Introduction
The widespread use of biological disease-modifying
anti-rheumatic drugs (DMARDs) has improved the management of
autoimmune diseases; however, these agents are also associated with
a number of adverse events, some of which impact on autoimmune
processes (1–3). Tumor necrosis factor (TNF) inhibitors
are the first biological agents used in rheumatoid arthritis (RA)
to have yielded satisfactory results, with significant decreases in
the clinical activity rates (majority of patients reaching a state
of clinical remission or low disease activity) and in structural
damage (minimal radiographic progression) (1–3), though
are also associated with a number of autoimmune systemic events
(lupus, vasculitis, sarcoidosis) and localized adverse events
[uveitis, psoriasis, interstitial lung disease, erythema multiforme
including the major form Stevens-Johnson syndrome (SJS)] (4–8).
Drug-induced lupus (DIL) is the most frequent
systemic autoimmune adverse event associated with the use of TNF
inhibitors in RA, and mucocutaneous manifestations including malar
rash, discoid lupus, oral ulcers, chilblain lupus and other lesions
are frequently associated with general manifestations and articular
symptoms (4–8). Severe manifestations of lupus
(nephritis, central nervous system involvement) are rare (4–8). It is
important to acknowledge that many patients with RA exhibit
positivity for antinuclear antibody prior to starting anti-TNF
treatment and between 15 and 80% (according to different reports)
develop positivity for antinuclear antibody during therapy, some of
which develop clinical manifestations, though only a minority
fulfill lupus classification criteria (less than 1%) (4–8).
Certain patients with lupus induced by TNF
inhibitors may develop positivity for antihistone antibodies (as in
other forms of DIL), but a particular aspect is the fact that
patients with lupus induced by TNF inhibitors may also frequently
develop anti-double-stranded DNA (dsDNA) antibodies (as in systemic
lupus erythematosus) (4–8). Hypocomplementemia is more frequently
observed in lupus induced by TNF inhibitors than in other forms of
drug-induced lupus (5,6).
The mechanisms underlying lupus induced by TNF
inhibitors are not fully understood. It is possible that TNF
inhibition leads to upregulation of interleukin (IL)-10 and B cell
hyperactivity or T helper 2 cell hyperactivity with B cell
activation (5,7,8). Another
mechanism proposed is the decreased apoptosis of cytotoxic T cells
(6). Common infections in patients
treated with TNF inhibitors may activate B cell activity (5,7,8). It has also been suggested that a
possible overlap of RA and underlying lupus pathology may be
propagated by therapy with TNF inhibitors into a complete form of
lupus (5–7). Some of the mucocutaneous manifestations
may be severe and must be differentiated from allergic reactions
and erythema multiforme (4–7). Stopping the application of TNF inhibitor
is usually sufficient for remission of symptoms but in certain
cases, glucocorticoid and immunosuppressive therapy are required
(4–7).
Lupus induced by TNF inhibitors has been reported also in other
immune mediated diseases including Crohn's disease (CD) and less
frequently in spondyloarthritis (8–12). The
current report presents the case of a patient with rheumatoid
arthritis who developed severe recurrent cutaneous reactions and
positive autoantibodies during TNF inhibitor treatment with
difficulties in differential diagnosis and treatment. A review of
the literature is also presented.
Case report
The current report presents the case of a
63-year-old female patient diagnosed with RA in the outpatient
department of County Hospital Tulcea (‘Spitalul Judetean Tulcea’,
Tulcea, Romania) in February 2016 (symmetrical arthritis on the
hands, wrists, knees, elbows and shoulders, morning stiffness for
~60 min, rheumatoid factor +, anti-citrullinated protein antibodies
+++) who was treated with methotrexate 10–15 mg weekly following
response failure to other DMARDs (leflunomide, hydroxychloroquine).
