Introduction
Adipose-derived mesenchymal stem cells (ADSCs) have
the potential to differentiate into numerous types of cells
including adipocyte, chondrocytes, osteocytes, cardiomyocytes,
vascular endotherial cells, pancreatic β cells and hepatocyte cells
(1–9).
ADSCs can be obtained in high yield with minimal discomfort under
local anesthesia (10,11). Therefore, ADSCs are considered a
useful source for regenerative therapy (12). However, in particular endodermal types
of cells, the differentiation method has not been well established
and differentiation efficiency is extremely low. Thus, the
development of an efficient differentiation method is extremely
important. Several studies suggested that conditioned medium
contains several undefined factors (8,12). These
undefined factors induce the stem cells to certain specific types
of cells (1).
In the present study, ADSCs were cultured in
hepatocyte-derived conditioned medium from mice of various ages and
conditioned medium from hepatocyte cells. To the best of our
knowledge, this is the first study to assess the differentiation of
ADSCs into bile cell lineages using murine fetal hepatocyte-derived
culture medium.
Materials and methods
Ethical statement
Animal studies were conducted in strict accordance
with the principles and procedures approved by the Committee on the
Ethics of Animal Experiments of Osaka University (Osaka,
Japan).
Isolation of mouse adipose-derived
stem cells
Mouse adipose tissue was obtained from 8 adult (8–12
weeks) C57BL/6JJcI mice. (Nihon Clea, Tokyo, Japan). Bilateral
inguinal subcutaneous fat pad was removed, minced into sections,
collected with ADSC-culture medium, centrifuged at 1,500 rpm for 5
min to remove cell debris, incubated in 0.1% collagenase type IV
(Worthington Biochemical Corp., Lakewood, NJ, USA) and agitated in
a water bath at 37°C for 30 min. Subsequently, the mixture was
added to ADSC-culture medium and centrifuged at 300 × g for 5 min
to remove cell debris. The cell pellets were suspended in
ASDS-culture medium and were plated at 500,000/ml following
filtration through a 70-µm cell strainer (Corning, Inc., Corning,
NY, USA). The cells were cultured at 37°C and 5% CO2. At
100% confluence, the cells were split. Culture media were replaced
every 2 days. The ADSCs until the fourth passage were used for
hepatic differentiation. The ADSC-culture medium consisted of
Dulbecco's modified Eagle's medium (DMEM) containing high glucose
(Nacalai Tesque, Inc., Kyoto, Japan) with 10% fetal bovine serum
(FBS) and 500 µg/ml of penicillin-streptomycin.
Isolation of mouse hepatocytes and
preparation of conditioned medium
Mouse hepatocytes were isolated from E13.5, E15.5,
E17.5 and E19.5 C57BL/6JJcI mice (Charles River Laboratories,
Willmington, MA, USA), either newborn (within 2 weeks) or adult (12
weeks) C57BL/6JJcI mouse (Nihon Clea). Briefly, when the mice were
sacrificed, the liver was removed, minced into small sections,
collected with hepatocyte-culture medium, centrifuged at 85 × g for
3 min to remove cell debris, incubated with 0.1% collagenase type
IV (Worthington Biochemical Corp.,) and agitated in a water bath at
37°C for 30 min. Subsequently, the mixtures were added to
hepatocyte culture medium followed by centrifugation at 800 rpm for
3 min to remove cell debris, damaged cells and non-parenchymal
cells. The remaining liver parenchyma was collected in hepatocyte
culture medium, passed through a 70-µm sterile filter (Corning
Inc.) and cultured at 500,000/ml at 37°C and 5% CO2. The
hepatocyte culture medium consisted of DMEM-high glucose with 10%
FBS, 500 µg/ml of penicillin-streptomycin, 0.5 µg/ml insulin
(Sigma-Aldrich, St. Louis, MO, USA), 1 µM dexamethasone
(Sigma-Aldrich), 10 ng/ml epidermal growth factor (Peprotech, Inc.,
Rocky Hill, NJ, USA) and 200 µM ascorbic acid (Sigma-Aldrich).
