 | 3460 | Controls
(n=281) | Patients | Controls |
|---|
| 1 | MT-ND1 | 3316G>A | 15 | 11 | 7 | 1 | 0 | 4 | 4.00 | 1.42 |
| 2 |
| 3376G>A | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 3 |
| 3394T>C | 19 | 11 | 7 | 0 | 0 | 8 | 4.00 | 2.85 |
| 4 |
|
3460G>A | 2 | 2 | 0 | 0 | 2 | 0 | 0.73 | 0.00 |
| 5 |
| 3497C>T | 14 | 7 | 4 | 0 | 0 | 7 | 2.55 | 2.50 |
| 6 |
| 3571C>T | 11 | 5 | 3 | 0 | 0 | 6 | 1.82 | 2.14 |
| 7 |
| 3635G>A | 1 | 1 | 0 | 0 | 0 | 0 | 0.36 | 0.00 |
| 8 |
| 3700G>A | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 9 |
| 3733G>A | 1 | 1 | 0 | 0 | 0 | 0 | 0.36 | 0.00 |
| 10 |
| 3866T>C | 4 | 4 | 1 | 0 | 0 | 0 | 1.45 | 0.00 |
| 11 |
| 4025C>T | 1 | 1 | 0 | 0 | 0 | 0 | 0.36 | 0.00 |
| 12 |
| 4171C>A | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 13 |
| 4216T>C | 11 | 3 | 0 | 0 | 0 | 8 | 1.09 | 2.85 |
| 14 | MT-ND2 | 4640C>A | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 15 |
| 5244G>A | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 16 | MT-ATP6 | 9101T>C | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 17 | MT-CO3 | 9804G>A | 2 | 1 | 1 | 0 | 0 | 1 | 0.36 | 0.36 |
| 18 | MT-ND3 | 10237T>C | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 19 | MT-ND4L | 10663T>C | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 20 |
| 10680G>A | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 21 | MT-ND4 | 11253T>C | 1 | 1 | 1 | 0 | 0 | 0 | 0.36 | 0.00 |
| 22 |
| 11696G>A | 18 | 12 | 10 | 1 | 0 | 6 | 4.36 | 2.14 |
| 23 |
|
11778G>A | 162 | 162 | 162 | 0 | 0 | 0 | 58.90 | 0.00 |
| 24 | MT-ND5 | 12338T>C | 19 | 5 | 2 | 0 | 0 | 14 | 1.82 | 4.98 |
| 25 |
| 12811T>C | 21 | 14 | 12 | 0 | 0 | 7 | 5.09 | 2.49 |
| 26 |
| 12848C>T | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 27 |
| 13051G>A | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 28 |
| 13528A>G | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 29 |
| 13637A>G | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 30 |
| 13730G>A | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 31 | MT-ND6 | 14279G>A | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 32 |
| 14325T>C | 2 | 0 | 0 | 0 | 0 | 2 | 0.00 | 0.71 |
| 33 |
| 14482C>A | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 34 |
| 14482C>G | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 35 |
|
14484T>C | 25 | 25 | 0 | 25 | 0 | 0 | 9.10 | 0.00 |
| 36 |
| 14495A>G | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 37 |
| 14498T>C | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 38 |
| 14502T>C | 18 | 11 | 8 | 3 | 0 | 7 | 4.00 | 2.49 |
| 39 |
| 14568C>T | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 40 |
| 14596A>T | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 41 | MT-CYB | 14831G>A | 5 | 1 | 0 | 0 | 0 | 4 | 0.36 | 1.42 |
| 42 |
| 15812G>A | 1 | 0 | 0 | 0 | 0 | 1 | 0.00 | 0.36 |
| 43 | MT-TM | 4435A>G | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
| 44 | MT-TT | 15951A>G | 5 | 3 | 1 | 2 | 0 | 2 | 1.09 | 0.71 |
| 45 | MT-TE | 14693A>G | 5 | 4 | 3 | 1 | 0 | 1 | 1.45 | 0.36 |
| 46 |
| 14727T>C | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 |
|
|
| Total |
|
| 162b | 25c |
|
|
|
|
|
|
| variants | 363 | 285 | (60) | (8) | 2 | 78 | 103.64 | 27.76 |
As 46 mutations of 13 mitochondrial genes were
selected from all populations of the world, variants from LHON
cases in the current study were deposited in 8 mitochondrial genes,
MT-ND1, MT-CO3, MT-ND4, MT-ND5, MT-ND6, MT-CYB, MT-TT and
MT-TE, with frequencies of 16.14, 0.35, 61.40, 6.67, 12.63,
0.35, 1.05 and 1.40%, respectively. Consistent with our previous
reports (8–10), MT-ND1, MT-ND4 and MT-ND6
were the hotspots associated with LHON, and almost cover 90% of
variants in the present study.