In December 2016 the patient began biological therapy (certolizumab
pegol; two subcutaneous injections of 200 mg followed by one
subcutaneous administration of 200 mg every 2 weeks) and in January
2017 was admitted to the Emergency Department of ‘Sfanta Maria’
Hospital (Bucharest, Romania), with complaints of aggravated
generalized erythematous rash, intense pruritus, dysphonia and
difficulty in swallowing. She reported the onset of dysphonia 2
days after the first administration of certolizumab pegol, which
was deemed as not notably discomforting at that time by the patient
and also following the second administration (3 weeks prior to
hospital presentation), but was associated with erythematous rash
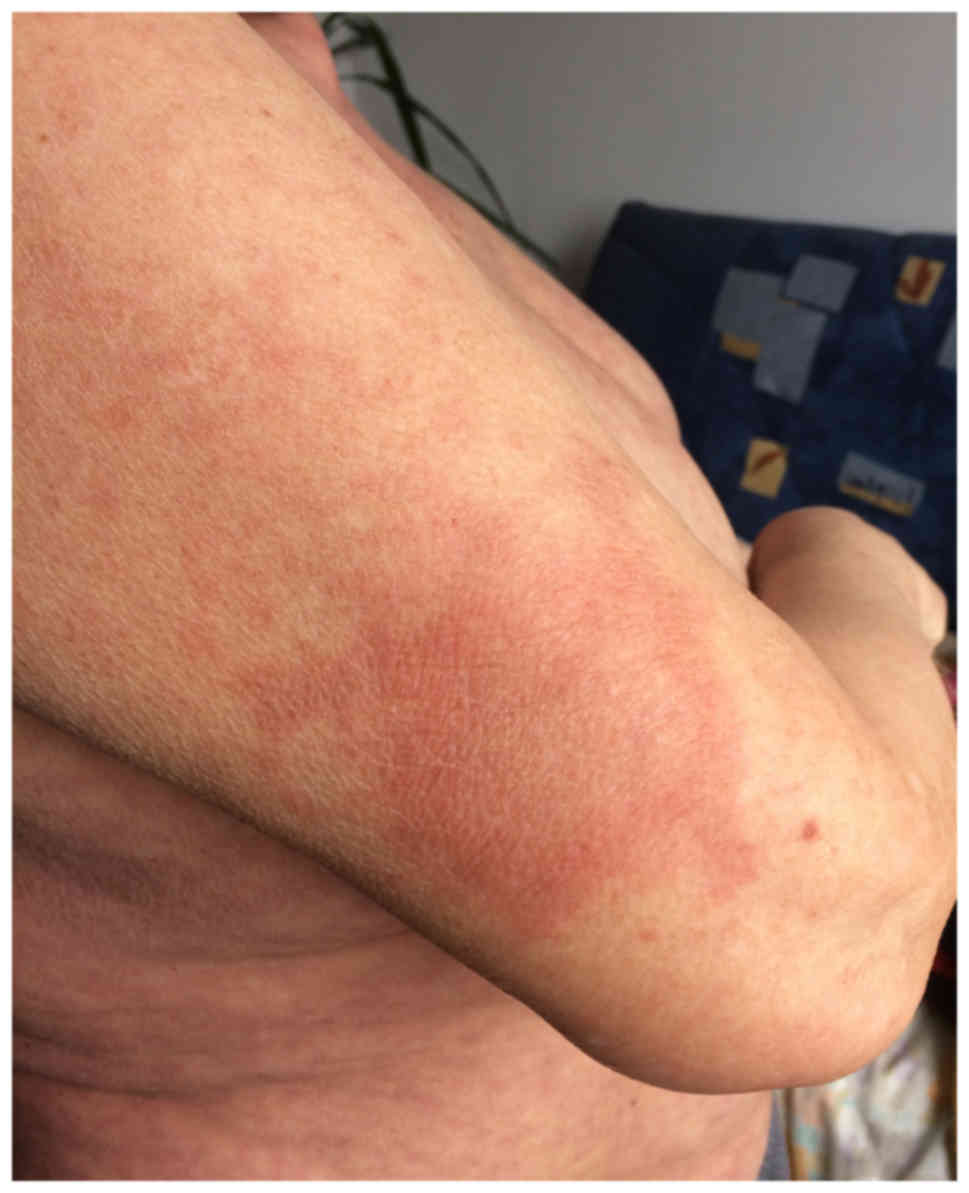
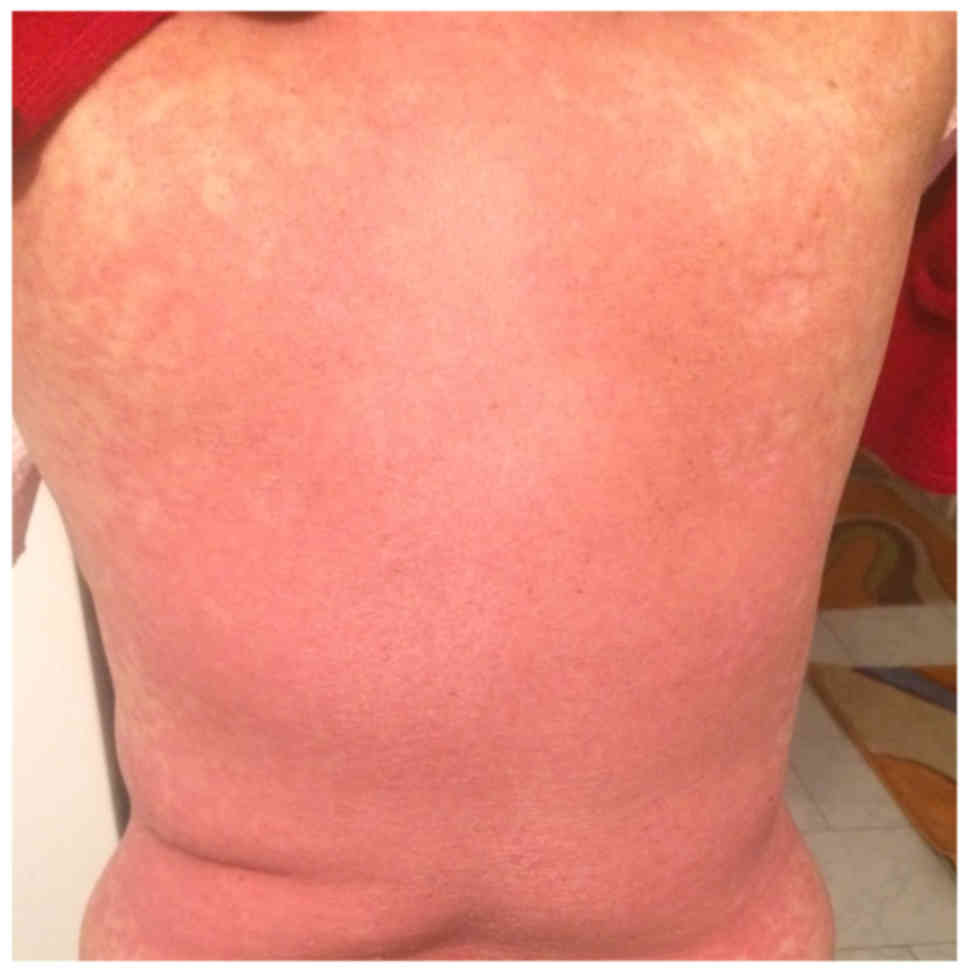
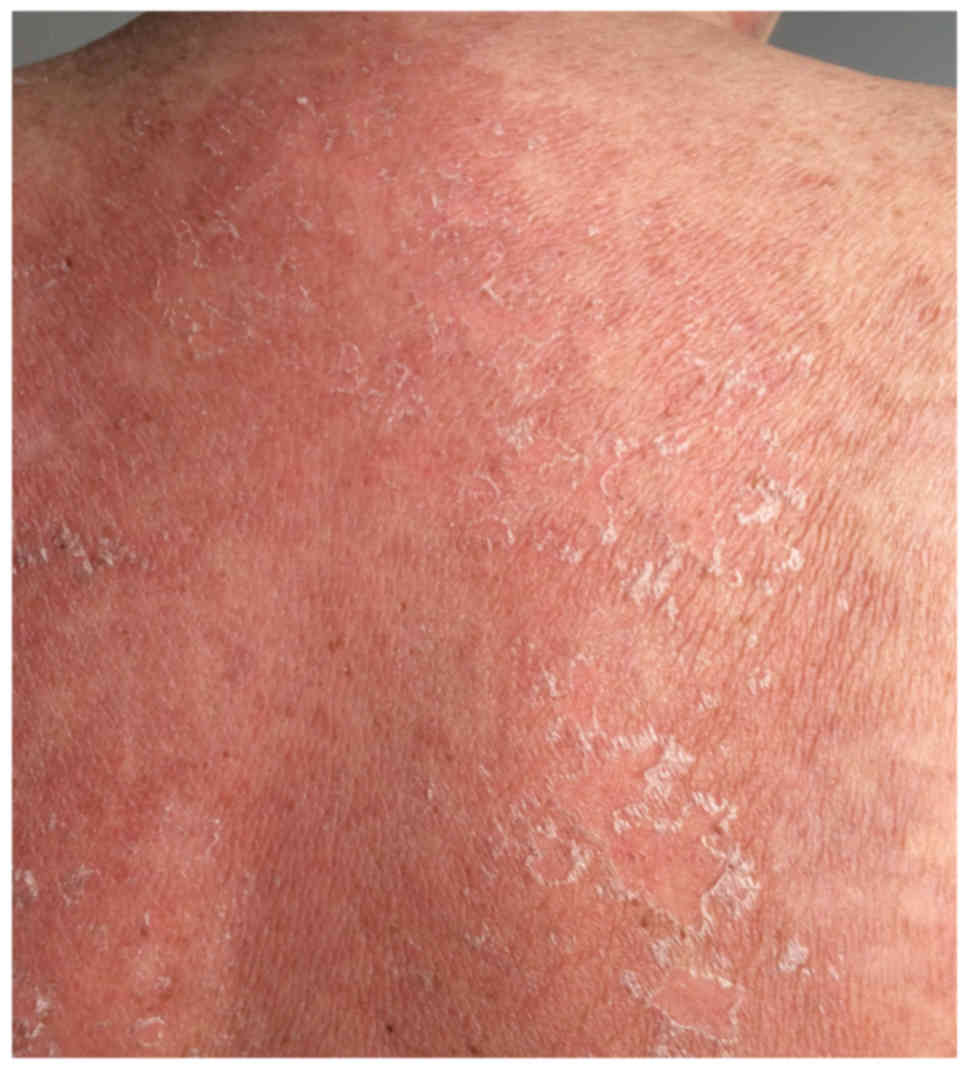