Culture media were replaced every 2 days. The conditioned medium
was generated according to the previous study by Kawamoto et
al (12). In detail, these
hepatocytes grew to ≤50% confluence with hepatocyte culture medium.
Subsequently, the hepatocyte culture medium was replaced with fresh
medium on day 2. After a 48-h culture period, the medium was
collected (#1) and replaced with fresh medium. Subsequently, CMs
were collected every 48 h incubation (#2). These conditioned media
(#1 and #2) were pooled and filtered using a bottle-top filter
(Corning, Inc.) to remove cells and debris. Conditioned medium
samples were frozen at −20°C for later use. Induction of
hepatogenic differentiation of mouse ADSCs was by
hepatocyte-conditioned medium. For evaluation of the hepatogenic
differentiation ability, mouse ADSCs were cultured with these types
of hepatocyte-conditioned medium at 37°C and 5% CO2.
These cells were maintained by media exchange every 2–3 days for 2
and 4 weeks, and subsequently they were collected for RNA
isolation.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from samples using TRIzoL
Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
following the manufacturer's protocol, and was treated with
ReverTra Ace (Toyobo Co., Ltd., Osaka, Japan) to generate cDNA.
Subsequently, PCR amplification was performed for mouse hepatocyte
nuclear factor 4, α (HNf4α), α-fetoprotein (Afp),
glucose-6-phosphatase (G6p), albumin (Alb),
cytokeratin 19 (Ck19), Ck7 and sex-determining
region-Y-box 9 (Sox9). The RT-PCR products ware analyzed by
1% agarose gel electrophoresis and visualized with ethidium
bromide.
Results
Differentiation of ADSCs
To investigate the effect of conditioned medium from
mouse hepatocyte, ADSCs were cultured using the conditioned medium
(Fig. 1). Subsequently the gene
expression pattern was analyzed. The hepatocyte marker genes
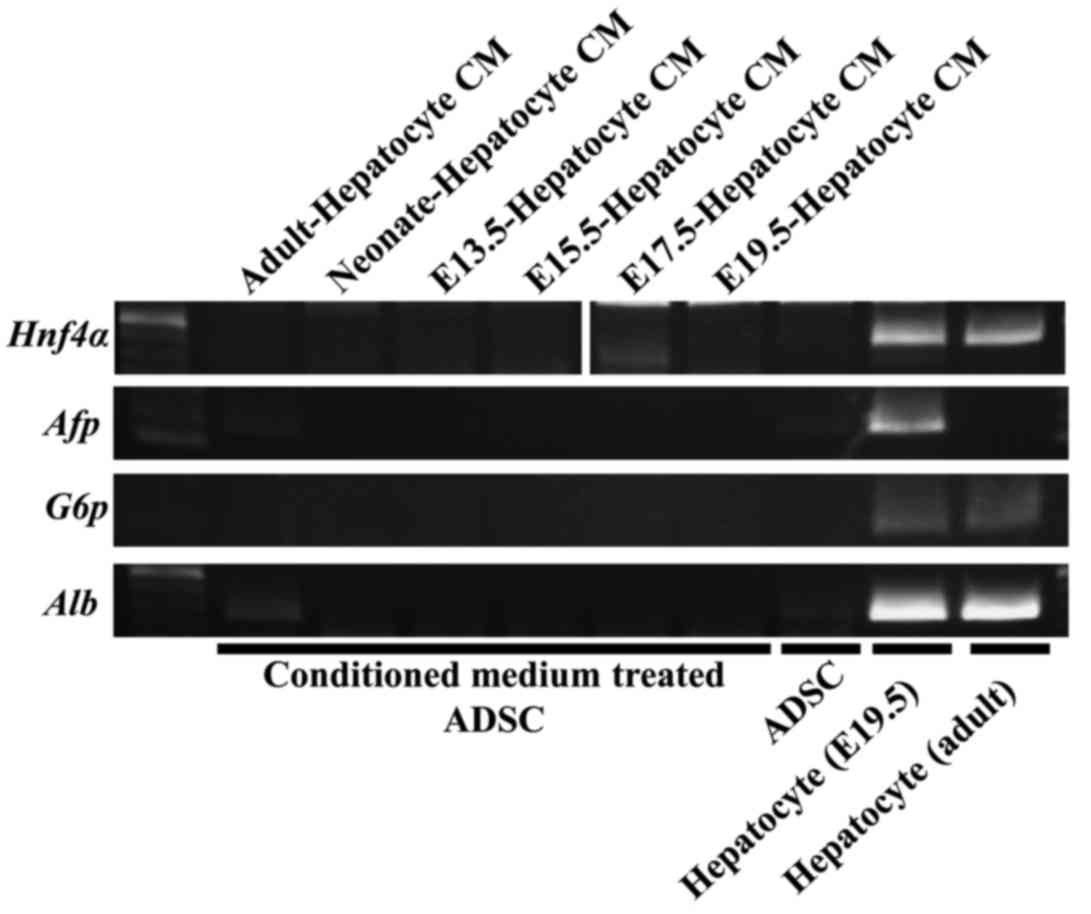
Hnf4α, Afp and G6P were not expressed in
undifferentiated and conditioned medium-treated ADSCs (Fig. 2). Alb expression can be observed at an
extremely low level in adult hepatocyte-derived conditioned
medium-treated ADSCs (Fig. 2). These
data suggested that the conditioned medium from mouse hepatocyte
could not induce mouse ADSCs to hepatocyte. By contrast, bile cells
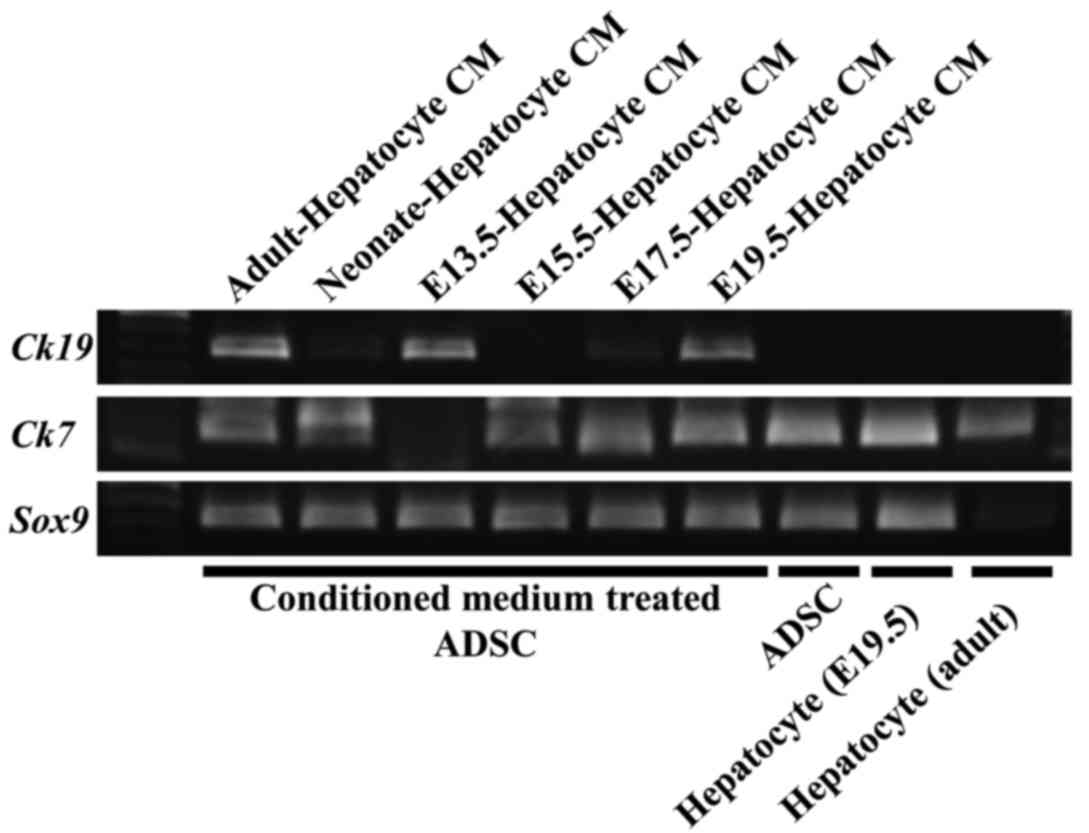
marker genes were expressed in conditioned medium-treated ADSCs.
