Introduction
Lactoferrin is a secretory protein present in
mammalian secretary fluids, and has roles in protecting the body
from external pathologies (1–6). It is also involved in prevention of
cancer (7) and anemia (8,9).
Lactoferrin has been demonstrated to alleviate
psychological stresses in rats: Intraperitoneal administration of
bovine lactoferrin suppressed the behavioral symptoms of stress in
fear-induced freezing and elevated plus-maze tests (10). However, research on lactoferrin as an
aid to manage psychological stresses has been limited to animal
studies. Therefore, it remains unclear whether lactoferrin is
capable of alleviating psychological stresses in humans. The
present study, therefore, examined the effect of bovine lactoferrin
ingestion on psychological stresses in human subjects. The
psychological stresses were induced by facing subjects with
calculation problems.
In the present study, the levels of psychological
stresses were evaluated by means of salivary stress markers, namely
the level of amylase activity (11,12) and
the concentration of chromogranin A (13–15). The
levels of these stress markers have been reported to be increased
in response to physical stresses, notably at an intensity greater
than 70% VO2max in healthy young individuals (16). Psychological stresses may also trigger
their production. For instance, it has been reported that amylase
activity was increased in subjects undergoing a written examination
(17) and the Trier Social Stress
Test (TSST) (11,18). Similarly, chromogranin A concentration
was increased in subjects receiving venipuncture (14), giving an oral presentation, or driving
a car (19).
Autonomic nervous activities may also be assessed by
frequency domain analysis of the R-R intervals of the heartbeat
(20). This analysis provides the
powers of low-frequency (LF; 0.04–0.15 Hz) and high-frequency (HF;
0.15–0.4 Hz) components (20). The
power of the frequency domain to the LF component indicates the
activities of the sympathetic and parasympathetic nervous systems,
whereas that to the HF component signifies parasympathetic
activity. Accordingly, increased LF/HF and LF/(LF+HF) ratios are
indicative of increased sympathetic activity (21).
To the best of our knowledge, the present study is
the first to investigate the potential of lactoferrin for managing
psychological stresses incurred during human activity, using the
aforementioned heart rate variables and salivary stress
markers.
Materials and methods
Subjects
A total of 18 healthy female college students were
recruited for the current study at Juntendo University, Inzai,
Japan between 1 and 10 September, 2015. Their salivary amylase
activities were confirmed to be elevated by the calculation task
used in the trial (described below) prior to the study. All
subjects read the guidelines detailing the purpose, methods and
ethical considerations (including possible adverse effects)
associated with the study, and provided written informed consent
prior to participation. Among the participants, 2 subjects dropped
out of the study for personal reasons. The remaining 16 subjects
completed the study. Their age, height, body mass and body mass
index were respectively 19.0±0.6 years, 158.6±5.0 cm, 54.5±4.3 kg
and 21.7±1.7 kg/m2 (mean ± standard deviation). The
research protocol was reviewed and approved by the research ethics
committee of the Graduate School of Health and Sports Science,
Juntendo University (Inzai, Chiba, Japan; approval no. 27–54). The
study was registered in the UMIN Clinical Trials Registry (UMIN ID:
UMIN000031319).
Study design
The present study employed a single-dose
administration, double-blinded placebo-controlled trial in a
crossover fashion. Subjects visited the institution on two
occasions that were separated by 4 days. On the day prior to each
experiment, subjects refrained from vigorous exercise and finished
dinner by 9:00 p.m., and fasted on water until the experiment.
On the experiment day (Fig. 1), subjects visited the institution at
8:30 a.m., wore a heart-rate monitor (RS 800CX; Polar Japan, Tokyo,
Japan) and rinsed their mouths with water. Each subject was quietly
seated for 15 min, while stabilizing the respiratory rate to
breathe once every 4 sec (equivalent to 0.25 Hz) using metronome
software (Art Metronome version 1.8; obtained from http://www.asahi-net.or.jp/~hb9t-ktd/music/Japan/Soft/metronome.html;
accessed July, 2015). Heart rate was recorded during this rest
period [Pre-condition (PRE); Fig. 1].
Saliva (~2 ml) was then collected (PRE) using Salivette®
(Sarstedt AG and Co., Nümbrecht, Germany), followed by an oral
ingestion of either bovine lactoferrin or a placebo (details
provided below). Following the ingestion, subjects remained seated
for another 15 min while maintaining the respiratory rate at 0.25
Hz, and began a calculation task. The calculation task comprised of
two sets of 15-min calculations with a 5-min interval between sets.
In each set of calculations, subjects attempted to solve 60
questions involving multiplications and divisions using pairs of
three-digit numbers. Heart rate was recorded again during the
second calculation set (Calc 2). Upon completion of the calculation
task, subjects rinsed their mouths with water and provided saliva
(~2 ml) post-condition (POST) using Salivette®.