(Figs. 1 and 2), located at the anterior and posterior
thorax, on the arms and in the lumbar region, which then associated
with epidermal detachment (Fig. 3).
This situation alerted the patient who presented to the Emergency
Department. The diagnosis was severe allergoderma and the patient
begun administration of glucocorticoid therapy (dexamethasone 8 mg,
intravenous, once/day for 5 days followed by oral prednisone 30
mg/day) and oral loratadine 10 mg/day. The progression was
favorable with initial marked improvement of dysphonia and skin
lesions and the patient was discharged with the recommendation of
tapering prednisone at 5 mg/week. However, after 6 weeks, the
patient presented again at the hospital with an aggravated general
condition: Malaise, an extended rash, intense arthralgia, pruritus,
dysphonia and difficulty in swallowing were evident. She was
urgently hospitalized and a physical examination revealed
generalized maculopapular rash, severe pruritus. The patient was
hemodynamically stable (normal arterial tension of 130/80 mm Hg and
a regular heart rate of 88 beats/min) at that time with no signs of
laryngeal edema.
Laboratory tests identified increased values for
erithrocyte sedimentation rate (ESR), 80 mm/h (normal range, 2–20
mm/h); C-reactive protein level (CRP), 101 mg/l (normal range, 0–5
mg/l); normal white blood cell (WBC) count (7,800/mmc; normal
range, 3,500–10,000/mmc) without any other signs of infection
(references ranges of ‘Sfanta Maria’ Hospital laboratory). An
extended antinuclear antibodies immunoblot profile (qualitative
serum immunoblot method; Synevo Central Labs, Belro Medical S.A.,