Ck19 was expressed in ADSCs cultured in adult, E13.5, and
E19.5 mouse hepatocyte derived conditioned medium (Fig. 3). Ck7 was expressed in ADSCs
cultured in adult, neonate, E13.5, E15.5 and E19.5 mouse
hepatocyte-derived conditioned medium (Fig. 3). Furthermore, Sox9, which was
expressed in various types of stem cells or progenitor cells, was
expressed in undifferentiated ADSCs and conditioned medium-treated
ADSCs (Fig. 3). These data suggested
that the conditioned culture medium from mouse fetal hepatocyte
could induce mouse ADSCs to differentiate to bile cell lineages
and/or their progenitor cells.
Discussion
The present study showed that conditioned medium
derived from hepatocytes could induce ADSCs to bile cells. In mouse
embryonic development, CCAAT-enhancer-binding protein α (C/EBPα) is
known as a critical transcription factor that induces hepatoblasts
into hepatocytes (13).
Downregulation of C/EBPα is the most important event in
differentiation into bile cells. C/EBPα-knockout mice could not
develop mature hepatocyte cells, and all the cells that should have
been hepatocytes were bile cells (14). Furthermore, it was reported that
transforming growth factor β (TGFβ)/activin and Notch signaling
were important for developing bile cells. Therefore, it was
considered that the conditioned medium derived from hepatocytes may
contain the C/EBPα inhibitor, TGFβ/activin or Notch (15–17). In
conclusion, analysis of the factors in the conditioned medium will
lead to the development of efficient bile cell differentiation
culture medium and regenerative therapy for the bile duct.
Acknowledgements
The authors thank all the members of the laboratory
for the discussion of the study and technical assistance. The
present study was supported in part by a Grant-in-Aid for
Scientific Research from the Ministry of Education, Culture,
Sports, Science, and Technology and P-DIRECT; a Grant-in-Aid from
the Ministry of Health, Labor and Welfare; a grant from the
National Institute of Biomedical Innovation; and a grant from the
Osaka University Drug Discovery Funds. Partial support was received
from the Suzuken Memorial Foundation (M.K.), the Yasuda Medical
Foundation (N.N.), the Pancreas Research Foundation (K.K.), the
Nakatani Foundation (H.I.), and the Nakatomi Foundation of Japan
(M.K.). Institutional endowments were received partially from Taiho
Pharmaceutical Co., Ltd., Evidence Based Medical Research Center,
Chugai Co., Ltd., Yakult Honsha Co., Ltd., and Merck Co., Ltd.
These funding bodies had no role in the main experimental materials
and methods, supplies or expenses, study design, data collection
and analysis, decision to publish, or preparation of the
manuscript.
References
|
1
|
Majumdar MK, Banks V, Peluso DP and Morris
EA: Isolation, characterization, and chondrogenic potential of
human bone marrow-derived multipotential stromal cells. J Cell
Physiol. 185:98–106. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Halvorsen YC, Wilkison WO and Gimble JM:
Adipose-derived stromal cells - their utility and potential in bone
formation. Int J Obes Relat Metab Disord. 24 Suppl 4:S41–S44. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Halvorsen YD, Franklin D, Bond AL, Hitt
DC, Auchter C, Boskey AL, Paschalis EP, Wilkison WO and Gimble JM:
Extracellular matrix mineralization and osteoblast gene expression
by human adipose tissue-derived stromal cells. Tissue Eng.
