Introduction
Sarcomatoid carcinoma (SC), also known as spindle
cell carcinoma, pseudosarcoma and carcinosarcoma, is a malignant
tumor type of unclear pathogenesis. Previous studies have reported
cases of SC in the skin, breast, urinary tract, lung, head and neck
mucosal region and small intestine (1–6). SC
rarely occurs in the liver, and has been detected in only 1.8% of
all surgically resected hepatocellular carcinomas (HCCs) and in
3.9–9.4% of autopsied cases (7,8). To the
best of our knowledge, the majority of hepatic SC (HSC) cases
reported in the English literature occurred with a simultaneous HCC
or cholangiocellular carcinoma (CCC). With regard to the
histogenesis of HSC, it has been suggested that sarcomatoid
elements in liver carcinomas are derived from a dedifferentiation
of HCC (7). HCC and CCC exhibit a
number of histological variations, and anaplastic sarcomatoid
changes are known to arise with a reported frequency of 2.2–2.9%
(9). Local recurrence, venous
invasion and intrahepatic, distant and lymph node metastasis occur
frequently. The case reported in the present study possessed two
noteworthy characteristics: First, it was a rare case of pure
primary HSC (PHSC). Secondly, the recurrence of the tumor was
recorded by imaging. The present study reports the primary
clinical, pathological and immunohistochemical observations of the
case, as well as the treatment and detection of tumor
recurrence.
Case report
A 60-year-old woman was admitted to the West China
Hospital of Sichuan University (Chengdu, China) with multiple
masses in her liver, but with no complaints of fever, abdominal
pain, jaundice or other symptoms. Serological markers for hepatitis
B and C virus were negative, as was the test for α fetoprotein
(AFP; 1.99<8 ng/ml). An abdominal enhanced magnetic resonance
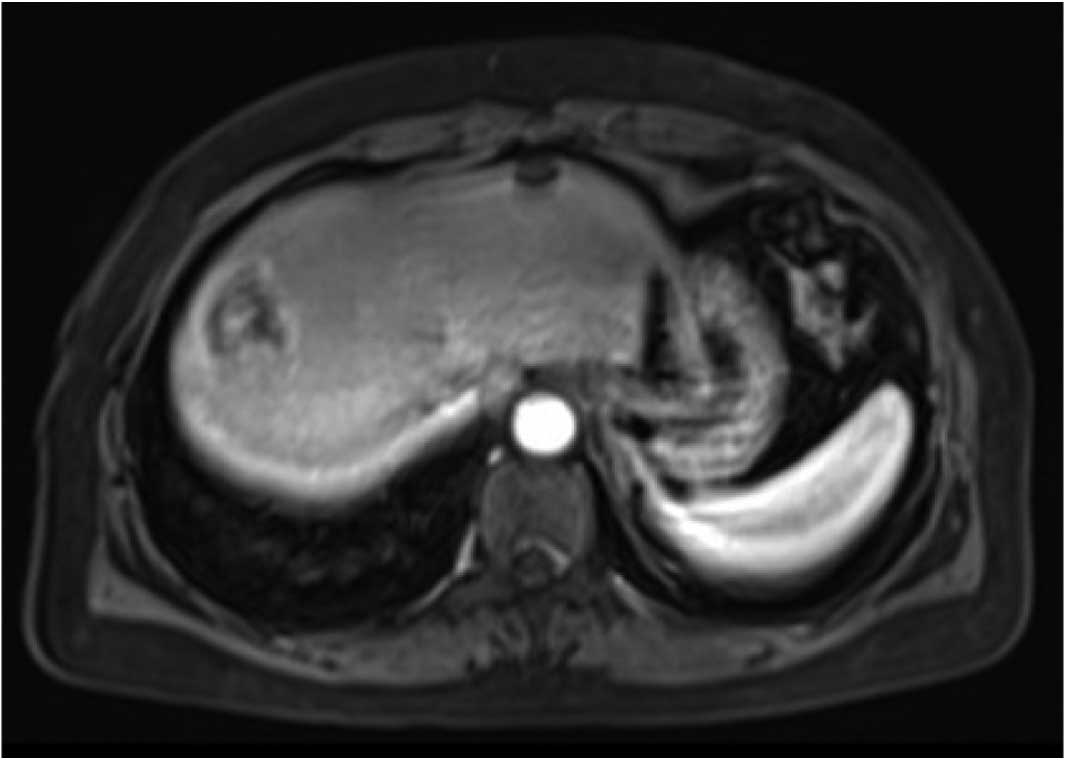
imaging (MRI) examination confirmed multiple lesions in the liver;
these were predominantly located in the right lobe, with evident
liquefactive necrosis (Fig. 1).
