Introduction
There is a higher incidence of acute kidney injury,
for several reasons including ischemia and hypoxia, poisoning,
infection, reperfusion, which may contribute to apoptosis or
necrosis of renal tubular epithelial cells by the pathogenesis of
inflammation, oxidative stress, calcium overload and metabolic
disorders of energy (1,2). Notch2 can be widely expressed in the
development process of kidney, and then turn silent when mature,
and can be activated to participate in the disease process in a
variety of external stimulation rapidly and largely such as sugar,
in ischemia reperfusion injury (3).
The Notch inhibitor DAPT (γ-inhibitor) has a protective effect on
acute kidney injury (4).
Jinguishenqi pill as an ancient prescription for tonifying kidney
and kidney ‘qi’, (5,6) has better application effect in acute
and chronic renal injury disease.
We analyzed the repairing mechanism of Shenqi pill
in vitro on acute injury of renal tubular epithelial cells,
to find the optimal blood concentration.
Materials and methods
Main materials and reagents
Rat proximal renal tubular epithelial cell strains
(NRK-52E) were purchased from Cell Resource of Shanghai Institutes
for Biological Sciences Center of Chinese Academy of Sciences
Committee on Type Culture Collection Cell Bank (Shanghai, China).
Dulbecco's modified Eagle's medium (DMEM)/F12 medium powder,
trypsin (Gibco, Grand Island, NY, USA), high pure nitrogen of
high-pressure (Shanxi Taiyuan Pharmaceutical Co., Ltd., Taiyuan,
China), total RNA extraction kit, polymerase chain reaction (PCR)
primers, TranScript cDNA first-strand synthesis kit, SYBR-Green I
real-time PCR kits, pre-stained protein molecular weight marker
(Beijing Zhongshan Science and Technology Co., Ltd., Beijing,
China), ECL chemiluminescence kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA), BCA protein assay kit (Kaiji Biological
Technology Development Co., Ltd., Nanjing, China), rabbit
anti-human Jag2/Notch2/hes1 and β-actin monoclonal antibody (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), horseradish
peroxidase-labeled goat anti-rabbit IgG (Sigma-Aldrich, St. Louis,
MO, USA) were purchased.
Main instruments
HEPA-CLASS 100 three gas tissue incubator (Thermo
Fisher Scientific, Waltham, MA, USA), inverted microscope (Leica,
Mannheim, Germany), ESP Elite flow cytometer (Beckman Coulter,
Fullerton, CA, USA), micropipette (Eppendorf AG, Hamburg, Germany),
SW-CJ-2FD type double-sided clean bench (Zhejiang Sujing
Purification Equipment Co., Ltd., Sujing, China), TGL-16B
high-speed desktop centrifuge (Shanghai Anting Scientific
Instrument Factory, Shanghai, China), cell culture flasks, culture
plates (Corning Inc., Acton, MA, USA), 721 spectrophotometer (Third
Shanghai Analytical Instrument Factory, Shanghai, China), DYCZ-40D
mini transfer electrophoresis and WD-9465B horizontal shaker
(Beijing Liuyi Instrument Factory, Beijing, China).
Animal model of
ischemia/reperfusion
Conventional recovery, culture, subculture, frozen
storage, cells which had been passaged 2–3 times and reached to 80%
cell confluence after replaced with serum-free DMEM/F12 medium for
24 h. The pure N2 was aerated continuously into
oxygen-free liquid for 30 min in advance, to reach saturation. The
three gas incubators were adjusted, and filled with 95%
N2 + 1% O2 + 4% CO2. The cell
culture medium was discarded, washed twice with phosphate-buffered
saline (PBS) and incubated for hypoxia cultivation. It was followed
by removal of liquid inside and oxygen-free liquid was added.
