Introduction
Osteosarcoma (OS) is a type of primary mesenchymal
tumor, which is histologically characterized as a malignant tumor
that directly produces osteoid or immature bones (1). Although OS remains the most common type
of primary bone tumor in children and adolescents (2,3), the
5-year survival rates of patients without metastases have reached
60–75% through the use of combined aggressive surgery and
neoadjuvant chemotherapy (4).
However, 40–50% of patients will develop metastatic disease, which
is associated with a poor prognosis, particularly in patients with
primary pulmonary metastases (5).
Therefore, there is an urgent clinical need for novel therapies for
the treatment of OS. Due to increased understanding regarding the
molecular pathogenesis of OS, numerous genetic alterations have
been associated with OS (6), which
may represent novel therapeutic targets for the diagnosis and
treatment of patients with OS.
Cancer testis (CT) antigens are a unique class of
tumor antigens that are aberrantly expressed in various
malignancies (7). It has been
suggested that the aberrant expression of CT antigens in tumors may
contribute to various malignant properties, including immortality,
migration, invasion and metastatic capacity (8). As a novel member of the CT antigen
family, sperm-associated antigen 9 (SPAG9) is a scaffolding protein
that facilitates interactions between mitogen-activated protein
kinases and their target transcription factors, in order to
activate specific signaling pathways (9,10). SPAG9
was first suggested as a potential target for immunotherapy in
human epithelial ovarian cancer (11). Previous studies have detected
overexpression of SPAG9 in various types of human cancer including
renal, breast, thyroid, colon and lung carcinomas (12–16), and
SPAG9 small interfering (si)RNA treatment successfully inhibited
tumor cell proliferation and invasion in a previous study (12). Furthermore, SPAG9 expression has been
demonstrated to be associated with circulating anti-SPAG9
antibodies in patients with early stage and low grade breast cancer
and cervical cancer, suggesting its potential application in the
early detection of the disease (17,18).
However, whether SPAG9 is involved in the development of OS remains
to be elucidated. The present study aimed to determine the effects
of SPAG9 knockdown on the proliferation, migration and invasiveness
of OS cells in an in vitro cell culture system.
Materials and methods
Cell culture and tissue samples
The U2OS human OS cell line, and human umbilical
vascular endothelial cells (HUVECs) were obtained from the Shanghai
Academy of Life Sciences (Shanghai, China). The cells were cultured
in Dulbecco's modified Eagle medium (DMEM) (HyClone; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a
humidified atmosphere containing 95% air and 5% CO2.
Four OS tissue samples and paired non-cancerous
tissue samples were harvested from patients with OS of the
extremities (three men, one woman; age, 48–74 years) who underwent
surgery at the Renmin Hospital of Wuhan University (Wuhan, China).
None of the patients had a history of previous treatment with
antitumor agents or radiotherapy. The patients provided informed
consent and the study was approved by the ethics committee of Enshi
Renmin Hospital of Wuhan University (Enshi, China).
siRNA transfection
U2OS cells were grown to 50% confluence prior to
transfection with nonspecific control siRNA (Qiagen, Inc.,
Mississauga, ON, Canada) or SPAG9 siRNA (Shanghai GenePharma Co.,
Ltd., Shanghai, China) using Lipofectamine® 2000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. The siRNA sequences were as follows:
Control, sense 5′-UUCUCCGAACGUGUCACGUTT-3′, anti-sense
5′-ACGUGACACGUUCGGAGAATT-3′; SPAG9, sense
5′-AGAUCUCAGUGGAUAUAAATT-3′, and anti-sense
5′-UUUAUAUCCACUGAGAUCUTT-3′.
Wound healing assay
For the wound healing assay, 1×105
cells/well were seeded on 6-well plates supplemented with culture
medium. Cells were cultured in serum-free medium 12 h prior to the
assay, and an artificial wound was subsequently scratched into the
confluent cell monolayer using a P200 pipette tip. Photomicrograph
images (TE2000; Nikon Corporation, Tokyo, Japan) were immediately
captured (time 0 h), and the cells were subsequently incubated in
DMEM supplemented with 1% FBS. The migration of the cells and the
closing of the scratch wound were observed and microphotographs
were captured every 4 h (TE2000; Nikon Corporation). The
experiments were performed in triplicate and the whole assay was
repeated three times.
