Introduction
Several studies have reported on the concentration
of gingerols in fresh ginger (Zingiber officinale var.
Roscoe) rhizomes, for instance, the methanol extract of Z.
officinale var. Roscoe rhizome cultivated in Hawaii contained
6-, 8- and 10-gingerol at concentrations of 2,100, 288 and 533
µg/g, respectively (1); the methylene
chloride extract of Z. officinale var. Roscoe rhizome
cultivated in America yielded 880, 93 and 120 µg/g of 6-, 8- and
10-gingerol, respectively (2); and
the fresh rhizome of Z. officinale var. Roscoe cultivated in
Taiwan contained 806 µg/g of 6-gingerol (3). Furthermore, phytochemical screening of
the chloroform extract of Z. officinale var. Roscoe rhizome
cultivated in Pakistan has given positive results on the presence
of alkaloid, phlobotannins, flavonoids, glycosides, saponins,
tannin and terpenoids, while indicating the absence of steroids
(4). Alkaloids, carbohydrates,
glycosides, proteins, saponins, steroids, flavonoids and terpenoids
has been identified in Z. officinale var. Roscoe, and
phenolic compounds including gingerol and shogaol (5–7), while
reducing sugars, tannins, oils and acid compounds were absent.
Similarly, results of proximate analysis of the rhizome have
indicated mostly carbohydrates (71.46%) and crude protein (8.83%)
with a small crude fibre content of 0.92% (5).
A comparative study of Z. officinale var.
Roscoe pulp and peel demonstrated that the hydro-alcoholic extract
of the peel exhibited marked inhibition of the growth of colon
cancer cells on MTT assay, while the pulp extract exhibited high
anti-inflammatory and antioxidant activities, allegedly due to
differing polyphenolic content and lipophilic composition (8). Data on the effects of Z.
officinale var. Roscoe have been reviewed, and it has been
concluded that the rhizome of this plant has potential as an
anti-inflammatory and anti-oxidative source, nonetheless further
research has been suggested prior to confirming its efficacy
(9). The medicinal properties of
Z. officinale var. Roscoe may be due to the presence of
gingerol, paradol and shogaols, among other compounds (10).
Gingerols are homologues of
1-(3-methoxy-4-hydroxyphenyl)-3-keto-5-hydroxyhexane, shogaols are
dehydration products of the gingerols, and paradols are β-ketone
hydroxyl deoxygenation products of gingerols (11). In previous studies Z.
officinale var. Roscoe has exhibited anti-inflammatory activity
(8,12–16).
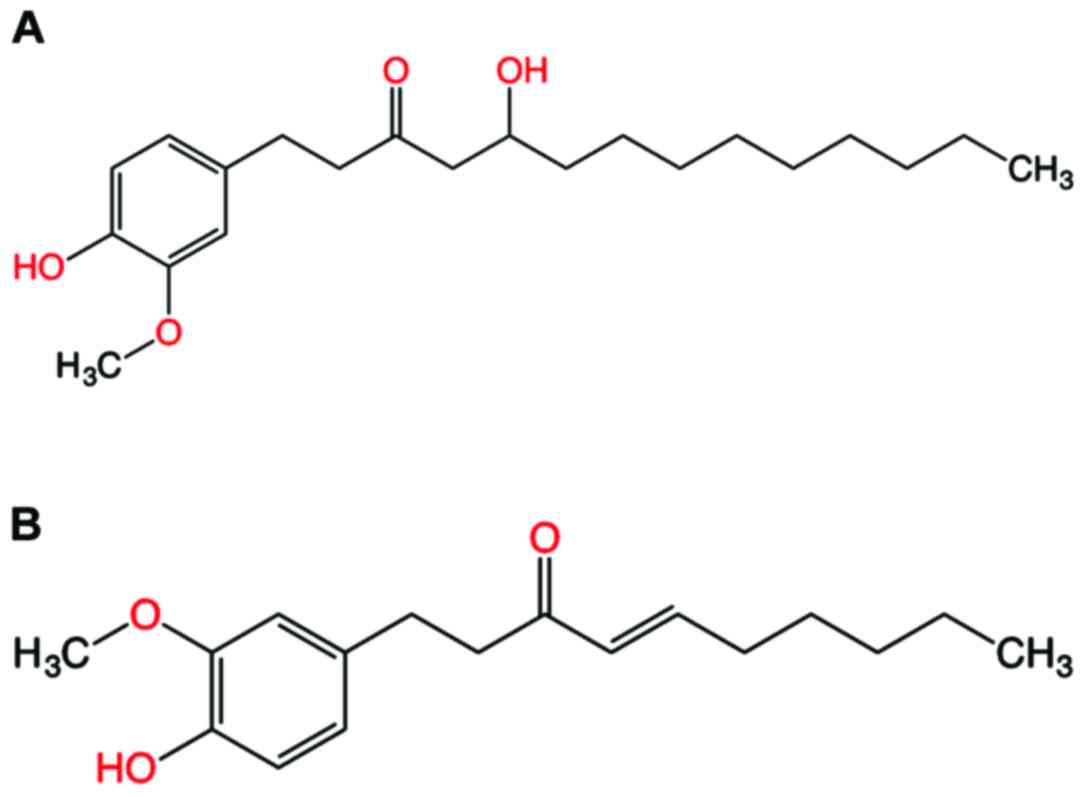
10-gingerol (Fig. 1A) has been
reported to exhibit the highest anti-inflammatory and anti-oxidant
activities compared with those of other gingerols (17). Other studies on 6-gingerol have
confirmed its inhibition of nitric oxide production in activated
J774.1 mouse macrophages and prevention of peroxynitrite-induced
oxidation and nitration reactions (18). This compound may also inhibit
cyclooxygenase (COX)-2 expression by blocking the activation of p38
mitogen-activated protein kinase and nuclear factor-κB in phorbol
ester-stimulated mouse skin (19).
However, studies on the effects of red ginger (Z. officinale
var. Rubrum) are limited.
