Introduction
In excess of 678,000 women were diagnosed with
cancer in 2007 (1). A total of 2% of
the cancer patients were <40 years old and at a prereproductive
or reproductive age (1). Although
effective chemotherapeutics and advanced treatment technology have
increased the chances of long-term survival in cancer patients and
improved the quality of life, the side effects of cancer treatments
remain a neglected problem. While chemotherapeutics provide an
effective cure method for female cancer patients aged <40 years,
they can also induce damage to the ovarian reserve function
(2).
Cisplatin (cis-diamine-dichloroplatinum II)
is a chemotherapy drug that is commonly and widely used for the
treatment of several types of cancer, including ovarian, breast and
endometrial cancer (3–5); however, while cisplatin exerts a
therapeutic effect in various types of cancer, its side effects
also receive considerable attention. The side effects induced by
cisplatin are associated with an increased production of free
radicals and reactive oxygen species (ROS), leading to oxidative
stress and lipoperoxidation (6). It
is reported that cisplatin can cause oocyte and granulosa cell
injury, as well as ovarian reserve insufficiency (7). Since ROS are closely associated with
ovarian failure, the abnormal production of ROS may be involved in
chemotherapy-induced ovarian damage.
Hydrogen has been reported to exert a therapeutic
antioxidant effect, by selectively reducing cytotoxic ROS, and
reduce levels of inflammation and apoptosis in several diseases
(8–13). Furthermore, hydrogen may alleviate
the nephrotoxicity induced by chemotherapy drugs without
compromising their anti-tumor activity (12). Our previous study demonstrated that
hydrogen treatment alleviated chemotherapy-induced ototoxicity by
reducing oxidative stress (10); the
aim of the present study was to investigate effect of hydrogen-rich
saline treatment on chemotherapy-induced ovarian damage.
Materials and methods
Experimental animals
Adult, virgin, female Sprague Dawley rats, weighing
180–220 g, were obtained from the Laboratory Animal Center of the
Academy of Military Medical Sciences (Beijing, China). The animals
were housed at 20–22°C with a 12-h light/dark cycle and fed
standard chow and water ad libitum. The procedures in this
study were approved by the Animal Care and Use Committee of Tianjin
Medical University (Tianjin, China) and performed in accordance
with the guidelines for the use of experimental animals from the
National Institutes of Health. Vaginal smears had been obtained
daily for >10 days to manifest ≥2 sequential, normal, 5-day
vaginal estrus cycles, in order to ensure the availability of
experimental animals in this study
Hydrogen-rich saline production
The hydrogen-rich saline was prepared as previously
described (13). Hydrogen was
dissolved in physiological saline for 4 h under 0.4 MPa pressure to
a saturated level using a self-designed, hydrogen-rich,
water-producing apparatus, which was stored under atmospheric
pressure at 4°C in an aluminum bag with no dead volume. To ensure a
concentration of >0.6 mmol/l, it was necessary to freshly
prepare the hydrogen-rich saline every week. A needle-type hydrogen
sensor (Unisense A/S, Aarhus, Denmark) was used to confirm the
content of hydrogen in the saline (14).
Animal model establishment and
grouping
A total of 240 animals were randomly divided into
four groups (n=60 per group): Control (Con), control +
hydrogen-rich saline (Con + H2), cisplatin-induced
ovarian injury (OI) and cisplatin-induced ovarian injury +
hydrogen-rich saline (OI + H2). The animal model was
established using a previously described method (15) with little change. Cisplatin was
diluted in saline immediately before use. In the OI and OI +
H2 groups, the rats received a single dose of cisplatin
[5 mg/kg; the lethal dose-50 of cisplatin in rats is 7.4 mg/kg
(16)] by intraperitoneal injection
on the 1st day. On the 7th day, the rats received another dose of
cisplatin in an identical manner, which established the rat models
of cisplatin-induced ovarian damage. An identical dose of saline
was injected in the Con and Con + H2 group rats using
the same method.
In the Con + H2 and OI + H2
groups, the rats were intraperitoneally injected with hydrogen-rich
saline (10 ml/kg body weight) once a day between the 1st and the
14th day. Vaginal smears were obtained from each rat at 8:00 a.m.
each day for ≥5 days after cisplatin injection to ensure the
ovarian injury model was successful.
Specimen collection
On the 14th, 28th and 42nd days (T1,
T2 and T3) after cisplatin injection, femoral
vein blood was collected from the rats (n=10 per group) and
centrifuged at 10,000 × g, 4°C for 10 min. At the same time,
ovarian tissue was collected, and homogenates were prepared via
centrifugation, using the aforementioned conditions. These sample
were stored at −80°C until the estrogen (E2),
follicle-stimulating hormone (FSH), superoxide dismutase (SOD),
catalase (CAT) and malondialdehyde (MDA) examination. At each
time-point, another 10 rats (n=10 per group) were sacrificed for
bilateral ovary removal; one was fixed in formalin for
follicle-counting analysis, while the other was used for nuclear
factor erythroid 2-related factor 2 (Nrf2) detection by western
blotting.