Cell migration and invasion assay
Modified two-chamber migration apparatus (pore size,
8 µm) was used to perform cell migration and invasion assays
(Corning Life Sciences, Lowell, MA, USA). The migration assay was
completed as follows, the U2OS cells transfected with control or
SPAG9 siRNA were seeded in serum-free medium in the upper chamber
(1×106 cells). Following 12 h incubation at 37°C, the
cells were carefully removed from the upper chamber using a cotton
swab and the cells that had traversed the membrane were fixed. For
the invasion assay, cells were trypsinized 48 h post-transfection
and 2×105 cells were transferred in 100 µl serum-free
medium to the upper Matrigel™ chamber (BD Biosciences,
Franklin Lakes, NJ, USA) and incubated for 24 h. Medium
supplemented with 10% FBS was added to the lower chamber as the
chemoattractant. Following incubation, the non-invaded cells were
removed from the upper membrane surface using a cotton tip, and the
cells that passed through the filter were fixed with 4%
paraformaldehyde and stained with hematoxylin (Sigma-Aldrich).
Cells were observed under a light microscope (BX-51; Olympus
Corporation, Tokyo, Japan) and images were captured using a Camedia
Master C-3040 digital camera (Olympus Corporation).
Cell proliferation assay
In order to quantitatively evaluate cell viability,
the effects of si-SPAG9 on the proliferation of HUVECs and U2OS
cells (2×104 cells/well) were detected using a CCK-8
assay. U2OS cells and HUVECs were seeded in a 96-well culture plate
and incubated at 37°C in a humidified atmosphere containing 5%
CO2 for 24 h. Subsequently, 10 µl CCK-8 solution was
added to each well and the cultures were incubated at 37°C for 1 h.
Absorbance was measured at 450 nm using an ELx800 spectrometer
reader (BioTek Instruments, Inc.).
Tube formation assay
A 96-well plate was pre-coated with
Matrigel™ and incubated at 37°C for 2 h prior to the
addition of 2×104 HUVECs/well suspended in 100 µl
conditioned medium. Following incubation at 37°C for 24 h, three
fields were chosen at random and the number of capillary-like tubes
were counted. Images were captured using an inverted microscope
(TE2000; Nikon Corporation).
Enzyme-linked immunosorbent assay
(ELISA) for vascular endothelial growth factor (VEGF)
A total of 1×106 U2OS cells/well were
plated in 6-well tissue culture plates and subsequently transfected
with control siRNA or siRNA-SPAG9 with serum starvation. The
supernatants were collected 24 h post-transfection and the
concentrations of VEGF were determined using Human VEGF Quantikine
ELISA kits according to the manufacturer's protocol (R&D
Systems, Inc., Minneapolis, MN, USA).
Reverse transcription
quantitative-polymerase chain reaction (RT-qPCR)
Tissue samples were initially homogenized and total
RNA was extracted from them using an mRNA Isolation kit (Qiagen,
Inc., Valencia, CA, USA). An RNeasy kit (Qiagen, Inc., Valencia,
CA, USA) was used to isolate mRNA from the cells. cDNA was
synthesized from RNA extracted from cells and tissue samples using
an iScript™ Select cDNA Synthesis kit (Bio-Rad Laboratories, Inc.,
Hercules, CA). PCR amplification of SPAG9, metalloproteinase
(MMP)-2, MMP-9 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
was performed as follows: 20 sec at 95°C, followed by 50 cycles at
95°C for 3 sec and annealing at 60°C for 30 sec, and a final
extension step at 72°C for 5 min using 2X PCR Master Mix (Beyotime
Institute of Biotechnology) and an ABI PRISM® 7500 Sequence
Detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The results were normalized to those of the GAPDH
housekeeping gene and are expressed as a ratio of the percentage of
individual genes to the GAPDH control. The specific primer
sequences for each gene were as follows: SPAG9 forward,
5′-TCATAGTAGAATTATTCAAGAG-3′; and reverse
5′-ATAGTAGAATTATCTCTTGAA-3′; MMP-2 forward,
5′-CTGGCTTCTGGCATCCTGTT-3′; and reverse,
5′-GACGAGGTCGGAATTGCAGA-3′; MMP-9 forward,
5′-CCTCTGGAGGTTCGACGTGA-3′; and reverse,
5′-TAGGCTTTCTCTCGGTACTGGAA-3′; and GAPDH forward,
TGAAGGTCGGAGTCAACGGATT; and reverse, CCTGGAAGATGGTGATGGGATT
(Shanghai GenePharma Co., Ltd.). Expression levels were analyzed
using the 2−ΔΔCq method (19).