Previous study by Fikri et al (20) concluded that the hot water extract of
Z. officinale var. Rubrum rhizome inhibited the rate of
prostaglandin production in vitro. Furthermore, a
computational study of gingerol and shogaol against COX enzymes
reported that 6-gingerol and 6-shogaol were preferential COX-2
inhibitors and therefore potential candidates for development into
anti-inflammatory drugs (21).
Pharmacokinetic studies of the dry extract of ginger rhizomes (at
doses of 100 mg to 2 g) in the plasma of healthy American
volunteers have been conducted (22,23).
However, there is a lack of reports on red ginger. Thus, the
present work studied the pharmacokinetics of 10-gingerol (Fig. 1A) and 6-shogaol (Fig. 1B) in the plasma of healthy Indonesian
volunteers treated with a single dose (2 g) of red ginger
suspension. The aims of this study were to: i) Determine if red
ginger suspension is absorbed and biotransformed in humans; and ii)
assess the human pharmacokinetics of 10-gingerol and 6-shogaol.
Materials and methods
Instruments
Instruments used in the study were a liquid
chromatography-mass spectrometry (LC-MS) XEVO-QTOF MS
(Waters-MassLynx 4.1 SCN719; PT Kromtekindo Utama, Jakarta,
Indonesia) equipped with an RP-C18 column (2.1 × 100 mm), a
UV-visible double beam spectrophotometer (Specord 200; Analitik
Jena AG, Jena, Germany), a digital analytical balance [Ohaus
Pioneer; Ohaus Instruments (Shanghai) Co., Ltd., Shanghai, China],
an Eppendorf Centrifuge 5424 R, a vortex mixer (Cole-Parmer, Vernon
Hills, IL, USA), a dipotassium ethylenediamine tetraacetic acid
(K2EDTA) tube (BD Vacutainer; BD Biosciences, Franklin Lakes, NJ,
USA) and chemical glasswares.
Chemicals and plant materials
10-gingerol of standard 98% purity (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany; CAS no. 23513-15-7) and 6-shogaol
of standard 98% purity (Sigma-Aldrich; CAS no. 555-66-8) were
purchased from Quartiz Indonesia (PT Indogen Intertama, Jakarta,
Indonesia); methanol hypergrade for LC-MS (LiChrosolv®;
CAS no. 67-56-1) and acetonitrile gradient grade for liquid
chromatography (LiChrosolv® CAS no. 75-05-08) were
purchased from the Central Laboratory of Padjadjaran University
(Jatinangor, West Java, Indonesia); and 1 kg of fresh rhizome of
Z. officinale var. Rubrum was purchased from the Research
Institute for Spices and Medicinal Plants (Balittro) Manoko Lembang
(Bandung, Indonesia). The fresh rhizome was taxonomically
identified at the Laboratory of Plant Taxonomy, Department of
Biology, Faculty of Mathematics and Natural Sciences, Padjadjaran
University, and the voucher specimen (no. 415/HB/08/2017) was
retained in our laboratory for future reference.
Preparation of red ginger (Zingiber
officinale var. Rubrum) suspension
The 1 kg of fresh rhizome of Z. officinale
var. Rubrum was thin-sliced (1–2 mm) and dried in a dehydrator at
50°C for 4 h. The dried rhizome was ground and each portion per
subject contained 2 g of the powder dissolved in 15 ml hot
distilled water (70°C).
Identification of 10-gingerol and
6-shogaol in Z. officinale var. Rubrum suspension
Identification of 10-gingerol and 6-shogaol in Z.
officinale var. Rubrum suspension was performed by dissolving
the suspension in 15 ml methanol hypergrade. The mixture was
vortexed and centrifuged at 20,440 × g at room temperature for 15
min. The supernatant was scanned at 200–380 nm in a double beam UV
spectrophotometer against the methanol hypergrade its maximum
wavelength (λmax) was compared with those of 10-gingerol and
6-shogaol.
Preparation of 10-gingerol and
6-shogaol standard solutions
A total of 2.5 mg of each of the 10-gingerol and
6-shogaol standards was dissolved in 100 ml methanol hypergrade in
a volumetric flask (25 µg/ml). The solutions were scanned at
200–380 nm in the double beam UV spectrophotometer against the
methanol hypergrade to obtain the λmax's of 10-gingerol and
6-shogaol. Various concentrations were prepared by diluting the
standard solution (Tables I and
II) (24).
 | Table I.Preparation of 10-gingerol standard
solutions. |
Table I.
Preparation of 10-gingerol standard
solutions.
| Blank plasma, µl | 10-gingerol, µl | Acetonitrile, µl | Final concentration,
ng/ml |
|---|
| 200 | – | 800.0 | Blank |
| 200 |
2.5 | 797.5 |
2.5 |
| 200 |
5.0 | 795.0 |
5.0 |
| 200 | 10.0 | 790.0 | 10.0 |
| 200 | 15.0 | 785.0 | 15.0 |
| 200 | 20.0 | 780.0 | 20.0 |
| 200 | 25.0 | 775.0 | 25.0 |
 | Table II.Preparation of 6-shogaol standard
solutions. |
Table II.
Preparation of 6-shogaol standard
solutions.
| Blank plasma,
µl | 6-shogaol, µl | Acetonitrile,
µl | Final
concentration, ng/ml |
|---|
| 200 | – | 800.0 | Blank |
| 200 |
2.5 | 797.5 |
2.5 |
| 200 |
5.0 | 795.0 |
5.0 |
| 200 | 10.0 | 790.0 | 10.0 |
| 200 | 15.0 | 785.0 | 15.0 |
| 200 | 20.0 | 780.0 | 20.0 |
| 200 | 25.0 | 775.0 | 25.0 |
Optimization of LC-MS analytical
conditions
The 10-gingerol and 6-shogaol standard solutions
(2.5, 5.0, 10.0, 15.0, 20.0 and 25.0 ng/ml) were filtered using
Millipore membranes (pore size 0.2 µm; EMD Millipore, Billerica,
MA, USA) and injected into the LC-MS RP-C18 column. The optimized
conditions of the LC-MS are presented in Table III.
 | Table III.LC-MS optimum analytical conditions
for 10-gingerol and 6-shogaol (N2 gas temperature 350°C;
drying N2 gas flow rate 10 l/min; nebulizer pressure 50
psi). |
Table III.