Staining and ovarian follicle-counting
analysis
Ovarian follicle populations were counted using
previously described methods (15).
The ovaries were fixed in 10% formalin for 6 h at room temperature,
embedded in paraffin and sectioned at a 5-µm thickness. Following
deparaffinization and rehydration, the sections were stained with
hematoxylin and eosin. The oocyte-containing follicles in the
developmental stage were classified, and the antral follicles were
counted in every 12th section by two experienced pathologists.
Follicle classification and counting was carried out according to
the criteria of Oktay et al (17).
Detection of E2 and FSH by
enzyme-linked immunosorbent assay (ELISA)
The serum E2 and FSH levels were measured
using ELISA kits from R&D Systems (Minneapolis, MN, USA). The
assays were performed according to the manufacturer's instructions.
The absorbance was read on a microplate reader (Denley Dragon
Wellscan MK 3; Thermo Fisher Scientific, Vantaa, Finland), and the
concentrations were calculated based on a standard curve. All
standards and samples were run in duplicate.
Detection of antioxidant enzymes (SOD
and CAT) and oxidation products (MDA)
To investigate the mechanism associated with the
effects of hydrogen-rich saline, the levels of antioxidant enzymes
and oxidation products in the serum and ovarian tissue were
measured. The activities of SOD, CAT and MDA were measured using
commercial kits purchased from Cayman Chemical Company (Ann Arbor,
MI, USA). According to the manufacturer's instructions, total SOD
activity was assayed at 450 nm and CAT at 540 nm.
Spectrophotometric readings of SOD and CAT were performed using a
DU-640B spectrophotometer (Beckman Coulter, Miami, FL, USA), while
readings for MDA were obtained using a microplate reader (CA94089;
Molecular Devices, Sunnyvale, CA, USA). All standards and samples
were run in duplicate.
Detection of Nrf2 by western
blotting
At each time-point, ovarian tissue was collected to
lyse on ice in 100 µl radioimmunoprecipitation assay buffer [50
mmol/l Tris-HCl (pH 7.4), 150 mmol/l NaCl, 1 mmol/l
phenylmethanesulfonyl fluoride, 1 mmol/l ethylenediaminetetraacetic
acid, 1% Triton X-100, 0.5% sodium deoxycholate and 0.1% sodium
dodecyl sulfate]. The lysates were cleared by centrifugation at
15,000 × g for 10 min, and the supernatant protein samples were
denatured at 100°C for 5 min, separated on 10% acrylamide gels and
then electrotransferred to polyvinylidene fluoride membranes.
Following the blocking of the membranes with 5% non-fat dried milk
in Tris buffer saline tween 20 buffer at room temperature for 2 h,
primary rabbit polyclonal antibodies against Nrf2 (1:200; ab92946;
Abcam, Cambridge, MA,USA) and β-actin (1:2,000; ab129348; Abcam)
were added for incubation overnight at 4°C. The immunoblots were
subsequently incubated with horseradish peroxidase-conjugated goat
anti-rabbit IgG (ab175773; Abcam, Cambridge, MA,USA and detected
using enhanced chemiluminescence reagent (Merck Millipore,
Molsheim, France). Images of the immunoblots were visualized and
captured using the Quantity One® quantitative gel analysis system
(Bio-Rad, Tokyo, Japan). All western blot analyses were carried out
≥3 times.
Statistical analysis
Differences between the groups were analyzed using
one-way analysis of variance followed by the Tukey comparison.
Results are expressed as the mean ± standard deviation of ≥3
independent experiments, and P<0.05 was considered to indicate a
statistically significant difference between groups.
Results
Hydrogen-rich saline regulates
E2 and FSH during the process of chemotherapy-induced
ovarian injury
Following the injection of two single doses of 5
mg/kg cisplatin in the rats, abnormal levels of the sex hormones
appeared in the serum. Compared with group Con, no significant
difference in the release of FSH was found in group Con +
H2 (P>0.05, Fig. 1);
however, the release of FSH was increased and E2 release
was reduced in group OI (P<0.05, Fig.
1). The administration of hydrogen-rich saline (10 ml/kg body
weight) for 2 weeks during cisplatin injection attenuated the FSH
release and elevated the level of E2 at T1,
T2 and T3 compared with group OI (P<0.05,
Fig. 1).