Zymographic analysis
Cells were grown to 70–80% confluence on 10 cm
plates, washed twice with phosphate-buffered saline and cultured in
serum-free medium for 36 h. Total protein was extracted from the
cells using radioimmunoprecipitation assay lysis buffer containing
60 µg/ml phenylmethylsulfonyl fluoiride (Beyotime Institute of
Biotechnology). Protein concentrations were determined using a
Bicinchoninic Acid Protein Assay kit (Boster Biological Technology,
Ltd., Wuhan, China). An appropriate volume of medium with an
equivalent amount of protein (80 µg) was subjected to
electrophoresis in 10% gel supplemented with 0.1% gelatin.
Following electrophoresis, the gelatin gel was washed twice with
2.5% Triton X-100 and incubated with Tris buffer (50 mM Tris-HCl;
200 mM NaCl; 0 mM CaCl2; pH 7.4) overnight at 37°C.
Subsequently, the gel was stained with 0.5% Coomassie Brilliant
Blue R-250 and destained using 5% acetic acid and 10% methanol (all
Invitrogen; Thermo Fisher Scientific, Inc.).
Western blot analysis
Tissue samples were homogenized, the homogenates
were centrifuged at 1,000 × g for 10 min at 4°C, and the
supernatants were collected. Total protein was extracted from the
cells and tissue supernatants using radioimmunoprecipitation assay
lysis buffer supplemented with 60 µg/ml phenylmethylsulfonyl
fluoride (Beyotime Institute of Biotechnology) 48 h
post-transfection with siRNA. Protein concentrations were assessed
using a Bicinchoninic Acid Protein Assay kit (Boster Biological
Technology, Ltd.). Proteins (80 µg) were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to
nitrocellulose membranes (EMD Millipore, Billerica, MA, USA) and
incubated overnight at 4°C with the following antibodies: Rabbit
anti-SPAG9 (1:1,000; cat. no. 5519), rabbit anti-MMP-2 (1:1,000;
cat. no. 4022), rabbit anti-MMP-9 (1:1,000; cat. no. 3852) (all
Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit
anti-VEGF (1:1,000; cat. no. sc-507) and mouse anti-β-actin (1:200;
cat. no. sc-47778) (both Santa Cruz Biotechnology, Inc., Dallas,
TX, USA). Blots were washed four times with Tris-buffered saline
supplemented with 0.1% Tween-20 and subsequently incubated with
horseradish peroxidase-conjugated anti-rabbit (1:2,000; cat. no.
7074) or anti-mouse (1:20,000; cat. no. 7076) secondary antibodies
(Cell Signaling Technology, Inc.) at 24°C for 2 h. The signals were
detected using a Super Signal West Pico Chemiluminescent Substrate
kit (Pierce Biotechnology, Inc., Rockford, IL, USA) according to
the manufacturer's protocol. Western blots were analyzed using
ImageJ 1.48u software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Data were expressed as the mean ± standard
deviation. Between group statistical differences were evaluated
using Instat software (version 5.0; GraphPad Software, Inc., La
Jolla, CA, USA) using one-way analysis of variance, whereas
Student's t-test was used to analyze the cell proliferation assays.
P<0.05 was considered to indicate a statistically significant
difference.
Results
SPAG9 expression levels were
upregulated in patients with osteosarcoma
In order to determine whether SPAG9 expression
levels are altered in human patients with OS, RT-qPCR and western
blotting were performed on four pairs of human OS tissue samples
and pair-matched noncancerous tissue samples. The results
demonstrated that SPAG9 expression levels were markedly increased
in OS tissues, as compared with the noncancerous tissue samples
(Fig. 1A and B).