LC-MS optimum analytical conditions
for 10-gingerol and 6-shogaol (N2 gas temperature 350°C;
drying N2 gas flow rate 10 l/min; nebulizer pressure 50
psi).
| LC-MS
parameter | Setting
condition |
|---|
| Merk/Type | Waters Xevo QTof
-MassLynx 4.1 SCN719 |
| Column | BEH Shield RP18 Ø
1.7 µm; 2.1 × 100 mm |
| Mobile phase | Phase A: 0.1% (v/v)
formic acid in water; phase B: acetonitrile |
| Time setting (ratio
phase A:phase B) | 0-6th min (38:62);
6–9th min (0:100); 9–15th min (38:62) |
| Flow rate;
retention time | 300 µl/min; 15
min |
| Mass spectrometer
mode/ion detection | Electrospray
ionization (ES+) ionization/multiple reaction monitoring mode |
Validation of analytical method
Selectivity
The selectivity of the method was investigated by
comparing the chromatogram of extracted blank plasma (3 ml)
obtained from six randomly selected participants described below,
each spiked with 25.0 ng/ml 6-shogaol.
Linearity
Linearity of the standard curves was calculated
using human plasma spiked with various concentrations (2.5, 5.0,
10.0, 15.0, 20.0 and 25.0 ng/ml) of 10-gingerol and 6-shogaol. The
concentrations were plotted against the area under curves (AUCs) of
the 10-gingerol and 6-shogaol chromatograms. The regression line
(y=ax+b) and the coefficient of correlation (r) of
the data were calculated.
Accuracy and precision
The accuracy and precision of the method were
evaluated by analyzing 10-gingerol and 6-shogaol in three sets of
quality control samples [lower concentration of quality control
(LCQC)=2.5 ng/ml; medium concentration of quality control (MCQC)=15
ng/ml; and higher concentration of quality control (HCQC)=25 ng/ml
each] within the same day. Each solution was injected into the
column in three replicates. The percentage recovery and standard
deviation (SD) were calculated to ascertain accuracy and precision
of the analytical method, respectively.
Limit of detection (LOD) and limit of
quantification (LOQ)
The LOD and LOQ were calculated by using:
LOD=(3×SD)/b; and LOQ=(10×SD)/b where b is the y-intercept
of the linear regression curve (25).
Subjects and treatment
A total of 21 participants (8 females and 13 males)
aged 21–30 years, with body mass indices (BMIs) of 18–25,
non-smokers and/or alcohol drinkers, were solicited by
advertisement from May to September 2017. All participants were
confirmed to be healthy by physical examination at Padjadjaran
Clinic, Padjadjaran University (Jatinangor, Indonesia; Table IV). Two of the participants (R14 and
R20) had low BP (90/60). Initially both were included in the study,
but one of them (R14) vomited during the treatment, and was
excluded. One of the participants (R21) was menstruating on the day
of preliminary health screening and was excluded. The remaining 19
subjects continued the study. All study procedures were
administered at the Faculty of Pharmacy, Padjadjaran University
following collection of signed written informed consent according
to the principles of the Declaration of Helsinki. The subject
protocols and treatment were approved by the Research Ethics
Committee of Padjadjaran University (approval nos.
1211/UN6.C.10/PN/2017 for 10-gingerol and 924/UN6.C.10/PN/2017 for
6-shogaol). The subjects were asked to avoid all foods containing
ginger within 7 days prior to the project and completed a food
checklist to verify that they were not consuming any ginger-rich
food or beverages. All subjects received the Sundanese standard
meal (one portion of white rice equivalent to 240 cal, 100 g of
fried chicken/tofu equivalent to 110–120 cal, hot tea equivalent to
70 cal, and a banana/orange 3 times/day at 24 h pre-study and for
breakfast on the day of the study.
 | Table IV.Preliminary health screening of the
participants. |
Table IV.
Preliminary health screening of the
participants.
|
|
| Health status |
|---|
|
|
|
|
|---|
| Subject code | Age, years | Height, cm | Weight, kg | Blood type | BP, mmHg | Result |
|---|
| R1 | 22 | 160 | 63 | A | 120/80 | Healthy |
| R2 | 21 | 164 | 61 | O | 120/80 | Healthy |
| R3 | 21 | 176 | 72 | AB | 110/70 | Healthy |
| R4 | 22 | 164 | 64 | B | 110/70 | Healthy |
| R5 | 22 | 175 | 63 | O | 120/80 | Healthy |
| R6 | 22 | 165 | 55 | A | 110/80 | Healthy |
| R7 | 22 | 172 | 56 | A | 110/70 | Healthy |
| R8 | 22 | 165.5 | 66 | O | 120/80 | Healthy |
| R9 | 22 | 164 | 55 | O | 100/60 | Healthy |
| R10 | 25 | 169 | 60 | B | 120/80 | Healthy |
| R11 | 26 | 168 | 65 | O | 120/80 | Healthy |
| R12 | 30 | 161 | 58 | A | 100/70 | Healthy |
| R13 | 22 | 164 | 54 | A | 100/70 | Healthy |
| R14 | 23 | 157 | 65 | O | 90/60 |
Excludeda |
| R15 | 22 |
160.5 | 49 | AB | 100/70 | Healthy |
| R16 | 22 | 155 | 48 | AB | 120/80 | Healthy |
| R17 | 25 | 157 | 48 | O | 100/70 | Healthy |
| R18 | 25 | 145 | 45 | O | 100/80 | Healthy |
| R19 | 23 | 159 | 51 | O | 100/70 | Healthy |
| R20 | 22 | 155 | 44 | A | 90/60 | Healthy |
| R21 | 23 | 157 | 62 | B | 110/80 |
Excludedb |
The red ginger suspension was administered the
subsequent morning after the standard breakfast had been received.