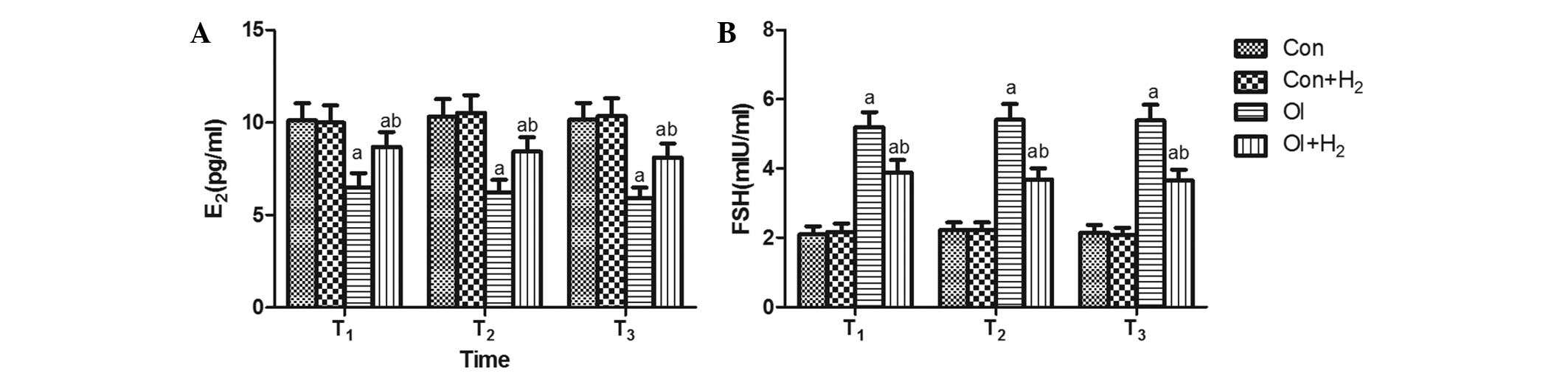 | Figure 1.Time-dependent effects of
hydrogen-rich saline on the release of sex hormones (E2
and FSH) during the process of chemotherapy-induced ovarian injury.
Cisplatin was injected twice, each time at 5 mg/kg, in group OI.
The same dose of saline was injected in group Con. The rats
received intraperitoneal hydrogen-rich saline at 10 ml/kg body
weight for 14 days. The levels of (A) FSH and (B) E2
were measured on the 14th, 28th and 42nd days (T1,
T2 and T3) after the cisplatin injection.
Results are presented as the mean ± standard deviation (n=10 per
group). aP<0.05 compared with group Con;
bP<0.05 compared with group OI. E2,
estrogen; FSH, follicle-stimulating hormone; Con, control; Con +
H2, control + hydrogen-rich saline; OI,
cisplatin-induced ovarian injury; OI + H2,
cisplatin-induced ovarian injury + hydrogen-rich saline. |
Effect of hydrogen-rich saline on
different developmental stages of the follicle during the process
of chemotherapy-induced ovarian injury in rats
All developmental stages of the follicles, and the
structure of the ovarian cortex and medulla, could be clearly seen
in group Con. Compared with group Con, few follicles were observed
in group OI; furthermore, the group OI rats exhibited significant
damage to the cortex, which could not be distinguished from the
medulla. Compared with group OI, the development of the follicles
in group OI + H2 was much more regular; all
developmental stages of the follicles could be seen, and the damage
to the cortex was less severe (P<0.05, Fig. 2).
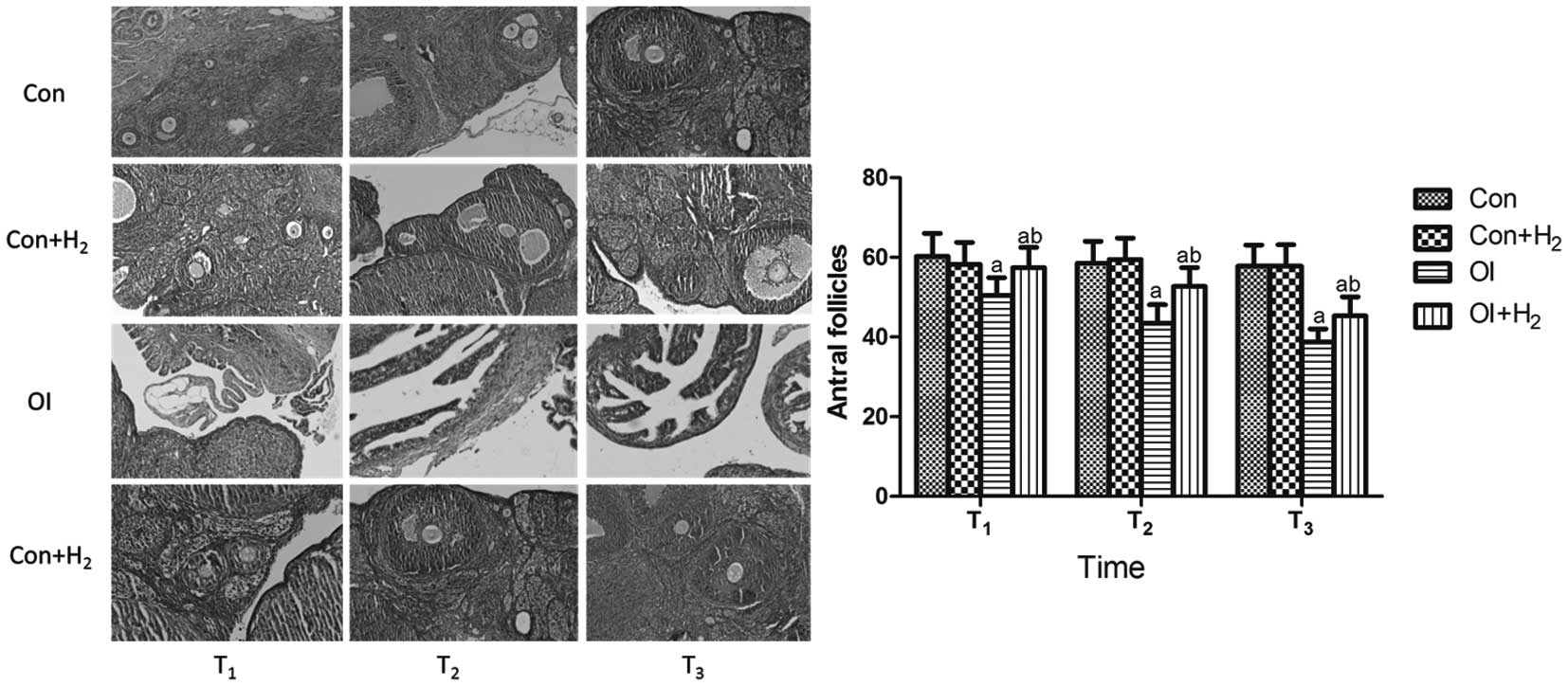 | Figure 2.Effect of hydrogen-rich saline on the