Further physical examination and other tests confirmed that no
lesions existed elsewhere in the body. The patient subsequently
underwent a right hepatectomy and cholecystectomy.
Intraoperatively, three hard lesions were detected; these lesions
were ~3.0 cm in diameter and were encapsulated in segments 7 and 8
of the liver. Overall, the resected specimens measured 14×10×6.5
cm, which included multiple gray nodules with a diameter of 0.5–3
cm. No obvious cirrhosis was observed surrounding the lesions.
Furthermore, there were no indications of ordinary HCC and CCC in
the resected specimens, and the surgical margins were free of
tumor. Histologically, the majority of the regions consisted of
spindle-cell components, while the epithelial-cell components
exhibited a focal distribution. Immunohistochemically, positive
results were obtained for cytokeratin 8 (CK8), pancytokeratin,
cluster of differentiation (CD) 117 and vimentin; however, the
sample was negative for Discovered on GIST-1, CD34, synaptophysin
and chromogranin A. The positive rate of Ki-67 detection was ~50%.
No hepatocytes were observed in the resected samples. The results
of a fluorescence in situ hybridization test indicated no
SS18 gene translocation. The result of the KIT/PDGFRA mutation test
indicated no KIT exon 9 and 13 mutations or PDGFRA exon 14 and 18
mutations. Finally, a diagnosis of poorly differentiated PHSC was
established. Postoperatively, the patient received thymosin (80 mg
qd) treatment, but no radiotherapy or chemotherapy was
administered.
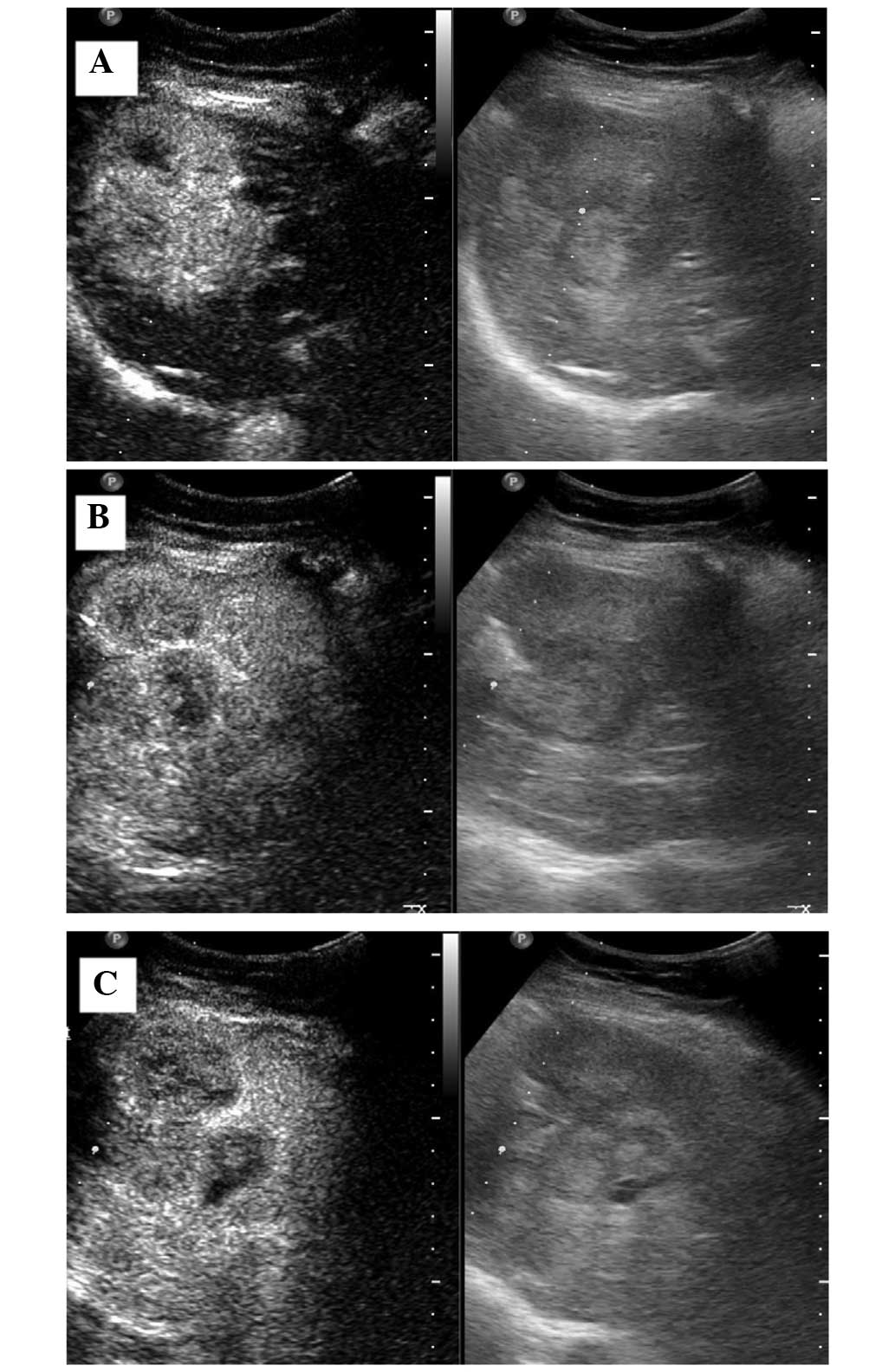
After a 3-month follow-up period, conventional
ultrasound examination indicated a weak-echo mass of 2 cm in the
residual liver. Two months later, the mass had developed into a
6.1×6.4-cm clutter-echo mass in the residual liver, and exhibited