Lactoferrin and placebo
A total of 8 lactoferrin tablets (Morinaga
Lactoferrin Original; Morinaga Milk Industry, Co., Ltd., Tokyo,
Japan), containing 100 mg of lactoferrin per tablet, were grained
and dissolved in 150 ml of soy beverage ‘soymilk drink malt coffee
calorie 50% off’ (Marusanai, Co., Ltd., Aichi, Japan). The same
amount of the soymilk was used as a placebo. The appearance and the
taste of the two test drinks were indistinguishable as judged by
the examiners.
Salivary analyses
Salivary amylase activity (U/ml) and chromogranin A
(pmol/ml) were analyzed with CicaLiquid-N AMY (Kanto Chemical, Co.,
Inc., Tokyo, Japan) and YK 070 Human Chromogranin A EIA (Yanaihara
Institute, Inc., Shizuoka, Japan) kits, respectively, according to
the manufacturer's protocols.
Frequency analysis of heart rate
variability
Heart rate variability was analyzed using MemCalc
version 2.0 software (GMS Co., Ltd., Tokyo, Japan) from R-R
intervals obtained from the heart rate monitor, with segment length
of 30 sec and segment interval of 2 sec. The average values of
power spectral density in LF (0.04–0.15 Hz) and HF (0.15–0.4 Hz)
components, and the ratios LF/HF and LF/(LF+HF) were
calculated.
Statistical analysis
Salivary amylase activity and chromogranin A
concentration as well as variables of heart-rate variability were
analyzed using a generalized linear model with the following
predictor variables: i) Subject variable was ‘subject ID’; and ii)
within-subject variables were ‘condition’ (lactoferrin or placebo),
‘experiment day’ (date) and ‘measurement’ (PRE, POST or PRE/Calc
2). Estimated marginal means of ‘condition × measurement’ were
compared. All interactions were confirmed and the model with the
smallest quasi-likelihood under independence model criterion was
adopted.
Changes in salivary amylase activity and
chromogranin A concentration (POST-PRE) and the ratio of heart-rate
variables (Calc 2/PRE) were also assessed in the same manner used
to compare the estimated marginal mean of ‘condition’.
All statistical analyses were performed using SPSS
version 23 software (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Salivary stress markers
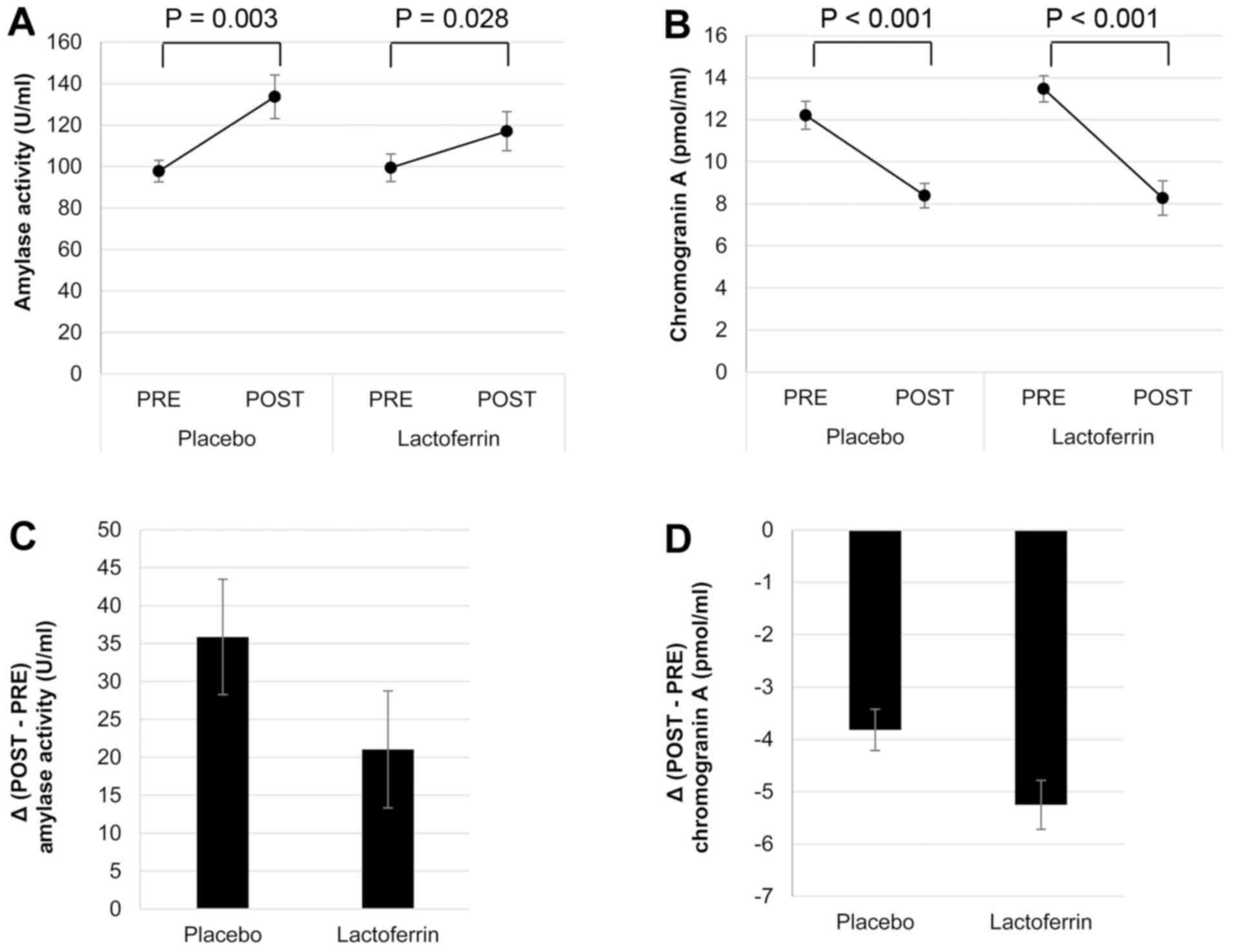
For both placebo and lactoferrin treatment
conditions, following the calculation task (POST), amylase activity
was elevated (P=0.003 and P=0.028, respectively; Fig. 2A), while chromogranin A concentration
was reduced (both P<0.001; Fig.