Twenty-three out of the 46 LHON-associated mutations
were detected in all subjects. These were as follows: 3316G>A,
3394T>C, 3460G>A, 3497C>T, 3571C>T, 3635G>A,
3733G>A, 3866T>C, 4025C>T and 4216T>C mutations in
MT-ND1, 9804G>A in MT-CO3, 11253T>C,
11696G>A and 11778G>A in MT-ND4, m.12338T>C and
12811T>C in MT-ND5, 14325T>C, 14484T>C and
14502T>C in MT-ND6, 14831G>A and 15812G>A in
MT-CYB, 15951A>G in MT-TT and 14693A>G in
MT-TE. The incidence of these three common mutations
m.11778G>A, m.14484T>C and m.3460G>A in this Chinese
cohort were 58.90, 9.10 and 0.73%, respectively. In addition, two
causative mutations were detected; m.3635G>A in 1 patient and
m.3866T>C in 4 patients (one case carrying both m.11778G>A
and m.3866T>C mutations), as reported previously (16). Notably, three mutations, m.3733G>A,
m.4025C>T and m.11253T>C, were observed in one LHON case
each, but absent in the control cohort. Whereas, m.4025C>T and
m.11253T>C were observed in the control population in our recent
studies (8,9).
Thirteen secondary mutations were identified in
30.55% patients. The incidence of these known secondary mutations,
m.12811T>C, m.11696G>A, m.3316G>A, m.3394T>C,
m.14502T>C, m.3497C>T, m.3571C>T, m.12338T>C,
m.14693A>G, m.4216T>C, m.15951A>G, m.14831G>A and
m.9804G>A were 5.09, 4.36, 4.00, 4.00, 4.00, 2.55, 1.82, 1.82,
1.45, 1.09, 1.09, 0.36 and 0.36%, respectively. A total of 88
variations from these 13 mutations were observed in patients. Among
these, 67 variations were concurrent with either m.11778G>A (59
variations, except 1 from the m.3866T>C mutation) or
m.14484T>C (8 variations). Besides the hotspots of MT-ND1,
MT-ND4 and MT-ND6, the MT-ND5 gene was frequently
accumulated in the distribution of secondary mutations with an
incidence of 6.91% in the patients. In addition, the incidence of
MT-ND1, MT-ND4 and MT-ND6 for secondary mutations,
were 13.45, 4.36 and 4%, respectively. The secondary mutations were
predominantly present in the patient and control populations. Of
those, mutations m.12338T>C m.4216T>C, m.3571C>T and
m.14831 G>A had higher incidences in the control cohort than in
the patients. Two LHON-associated mutations (m.14325T>C and
m.15812G>A) only arose in the control population in the present
study.
Discussion
The present study evaluated the distribution of
mitochondrial genes and mutations among 275 Chinese LHON patients,
in parallel with a control cohort of 281 subjects, using a
multi-gene panel. Mutations of the multi-gene panel were designed
from the Mitomap database, previous reports and our previous study
in a Chinese Han population (8–10).