Waterloo, Belgium) revealed positivity for
anti-Sjögren's-syndrome-related antigen A (SS-A) and
anti-Sjögren's-syndrome-related antigen B (SS-B), and anti-histone
antibodies. A dermatological assessment of skin lesions concluded a
diagnosis of the major form of erythema multiforme, which further
prompted a cutaneous biopsy for diagnosis of SJS and
differentiation from other possible diseases including Rowel
syndrome, pemphigus vulgaris and bullous pemphigoid or disseminated
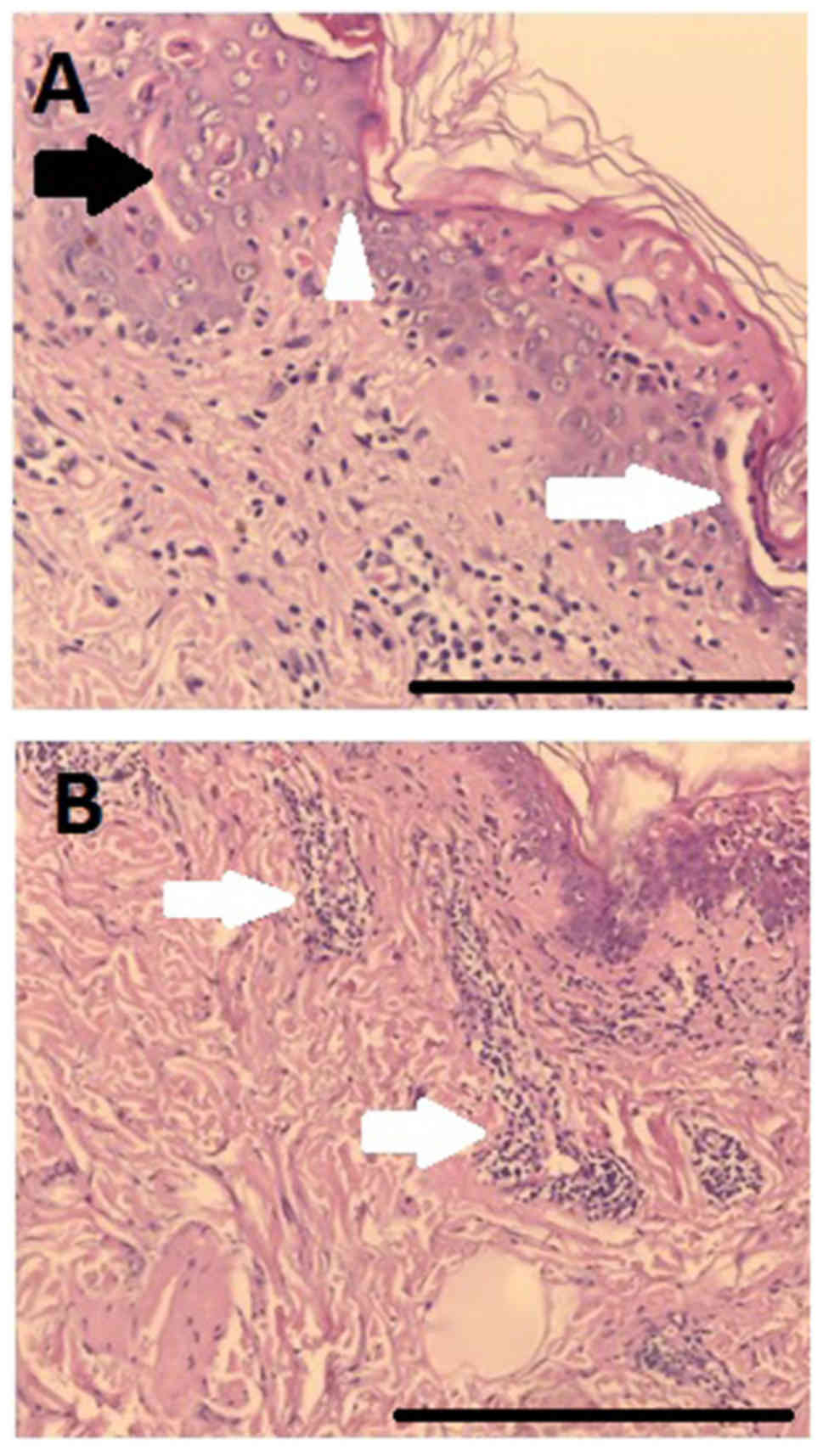
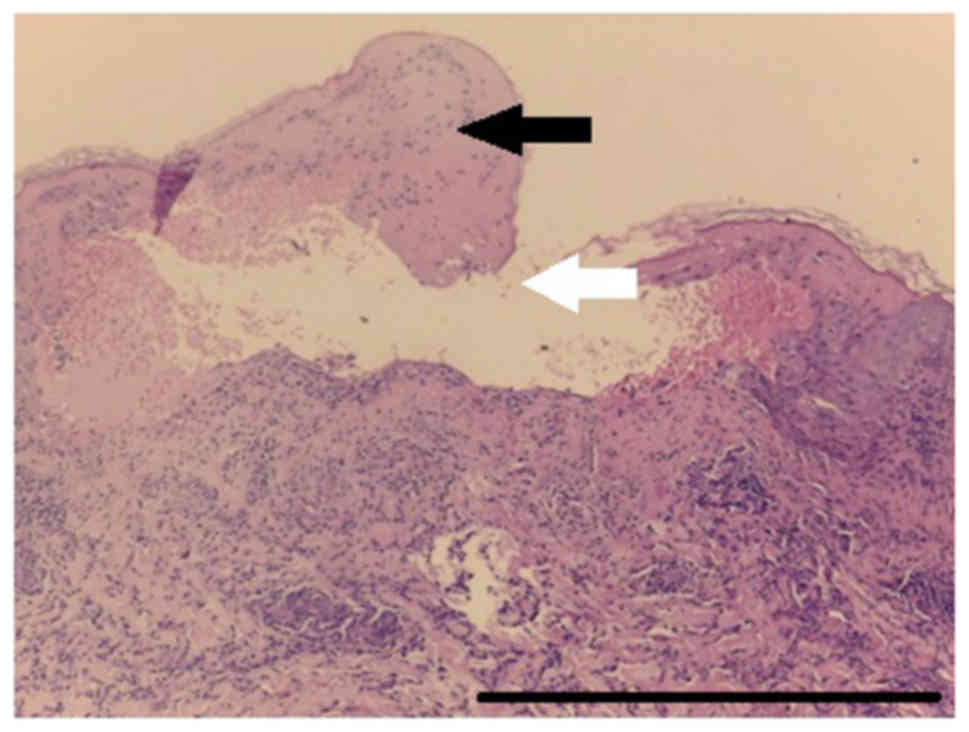
fixed bullous drug eruption. The result of the skin biopsy
employing hematoxylin and eosin staining (Figs. 4 and 5)
sustained the diagnosis of SJS following anti-TNF treatment. The
severity-of-illness score for toxic epidermal necrolysis (SCORTEN)
was 2.