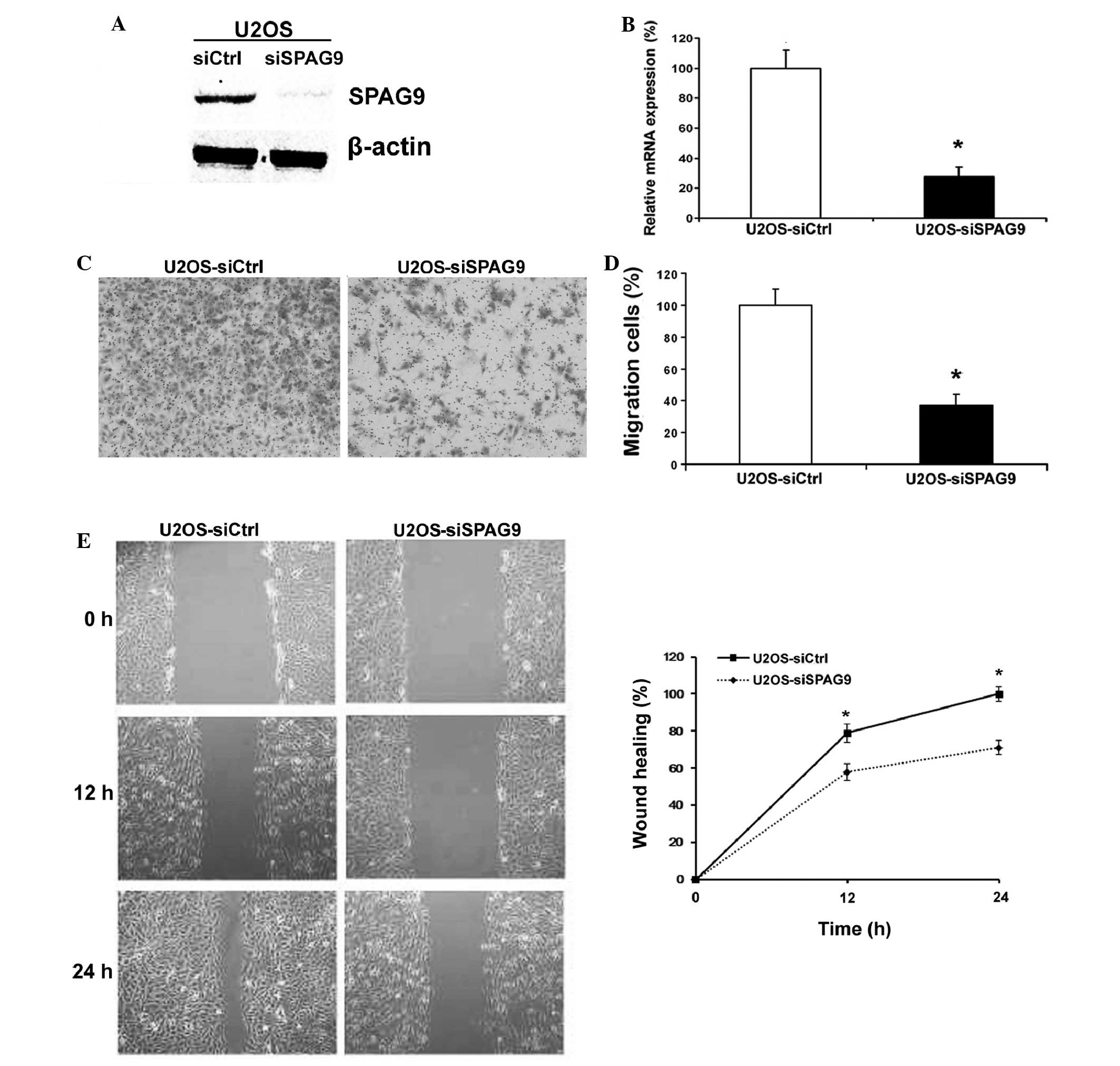
Knockdown of SPAG9 by siRNA in U2OS
cells inhibits cell motility
In order to explore the biological function of SPAG9
in OS cells, siRNA knockdown was performed in U2OS cell lines, and
western blotting and RT-qPCR were used to analyze SPAG9 expression
levels. Protein and mRNA expression levels of SPAG9 were markedly
decreased 48 h following siRNA transfection (Fig. 2A and B). Therefore, all subsequent
experiments were performed using cells transfected with SPAG9 or
control siRNA.
The results of the cell migration assay demonstrated
that transfection with SPAG9 siRNA decreased the migratory ability
of U2OS cells through the Boyden chamber by 64% (Fig. 2C and D). Furthermore, the wound
healing assay data demonstrated that there was a significant delay
in wound closure following treatment with SPAG9 siRNA (Fig. 2E).
Knockdown of SPAG9 by siRNA in U2OS
cells inhibits cell invasion
Invasion is the crucial step in the progression of
cancer metastasis. The capacity of OS cells to invade through
Matrigel™, an artificial extracellular matrix (ECM), was measured
following transfection with control or SPAG9 siRNA. Knockdown of
SPAG9 expression resulted in a 48% reduction in cell invasion
(Fig. 3A), and histogram analysis
demonstrated that significantly fewer cells (P<0.05) passed
through the filters coated with an artificial ECM, suggesting that
the invasive potential of SPAG9 siRNA-transfected OS cells was
severely impaired. Downregulation of SPAG9 had no effect on the
proliferation of OS cells (Fig.