2B).
It appeared that the magnitude of change in amylase
activity following the calculation task was smaller (P=0.33;
Fig. 2C;), and of the change in
chromogranin A concentration greater (P=0.093; Fig. 2D) for the lactoferrin treatment
compared with the placebo; however, these differences were not
statistically significant.
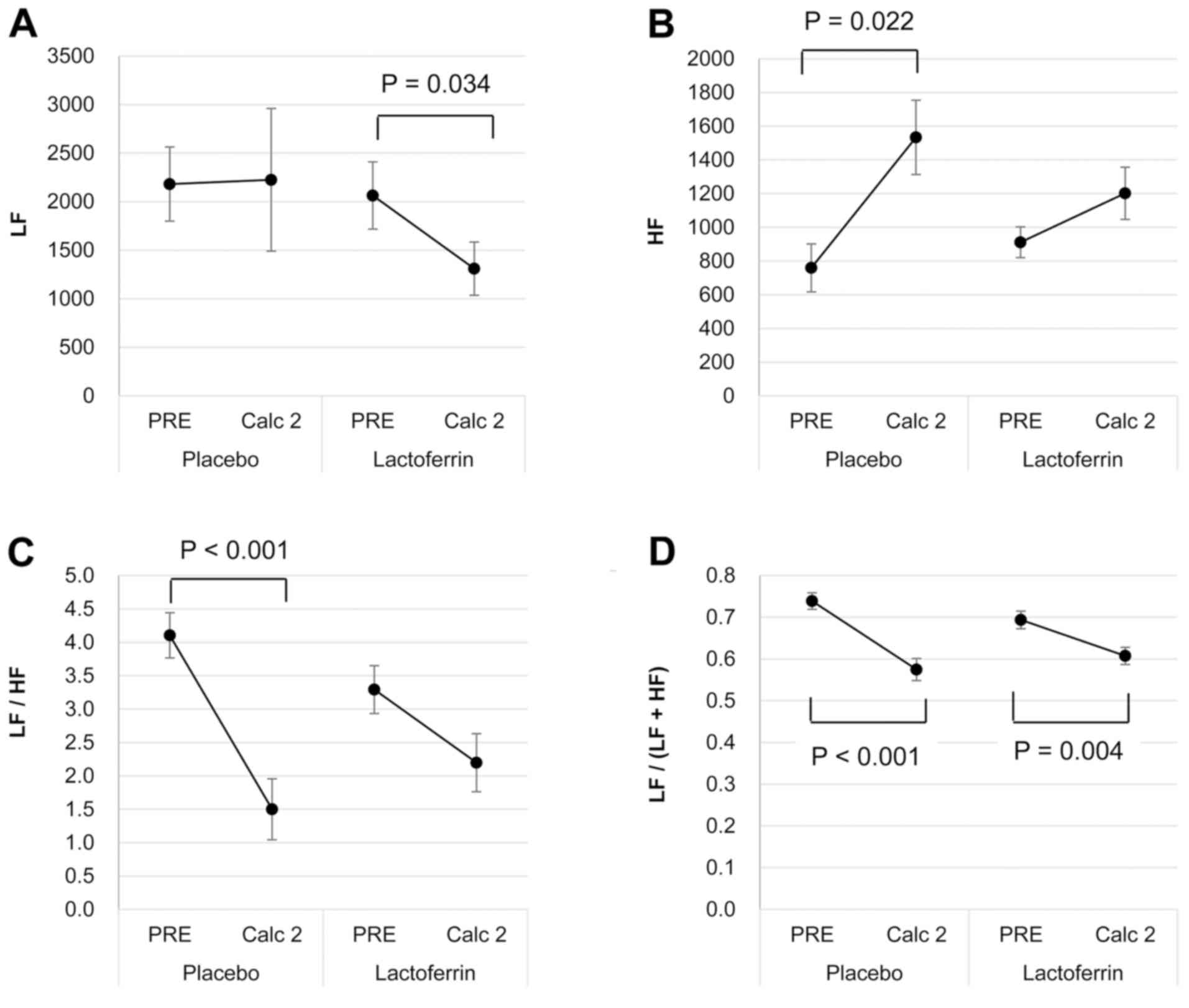
Heart-rate variability
With the placebo, the calculation task had no effect
on the LF component (Fig. 3A),
whereas it increased the HF component (P=0.022; Fig. 3B). Consequently, the LF/HF and
LF/(LF+HF) ratios were reduced (both P<0.001; Fig. 3C and D). By contrast, with the
lactoferrin ingestion, the LF component decreased (P=0.034;
Fig. 3A), while the HF component
remained similar (Fig. 3B) following
the calculation task. Accordingly, only the LF/(LF+HF) ratio
decreased (P=0.004; Fig. 3D), without
a significant change in the LF/HF ratio (Fig. 3C).