A lymphoblastic transformation test (LTT; Synevo
Central Labs, Belro Medical S.A.) to certolizumab pegol was
negative. Patient management included supportive care and the dose
of systemic glucocorticoid was increased to 40 mg predenisone/day
with which a partial response to treatment was observed. Although
there was some remission of the lesion, the subsequent recurrence
required prolonged hospitalization for 4 weeks (May 11 to June 9,
2017). The patient experienced several episodes of recurrence of
lesions on attempt at lowering the dose of prednisone to 10 mg/day.
While this was not a life-threatening case at admission, the
recurrence of the skin lesion was a notable problem.
As glucocorticoid treatment was unable to attain
remission of the lesions at daily doses under 15 mg prednisone, a
second skin biopsy was performed that raised the suspicion of lupus
erythematosus.
Based on this biopsy, a further dermatological
evaluation raised the suspicion of lupus induced by TNF inhibitor
therapy (DIL).
Reintroducing methotrexate (stopped during the most
acute phase of skin lesions) had positive effect and the
progression of the patient was favorable with the disappearance of
cutaneous lesions, allowing a decrease of prednisone dose to 5 mg
daily. If the activity of RA increases, it should be proposed that
the patient undergoes rituximab treatment (anti lymphocyte B).
Discussion
Lupus induced by TNF inhibitors is among the most
frequent autoimmune adverse events of anti-TNF therapy (4–9). Patients
with RA and CD appear to be more affected than patients with
spondyloarthritis by this form of DIL (4–12).
Mucocutaneous manifestations, articular symptoms and general
manifestations are the most common manifestations, and patients may
develop positivity for antinuclear, antihistone and anti-dsDNA
antibodies (4–9). The emergence of anti-dsDNA antibodies is
more frequent in lupus induced by TNF inhibitors than in other
forms of DIL (4–7). Usually the major manifestations of
systemic lupus (nephritis, central nervous system involvement) are
rare, but certain patients may develop severe forms of
mucocutaneous manifestations (as in the presented patient)
mimicking severe allergic reactions or erythema multiforme
(4–7).
Expert opinion and the conclusions of the skin
biopsies may be misleading. Laboratory tests demonstrating the
occurrence of antinuclear, anti-histone and, most importantly,
anti-dsDNA antibodies may orientate the diagnosis to lupus induced
by TNF inhibitors. Discontinuation of the TNF inhibitor treatment
is key and in some cases sufficient to stop the symptoms (4–8). However,
in certain cases systemic glucocorticoid therapy and
immunosuppressive treatment are required as was the situation for
the presented patient.
In patients with RA who develop lupus induced by TNF
inhibitors, following termination of the treatment and management
of the lupus symptoms, in certain cases biological treatment is
required for the reactivation of RA, and the use of a different
class of biological treatment is a common decision (4,5).
The alternative diagnosis that was discussed for the
present patient was a form of SJS. SJS is a rare but severe
cutaneous adverse reaction related to a variety of medications
(sulfonamides, NSAIDs, antimalarials, anticonvulsants, allopurinol)
that predominantly involves the skin and mucous membranes (13–15). SJS
may be fatal and is considered a medical emergency associated with
significant morbidity and mortality (13). The extent of skin involvement is a
major prognostic factor (14). The
damage to the skin is considered to be mediated by cytotoxic T
lymphocytes and mononuclear cells that induce apoptosis in
keratinocytes expressing drug-derived antigens at their surface
(15).