3B).
Knockdown of SPAG9 inhibits
angiogenesis in vitro
In order to further elucidate the functional role of
SPAG9 on the angiogenic potential of human OS cells, tube formation
and HUVEC proliferation were investigated in vitro. When
cultured in conditioned medium from SPAG9 siRNA cells the
percentage of proliferating HUVECs was significantly reduced (52%;
P<0.05), as compared with the control (Fig. 4A). Furthermore, the average number of
complete tubular structures formed by HUVECs cultured in
conditioned medium from U2OS si-SPAG9 cells was significantly
decreased (P<0.05), as compared with the control group (Fig. 4B).
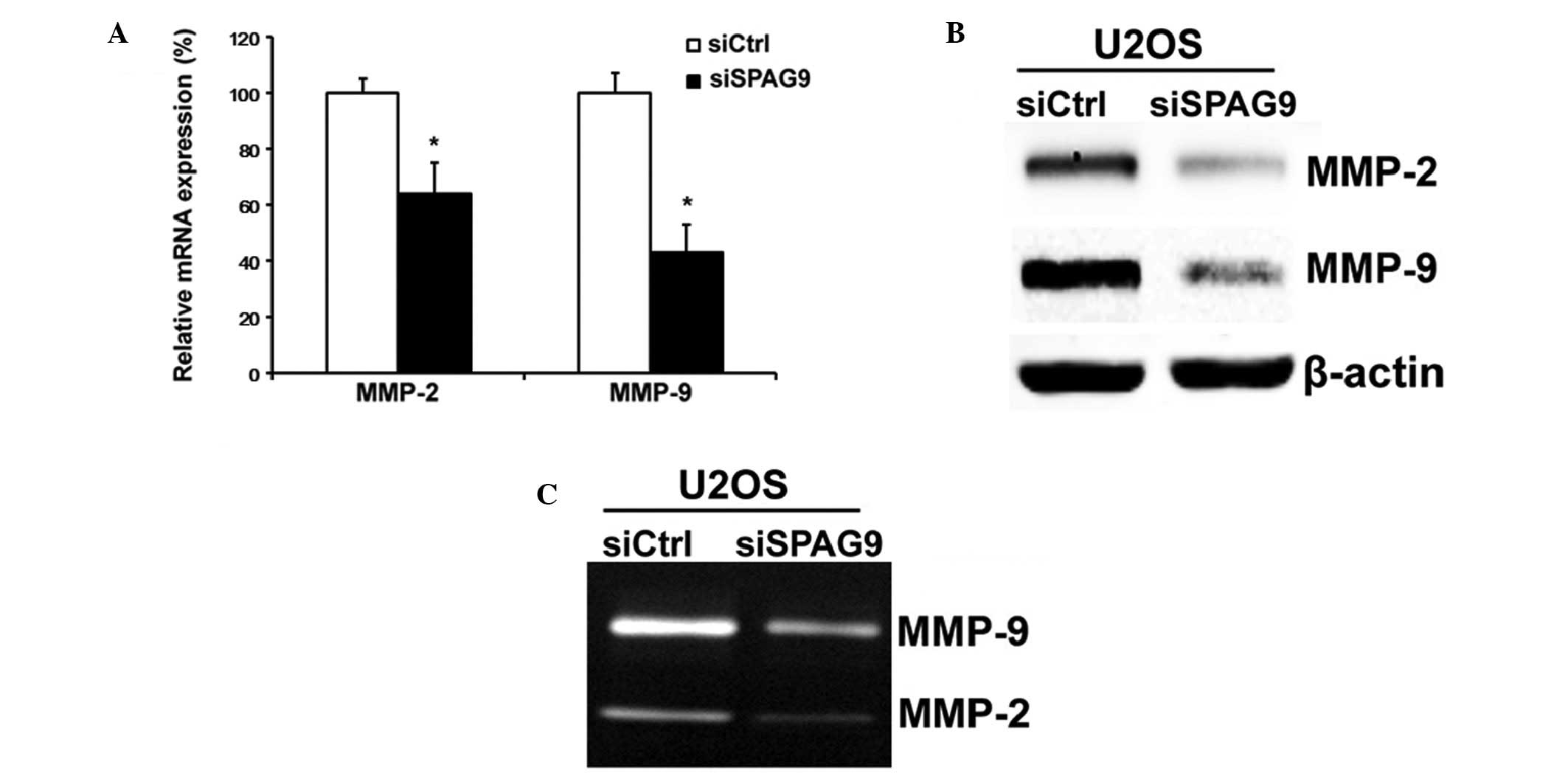
Knockdown of SPAG9 suppresses MMP-2
and MMP-9 expression levels and activity in OS cells
MMPs, in particular MMP-2 and MMP-9, have important
roles in the invasion and metastasis of OS cells. Therefore,
RT-qPCR, western blot and gelatin zymography analyses were employed
in order to determine the expression levels of MMP-2 and MMP-9 in
si-SPAG9-transfected cancer cells. The results of the present study
demonstrated that MMP-2 and MMP-9 mRNA and protein expression
levels and activity were markedly reduced in U2OS cells transfected
with si-SPAG9 (Fig. 5A–C), as
compared with the control.