7:729–741. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rangappa S, Fen C, Lee EH, Bongso A and
Sim EK: Transformation of adult mesenchymal stem cells isolated
from the fatty tissue into cardiomyocytes. Ann Thorac Surg.
75:775–779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Planat-Benard V, Silvestre JS, Cousin B,
André M, Nibbelink M, Tamarat R, Clergue M, Manneville C,
Saillan-Barreau C, Duriez M, et al: Plasticity of human adipose
lineage cells toward endothelial cells: Physiological and
therapeutic perspectives. Circulation. 109:656–663. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Konno M, Hamazaki TS, Fukuda S, Tokuhara
M, Uchiyama H, Okazawa H, Okochi H and Asashima M: Efficiently
differentiating vascular endothelial cells from adipose
tissue-derived mesenchymal stem cells in serum-free culture.
Biochem Biophys Res Commun. 400:461–465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Konno M, Hamabe A, Hasegawa S, Ogawa H,
Fukusumi T, Nishikawa S, Ohta K, Kano Y, Ozaki M, Noguchi Y, et al:
Adipose-derived mesenchymal stem cells and regenerative medicine.
Dev Growth Differ. 55:309–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Banas A, Teratani T, Yamamoto Y, Tokuhara
M, Takeshita F, Quinn G, Okochi H and Ochiya T: Adipose
tissue-derived mesenchymal stem cells as a source of human
hepatocytes. Hepatology. 46:219–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Casteilla L and Dani C: Adipose
tissue-derived cells: From physiology to regenerative medicine.
Diabetes Metab. 32:393–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D'Andrea F, De Francesco F, Ferraro GA,
Desiderio V, Tirino V, De Rosa A and Papaccio G: Large-scale
production of human adipose tissue from stem cells: A new tool for
regenerative medicine and tissue banking. Tissue Eng Part C
Methods. 14:233–242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawamoto K, Yabe S, Konno M, Ishii H,
Nishida N, Koseki J, Fukuda S, Tomimaru Y, Hama N, Wada H, et al:
Murine insulinoma cell-conditioned medium with BETA2/Neurod1
transduction efficiently induces the differentiation of
adipose-derived mesenchymal stem cells into β-Like cells both in
vitro and in vivo. J Stem Cell Res Ther. 4:10002212014. View Article : Google Scholar
|
|
13
|
Shiojiri N, Takeshita K, Yamasaki H and
Iwata T: Suppression of C/EBP alpha expression in biliary cell
differentiation from hepatoblasts during mouse liver development. J
Hepatol. 41:790–798. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamasaki H, Sada A, Iwata T, Niwa T,
Tomizawa M, Xanthopoulos KG, Koike T and Shiojiri N: Suppression of
C/EBPalpha expression in periportal hepatoblasts may stimulate
biliary cell differentiation through increased Hnf6 and Hnf1b
expression. Development. 133:4233–4243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCright B, Lozier J and Gridley T: A
mouse model of Alagille syndrome: Notch2 as a genetic modifier of
Jag1 haploinsufficiency. Development. 129:1075–1082.
2002.PubMed/NCBI
|
|
16
|
Tanimizu N and Miyajima A: Notch signaling
controls hepatoblast differentiation by altering the expression of
liver-enriched transcription factors. J Cell Sci. 117:3165–3174.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clotman F, Jacquemin P, Plumb-Rudewiez N,
Pierreux CE, Van der Smissen P, Dietz HC, Courtoy PJ, Rousseau GG
and Lemaigre FP: Control of liver cell fate decision by a gradient
of TGF beta signaling modulated by Onecut transcription factors.
Genes Dev. 19:1849–1854. 2005. View Article : Google Scholar : PubMed/NCBI
|