Initial symptoms may be unspecific (fever, stinging
eyes and discomfort upon swallowing) and precede cutaneous
manifestations by a few days. The early skin lesions include
erythematous and livid macules, which may or may not be slightly
infiltrated, and have a tendency to rapidly coalescence. In the
second phase, hemorrhagic erosions and erythema typically develop,
followed by varying levels of epidermal detachment, which present
as blisters and areas of denuded skin. The diagnosis relies on
clinical symptoms and on histological examination revealing
widespread epidermal necrosis involving all layers (13–15).
Reports of SJS in patients treated with certolizumab
pegol, a recombinant Fab antibody fragment against TNF-α conjugated
to an ~40-kDa polyethylene glycol, are rare, but it seems to
particularly affect female patients with RA of ≥60 years of age who
have been taking the drug for <1 month (16).
If the SJS physiopathology remains unclear, a
specific immune response to one or more drugs is typically
involved, constituting a form of delayed-type hypersensitivity
(15). The LTT, which measures the
proliferation of T cells in response to a drug in vitro, has
indicated a sensitivity of 60–70% for patients allergic to β-lactam
antibiotics (17).
The earlier the causative drug is withdrawn, the
better the prognosis; while patients exposed to causative drugs
with long half-lives have an increased risk of mortality (18).
To standardize the evaluation of risk and prognosis,
the severity-of-illness score for toxic epidermal necrolysis
(SCORTEN) scale is now a widely used scoring system that evaluates
the following parameters: Age, malignancy, tachycardia, initial
body surface area of epidermal detachment, serum urea, glucose and
bicarbonate (19,20). In the case presented the SCORTEN was
2, associated with 12.1% mortality rate.
SJS related to administration of certolizumab pegol
is rare, and conversely, there are certain case reports that have
identified sustained efficacy of other anti-TNF therapy (infliximab
and etanercept) in the treatment of patients with severe epidermal
necrolysis (21,22), though there is a lack of data for
certolizumab.
It may be speculated that in the case presented, the
patient developed various autoantibodies (antinuclear, antihistone
and anti-dsDNA) under TNF-inhibitor treatment, but only following
SJS development (potentially also induced by TNF inhibitor), the
release of marked quantities of antigens from the mucocutaneous
lesions may have converted the subclinical form into a clinical
form of DIL.
At present, no treatment modality has been
established as a standard for these patients with DIL induced by
anti-TNF therapies; therefore, after stopping application of the
causative drug, therapy is primarily supportive and
anti-symptomatic for mild forms, while in moderate to severe forms,
systemic glucocorticoids and sometimes immunosuppressive drugs and
a multidisciplinary approach are required.
Acknowledgements
The authors are thankful to Professor Calin
Giurcaneanu and Associate Professor Roxana Bumbacea at the
Dermatology and Allergology Department of ‘Elias’ Hospital
(Bucharest, Romania) and to Professor Olga Simionescu at the
Dermatology Department of Colentina Hospital (Bucharest, Romania)
for their dermatologic and allergologic evaluations of the patient
and skin biopsies.
Funding
No funding was received.
Availability of data and materials
All data described in the current report are
available from the corresponding author on reasonable request.
Authors' contributions
VCB, MG and ARB were responsible for clinical
evaluation and therapeutic management of the patient, and aided the
literature search. AB and CII were responsible for the processing
and histological assessment of skin biopsies. SMB performed
cardiological evaluation and aided the literature search. MB
provided a secondary clinical opinion, oversaw manuscript writing
and provided corrections to the manuscript and figures, and aided
the literature search.
Ethics approval and consent to
participate
The report was approved by the Ethics Committee of
‘Sfanta Maria’ Hospital, Bucharest, Romania (approval no. 5084/12;
March 2018).
Consent for publication
The patient provided written consent for case
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Simsek I: TNF inhibitors for rheumatoid
arthritis - a year in review. Bull NYU Hosp Jt Dis. 69:220–224.
2011.PubMed/NCBI
|
|
2
|
Ma X and Xu S: TNF inhibitor therapy for
rheumatoid arthritis. Biomed Rep. 1:177–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smolen JS, Landewé R, Breedveld FC, Buch
M, Burmester G, Dougados M, Emery P, Gaujoux-Viala C, Gossec L, Nam
J, et al: EULAR recommendations for the management of rheumatoid
arthritis with synthetic and biological disease-modifying
antirheumatic drugs: 2013 update. Ann Rheum Dis. 73:492–509. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perez-Alvarez R, Pérez-de-Lis M and
Ramos-Casals M; BIOGEAS study group: Biologics-induced autoimmune
diseases. Curr Opin Rheumatol. 25:56–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vaz JL, Andrade CA, Pereira AC, Martins
Mde F and Levy RA: Systematic review of infliximab-induced
autoantibodies and systemic lupus erythematosus. Rev Bras
Rheumatol. 53:358–364. 2013.(In English, Portuguese).