SPAG9 regulates VEGF expression and
secretion in OS cells
VEGF is a key mitogen and survival factor in
endothelial cells, as endothelial cells enter an active
proliferative state in response to angiogenic stimulation. To
evaluate whether depletion of SPAG9 contributes to VEGF expression
and secretion in OS cells, a western blot analysis was performed in
order to evaluate protein expression levels. A subsequent ELISA
analysis was used to measure the secretion of VEGF in the
conditioned medium from U2OS cells transfected with SPAG9 siRNA. A
reduction in VEGF expression and secretion was detected in the
conditioned medium from U2OS cells transfected with SPAG9 siRNA, as
compared with the control cells (Fig. 6A
and B).
Discussion
SPAG9 is localized on the surface of the sperm and
is only expressed in haploid germ cells during spermatogenesis
(9). Previous studies have
demonstrated that SPAG9 may promote sperm-egg fusion, which is
characterized by an increase in intracellular Ca2+ and
pH, and the tyrosine phosphorylation of several proteins (20,21).
Since SPAG9 is highly expressed in cancer tissues, the role of
SPAG9 in cancer has been well-studied. Previous studies have
suggested that SPAG9 may be useful as a tumor marker for various
types of human cancer, including breast, thyroid, colorectal and
renal cell carcinoma (12,17,22). In
addition, a previous study demonstrated that knockdown of SPAG9
inhibited tumor growth of renal cell carcinoma in vivo,
indicating its potential role in the regulation of tumor
development and metastasis (12).
These observations indicated that SPAG9 may have an important role
in the tumorigenesis of human cancer; however, the association
between SPAG9 and OS has yet to be examined. In order to elucidate
the role of SPAG9 in OS metastasis, tissue samples and an in
vitro cell model were used to investigate whether SPAG9 is
associated with the metastasis of OS. The results of the present
study demonstrated that SPAG9 was highly expressed in OS tissues
and knockdown of SPAG9 by siRNA in OS cells reduced cell migration,
invasion and angiogenesis.
Metastasis is a complex multistep process that
requires detachment from the primary tumor mass, migration through
the ECM and the colonization of surrounding sites; therefore, cell
motility and invasion are essential for this process (23). The present study demonstrated that
knockdown of SPAG9 suppressed the motility and invasion of OS
cells, as assessed by wound healing and transwell assays. One of
the key factors associated with the invasion and metastasis of
cancer cells is the degradation of ECM, as this allows cancer cells
to invade and migrate. MMPs have an important role in this process
(24), and MMP-2 and MMP-9 are
postulated to promote the invasion and metastasis of OS cells to
the lymph nodes (25). In order to
determine how SPAG9 siRNA inhibits the motility and invasion of OS
cells, the present study aimed to elucidate the effects of SPAG9
siRNA on MMP-2 and MMP-9 activity levels. Knockdown of SPAG9
expression significantly inhibited the expression levels and
bioactivities of MMP-2 and MMP-9 in OS cells. The results of the
present study indicated that SPAG9 may promote cell motility and
invasion by increasing MMP-2 and MMP-9 expression levels and enzyme
activity. However, it remains to be elucidated how SPAG9 regulates
MMP-2 and MMP-9 expression and activity levels, and signaling
pathway in order to regulate OS cell metastasis.
Angiogenesis is essential for the growth and
metastasis of cancer cells. Previous studies have indicated that
angiogenesis is associated with tumor metastasis in various types
of cancer, including breast, lung, prostate, head, neck and
melanoma (26–28). Therefore, the suppression of
angiogenesis is emerging as a promising therapeutic strategy for
the treatment of cancer (29).