different developmental stages of follicles during the process of
chemotherapy-induced ovarian injury in rats (hematein eosin
staining; magnification, ×100). Cisplatin was injected at 5 mg/kg
in group OI. Rats received intraperitoneal hydrogen-rich saline at
10 ml/kg body weight for 14 days. The number of antral follicles
was counted on the 14th, 28th and 42nd days (T1,
T2 and T3) after the cisplatin injection. All
levels of the follicles, as well as the clear structure of the
cortex, could be observed in group Con. Few follicles could be seen
in group OI. In group OI + H2 the development of the
follicles was much more regular, all levels of follicles could be
determined and the damage to the cortex was less severe. Results
are presented as the mean ± standard deviation (n=10 per group).
aP<0.05 compared with group Con;
bP<0.05 compared with group OI. Con, control; Con +
H2, control + hydrogen-rich saline; OI,
cisplatin-induced ovarian injury; OI + H2,
cisplatin-induced ovarian injury + hydrogen-rich saline. |
Effect of hydrogen-rich saline on
antioxidant enzymes (SOD and CAT) and the oxidation product MDA
during chemotherapy in rats
To determine the underlying mechanism of the effects
observed, antioxidant enzymes and oxidation products were detected
in the serum and ovarian tissue of the rats at the T1,
T2 and T3 time-points. As shown in Fig. 3, when compared with group Con,
cisplatin induced an excessive release of MDA and inhibited the
activity of SOD and CAT in the serum and ovarian tissue of group OI
(P<0.05). Hydrogen-rich saline significantly reduced the levels
of MDA and elevated the activity of SOD and CAT in the serum and