Twenty-seven amplicons, the specific primers for the point
mutations, covered those of 46 mutations, as well as other
substitutions in the amplicons, such as pathogenic mutations,
m.3697G>A, m.10197G>A and m.14459G>A in the fragments of
amplicon 4, 13 and 23, respectively. None of these three rare
causative mutations were recorded among subjects in the test. Of
the 46 mutations, the m.14727T>C variant in the MT-TE
gene reported in encephalomyopathy patients (17), was set as a negative control variant
in the panel and was absent in all of the subjects. The panel with
high-throughput sequencing makes it possible to screen multi-genes
or multi-single nucleotide polymorphisms (SNPs) for subjects in a
run, and provide more information than traditional Sanger
sequencing. Certainly, sequencing the whole mtDNA genome is an
optional selection using next generation sequencing. The
information from the primary and secondary mutations may be
indicative regarding the incomplete penetrance and other clinical
symptoms.
It is generally accepted that LHON-associated
mutations and their incidence are varied in populations with
different ethnic backgrounds (18).
Consistent results were confirmed in our multi-gene panel
screening. Twenty-three mutations in the panels were absent in the
patients and control cohort in the current study. Those mutations
reported as rare causative mutations in a European LHON population,
m.3376G>A, m.3700G>A and m.4171C>A in the MT-ND1
gene (19–21), m.10663T>C in the MT-ND4L
(20), m.13051G>A in the
MT-ND5 gene (22),
m.14482C>G/A, m.14495A>G and m.14568C>T in the
MT-ND6 gene (8,20,23), were
undetected in the present study. However, 6 causative mutations,
m.11778G>A, m.14484T>C, m.3460G>A, m.3635G>A,
m.3866T>C and m.3733G>A were observed in 194 LHON cases with
their contribution of 83.51, 12.89, 1.04, 0.52, 2.06 and 0.52. Of
these, the incidence of m.3460G>A was markedly lower in this
cohort than that in a Caucasian population reported by Mackey et
al (24). Additionally, the
spectrum of secondary mutations associated with LHON was also
dependent on their ethnic background, and were distinct between the
Chinese and Caucasian cohorts. This panel screening demonstrated
that the secondary mutations, m.12811T>C, m.11696G>A,
m.3394T>C, m.3316G>A, m.14502T>C and m.12338T>C had
higher frequencies in the patient cohort, as these mutations were
assigned to Asian mtDNA lineage, including the macro-haplo group of
M and N. Certainly, m.12811T>C is considered to be a polymorphic
variant in sub-haplo groups of M7. While, mutations, m.3394T>C
and m.11696G>A are categorized as haplo group-specific variants
of M9a and D4j, respectively (25,26).
Congruent results were obtained in our previous reports (27).
Usually, secondary mutations, proposed to increase
the penetrance of LHON (25–27), are observed in LHON cases associated
with m.11778G>A or m.14484T>C mutations. In the present
study, 77.27% of variations of secondary mutations were coexistent
with one of the primary mutations, m.11778G>A and m.14484T>C.
Their detailed distribution was illustrated in Table V. Secondary mutations m.12811T>C,
m.11696G>A, m.14502T>C, m.3394T>C and m.3316G>A
exhibited the most co-occurrence with m.11778G>A. Meanwhile,
three LHON cases carried m.14484T>C and m.14502T>C together.
Notably, the m.14502T>C mutation was evidenced as a modifier in
the phenotypic manifestation of LHON (28), although it was reported as a causative
mutation elsewhere (https://www.mitomap.org/foswiki). Furthermore, more
than one secondary mutation co-occurred with m.11778G>A, but not
with m.14484T>C in this panel. m.3497C>T and m.3571C>T,
which belong to the haplo group variants of B4c1, arose in three
cases associated with m.11778G>A. In addition, m.9804G>A and
m.14831G>A, reported as LHON-associated mutations in Caucasian
cases, were common in the present control cohort according to the
panel analysis. It was confirmed that the spectrum of mutations
varied between ethnic backgrounds and indicated that the selected
SNPs of the panel would be optimized for a Han population in the
future.
 | Table V.Distribution of secondary mutations
with m.11778 G>A and m.14484 T>C. |
Table V.