an irregular internal echo-free zone under conventional ultrasound.
Contrast-enhanced ultrasound (CEUS) was immediately conducted,
which revealed that the mass was rapidly and markedly enhanced in
the arterial phase, while weakly enhanced in the portal and delayed
phases (Fig. 2). Thus, tumor
recurrence was diagnosed and the patient received chemotherapy as
opposed to surgical treatment. After ~6 months, the patient was
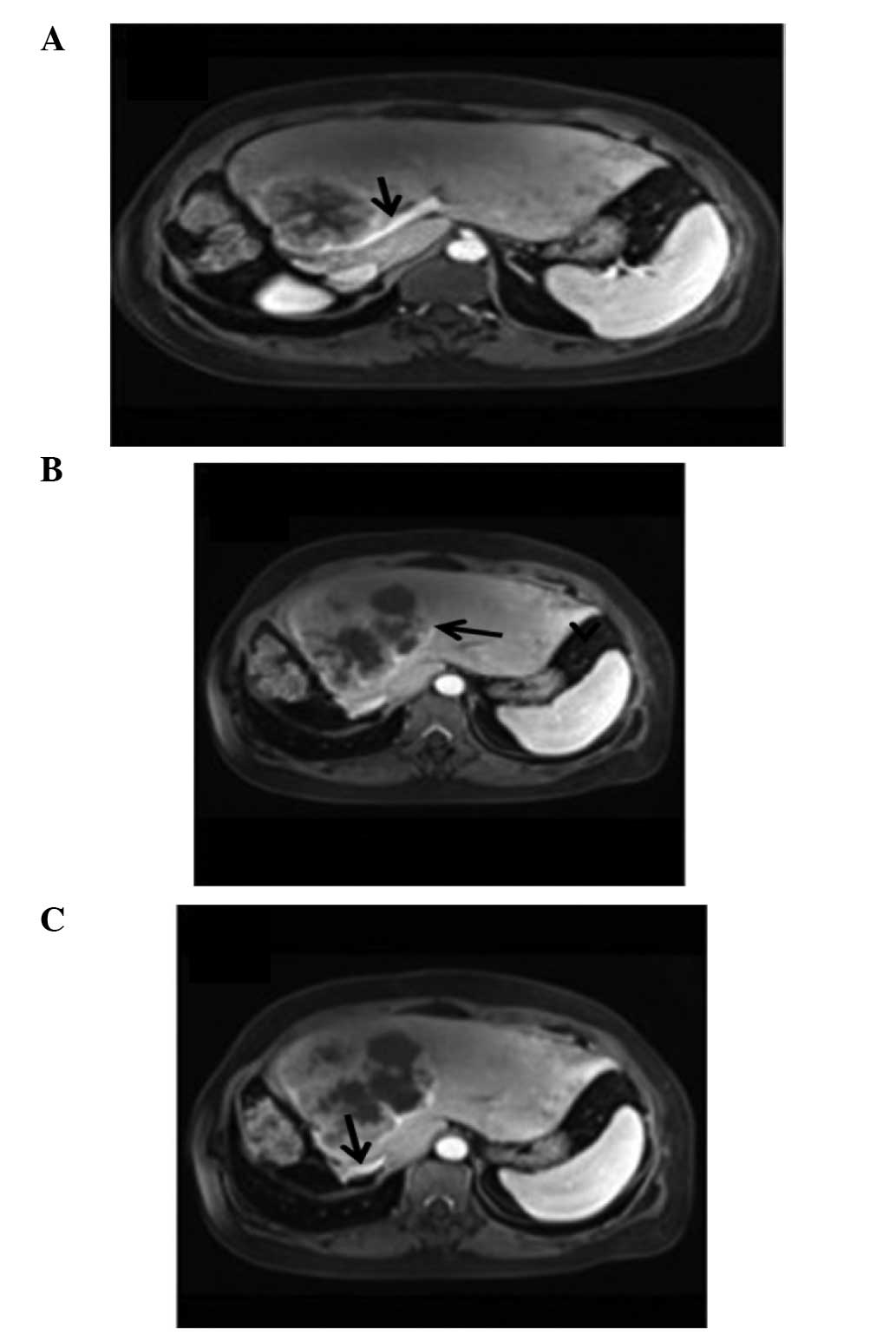
referred for a further review. An MRI examination revealed that the
residual liver was enlarged, and multiple nodules and masses with
unequal size were evident, with a reduced T1 signal and an elevated
T2 signal. The largest mass, which was lobulated and had a maximum
cross-section of ~10.3×10.7 cm, was located in the left inner lobe.
Enhanced MRI scanning indicated that the lesions were
ring-enhanced, with no enhancement of central necrosis. The largest
lesion had no clear boundaries with the inferior vena cava near the
second hepatic hilum or the left branch of the portal vein trunk.
The left hepatic vein mixed with the left external lobe lesions
(Fig. 3). Thus, the recurrence and
widespread intrahepatic metastasis of the PHSC was confirmed.
Furthermore, the tumors had invaded the inferior vena cava, near
the second hepatic hilum, left portal vein trunk and left hepatic
vein. The patient and her family elected to forgo further treatment
and the patient succumbed to the PHSC 2 months later due to her
poor general condition. Written, informed consent was obtained from
the patient's family.
Discussion
PHSC is a rare malignant tumor type of unclear
pathogenesis. The present study reports a rare case of pure PHSC
with a rapid clinical course; HSC has been reported in 1.8% of all
surgically resected HCCs and in 3.9–9.4% of the autopsied cases