When changes in heart-rate variability parameters
were expressed as ratios (Calc 2/PRE; Fig. 4), lactoferrin ingestion was identified
to significantly attenuate the elevation in the HF component
(P=0.007; Fig. 4B) and the reduction
in the LF/(LF+HF) ratio (P=0.026; Fig.
4D) compared with the placebo treatment.
Discussion
The current study investigated the effect of oral
lactoferrin ingestion on changes in salivary stress markers and
autonomic nervous activities before and after solving calculation
problems. For both lactoferrin and placebo treatments, the
calculation task increased salivary amylase activity and decreased
chromogranin A concentration, with the magnitudes of changes in
these salivary markers being similar between the two treatments.
Despite this, autonomic nervous activities appeared to be
influenced by the ingestion of lactoferrin. With the placebo,
heart-rate variability exhibited an increase in the HF component
and decreases in the LF/HF and LF/(LF+HF) ratios, representing an
upregulation of parasympathetic activity and a downregulation of
sympathetic activity. Lactoferrin ingestion, however, made the
changes in the HF component and LF/(LF+HF) ratio significantly
smaller compared with placebo.
The present study identified a decrease in
chromogranin A concentration following the calculation task, which
was contradictory to what was expected based on previous studies
(13–15). The observed discrepancy may be due to
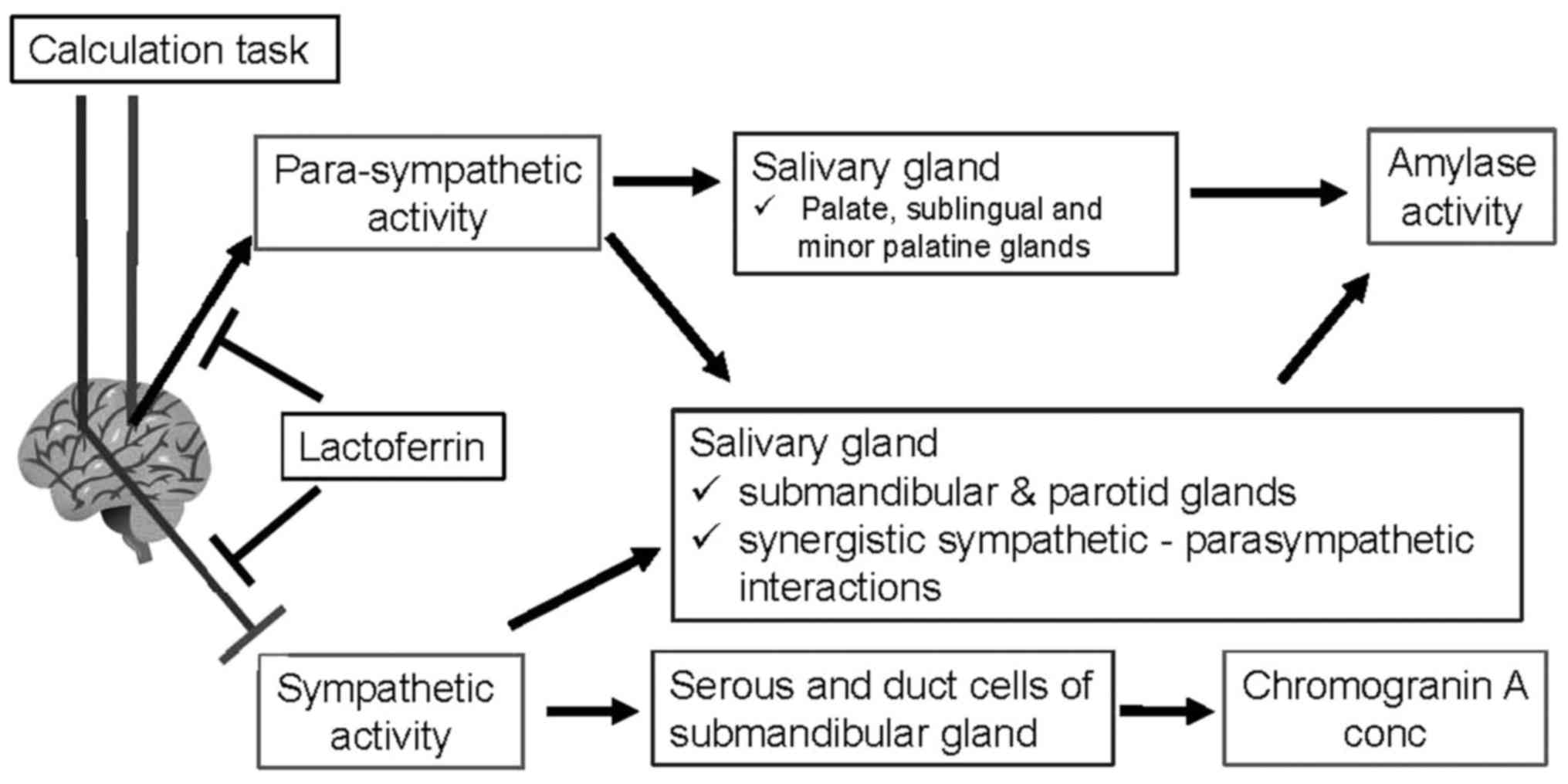
the difference in secretory mechanisms (Fig. 5). The release of salivary amylase is
affected by noradrenergic stimuli; both the sympathetic nervous
system and hypothalamic-pituitary-adrenal axis (12), as well as parasympathetic nervous
activity (22). An association of
parasympathetic activity and amylase activity has been identified
in previous study: A rise in amylase activity was observed during
relaxation (23). Subsequently,
exercise and psychological stress were also observed to increase
amylase activity (12,16) Predominantly, parasympathetic nerves
stimulate amylase release from the palate, sublingual and minor
palatine glands, whereas parasympathetic and sympathetic nerves