Distribution of secondary mutations
with m.11778 G>A and m.14484 T>C.
|
| Secondary mutation
(cases harboring successive secondary mutations, n) |
|
|---|
|
|
|
|
|---|
| Primary
mutation | 1 | 2 | 3 | Samples, n |
|---|
|
| 12811T>C | − | − | 12 |
|
| 11696G>A | 3394 T>C
(1) | − | 10 |
|
| 14502T>C | 14693 A>G
(1) | 9804 G>A
(1) | 8 |
|
|
| 3866 T>C
(1) | − |
|
|
| 3316G>A | − | − | 7 |
| 11778 G>A | 3394T>C | 11696 G>A
(1) | − | 7 |
|
| 3497C>T | 3571 C>T
(3) | − | 4 |
|
| 14693A>G | 14502 T>C
(1) | − | 3 |
|
| 3571C>T | 3497 C>T
(3) | − | 3 |
|
| 12338T>C | − | − | 2 |
|
| 9804G>A | 14502 T>C
(1) | 14693 A>G
(1) | 1 |
|
| 11253T>C | − | − | 1 |
|
| 15951A>G | − | − | 1 |
| 14484 T>C | 14502T>C | − | − | 3 |
|
| 15951A>G | − | − | 2 |
|
| 11696G>A | − | − | 1 |
|
| 3316G>A | − | − | 1 |
|
| 14693A>G | − | − | 1 |
In conclusion, the current data indicates that the
spectrum and incidence of mtDNA mutation-associated LHON cases in
the Han population are different to those in a Caucasian
population. Here, the causative mutations associated with LHON,
m.11778G>A, m.14484T>C, m.3460G>A, m.3635G>A,
m.3866T>C and m.3733G>A, were observed in 70.55% of the
patient cohort. The common secondary mutations in the Chinese LHON
population were m.12811T>C, m.11696G>A, m.3394T>C,
m.3316G>A, m.14502T>C and m.12338T>C. Furthermore, besides
the three hotspots genes MT-ND1, MT-ND4 and MT-ND6,
MT-ND5 also had a high incidence of secondary mutations
including m.12811T>C and m.12338T>C; this finding was
comparable with a previous study (29). The primary and secondary
mutation-associated LHON cases in the present multi-gene panel will
advance current understanding of the clinical phenotype of LHON,
and offer valuable information for the early diagnosis and
subsequent options of intervention for mitigating risk of
additional vision loss in LHON patients.
Acknowledgements
The present study was supported by the National
Technologies R&D Program (grant no. 2012BAI09B03) and a grant
from the National Natural Science Foundation of China (grant no.
31671303).
Glossary
Abbreviations
Abbreviations:
|
LHON
|
Leber's hereditary optic
neuropathy
|
|
mtDNA
|
mitochondrial DNA
|
References
|
1
|
Y-W-Man P, Griffiths PG, Brown DT, Howell
N, Turnbull DM and Chinnery PF: The epidemiology of Leber
hereditary optic neuropathy in the North East of England. Am J Hum
Genet. 72:333–339. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saracchi E, Difrancesco JC, Brighina L,
Marzorati L, Curtò NA, Lamperti C, Carrara F, Zeviani M and
Ferrarese C: A case of Leber hereditary optic neuropathy plus
dystonia caused by G14459A mitochondrial mutation. Neurol Sci.
34:407–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Howell N and Mackey DA: Low-penetrance
branches in matrilineal pedigrees with Leber hereditary optic
neuropathy. Am J Hum Genet. 63:1220–1224. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fraser JA, Biousse V and Newman NJ: The
neuro-ophthalmology of mitochondrial disease. Surv Ophthalmol.