(7,8). Using immunohistochemistry, Kakizoe
et al (7) concluded that the
appearance of sarcomatoid components represented a sarcomatoid
change in the HCC, rather than a complication of HCC and sarcoma.
Thus, a diagnosis of sarcomatoid change in HCC is feasible based on
previous literature, particularly for patients with HCC following
artery embolization, anticancer treatment, radio frequency ablation
or liver transplantation (10–12).
These patients usually possess a history of hepatitis B or C, or a
foundation disease of liver cirrhosis due to various causes, such
as alcohol. The present case, by contrast, did not present with
these features (13). Comparable
with ordinary HCC, the clinical symptoms associated with PHSC are
typically abdominal pain, weight loss, anorexia and fatigue;
however, fever and abdominal pain are considered to be more
specific manifestations of SC (14).
The AFP expression levels in HSC patients may be normal or slightly
elevated; this differs from HCC, in which AFP is considered to be a
characteristic diagnostic indicator.
Imaging examinations serve a crucial function in the
diagnosis of PHSC. PHSC typically presents as a mass with
peripheral enhancement, central necrosis, variable enhancement of
the solid portion with or without tumor capsule, and intrahepatic
metastasis in enhanced computed tomography and MRI (15). In the present case, the findings from
CEUS and enhanced MRI were highly consistent. If a patient with a
low-level of AFP and no history of viral hepatitis presents with
the aforementioned imaging characteristics, a diagnosis of PHSC
should be considered. Specifically, central necrosis and peripheral
enhancement are considered to be among the most valuable diagnostic
characteristics of PHSC (15), and
vascular invasion is another common diagnostic feature.