ovarian tissue of the cisplatin-challenged rats (P<0.05). The
results revealed that cisplatin led to oxidative stress by
increasing the levels of oxidation products and attenuating the
activity of antioxidant enzymes, which could be reversed by
hydrogen-rich saline treatment.
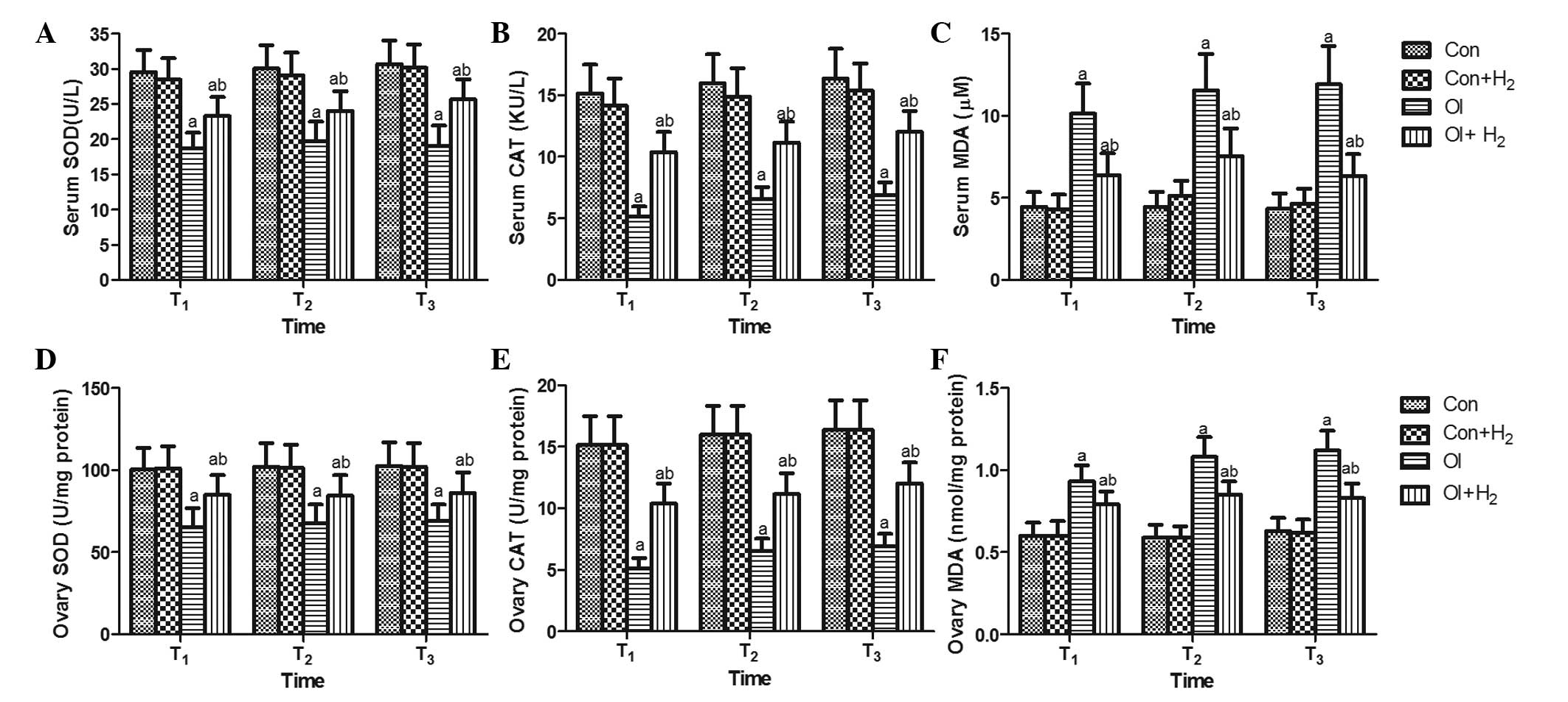 | Figure 3.Hydrogen-rich saline regulates
cisplatin-induced oxidative stress by increasing the activity of
SOD and CAT and reducing the level of MDA in the serum and ovarian
tissue of rats. Cisplatin was injected at 5 mg/kg in group OI. Rats
received intraperitoneal hydrogen-rich saline at 10 ml/kg body
weight for 14 days. On the 14th, 28th and 42nd days (T1,
T2 and T3) after the cisplatin injection, the
(A-C) blood and (D-F) tissue samples were collected for the
measurement of (A and D) SOD, (B and E) CAT and (C and F) MDA.
Values are expressed as the mean ± standard deviation (n=10 per
group). aP<0.05 compared with group Con;
bP<0.05 compared with group OI. SOD, superoxide
dismutase; CAT, catalase; MDA, malondialdehyde; Con, control; Con +
H2, control + hydrogen-rich saline; OI,
cisplatin-induced ovarian injury; OI + H2,
cisplatin-induced ovarian injury + hydrogen-rich saline. |
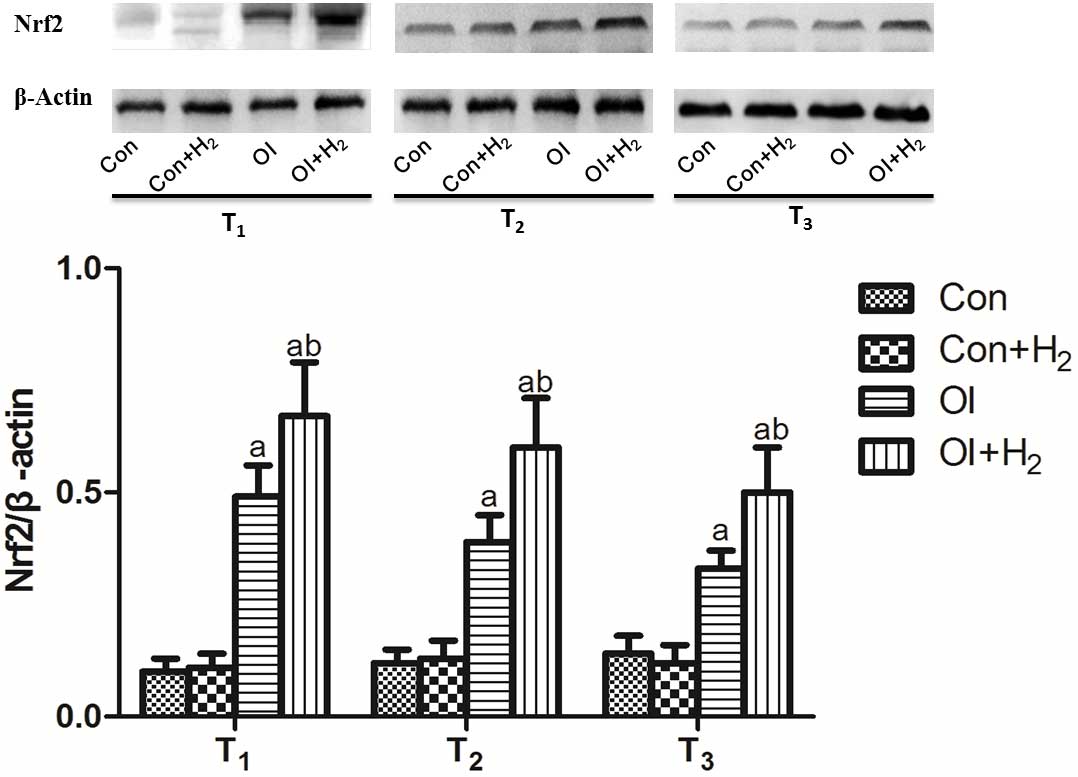
Hydrogen-rich saline elevates Nrf2
expression in the ovarian tissue of the chemotherapy-induced
rats
Nrf2 is the key molecule of the Nrf2/antioxidant
response element (ARE) signaling pathway, which is a relatively
conservative and important endogenous antioxidative system
(18). Cisplatin induced Nrf2
expression in the ovarian tissue of the rats between T1
and T3 (P<0.05, Fig.