55:299–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu-Wai-Man P, Griffiths PG and Chinnery
PF: Mitochondrial optic neuropathies – disease mechanisms and
therapeutic strategies. Prog Retin Eye Res. 30:81–114. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim SH and Kim JS: Leber's ‘Plus’ in a
Korean patient with 14484/ND6 mutation. J Clin Neurol. 12:512–514.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jia X, Li S, Xiao X, Guo X and Zhang Q:
Molecular epidemiology of mtDNA mutations in 903 Chinese families
suspected with Leber hereditary optic neuropathy. J Hum Genet.
51:851–856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji Y, Liang M, Zhang J, Zhu L, Zhang Z, Fu
R, Liu X, Zhang M, Fu Q, Zhao F, et al: Mitochondrial ND1 variants
in 1281 Chinese subjects with Leber's hereditary optic neuropathy.
Invest Ophthalmol Vis Sci. 57:2377–2389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang P, Liang M, Zhang J, Gao Y, He Z, Yu
H, Zhao F, Ji Y, Liu X, Zhang M, et al: Prevalence of mitochondrial
ND4 mutations in 1281 Han Chinese subjects with Leber's hereditary
optic neuropathy. Invest Ophthalmol Vis Sci. 56:4778–4788. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang M, Jiang P, Li F, Zhang J, Ji Y, He
Y, Xu M, Zhu J, Meng X, Zhao F, et al: Frequency and spectrum of
mitochondrial ND6 mutations in 1218 Han Chinese subjects with
Leber's hereditary optic neuropathy. Invest Ophthalmol Vis Sci.
55:1321–1331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu-Wai-Man P and Chinnery PF: Leber
hereditary optic neuropathyGeneReviews® [Internet].
Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH,
Bird TD, Fong CT, Mefford HC and Smith RJH: University of
Washington; Seattle, WA: pp. 1993–2016
|
|
12
|
Qu J, Li R, Zhou X, Tong Y, Lu F, Qian Y,
Hu Y, Mo JQ, West CE and Guan MX: The novel A4435G mutation in the
mitochondrial tRNAMet may modulate the phenotypic expression of the
LHON-associated ND4 G11778A mutation. Invest Ophthalmol Vis Sci.
47:475–483. 2006. View Article : Google Scholar
|
|
13
|
Li X, Liu L, Xi Q, Zhao X, Fang M, Ma J,
Zhu Z, Wang X, Shi C, Wang J, et al: Short-term serum deprivation
causes no significant mitochondrial DNA mutation in vascular smooth
muscle cells revealed by a new next generation sequencing
technology. Acta Biochim Biophys Sin (Shanghai). 48:862–864. 2016.
View Article : Google Scholar
|
|
14
|
Li H and Durbin R: Fast and accurate short
read alignment with Burrows-Wheeler transform. Bioinformatics.
25:1754–1760. 2009. View Article : Google Scholar
|
|
15
|
Andrews RM, Kubacka I, Chinnery PF,
Lightowlers RN, Turnbull DM and Howell N: Reanalysis and revision
of the Cambridge reference sequence for human mitochondrial DNA.
Nat Genet. 23:1471999. View
Article : Google Scholar
|
|
16
|
Zhou X, Qian Y, Zhang J, Tong Y, Jiang P,
Liang M, Dai X, Zhou H, Zhao F, Ji Y, et al: Leber's hereditary
optic neuropathy is associated with the T3866C mutation in
mitochondrial ND1 gene in three Han Chinese families. Invest
Ophthalmol Vis Sci. 53:4586–4594. 2012. View Article : Google Scholar
|
|
17
|
Truong HT, Nguyen VA, Nguyen LV, Pham VA
and Phan TN: Screening of common point-mutations and discovery of
new T14727C change in mitochondrial genome of Vietnamese
encephalomyopathy patients. Mitochondrial DNA A DNA Mapp Seq Anal.