Each subject was given one portion of red ginger suspension (2 g in
15 ml of water) as a single oral dose, and 3 ml blood samples were
taken from the subjects at baseline (0 min), 30, 60, 90, 120 and
180 min. The blood was put into the K2EDTA vacutainer tubes. Plasma
was separated by centrifuging at 20,440 × g for 15 min at room
temperature and kept at −20°C until assayed.
Pharmacokinetics of 10-gingerol and
6-shogaol
Analysis of 10-gingerol and 6-shogaol was performed
by dissolving 200 µl of the subjects' plasma in 800 µl
acetonitrile. The mixture was vortexed and centrifuged at 20,440 ×
g for 15 min at room temperature. The supernatant was filtered
using Millipore membrane (pore size 0.2 µm) and injected into the
RP-C18 column embedded in the LC-MS XEVO-QTOFMS.
Results and Discussion
Identification of 10-gingerol and
6-shogaol in Z. officinale var. Rubrum suspension
Gingerol and/or shogaol were indicated to be present
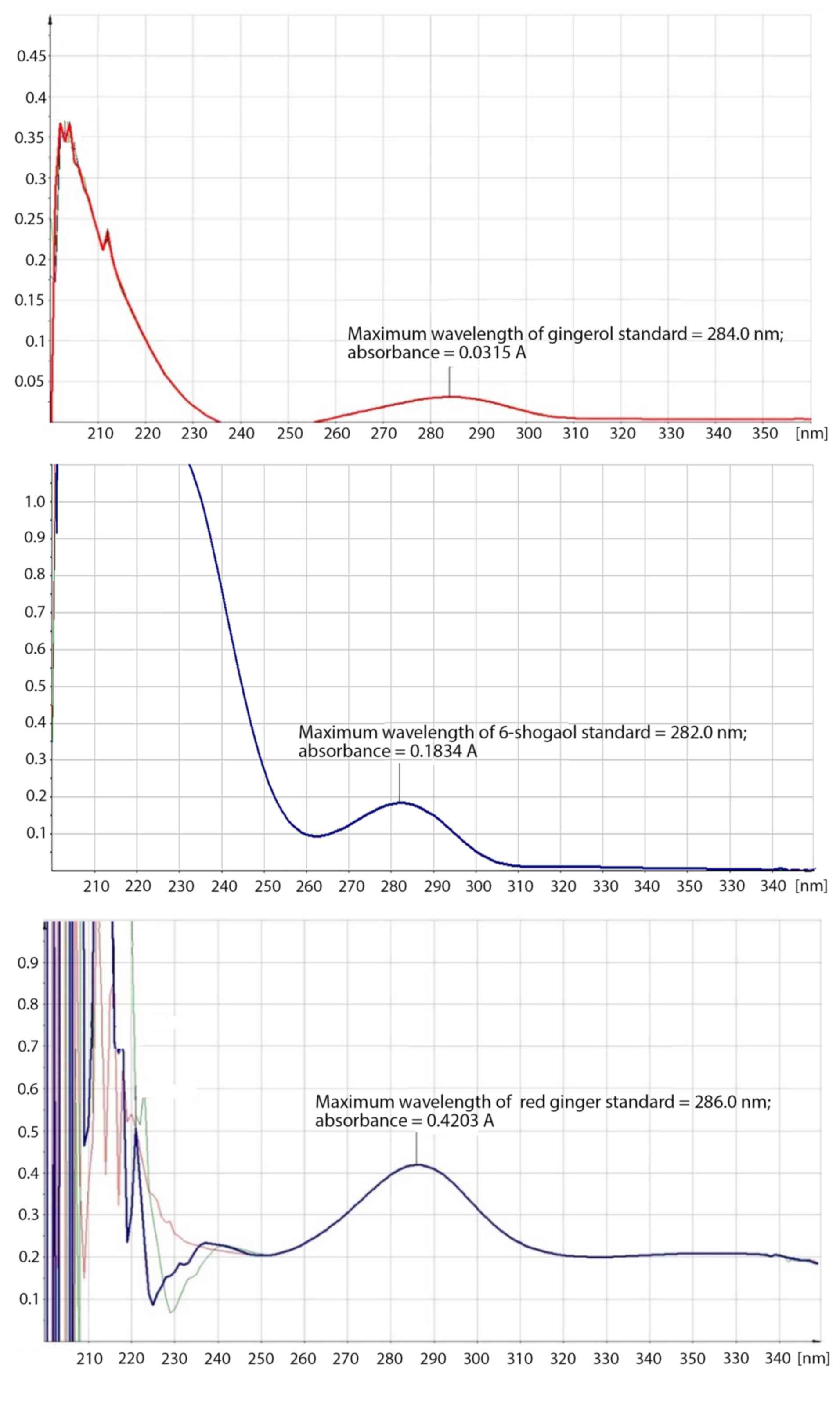
in the red ginger suspension by the λmax of red ginger suspension
in methanol (λmax=286 nm) which was similar to those of 10-gingerol
(λmax=284 nm) and 6-shogaol (λmax=282 nm; Fig. 2).
Ethanol can extract gingerols more effectively than
water; moreover, hot water is reportedly more effective than cold
water (26). Ghasemzadeh et al
(27) when working on the
optimization procedure for the extraction of 6-gingerol and
6-shogaol (focusing on temperature 50–80°C and time 2–4 h) reported
that increasing the extraction temperature (up to 76.9°C) and time
(3. h) induced the highest yield of 6-gingerol (2.74 mg/g dry
weight) and 6-shogaol (1.59 mg/g dry weight) from Z.
officinale var. Rubrum Theilade (27). The present work employed hot distilled
water (70°C) to extract 2 g of the red ginger (Z. officinale
var. Rubrum) powder.