An exact diagnosis of PHSC depends on a number of
pathological and immunohistochemical observations. Histologically,
PHSC lesions contain spindle- and epithelial-cell components. The
spindle-cell components consist of spindle-shaped cells, which
exhibit oval and elongated nuclei with conspicuous nucleoli and
spindle-shaped eosinophilic cytoplasms (13). Furthermore, these spindle cells form
interlacing bundles and a local storiform pattern, and mitotic
figures are frequently observed. Using immunohistochemical
staining, the detection of CK, epithelial membrane antigen (EMA)
and vimentin may be useful for the diagnosis of PHSC. CK is
regarded as an epithelial marker, while vimentin is a mesenchymal
marker. Sarcomatoid lesions are positive for CK and vimentin. CK8
has been reported to be among the most critical indicators of SC
(16). In addition, a number of
other markers, including anion exchanger (AE) 1/AE3, 34βE12, CAM
5.2, c-Kit, S-100 protein, HHF-35, kinesin-like protein-1, CD34 and
HAM-56, may indicate a positive diagnosis of PHSC in certain cases.
In the present case, the patient tested positive for CK8, vimentin
and Ki-67 detection rate. Genetically, p53 has been shown to
accumulate in the nuclei of undifferentiated spindle cells,
indicating that the p53 gene mutation may be common in these cells
(17). Thus, the mutation of the p53
gene may be involved in the progression of SC. Due to the
complexity of the diagnosis, methods of differentiating PHSC from
other types of liver sarcoma, such as chondrosarcoma,
rhabdomyosarcoma, fibrosarcoma, leiomyosarcoma and malignant
fibrous histiocytoma, are required. Tests for CK and EMA in these
tumors are usually negative. In the present case, a distinction was
made primarily between metastatic gastrointestinal stromal tumors
(GISTs) and synovial sarcoma. These tumors are spindle-cell tumor
lines with comparable morphology; however, the majority of GISTs
express c-Kit protein and exhibit activating mutations in the KIT
or PDGFRA proto-oncogenes (18).
Furthermore, >95% of synovial sarcomas (SSs) are characterized
by the reciprocal chromosomal translocation t(X;18)(p11.2;q11.2),
which results in an SS-specific SYT-SSX fusion gene (19). In the present case, tests for the KIT
gene and the SS18 gene mutation were negative, and the final
diagnosis was PHSC.
The preferred treatment for PHSC is surgical
resection. As this tumor type is rare and there are few
large-sample studies, the exact effect of radiotherapy and
chemotherapy is not clear. The prognosis of PHSC is particularly
poor due to its high malignancy and local recurrence rates, venous
invasion and intrahepatic, distant and lymph node metastasis
(8). In particular, lymph node
metastasis occurs almost twice as frequently in cases of PHSC as it
does in HCC without sarcomatous change (14). The patient described in this study
presented with recurrence of intrahepatic metastasis and venous
invasion within 1 year. Extrahepatic metastasis can occur in the
skin, pelvis, lungs and bladder, amongst other organs (20). The survival curve of patients with
PHSC following hepatic resection is significantly worse than that
of patients with ordinary HCC (8).
The median survival time and 3-year survival rate of patients with
SC have been found to be 9.6 months and 17%, respectively (21). A Ki-67 proliferative index >35% in
patients with SC has been associated with poor prognosis (4); in the present case, the Ki-67 index was
found to be 50%.
In conclusion, PHSC is an aggressive tumor type,
which is characterized by a rapid clinical course; a prompt
diagnosis using histological and immunohistochemical analysis is
therefore required. Early detection and treatment through
appropriate examination and radical resection may improve patient
prognosis. The present case of a rare, pure PHSC may improve the
current understanding of this tumor type.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81271585).
References
|
1
|
Morgan MB, Purohit C and Anglin TR:
Immunohistochemical distinction of cutaneous spindle cell
carcinoma. Am J Dermatopathol. 30:228–232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nonnis R, Paliogiannis P, Giangrande D,
Marras V and Trignano M: Low-grade fibromatosis-like spindle cell
metaplastic carcinoma of the breast: A case report and literature
review. Clin Breast Cancer. 12:147–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chrysikos D, Zagouri F, Sergentanis TN, et
al: Mucinous tubular and spindle cell carcinoma of the kidney: A
case report. Case Rep Oncol. 5:347–353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Travis WD: Sarcomatoid neoplasms of the
lung and pleura. Arch Pathol Lab Med. 134:1645–1658.