collaboratively promote amylase secretion from the submandibular
and parotid glands (22). By
contrast, chromogranin A is stored in the serous and duct cells of
the submandibular gland (24), and is
secreted into saliva by sympathetic noradrenergic stimulation.
Therefore, the secretions of amylase and chromogranin A are
controlled by differing innervation patterns.
The present study demonstrated that, without
lactoferrin, the calculation task raised HF (parasympathetic
activity), but did not influence LF, resulting in a reduced LF/HF
ratio (sympathetic activity). These results, including the change
in amylase activity, were in agreement with a previous report using
the TSST to induce psychological stress (18). Therefore, the calculation task was
assumed to stimulate parasympathetic activity to upregulate amylase
activity, and suppress sympathetic activity to downregulate
chromogranin A secretion (Fig. 5).
Oral lactoferrin ingestion, however, diminished LF activity and
suppressed the rise in HF (parasympathetic) activity, resulting in
a smaller decline in LF/HF ratio (sympathetic activity).
Accordingly, this made the changes (Calc 2/PRE) in parasympathetic
(HF) and sympathetic (LF/(LF+HF) ratio) activities smaller than the
placebo. Therefore, oral lactoferrin ingestion may have alleviated
the changes in autonomic nervous activity evoked by the calculation
task (Fig. 5).
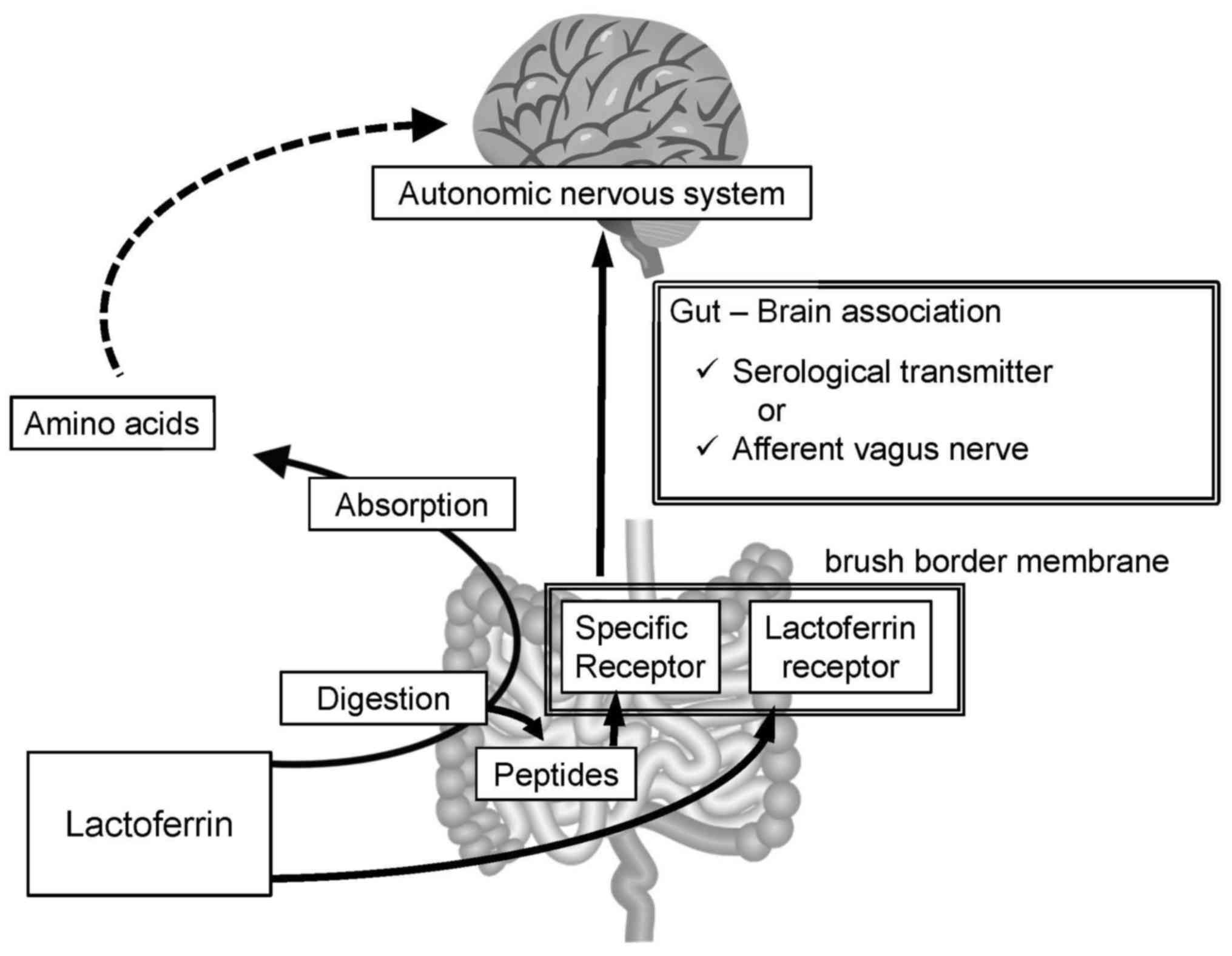
As lactoferrin is a protein with a molecular weight
of 80 kDa, it should be digested into smaller peptides or amino
acids prior to absorption. Certain reports have described
milk-derived peptides exerting biological activities other than
nutritive value (25–28). There may be a peptide derived from
lactoferrin that binds to a specific receptor to modulate autonomic
nervous activity; however, the absorbed amino acids are unlikely to
have such specific roles (Fig.
6).
In the human intestine, lactoferrin has a specific
receptor on the brush border membrane (29), namely intelectin-1, a
carbohydrate-binding protein with a fibrinogen-like fold and
calcium-binding site (30).
Intestinal introduction of Lactobacillus rhamnosus (L.
rhamnosus) has been demonstrated to augment afferent vagus