Validation of analytical method
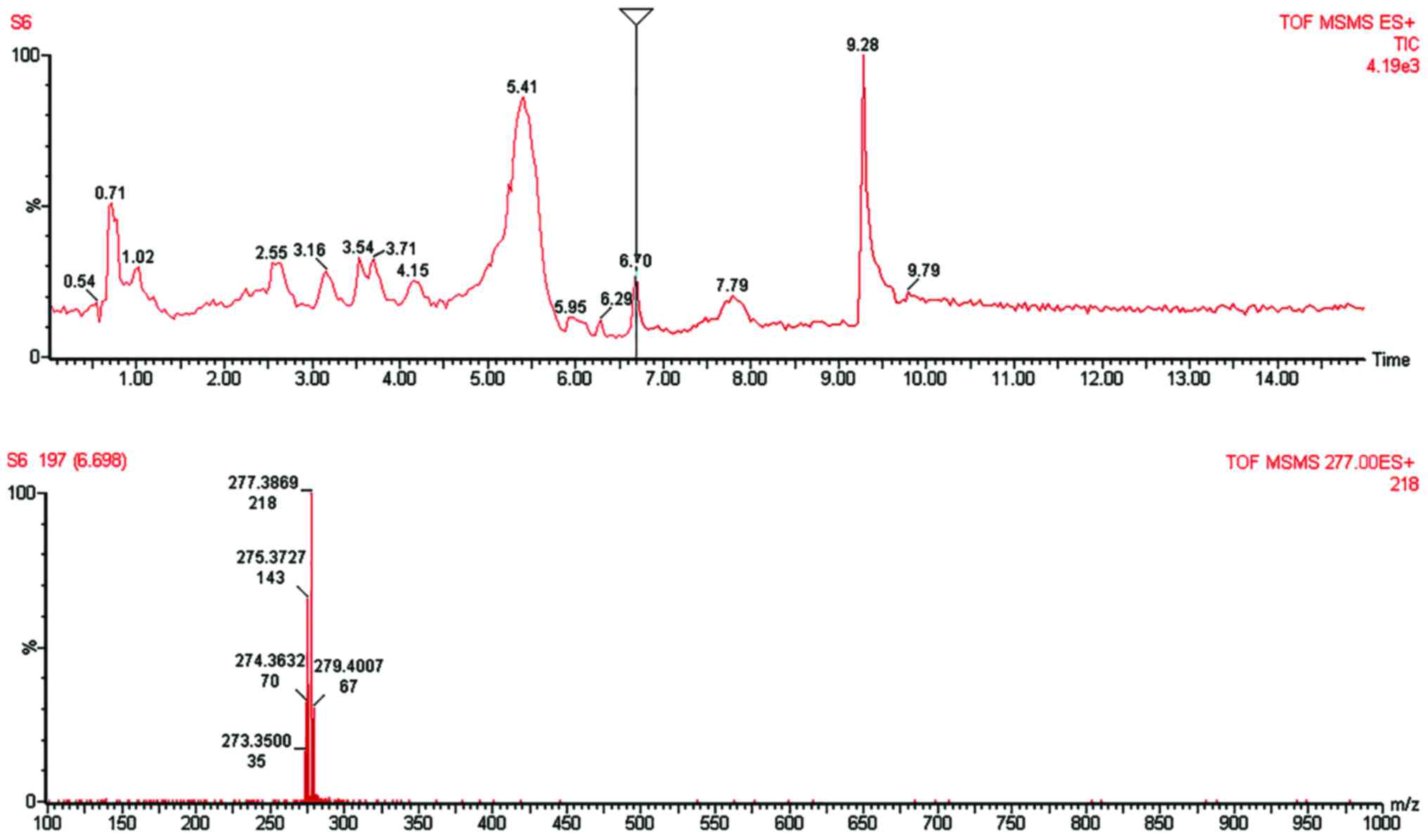
The LC-MS analytical method was selective for both
6-shogaol (Fig. 3) and 10-gingerol
(Fig. 4) as proven by the respective
chromatogram peaks that were free of interference at the retention
time of 6-shogaol (6.70 min) and 10-gingerol (8.26 min). The MS
spectrum confirmed and correlated the chromatogram peak with the
mass-to-charge ratio (m/z). Our MS spectra indicated the base peak
of 6-shogaol [M+1] (m/z=277.3869; MW 6-shogaol=276.376); and the
base peak of 10-gingerol [M+1] (m/z=351.2959; MW
10-gingerol=350.4923).
Linearity of the standard curves was constructed
using human plasma spiked with various concentrations (2.5, 5.0,
10.0, 15.0, 20.0 and 25.0 ng/ml) of 10-gingerol and 6-shogaol
(Fig. 5). The LCQC (2.5 ng/ml), MCQC
(15.0 ng/ml) and HCQC (25.0 ng/ml) of the ginger analytes were
assayed on an intra-day basis to determine the accuracy and
precision of the method. The intra-day values for the SD ranged
from 0.16–0.69 for 10-gingerol and 0.74–1.49 for 6-shogaol, whereas
the recovery of the analytes was 100.64–104.44% for 10-gingerol and
103.40–104.22% for 6-shogaol. The data (plot of AUC of the
chromatogram peak against the concentration of
10-gingerol/6-shogaol; not shown) correlated as indicated by r
values >0.99 (Table V).
 | Table V.Validation of the 10-gingerol and
6-shogaol analytical method. |
Table V.