However, the effects of SPAG9 on angiogenesis are yet to be
reported. The present study demonstrated that the transfection of
SPAG9 siRNA reduced the capacity of OS cell supernatant to
stimulate tube formation and proliferation of HUVECs, as compared
with the control cells, suggesting that knockdown of SPAG9
expression levels significantly impaired the angiogenic potential
of OS cells in vitro. However, the molecular bases for this
hypothesis remain unclear. VEGF is considered a key mediator of
tumor angiogenesis associated with the development of tumor blood
supply and the progression of cancer (30). VEGF expression levels are
downregulated by tumor suppressor genes, including p53, p75 and von
Hippel-Lindau, which most likely occurs via the formation of
complexes with Sp1, and the inhibition of its binding and
subsequent transcriptional activation of the VEGF promoter
(31,32). The present study investigated whether
SPAG9 regulated VEGF expression and activity levels in
vitro. The results demonstrated that VEGF expression and
secretion levels were decreased following SPAG9 knockdown,
suggesting that decreased SPAG9 expression suppresses blood vessel
formation by regulating VEGF expression and activity levels.
Previous studies have demonstrated that SPAG9 participates in c-Jun
N-terminal kinases (JNK) pathway activation, and JNK signaling may
induce c-Jun phosphorylation at the VEGF promoter and result in its
activation (12,33); therefore it is possible that JNK
signaling is involved in the angiogenesis-promoting function of
SPAG9.
In conclusion, the key findings of the present study
demonstrated that SPAG9 may be involved in promoting OS cell
motility, invasion and angiogenesis. Silencing of SPAG9 led to the
inhibition of motility and invasion in OS cells due to the
inactivation of MMP-2 and MMP-9. Furthermore, knockdown of SPAG9
suppressed blood vessel formation and the proliferation of HUVECs,
which may be associated with decreased secretion and expression of
VEGF. These findings will not only advance our knowledge of OS
biology, but may also help determine if SPAG9 has potential as a
novel therapeutic target for the treatment of patients with OS.
References
|
1
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age, State
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nau JY: New techniques in bone and joint
imaging (2). Rev Med Suisse. 1:6512005.(In French). PubMed/NCBI
|
|
3
|
Klein MJ and Siegal GP: Osteosarcoma:
Anatomic and histologic variants. Am J Clin Pathol. 125:555–581.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hawkins DS and Arndt CA: Pattern of
disease recurrence and prognostic factors in patients with
osteosarcoma treated with contemporary chemotherapy. Cancer.
98:2447–2456. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu PK, Chen WM, Chen CF, Lee OK, Haung CK
and Chen TH: Primary osteogenic sarcoma with pulmonary metastasis,
Clinical results and prognostic factors in 91 patients. Jpn J Clin
Oncol. 39:514–522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan ML, Choong PF and Dass CR:
Osteosarcoma. Conventional treatment vs. Histopathology. Cancer
Biol Ther. 8:106–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simpson AJ, Caballero OL, Jungbluth A,
Chen YT and Old LJ: Cancer/testis antigens, gametogenesis and
cancer. Nat Rev Cancer. 5:615–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zendman AJ, Ruiter DJ and Van Muijen GN:
Cancer/testis-associated, genes. Identification, expression profile
and putative function. J Cell Physiol. 194:272–288. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jagadish N, Rana R, Selvi R, Mishra D,
Garg M, Yadav S, Herr JC, Okumura K, Hasegawa A, Koyama K and Suri
A: Characterization of a novel human sperm-associated antigen 9
(SPAG9) having structural homology with c-Jun N-terminal
kinase-interacting protein. Biochem J. 389:73–82. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jagadish N, Rana R, Mishra D, Kumar M and
Suri A: Sperm associated antigen 9 (SPAG9): A new member of c-Jun
NH2 -terminal kinase (JNK) interacting protein exclusively
expressed in testis. Keio J Med. 54:66–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garg M, Chaurasiya D, Rana R, Jagadish N,
Kanojia D, Dudha N, Kamran N, Salhan S, Bhatnagar A, Suri S, et al:
Sperm-associated antigen 9, a novel cancer testis antigen, is a
potential target for immunotherapy in epithelial ovarian cancer.