activity within minutes, though this effect was abolished if
subdiaphragmatic vagotomy had already been performed (31). The bacterium was considered to
stimulate transmission of signal from the intestinal lumen to the
autonomic nervous system via the afferent vagus nerve. As the
lactoferrin receptor, intelectin-1, has a carbohydrate-binding
activity (30), the cell wall of
introduced L. rhamnosus may bind to intelectin-1. Therefore,
intelectin-1 may transmit the signal from lactoferrin and L.
rhamnosus, or there may be other receptors on the brush border
membrane.
Intestinal cells transmit signals via serological
messengers or nervous activity. Intestinal cells secrete hormones
including ghrelin, cholecystokinin and glucagon-like peptide-1 in
response to nutrients in the intestinal tract (32), whereas the afferent vagus nerve
transmits the signal directly to the brain (31). Therefore, lactoferrin or
lactoferrin-derived peptides may bind to specific receptors and
modulate the autonomic nervous activity (Fig. 6).
Limitations to the current study must be considered.
Firstly, lactoferrin did not exert apparent effects on salivary
stress markers. A previous report demonstrated that salivary
amylase activity increased when subjects viewed a stressful video,
but normalized immediately following the end of viewing (33). The current study collected saliva
following the second set of calculation tests. The time schedule
applied may have been unsuited to observe the effects of the
calculation stimuli. Salivary amylase activity and chromogranin A
concentration also have distinct circadian rhythms (34,35), which
may have confounded the current results. Secondly, the intestinal
microbiota was not assessed, despite its importance in the
gut-brain association (36–38), and the fact that lactoferrin may exert
effects as a bactericidal protein (1–4). Thirdly,
while certain possible mechanisms by which lactoferrin may
influence autonomic nervous activity have been considered, the
actual mechanisms remain to be elucidated. Finally, subjects in the
study were healthy female college students with homogeneous
background characteristics, which potentially limits the
generalizability of the conclusions.