Validation of the 10-gingerol and
6-shogaol analytical method.
| Concentration of
analyte, ng/ml | Mean concentration,
ng/ml | Standard
deviation | Recovery, % | Coefficient of
correlation (r) | Slope |
|---|
| 10-gingerol |
|
2.5 |
2.53 | 0.16 | 101.28 |
|
|
|
15.0 | 15.67 | 0.57 | 104.44 | 0.9991 | 6.3195 |
|
25.0 | 26.16 | 0.69 | 100.64 |
|
|
| 6-shogaol |
|
2.5 |
2.59 | 0.74 | 103.40 |
|
|
|
15.0 | 15.63 | 1.49 | 104.22 | 0.9987 | 2.7070 |
|
25.0 | 26.04 | 0.78 | 104.14 |
|
|
The LODs in human plasma for 10-gingerol and
6-shogaol were 1.31 and 1.51 ng/ml, respectively, and the LOQs were
4.36 and 5.03 ng/ml, respectively.
Characteristics of the subjects
All 19 subjects who completed the study were proven
healthy by a physical examination upon enrollment (Table IV); however, 3–6 months prior to the
start of the study, several subjects had suffered from gastritis
(1/19; 5.26%), allergy (7/19; 3.68%; cold urticaria and/or seafood
allergy), joint arthritis (1/19; 5.26%) and dengue hemorrhagic
fever (1/19; 5.26%). The subjects also confirmed their weekly
exercise as jogging and walking (7/19; 3.68%), football (2/19;
10.53%) and badminton (1/19; 5.26%).
Pharmacokinetics of 10-gingerol and
6-shogaol
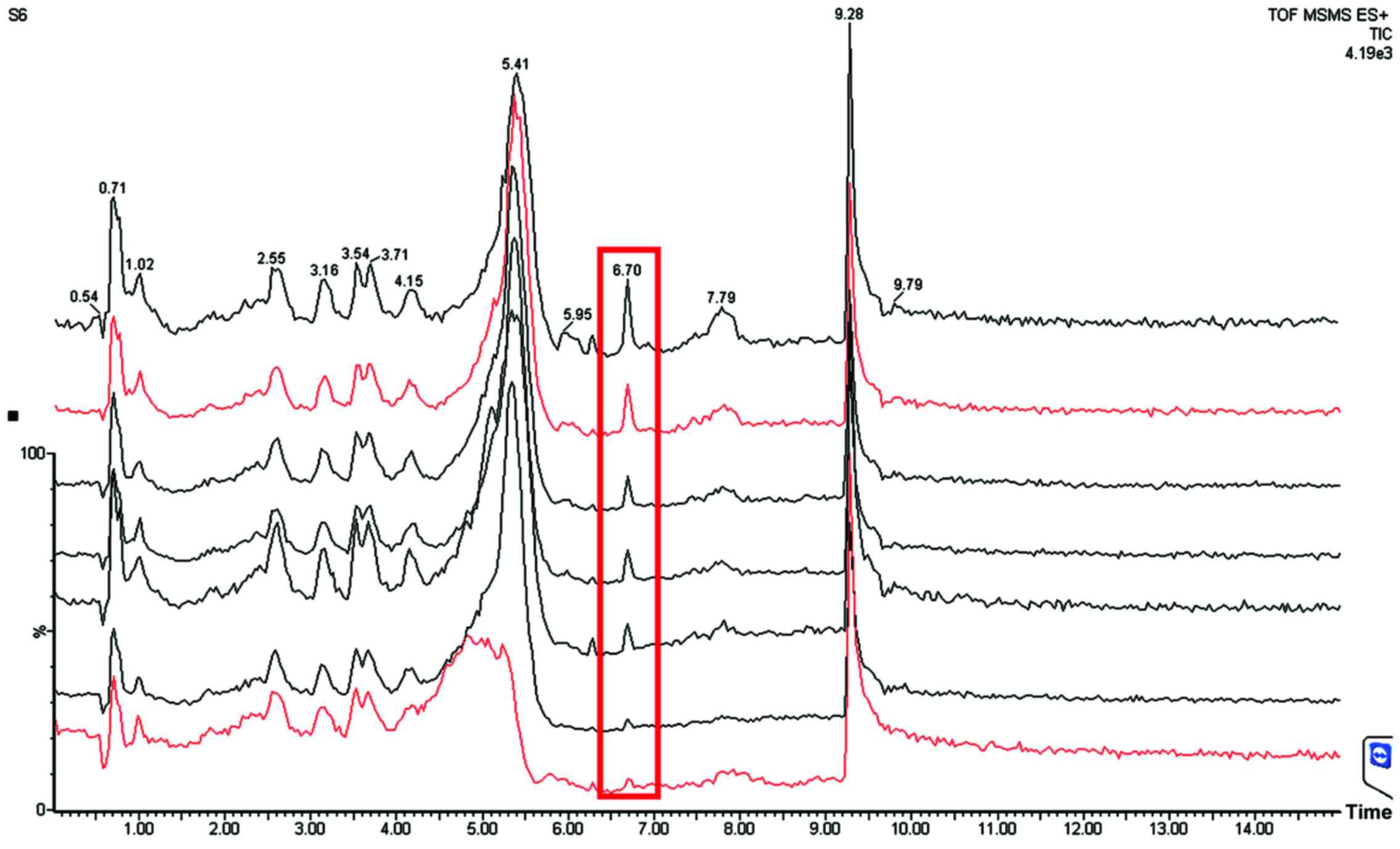
10-gingerol and 6-shogaol could be quantified in the
plasma of healthy subjects treated with a single dose of red ginger
suspension. They were eluted at 6.80 min (6-shogaol) and 8.0 min
(10-gingerol; Fig. 6), compared with
the pure compounds (for 6-shogaol, 6.70 min and for 10-gingerol,
8.26 min; Figs. 3 and 4), respectively. There was a slight
difference of elution time between the standards (pure 6-shogaol
and 10-gingerol compounds) and that of red ginger suspension due to
different conditions; the standards were spiked in vitro
into human plasma, while the red ginger suspension was administered
orally and taken ex vivo from the subjects' blood. The
pharmacokinetic profile of each of the red ginger compounds is
presented in Table VI.
 | Table VI.Pharmacokinetic profile of
10-gingerol and 6-shogaol in the plasma of healthy subjects treated
with a single dose of red ginger suspension. |
Table VI.