Clin Cancer Res. 13:1421–1428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garg M, Kanojia D, Khosla A, Dudha N, Sati
S, Chaurasiya D, Jagadish N, Seth A, Kumar R, Gupta S, et al:
Sperm-associated antigen 9 is associated with tumor growth,
migration and invasion in renal cell carcinoma. Cancer Res.
68:8240–8248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sinha A, Agarwal S, Parashar D, Verma A,
Saini S, Jagadish N, Ansari AS, Lohiya NK and Suri A: Down
regulation of SPAG9 reduces growth and invasive potential of
triple-negative breast cancer cells, Possible implications in
targeted therapy. J Exp Clin Cancer Res. 32:692013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Volard B, Krieger S, Planchard G, Hardouin
A, Vaur D, Rame JP and Bardet S: Assessment of SPAG9 transcript in
fine needle aspirates of thyroid nodules. Eur Thyroid J. 1:118–121.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanojia D, Garg M, Gupta S, Gupta A and
Suri A: Sperm-associated antigen 9 is a novel biomarker for
colorectal cancer and is involved in tumor growth and
tumorigenicity. Am J Pathol. 178:1009–1020. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Dong Q, Miao Y, Fu L, Lin X and
Wang E: Clinical significance and biological roles of SPAG9
overexpression in non-small cell lung cancer. Lung Cancer.
81:266–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanojia D, Garg M, Gupta S, Gupta A and
Suri A: Sperm-associated antigen 9, a novel biomarker for early
detection of breast cancer. Cancer Epidemiol Biomarkers Prev.
18:630–639. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garg M, Kanojia D, Suri S and Suri A:
Small interfering RNA-mediated down-regulation of SPAG9 inhibits
cervical tumor growth. Cancer. 115:5688–5699. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burks DJ, Carballada R, Moore HD and
Saling PM: Interaction of a tyrosine kinase from human sperm with
the zona pellucida at fertilization. Science. 269:83–86. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luconi M, Krausz C, Forti G and Baldi E:
Extracellular calcium negatively modulates tyrosine phosphorylation
and tyrosine kinase activity during capacitation of human
spermatozoa. Biol Reprod. 55:207–216. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garg M, Kanojia D, Salhan S, Suri S, Gupta
A, Lohiya NK and Suri A: Sperm-associated antigen 9 is a biomarker
for early cervical carcinoma. Cancer. 115:2671–2683. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hynes RO: Metastatic potential: Generic
predisposition of the primary tumor or rare metastatic variants-or
both? Cell. 113:821–823. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Halbersztadt A, Haloń A, Pajak J,
Robaczynski J, Rabczynski J and St Gabryś M: The role of matrix
metalloproteinases in tumor invasion and metastasis. Ginekol Pol.
77:63–71. 2006.(In Polish). PubMed/NCBI
|
|
25
|
Zhang XY, Hong BF, Chen GF, Lu YL and
Zhong M: Significance of MMP2 and MMP9 expression in prostate
cancer. Zhonghua Nan Ke Xue. 11:359–361. 2005.(In Chinese).
PubMed/NCBI
|
|
26
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:9–19.
1995.PubMed/NCBI
|
|
28
|
Czubayko F, Schulte AM, Berchem GJ and
Wellstein A: Melanoma angiogenesis and metastasis modulated by
ribozyme targeting of the secreted growth factor pleiotrophin. Proc
Natl Acad Sci USA. 93:14753–14758. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Folkman J: Tumor angiogenesis: T
herapeutic implications. N Engl J Med. 285:1182–1186. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao YEG, Wang E, Pal K, Dutta SK, Bar-Sagi
D and Mukhopadhyay D: VEGF exerts an angiogenesis-independent
function in cancer cells to promote their malignant progression.
Cancer Res. 72:3912–3918. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie K, Wei D, Shi Q and Huang S:
Constitutive and inducible expression and regulation of vascular
endothelial growth factor. Cytokine Growth Factor Rev. 15:297–324.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pal S, Datta K and Mukhopadhyay D: Central
role of p53 on regulation of vascular permeability factor/vascular
endothelial growth factor (VPF/VEGF) expression in mammary
carcinoma. Cancer Res. 61:6952–6957. 2001.PubMed/NCBI
|
|
33
|
Guma M, Rius J, Duong-Polk KX, Haddad GG,
Lindsey JD and Karin M: Genetic and pharmacological inhibition of
JNK ameliorates hypoxia-induced retinopathy through interference
with VEGF expression. Proc Natl Acad Sci USA. 106:8760–8765. 2009.
View Article : Google Scholar : PubMed/NCBI
|