A single-dose cross-over study was conducted to
assess the influence of oral lactoferrin on psychological stresses
incurred by a calculation task. The calculation task resulted in
upregulated parasympathetic activity that increased salivary
amylase activity, and downregulated sympathetic activity that
reduced chromogranin A concentration. Oral lactoferrin ingestion
suppressed the changes in parasympathetic and sympathetic
activities evoked by the calculation task. These findings indicate
the possible application of lactoferrin in managing psychological
stress.
Acknowledgements
The images of brain and intestine in Figs. 5 and 6
were provided by DBCLS Togo Picture Gallery (© 2016
DBCLS TogoTV; http://togotv.dbcls.jp/ja/pics.html, accessed on Feb
16, 2018). The authors would like to thank Dr Akihiro Sakamoto for
his invaluable advice.
Funding
The present study was supported by the
Cross-Ministerial Strategic Innovation Promotion Program of the
Cabinet Office, Government of Japan (grant no. 14532924).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST analyzed and interpreted the data and wrote the
draft manuscript. TI and MK collected the data. KS and AN were
involved in data collection and critically edited the manuscript.
YA analyzed the salivary chromogranin A concentrations. YS designed
and managed the study. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The research protocol was reviewed and approved by
the research ethics committee of the Graduate School of Health and
Sports Science, Juntendo University (Inzai, Japan; approval no.
27–54).
Consent for publication
All subjects provided written informed consent to
participate in the study and permitting publication of relevant
data following anonymization of personally identifiable
information.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Arnold RR, Cole MF and McGhee JR: A
bactericidal effect for human lactoferrin. Science. 197:263–265.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold RR, Brewer M and Gauthier JJ:
Bactericidal activity of human lactoferrin: Sensitivity of a
variety of microorganisms. Infect Immun. 28:893–898.
1980.PubMed/NCBI
|
|
3
|
Kalmar JR and Arnold RR: Killing of
Actinobacillus actinomycetemcomitans by human lactoferrin. Infect
Immun. 56:2552–2557. 1988.PubMed/NCBI
|
|
4
|
Yamauchi K, Tomita M, Giehl TJ and Ellison
RT III: Antibacterial activity of lactoferrin and a pepsin-derived
lactoferrin peptide fragment. Infect Immun. 61:719–728.
1993.PubMed/NCBI
|
|
5
|
Vitetta L, Coulson S, Beck SL, Gramotnev
H, Du S and Lewis S: The clinical efficacy of a bovine
lactoferrin/whey protein Ig-rich fraction (Lf/IgF) for the common
cold: A double blind randomized study. Complement Ther Med.
21:164–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishikado A, Uesaki S, Suido H, Nomura Y,
Sumikawa K, Maeda M, Miyauchi M, Takata T and Makino T: Human trial
of liposomal lactoferrin supplementation for periodontal disease.
Biol Pharm Bull. 33:1758–1762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuhara T, Iigo M, Itoh T, Ushida Y, Sekine
K, Terada N, Okamura H and Tsuda H: Orally administered lactoferrin
exerts an antimetastatic effect and enhances production of IL-18 in
the intestinal epithelium. Nutr Cancer. 38:192–199. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koikawa N, Nagaoka I, Yamaguchi M, Hamano
H, Yamauchi K and Sawaki K: Preventive effect of lactoferrin intake
on anemia in female long distance runners. Biosci Biotechnol
Biochem. 72:931–935. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paesano R, Torcia F, Berlutti F, Pacifici
E, Ebano V, Moscarini M and Valenti P: Oral administration of
lactoferrin increases hemoglobin and total serum iron in pregnant
women. Biochem Cell Biol. 84:377–380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamemori N, Takeuchi T, Hayashida K and
Harada E: Suppressive effects of milk-derived lactoferrin on
psychological stress in adult rats. Brain Res. 1029:34–40. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rohleder N, Wolf JM, Maldonado EF and
Kirschbaum C: The psychosocial stress-induced increase in salivary
alpha-amylase is independent of saliva flow rate. Psychophysiology.
43:645–652. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Granger DA, Kivlighan KT, el-Sheikh M,
Gordis EB and Stroud LR: Salivary α-amylase in biobehavioral
research: Recent developments and applications. Ann N Y Acad Sci.
1098:122–144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haririan H, Bertl K, Laky M, Rausch WD,
Böttcher M, Matejka M, Andrukhov O and Rausch-Fan X: Salivary and
serum chromogranin A and α-amylase in periodontal health and
disease. J Periodontol. 83:1314–1321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee T, Shimizu T, Iijima M, Obinata K,
Yamashiro Y and Nagasawa S: Evaluation of psychosomatic stress in
children by measuring salivary chromogranin A. Acta Paediatr.
95:935–939. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanno T, Asada N, Yanase H, Iwanaga T and
Yanaihara N: Salivary Secretion of Chromogranin A Control by
Autonomic Nervous SystemChromogranins: Functional and Clinical
Aspects. Helle KB and Aunis D: Springer US; Boston, MA: pp.
143–151. 2002, View Article : Google Scholar
|
|
16
|
Koibuchi E and Suzuki Y: Exercise
upregulates salivary amylase in humans (Review). Exp Ther Med.