Pharmacokinetic profile of
10-gingerol and 6-shogaol in the plasma of healthy subjects treated
with a single dose of red ginger suspension.
| Parameter
(n=19) | 10-gingerol | 6-shogaol |
|---|
| AUC, ng/ml/min |
948.750 |
268.140 |
| Cmax, ng/ml |
160.49 |
453.40 |
| Tmax, min | 38 | 30 |
| T½ elimination,
min | 336 | 149 |
According to elimination half-lives (T½),
10-gingerol and 6-shogaol were eliminated from the plasma at 336
and 149 min, respectively (Table
VI). The maximum plasma concentration (Cmax) of 10-gingerol
(160.49 ng/ml) was lower than that of 6-shogaol (453.40 ng/ml;
Table VI).
These findings were different to those of Zick et
al (22) who studied the
pharmacokinetic profile of 6-, 8- and 10-gingerol and 6-shogaol in
dry extract of ginger at doses of 100 mg to 2 g in 27 healthy
American participants (9 males, 22 females, mostly Caucasians).
They identified that no free 6-, 8-, 10-gingerol or 6-shogaol was
present, but 6-, 8- and 10-gingerol and 6-shogaol glucuronides were
detected. Furthermore, in the plasma of healthy Caucasians, the
concentration of 10-gingerol has been quantified higher than that
of 6-shogaol (23).
The present study used a single dose (2 g) of red
ginger (Z. officinale var. Rubrum) suspension, administered
orally to 19 healthy Indonesian participants (13 males and 6
females). Both 10-gingerol and 6-shogaol could be detected and
quantified. In the plasma of the healthy Indonesians, 10-gingerol
was quantified to a lower level than 6-shogaol.
10-gingerol and 6-shogaol exhibited slower
elimination in Indonesian subjects compared with that in Caucasians
(T½ elimination <120 min). In the current study, no serious
adverse effects were reported following ingestion of the red ginger
suspension, though several female subjects complained of the potent
pungent odor and spicy taste that caused stomach discomfort. These
mild adverse effects were consistent with those reported by Zick
et al (22) and in a previous
review study by Chrubasik et al (28), who documented that the majority of the
disadvantages were transient gastrointestinal reactions, such as
gas and bloating.
In conclusion, 10-gingerol and 6-shogaol were
absorbed after per oral single dose of red ginger suspension and
could be quantified in the plasma of healthy Indonesian subjects.
The Cmax of 10-gingerol (160.49 ng/ml) was lower than that of
6-shogaol (453.40 ng/ml). Notably, the two red ginger analytes
exhibited relatively slow elimination half-lives. In the present
trial, no serious adverse effects were reported following ingestion
of the red ginger suspension, but several female subjects
complained about the potent pungent odor and spicy taste that
caused stomach discomfort. Overall, the present pharmacokinetic
findings of 10-gingerol and 6-shogaol in Indonesian subjects
confirmed that different ethnicities may contribute to different
pharmacokinetic profiles. Identification of these differences may
lead to personalized-medicine, and thus contribute in a clinical
context, particularly in the discovery and development of
anti-inflammatory drugs.
Acknowledgements
The authors would like to thank Professor Tri
Hanggono Achmad, Rector of Universitas Padjadjaran (West Java,
Indonesia) for facilitating and supporting this research project.
The present work was conducted in the framework of the master
theses of DMS and RDS at the Faculty of Pharmacy, Padjadjaran
University, Jatinangor, West Java, Indonesia. The authors are also
thankful to Dr. Joko Kusmoro at the Laboratory of Plant Taxonomy,
Department of Biology, Faculty of Mathematics and Natural Sciences,
Padjadjaran University, for identifying the plant specimen.
Funding
The present study was funded by the
Academic-Leadership Grant Universitas Padjadjaran Batch-II 2017,
research contract no. 872/UN6.3.1/LT/2017.
Availability of data and materials
The datasets used and/or analyzed in the current
work are available from the corresponding author on reasonable
request.
Authors' contributions
JL and AD were principally responsible for
conception and design of the study. JL, MM and AD participated in
the acquisition of the reported data. DMS, RDS, SM and MF
participated in the processing, analysis and interpretation of the
reported data. JL and SM contributed to the writing and revising of
the manuscript. All authors read and approved the final manuscript
to be published.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants. Research Ethics Committee of Padjadjaran University
(Bandung, Indonesia) approved the study procedures (approval nos.
1211/UN6.C.10/PN/2017 for 10-gingerol and 924/UN6.C.10/PN/2017 for
6-shogaol).
Patient consent for publication
Informed consent was obtained for publication of the
participants' data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang X, Iwaoka WT, Huang AS, Nakamoto ST
and Wong R: Gingerol decreases after processing and storage of
ginger. J Food Sci. 59:1338–1340. 1994. View Article : Google Scholar
|
|
2
|
Hiserodt RD, Franzblau SG and Rosen RT:
Isolation of 6-, 8-, and 10-gingerol from ginger rhizome by HPLC
and preliminary evaluation of inhibition of Mycobacterium
avium and Mycobacterium tuberculosis. J Agric Food Chem.
46:2504–2508. 1998. View Article : Google Scholar
|
|
3
|
Young HY, Chiang CT, Huang YL, Pan FP and
Chen GL: Analytical and stability studies of ginger preparations.