2010.PubMed/NCBI
|
|
5
|
Lee SE and Park SY: Sarcomatoid carcinoma
of the small intestine: A rare and highly aggressive tumor. J
Korean Surg Soc. 83:321–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Viswanathan S, Rahman K, Pallavi S, et al:
Sarcomatoid (spindle cell) carcinoma of the head and neck mucosal
region: A clinicopathologic review of 103 cases from a tertiary
referral cancer centre. Head Neck Pathol. 4:265–275. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kakizoe S, Kojiro M and Nakashima T:
Hepatocellular carcinoma with sarcomatous change. Clinicopathologic
and immunohistochemical studies of 14 autopsy cases. Cancer.
59:310–316. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maeda T, Adachi E, Kajiyama K, Takenaka K,
Sugimachi K and Tsuneyoshi M: Spindle cell hepatocellular
carcinoma. A clinicopathologic and immunohistochemical analysis of
15 cases. Cancer. 77:51–57. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shinoda M, Mukai M, Tanabe M, Hashiguchi
N, Oda M and Kitajima M: Spindle cell carcinoma of the intrahepatic
bile duct in a patient with primary sclerosing cholangitis. J
Gastroenterol. 38:1091–1096. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakanishi C, Sato K and Ito Y: Combined
hepatocellular carcinoma and neuroendocrine carcinoma with
sarcomatous change of the liver after transarterial
chemoembolization. Hepatol Res. 42:1141–1145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koda M, Maeda Y, Matsunaga Y, Mimura K,
Murawaki Y and Horie Y: Hepatocellular carcinoma with sarcomatous
change arising after radiofrequency ablation for
well-differentiated hepatocellular carcinoma. Hepatol Res.
27:163–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Da Ines D, Bailly A, Lannareix V, et al:
Hepatocellular carcinoma with sarcomatous change: Prompt and fatal
intraabdominal recurrence after liver transplantation.
Gastroenterol Clin Biol. 33:590–593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giunchi F, Vasuri F, Baldin P, Rosini F,
Corti B and D'Errico-Grigioni A: Primary liver sarcomatous
carcinoma: Report of two cases and review of the literature. Pathol
Res Pract. 209:249–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eriguchi N, Aoyagi S, Hara M, Okuda K,
Fukuda S, Tamae T and Kanazawa N: Malignant sarcomatoid tumor of
the liver: Report of a case. Surg Today. 31:170–173. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koo HR, Park MS, Kim MJ, Lim JS, Yu JS,
Jin H and Kim KW: Radiological and clinical features of sarcomatoid
hepatocellular carcinoma in 11 cases. J Comput Assist Tomogr.
32:745–749. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haratake J and Horie A: An
immunohistochemical study of sarcomatoid liver carcinomas. Cancer.
68:93–97. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Murata M, Miyoshi Y, Iwao K, et al:
Combined hepatocellular/cholangiocellular carcinoma with
sarcomatoid features: Genetic analysis for histogenesis. Hepatol
Res. 21:220–227. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Agaram NP, Baren A, Arkun K, Dematteo RP,
Besmer P and Antonescu CR: Comparative ultrastructural analysis and
KIT/PDGFRA genotype in 125 gastrointestinal stromal tumors.
Ultrastruct Pathol. 30:443–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawauchi S, Ihara K, Nishikawa K, Sugino
N, Takahashi M and Sasaki K: Synovial sarcoma arising in the vulva
cytogenetically confirmed by SYT break-apart rearrangement
fluorescence in situ hybridization: A case report and
discussion of diagnostic methods. Oncol Lett. 4:955–959.
2012.PubMed/NCBI
|
|
20
|
Nishie W, Iitoyo M, Koshiyama T and Kusama
T: Sarcomatoid carcinoma of the liver with skin and pleural
metastases. Br J Dermatol. 148:1069–1071. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang QB, Cui BK, Weng JM, Wu QL, Qiu JL
and Lin XJ: Clinicopathological characteristics and outcome of
primary sarcomatoid carcinoma and carcinosarcoma of the liver. J
Gastrointest Surg. 16:1715–1726. 2012. View Article : Google Scholar : PubMed/NCBI
|