7:773–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chatterton RT Jr, Vogelsong KM, Lu YC,
Ellman AB and Hudgens GA: Salivary alpha-amylase as a measure of
endogenous adrenergic activity. Clin Physiol. 16:433–448. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nater UM, La Marca R, Florin L, Moses A,
Langhans W, Koller MM and Ehlert U: Stress-induced changes in human
salivary alpha-amylase activity - associations with adrenergic
activity. Psychoneuroendocrinology. 31:49–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakane H, Asami O, Yamada Y, Harada T,
Matsui N, Kanno T and Yanaihara N: Salivary chromogranin A as an
index of psychosomatic stress response. Biomed Res. 19:401–406.
1998. View Article : Google Scholar
|
|
20
|
Malik M, Bigger J, Camm A, Kleiger R,
Malliani A, Moss AJ and Schwartz PJ: Task Force of the European
Society of Cardiology and the North American Society of Pacing and
Electrophysiology: Heart rate variability. Standards of
measurement, physiological interpretation, and clinical use. Eur
Heart J. 17:354–381. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Acharya Rajendra U, Joseph Paul K,
Kannathal N, Lim CM and Suri JS: Heart rate variability: A review.
Med Biol Eng Comput. 44:1031–1051. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bosch JA, Veerman ECI, de Geus EJ and
Proctor GB: α-Amylase as a reliable and convenient measure of
sympathetic activity: Don't start salivating just yet!
Psychoneuroendocrinology. 36:449–453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morse DR, Schacterle GR, Furst ML,
Esposito JV and Zaydenburg M: Stress, relaxation and saliva:
Relationship to dental caries and its prevention, with a literature
review. Ann Dent. 42:47–54. 1983.PubMed/NCBI
|
|
24
|
Kanno T, Asada N, Yanase H, Iwanaga T and
Yanaihara N: Salivary secretion of chromogranin A. Control by
autonomic nervous system. Adv Exp Med Biol. 482:143–151. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takeuchi T, Hayashida K, Inagaki H,
Kuwahara M, Tsubone H and Harada E: Opioid mediated suppressive
effect of milk-derived lactoferrin on distress induced by maternal
separation in rat pups. Brain Res. 979:216–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zimecki M and Kruzel ML: Milk-derived
proteins and peptides of potential therapeutic and nutritive value.
J Exp Ther Oncol. 6:89–106. 2007.PubMed/NCBI
|
|
27
|
Mohanty DP, Mohapatra S, Misra S and Sahu
PS: Milk derived bioactive peptides and their impact on human
health - A review. Saudi J Biol Sci. 23:577–583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marcone S, Belton O and Fitzgerald DJ:
Milk-derived bioactive peptides and their health promoting effects:
A potential role in atherosclerosis. Br J Clin Pharmacol.
83:152–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawakami H and Lönnerdal B: Isolation and
function of a receptor for human lactoferrin in human fetal
intestinal brush-border membranes. Am J Physiol. 261:G841–G846.
1991.PubMed/NCBI
|
|
30
|
Suzuki YA, Shin K and Lönnerdal B:
Molecular cloning and functional expression of a human intestinal
lactoferrin receptor. Biochemistry. 40:15771–15779. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perez-Burgos A, Wang B, Mao YK, Mistry B,
McVey Neufeld KA, Bienenstock J and Kunze W: Psychoactive bacteria
Lactobacillus rhamnosus (JB-1) elicits rapid frequency
facilitation in vagal afferents. Am J Physiol Gastrointest Liver
Physiol. 304:G211–G220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Steinert RE, Feinle-Bisset C, Asarian L,
Horowitz M, Beglinger C and Geary N: Ghrelin, CCK, GLP-1, and
PYY(3-36): Secretory Controls and Physiological Roles in Eating and
Glycemia in Health, Obesity, and After RYGB. Physiol Rev.
97:411–463. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takai N, Yamaguchi M, Aragaki T, Eto K,
Uchihashi K and Nishikawa Y: Effect of psychological stress on the
salivary cortisol and amylase levels in healthy young adults. Arch
Oral Biol. 49:963–968. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nater UM, Rohleder N, Schlotz W, Ehlert U
and Kirschbaum C: Determinants of the diurnal course of salivary
alpha-amylase. Psychoneuroendocrinology. 32:392–401. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Den R, Toda M, Nagasawa S, Kitamura K and
Morimoto K: Circadian rhythm of human salivary chromogranin A.
Biomed Res. 28:57–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grenham S, Clarke G, Cryan JF and Dinan
TG: Brain-gut-microbe communication in health and disease. Front
Physiol. 2:942011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bengmark S: Gut microbiota, immune
development and function. Pharmacol Res. 69:87–113. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Forsythe P and Kunze WA: Voices from
within: Gut microbes and the CNS. Cell Mol Life Sci. 70:55–69.
2013. View Article : Google Scholar : PubMed/NCBI
|