27:441–448. 2016. View Article : Google Scholar
|
|
18
|
Wallace DC and Lott MT: Leber hereditary
optic neuropathy: Exemplar of an mtDNA disease. Handb Exp
Pharmacol. 240:339–376. 2017. View Article : Google Scholar
|
|
19
|
Blakely EL, de Silva R, King A, Schwarzer
V, Harrower T, Dawidek G, Turnbull DM and Taylor RW: LHON/MELAS
overlap syndrome associated with a mitochondrial MTND1 gene
mutation. Eur J Hum Genet. 13:623–627. 2005. View Article : Google Scholar
|
|
20
|
Achilli A, Iommarini L, Olivieri A, Pala
M, Kashani B Hooshiar, Reynier P, La Morgia C, Valentino ML,
Liguori R, Pizza F, et al: Rare primary mitochondrial DNA mutations
and probable synergistic variants in Leber's hereditary optic
neuropathy. PLoS One. 7:e422422012. View Article : Google Scholar
|
|
21
|
Kim JY, Hwang JM and Park SS:
Mitochondrial DNA C4171A/ND1 is a novel primary causative mutation
of Leber's hereditary optic neuropathy with a good prognosis. Ann
Neurol. 51:630–634. 2002. View Article : Google Scholar
|
|
22
|
Fauser S, Leo-Kottler B, Besch D and
Luberichs J: Confirmation of the 14568 mutation in the
mitochondrial ND6 gene as causative in Leber's hereditary optic
neuropathy. Ophthalmic Genet. 23:191–197. 2002. View Article : Google Scholar
|
|
23
|
Chinnery PF, Brown DT, Andrews RM,
Singh-Kler R, Riordan-Eva P, Lindley J, Applegarth DA, Turnbull DM
and Howell N: The mitochondrial ND6 gene is a hot spot for
mutations that cause Leber's hereditary optic neuropathy. Brain.
124:209–218. 2001. View Article : Google Scholar
|
|
24
|
Mackey DA, Oostra RJ, Rosenberg T,
Nikoskelainen E, Bronte-Stewart J, Poulton J, Harding AE, Govan G,
Bolhuis PA and Norby S: Primary pathogenic mtDNA mutations in
multigeneration pedigrees with Leber hereditary optic neuropathy.
Am J Hum Genet. 59:481–485. 1996.
|
|
25
|
Zhang M, Zhou X, Li C, Zhao F, Zhang J,
Yuan M, Sun YH, Wang J, Tong Y, Liang M, et al: Mitochondrial
haplogroup M9a specific variant ND1 T3394C may have a modifying
role in the phenotypic expression of the LHON-associated ND4
G11778A mutation. Mol Genet Metab. 101:192–199. 2010. View Article : Google Scholar
|
|
26
|
Qu J, Li R, Zhou X, Tong Y, Yang L, Chen
J, Zhao F, Lu C, Qian Y, Lu F and Guan MX: Cosegregation of the ND4
G11696A mutation with the LHON-associated ND4 G11778A mutation in a
four generation Chinese family. Mitochondrion. 7:140–146. 2007.
View Article : Google Scholar
|
|
27
|
Sudoyo H, Suryadi H, Lertrit P,
Pramoonjago P, Lyrawati D and Marzuki S: Asian-specific mtDNA
backgrounds associated with the primary G11778A mutation of Leber's
hereditary optic neuropathy. J Hum Genet. 47:594–604. 2002.
View Article : Google Scholar
|
|
28
|
Jiang P, Liang M, Zhang C, Zhao X, He Q,
Cui L, Liu X, Sun YH, Fu Q, Ji Y, et al: Biochemical evidence for a
mitochondrial genetic modifier in the phenotypic manifestation of
Leber's hereditary optic neuropathy-associated mitochondrial DNA
mutation. Hum Mol Genet. 25:3613–3625. 2016. View Article : Google Scholar
|
|
29
|
Seong MW, Choi J, Park SS, Kim JY and
Hwang JM: Novel MT-ND5 gene mutation identified in Leber's
hereditary optic neuropathy patient using mitochondrial genome
sequencing. J Neurol Sci. 375:301–303. 2017. View Article : Google Scholar
|