4). Compared with group OI, hydrogen-rich saline treatment
further increased the Nrf2 expression between T1 and
T3. Based on the results, it was suggested that
hydrogen-rich saline may regulate the Nrf2/ARE signaling pathway to
protect cells from immoderate oxidative stress.
Discussion
In the present study, cisplatin stimulation was used
to establish a model of ovarian injury in young female rats.
Compared with group OI, it was found that hydrogen-rich saline
treatment regulated the release of sex hormones and markedly
improved the pathological condition and antral follicle counts.
Hydrogen-rich saline additionally improved the production of
antioxidant enzymes and attenuated the levels of oxidation products
following cisplatin injection. These results indicate that
hydrogen-rich saline treatment exerts a protective effect against
ovarian injury in rats by regulating the balance of the redox
system.
Cisplatin is a commonly used chemotherapeutic agent
that exerts a therapeutic effect in various types of cancer and is
often used to treat female patients at a reproductive age; however,
despite its therapeutic effect, the side effects of cisplatin have
also received considerable attention. The side effects of cisplatin
are associated with an excessive production of free radicals and
ROS, such as superoxide anions or H2O2, in
different kidney cells (19) and in
ovarian cancer (6,20). To investigate the side effects of
cisplatin on the ovaries in the present study, an intraperitoneal
injection of cisplatin was used to induce ovarian injury in female
rats of a reproductive age. The results revealed that cisplatin
attenuated E2 secretion, increased FSH secretion by
negative feedback and damaged the structure of the cortex in female
rats with ovarian injury.
It has previously been found that hydrogen exerts
protective effects against various diseases. Hydrogen selectively
alleviates hydroxyl radicals, other ROS and oxidation products and
has been shown to improve the activity of antioxidants in a number
of disease models, including shock, multiple organ dysfunction
syndrome and ischemia-reperfusion (8–13). In
addition, hydrogen exerts a protective effect against
chemotherapy-induced organ injury, and studies using dynamic
contrast-enhanced computed tomography (19) and blood oxygenation level-dependent
magnetic resonance imaging (11)
have demonstrated that hydrogen-rich water protects the kidneys
against cisplatin-induced nephrotoxicity without compromising the
anti-tumor activity (12). Hydrogen
additionally exerts an antitumor effect. It has been reported that
hydrogen reduced the size of skin tumors of squamous cell carcinoma
in hairless albino mice (21), and
platinum nanocolloid-supplemented hydrogen-rich water inhibited the
clonal growth of human tongue carcinoma cells (22). These studies suggest that hydrogen
has the potential ability to protect ovarian tissue from
cisplatin-induced injury and may exert an antitumor effect in
cancer patients. In the present study, hydrogen-rich saline
treatment increased E2 secretion and reduced FSH
secretion in ovarian injury rats. The saline also had a protective
effect against damaged cells and tissue, either by slowing the cell
damage process or activating certain signaling pathways in the
cells.
It has previously been shown that the follicle
microenvironment consists of a dynamic balance of oxidation and
antioxidation, including antioxidant enzymes in the follicular
fluid (such as SOD and CAT), which has a close association with
follicle maturation (15). High
levels of oxidation products (MDA) and/or a decreased antioxidative
ability in oocytes (such as low SOD activity) may, therefore,
disrupt the redox system balance and lead to oxidative stress.
Oxidative stress can induce the blockade of follicular development
and ovulation and result in menstrual disorder (amenorrhea,
oligomenorrhea) and even infertility (23–26). SOD
as an antioxidant enzyme can eliminate oxygen radicals by
accelerating the dismutation of superoxide to
H2O2, and CAT catalyzes the degradation of
H2O2 into H2O and O2.
SOD and CAT therefore act as mutually supportive antioxidative
enzymes that actively defend against ROS (27). Matzuk et al (28) reported that sub-fertile or infertile
SOD1-knockout mice had ovarian defects and suppressed FSH and
luteinizing hormone levels; therefore, SOD has a vital role in
ovarian injury. The present results showed that cisplatin induced
an excessive production of MDA and reduced the SOD and CAT
activity. Hydrogen treatment reversed the effect of cisplatin on
MDA, SOD and CAT, in addition to regulating the sex hormone
secretion.