|
|
6
|
Katz U and Zandman-Goddard G: Drug-induced
lupus: An update. Autoimmun Rev. 10:46–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramos-Casals M, Brito-Zerón P, Muñoz S,
Soria N, Galiana D, Bertolaccini L, Cuadrado MJ and Khamashta MA:
Autoimmune diseases induced by TNF-targeted therapies: Analysis of
233 cases. Medicine (Baltimore). 86:242–251. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Atzeni F, Talotta R, Salaffi F, Cassinotti
A, Varisco V, Battellino M, Ardizzone S, Pace F and Sarzi-Puttini
P: Immunogenicity and autoimmunity during anti-TNF therapy.
Autoimmun Rev. 12:703–708. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wetter DA and Davis MDP: Lupus-like
syndrome attributable to anti-tumor necrosis factor α therapy in 14
patients during an 8-year period at Mayo Clinic. Mayo Clin Proc.
84:979–984. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sampaio-Barros PD and van der
Horst-Bruinsma IE: Adverse effects of TNF inhibitors in SpA: Are
they different from RA? Best Pract Res Clin Rheumatol. 28:747–763.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mocci G, Marzo M, Papa A, Armuzzi A and
Guidi L: Dermatological adverse reactions during anti-TNF
treatments: Focus on inflammatory bowel disease. J Crohn's Colitis.
7:769–779. 2013. View Article : Google Scholar
|
|
12
|
Oter-López B, Llamas-Velasco M,
Sánchez-Pérez J and Dauden E: Induction of autoantibodies and
autoimmune diseases in patients with psoriasis receiving tumor
necrosis factor inhibitors. Actas Dermosifiliogr. 108:445–456.
2017.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mockenhaupt M: Stevens-Johnson syndrome
and toxic epidermal necrolysis: Clinical patterns, diagnostic
considerations, etiology, and therapeutic management. Semin Cutan
Med Surg. 33:10–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Palmieri TL, Greenhalgh DG, Saffle JR,
Spence RJ, Peck MD, Jeng JC, Mozingo DW, Yowler CJ, Sheridan RL,
Ahrenholz DH, et al: A multicenter review of toxic epidermal
necrolysis treated in U.S. burn centers at the end of the twentieth
century. J Burn Care Rehabil. 23:87–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fritsch PO and Sidoroff A: Drug-induced
Stevens-Johnson syndrome/toxic epidermal necrolysis. Am J Clin
Dermatol. 1:349–360. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flendrie M, Vissers WH, Creemers MC, de
Jong EM, van de Kerkhof PC and van Riel PL: Dermatological
conditions during TNF-alpha-blocking therapy in patients with
rheumatoid arthritis: A prospective study. Arthritis Res Ther.
7:R666–R676. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pichler WJ and Tilch J: The lymphocyte
transformation test in the diagnosis of drug hypersensitivity.
Allergy. 59:809–820. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garcia-Doval I, LeCleach L, Bocquet H,
Otero XL and Roujeau JC: Toxic epidermal necrolysis and
Stevens-Johnson syndrome: Does early withdrawal of causative drugs
decrease the risk of death? Arch Dermatol. 136:323–327. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bastuji-Garin S, Fouchard N, Bertocchi M,
Roujeau JC, Revuz J and Wolkenstein P: SCORTEN: A
severity-of-illness score for toxic epidermal necrolysis. J Invest
Dermatol. 115:149–153. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schneider JA and Cohen PR: Stevens-Johnson
syndrome and toxic epidermal necrolysis: A concise review with a
comprehensive summary of therapeutic interventions emphasizing
supportive measures. Adv Ther. 34:1235–1244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hunger RE, Hunziker T, Buettiker U,
Braathen LR and Yawalkar N: Rapid resolution of toxic epidermal
necrolysis with anti-TNF-alpha treatment. J Allergy Clin Immunol.
116:923–924. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meiss F, Helmbold P, Meykadeh N, Gaber G,
Marsch WCh and Fischer M: Overlap of acute generalized
exanthematous pustulosis and toxic epidermal necrolysis: response
to antitumour necrosis factor-alpha antibody infliximab: report of
three cases. J Eur Acad Dermatol Venereol. 21:717–719.
2007.PubMed/NCBI
|