Yao Wu Shi Pin Fen Xi. 10:149–153. 2002.
|
|
4
|
Riaz H, Begum A, Raza SA, Khan ZMUD,
Yousaf H and Tariq A: Antimicrobial property and phytochemical
study of ginger found in local area of Punjab, Pakistan. Int Curr
Pharm J. 4:405–409. 2015. View Article : Google Scholar
|
|
5
|
Ugwoke CEC and Nzekwe U: Phytochemistry
and proximate composition of ginger (Zingiber officinale). J
Pharm Allied Sci. 7:No. 5. 2010.
|
|
6
|
Bhargava S, Dhabhai K, Amla Batra A,
Sharma A and Malhotra B: Zingiber officinaleChemical and
phytochemical screening and evaluation of its antimicrobial
activities. J Chem Pharm Res. 4:360–364. 2012.
|
|
7
|
Prasad S and Tyagi AK: Ginger and its
constituents: Role in prevention and treatment of gastrointestinal
cancer. Gastroenterol Res Pract. 2015:1429792015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marrelli M, Menichini F and Conforti F: A
comparative study of Zingiber officinale Roscoe pulp and
peel: Phytochemical composition and evaluation of antitumour
activity. Nat Prod Res. 29:2045–2049. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mashhadi NS, Ghiasvand R, Askari G, Hariri
M, Darvishi L and Mofid MR: Anti-oxidative and anti-inflammatory
effects of ginger in health and physical activity: Review of
current evidence. Int J Prev Med. 4 Suppl 1:S36–S42.
2013.PubMed/NCBI
|
|
10
|
Dhanik J, Arya N and Nand V: A review on
Zingiber officinale. J Pharmacogn Phytochem. 6:174–184.
2017.
|
|
11
|
Jiang H, Sólyom AM, Timmermann BN and Gang
DR: Characterization of gingerol-related compounds in ginger
rhizome (Zingiber officinale Rosc.) by high-performance
liquid chromatography/electrospray ionization mass spectrometry.
Rapid Commun Mass Spectrom. 19:2957–2964. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tjendraputra E, Tran VH, Liu-Brennan D,
Roufogalis BD and Duke CC: Effect of ginger constituents and
synthetic analogues on cyclooxygenase-2 enzyme in intact cells.
Bioorg Chem. 29:156–163. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Afzal M, Al-Hadidi D, Menon M, Pesek J and
Dhami MSI: Ginger: An ethnomedical, chemical and pharmacological
review. Drug Metab Drug Interact. 18:159–190. 2001. View Article : Google Scholar
|
|
14
|
Grzanna R, Phan P, Polotsky A, Lindmark L
and Frondoza CG: Ginger extract inhibits beta-amyloid
peptide-induced cytokine and chemokine expression in cultured THP-1
monocytes. J Altern Complement Med. 10:1009–1013. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lantz RC, Chen GJ, Sarihan M, Sólyom AM,
Jolad SD and Timmermann BN: The effect of extracts from ginger
rhizome on inflammatory mediator production. Phytomedicine.
14:123–128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghasemzadeh A, Jaafar HZ and Rahmat A:
Variation of the phytochemical constituents and antioxidant
activities of Zingiber officinale var. Rubrum Theilade
associated with different drying methods and polyphenol oxidase
activity. Molecules. 21:7802016. View Article : Google Scholar
|
|
17
|
Dugasani S, Pichika MR, Nadarajah VD,
Balijepalli MK, Tandra S and Korlakunta JN: Comparative antioxidant
and anti-inflammatory effects of [6]-gingerol, [8]-gingerol,
[10]-gingerol and [6]-shogaol. J Ethnopharmacol. 127:515–520. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ippoushi K, Azuma K, Ito H, Horie H and
Higashio H: [6]-Gingerol inhibits nitric oxide synthesis in
activated J774.1 mouse macrophages and prevents
peroxynitrite-induced oxidation and nitration reactions. Life Sci.
73:3427–3437. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim SO, Kundu JK, Shin YK, Park JH, Cho
MH, Kim TY and Surh YJ: [6]-Gingerol inhibits COX-2 expression by
blocking the activation of p38 MAP kinase and NF-kappaB in phorbol
ester-stimulated mouse skin. Oncogene. 24:2558–2567. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fikri F, Saptarini NM, Levita J, Nawawi A,
Mutalib A and Ibrahim S: The inhibitory activity on the rate of
prostaglandin production by Zingiber officinale var. Rubrum.
Pharmacol Clin Pharm Res. 1:33–40. 2016.
|
|
21
|
Saptarini NM, Sitorus EY and Levita J:
Structure-based in silico study of 6-gingerol, 6-ghogaol, and
6-paradol, active compounds of ginger (Zingiber officinale)
as COX-2 inhibitors. Int J Chem. 5:12–18. 2013. View Article : Google Scholar
|
|
22
|
Zick SM, Djuric Z, Ruffin MT, Litzinger
AJ, Normolle DP, Alrawi S, Feng MR and Brenner DE: Pharmacokinetics
of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate
metabolites in healthy human subjects. Cancer Epidemiol Biomarkers
Prev. 17:1930–1936. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu Y, Zick S, Li X, Zou P, Wright B and
Sun D: Examination of the pharmacokinetics of active ingredients of
ginger in humans. AAPS J. 13:417–426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zick SM, Ruffin MT, Djuric Z, Normolle D
and Brenner DE: Quantitation of 6-, 8-, and 10-gingerols and
6-shogaol in human plasma by high-performance liquid chromatography
with electrochemical detection. Int J Biomed Sci. 6:233–240.
2010.PubMed/NCBI
|
|
25
|
Şengül Ü: Comparing determination methods
of detection and quantification limits for aflatoxin analysis in
hazelnut. Yao Wu Shi Pin Fen Xi. 24:56–62. 2016.
|
|
26
|
Wohlmuth H: Phytochemistry and
pharmacology of plants from the ginger family, Zingiberaceae
(unpublished PhD thesis). Southern Cross University; Lismore, New
South Wales: 2008
|
|
27
|
Ghasemzadeh A, Jaafar HZ and Rahmat A:
Optimization protocol for the extraction of 6-gingerol and
6-shogaol from Zingiber officinalevar. rubrum
Theilade and improving antioxidant and anticancer activity using
response surface methodology. BMC Complement Altern Med.
15:2582015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chrubasik S, Pittler MH and Roufogalis BD:
Zingiberis rhizoma: A comprehensive review on the ginger effect and
efficacy profiles. Phytomedicine. 12:684–701. 2005. View Article : Google Scholar : PubMed/NCBI
|