Nrf2, as a member of the capncollar basic leucine
zipper subfamily of leucine zipper transcription factors, is one of
the key factors involved in initiating the endogenous protective
effect against oxidative stress. Following stimulation by chemical
toxicity, carcinogenesis and pathological processes, Nrf2 and its
cytoplasmic binding protein, Kelch-like ECH-associated protein 1,
become uncoupled, and Nrf2 is transferred into the nucleus. Nrf2
combines with ARE to induce two sets of target genes: Phase II
detoxification enzymes and antioxidant enzymes, which play an
essential role in cellular protection (29,30). Hu
et al (31) previously
demonstrated that Nrf2 serves as an essential sensor and regulator
of chemical homeostasis in ovarian cells, protecting the cells from
toxic chemicals by controlling metabolic detoxification, ROS
defense and forkhead box protein O3 expression. Without Nrf2
expression in the mice, the host became more sensitive to harmful
stimulation, facilitating organ injury (31). In the present study it was found that
Nrf2 expression was increased following the use of cisplatin to
induce a model of ovarian injury in rats, and this may have been
due to Nrf2 acting as a stress response following stimulation by
chemotherapeutic agents. Hydrogen-rich saline treatment further
increased the Nrf2 expression in the chemotherapy-induced ovarian
injury rats.
There were several limitations in the present study.
First, the changes in oxidation product and antioxidative enzymes
were measured in vivo; the expression levels and changes
should therefore be verified in vitro using ovarian cells.
Secondly, the role of Nrf2 should be more comprehensively
investigated, using an inhibitor of Nrf2 or Nrf2-knockdown rats;
this will be the aim of our next experiment.
In conclusion, exposure to certain harmful factors
can lead to the destruction of ovarian follicles in animal models.
Cisplatin induces abnormal sex hormone secretion in a rat model of
ovarian injury. Hydrogen-rich saline exerts a protective effect
against cisplatin-induced ovarian injury by reducing MDA and
increasing SOD and CAT activity. Furthermore, high Nrf2 expression
is associated with ovarian injury.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81372033 and 81101409), the
Natural Science Foundation of the Tianjin Science Committee (grant
nos. 11JCYBJC12900 and 13JCQNJC11400) and the Foundation of Tianjin
Bureau of Public Health (grant no. 2011KZ108).
Glossary
Abbreviations
Abbreviations:
|
CAT
|
catalase
|
|
E2
|
estrogen
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
FSH
|
follicle stimulating hormone
|
|
MDA
|
malondialdehyde
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
ROS
|
reactive oxygen species
|
|
SOD
|
superoxide dismutase
|
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee HJ, Selesniemi K, Niikura Y, Niikura
T, Klein R, Dombkowski DM and Tilly JL: Bone marrow transplantation
generates immature oocytes and rescues long-term fertility in a
preclinical mouse model of chemotherapy-induced premature ovarian
failure. J Clin Oncol. 25:3198–3204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Cheng Y, Ren X, Zhang L, Yap KL,
Wu H, Patel R, Liu D, Qin ZH, Shih IM and Yang JM: NAC1 modulates
sensitivity of ovarian cancer cells to cisplatin by altering the
HMGB1-mediated autophagic response. Oncogene. 31:1055–1064. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Silver DP, Richardson AL, Eklund AC, Wang
ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A, et
al: Efficacy of neoadjuvant Cisplatin in triple-negative breast
cancer. J Clin Oncol. 28:1145–1153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bae-Jump VL, Zhou C, Boggess JF and Gehrig
PA: Synergistic effect of rapamycin and cisplatin in endometrial
cancer cells. Cancer. 115:3887–3896. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu J and Friedman E: Depleting Mirk kinase
increases cisplatin toxicity in ovarian cancer cells. Genes Cancer.
1:803–811. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morgan S, Lopes F, Gourley C, Anderson RA
and Spears N: Cisplatin and doxorubicin induce distinct mechanisms
of ovarian follicle loss; imatinib provides selective protection
only against cisplatin. PLoS One. 8:e701172013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie K, Yu Y, Pei Y, Hou L, Chen S, Xiong L
and Wang G: Protective effects of hydrogen gas on murine
polymicrobial sepsis via reducing oxidative stress and HMGB1
release. Shock. 34:90–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie K, Yu Y, Zhang Z, Liu W, Pei Y, Xiong
L, Hou L and Wang G: Hydrogen gas improves survival rate and organ
damage in zymosan-induced generalized inflammation model. Shock.
34:495–501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qu J, Li X, Wang J, Mi W, Xie K and Qiu J:
Inhalation of hydrogen gas attenuates cisplatin-induced ototoxicity
via reducing oxidative stress. Int J Pediatr Otorhinolaryngol.
76:111–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsushita T, Kusakabe Y, Kitamura A,
Okada S and Murase K: Investigation of protective effect of
hydrogen-rich water against cisplatin-induced nephrotoxicity in
rats using blood oxygenation level-dependent magnetic resonance
imaging. Jpn J Radiol. 29:503–512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakashima-Kamimura N, Mori T, Ohsawa I,
Asoh S and Ohta S: Molecular hydrogen alleviates nephrotoxicity
induced by an anti-cancer drug cisplatin without compromising
anti-tumor activity in mice. Cancer Chemother Pharmacol.
64:753–761. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng X, Mao Y, Cai J, Li Y, Liu W, Sun P,
Zhang JH, Sun X and Yuan H: Hydrogen-rich saline protects against
intestinal ischemia/reperfusion injury in rats. Free Radic Res.
43:478–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayashida K, Sano M, Ohsawa I, Shinmura K,
Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, et
al: Inhalation of hydrogen gas reduces infarct size in the rat
model of myocardial ischemia-reperfusion injury. Biochem Biophys
Res Commun. 373:30–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Yang S, Lv X, Sun H, Weng J, Liang Y
and Zhou D: The mechanism of mesna in protection from
cisplatin-induced ovarian damage in female rats. J Gynecol Oncol.
24:177–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dale O, Mortensen B, Thommesen L and Hagen
B: Cisplatin toxicity in the rat may be influenced by anaesthetic
agents. Acta Anaesthesiol Scand. 44:7702000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oktay K, Schenken RS and Nelson JF:
Proliferating cell nuclear antigen marks the initiation of
follicular growth in the rat. Biol Reprod. 53:295–301. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng X, Ku CH and Siow RC: Regulation of
the Nrf2 antioxidant pathway by microRNAs: New players in
micromanaging redox homeostasis. Free Radic Biol Med. 64:4–11.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Li X, Wong YS, Chen T, Zhang H, Liu
C and Zheng W: The reversal of cisplatin-induced nephrotoxicity by
selenium nanoparticles functionalized with 11-mercapto-1-undecanol
by inhibition of ROS-mediated apoptosis. Biomaterials.
32:9068–9076. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Belotte J, Fletcher NM, Awonuga AO, Alexis
M, Abu-Soud HM, Saed MG, Diamond MP and Saed GM: The role of
oxidative stress in the development of cisplatin resistance in
epithelial ovarian cancer. Reprod Sci. 21:503–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dole M, Wilson FR and Fife WP: Hyperbaric
hydrogen therapy: A possible treatment for cancer. Science.
190:152–154. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saitoh Y, Yoshimura Y, Nakano K and Miwa
N: Platinum nanocolloid-supplemented hydrogen-dissolved water
inhibits growth of human tongue carcinoma cells preferentially over
normal cells. Exp Oncol. 31:156–162. 2009.PubMed/NCBI
|
|
23
|
Oyawoye O, Gadir Abdel A, Garner A,
Constantinovici N, Perrett C and Hardiman P: Antioxidants and
reactive oxygen species in follicular fluid of women undergoing
IVF: Relationship to outcome. Hum Reprod. 18:2270–2274. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Crha I, Hrubá D, Ventruba P, Fiala J,
Totusek J and Visnová H: Ascorbic acid and infertility treatment.
Cent Eur J Public Health. 11:63–67. 2003.PubMed/NCBI
|
|
25
|
Carbone MC, Tatone C, Delle Monache S,
Marci R, Caserta D, Colonna R and Amicarelli F: Antioxidant
enzymatic defences in human follicular fluid: Characterization and
age-dependent changes. Mol Hum Reprod. 9:639–643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Török A, Belágyi J, Török B, Tinneberg HR
and Bódis J: Scavenger capacity of follicular fluid, decidua and
culture medium with regard to assisted reproduction: An in vitro
study using electron paramagnetic resonance spectroscopy. Gynecol
Obstet Invest. 55:178–182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cohen M, Lippman M and Chabner B: Role of
pineal gland in aetiology and treatment of breast cancer. Lancet.
2:814–816. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matzuk MM, Dionne L, Guo Q, Kumar TR and
Lebovitz RM: Ovarian function in superoxide dismutase 1 and 2
knockout mice. Endocrinology. 139:4008–4011. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nguyen T, Yang CS and Pickett CB: The
pathways and molecular mechanisms regulating Nrf2 activation in
response to chemical stress. Free Radic Biol Med. 37:433–441. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dinkova-Kostova AT, Holtzclaw WD, Cole RN,
Itoh K, Wakabayashi N, Katoh Y, Yamamoto M and Talalay P: Direct
evidence that sulfhydryl groups of Keap1 are the sensors regulating
induction of phase 2 enzymes that protect against carcinogens and
oxidants. Proc Natl Acad Sci USA. 99:11908–11913. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu X, Roberts JR, Apopa PL, Kan YW and Ma
Q: Accelerated ovarian failure induced by 4-vinyl cyclohexene
diepoxide in Nrf2 null mice. Mol Cell Biol. 26:940–954. 2006.
View Article : Google Scholar : PubMed/NCBI
|