Hypoxic cells were removed after 12 h, oxygen-free supernatants
were collected and washed with the PBS, then added into complete
DMEM/F12 medium. The cells were cultivated with reoxygenation in
the three gas incubators adjusted to 95% air + 5% CO2,
after 12 h cells and oxygen culture supernatant were collected.
Grouping
The animals were randomly divided into the control,
model, low concentration (5 µg/ml), moderate concentration (10
µg/ml) and high concentrations (20 µg/ml) groups. After dissolving
kidney pills in saline, the different concentration criterions were
adjusted in terms of quality, experiment was co-cultured with 1 ml
medicine. The control group was normal renal tubular epithelial
cells, and the model group was applied with 1 ml saline. The
apoptotic rate was measured with flow cytometry and
Jag2/Notch2/hes1 mRNA as well as protein expression with
quantitative PCR and western blot analysis detection, respectively,
at 1, 3 and 7 days. Five samples were selected at each time-point,
and results are the average values.
Detection of cell apoptosis by flow
cytometry
The NRK-52E cells were seeded into six pore plates
in accordance with 5×105 cells/pore density. The
pancreatic cells (200 µl) were added and centrifuged for 5 min at
800 × g. The supernatant was discarded; washed with pre-cold PBS
and centrifuged for 5 min at 800 × g, and was repeated twice,
followed by removal of the supernatant. Subsequently, 500-µl
binding buffer suspension cells were added, 5 µl Annexin V-FITC was
added and mixed well. The cells were then incubated for 15 min at
4°C, and 5 µl PI was added to homogenize the content in the dark at
room temperature for 5–15 min, before detection within 1 h.
Quantitative PCR detection
Conventional TRIzol extraction of total RNA,
spectrophotometer purity and concentration, led to the production
of cDNA. The primer sequences were: Jag2 forward,
5′-TGTGGTTGGAAGCCTTGTCTG-3′ and reverse,
5′-TGTCCAAATGAGTGGTCCGT-3′; Notch2 forward,
5′-CACCAACTTACGCATTAAACAAGAC-3′ and reverse,
5′-TCCGATTCACTGACAACAGACAC-3′; hes1 forward,
5′-TGCTTTCCTCATCCCCAATG-3′ and reverse, 5′-GAAGGCGACACTGCGTTAGG-3′;
the internal control β-actin forward, 5′-CGTTGACATCCGTAAAGACCTC-3′
and reverse, 5′-TAGGAGCCAGGGCAGTAATCT-3′. The reaction system
consisted of cDNA (5 µl), target gene downstream primer (100
pmol/l, 2 µl), 10X buffer (2.5 µl), 10 mmol dBTPs (3 µl), Taq
enzyme (1 µl), ultra-pure water to total volume of 25 µl. The
reaction parameters included denaturation at 95°C for l min, 95°C
for 15 sec, 58°C for 20 sec, 72°C for 20 sec for 40 cycles in
total, and a final extension at 72°C for 5 min. The final
solubility curve results were expressed in the ratio of the target
gene and reference gene, using the 2−∆∆Cq formula.
Western blot assay
Total protein was extracted and quantified with
Bradford assay. Western blotting was performed as described
elsewhere (7). The primary antibody
was used for incubation overnight at 4°C. The following day, the
membrane was washed three times with TBST at room temperature and
the secondary antibody diluted with TBST 3,000 times, was used for
incubation at room temperature 30 min. It was then washed three
times with TBST at room temperature. Subsequently, the
chemiluminescence reaction was used for developing the protein
images. Rabbit polyclonal JAG2 antibody (dilution, 1:500; cat. no.
ab109627), rabbit polyclonal Notch2 antibody (dilution, 1:500; cat.
no. ab8926), rabbit polyclonal Hes1 antibody (dilution, 1:500; cat.
no. ab71559) and secondary goat anti-rabbit (HRP) IgG antibody
(dilution, 1/2000; cat. no. ab6721) were all purchased from Abcam
(Cambridge, MA, USA).
Statistical analysis
SPSS 19.0 (Chicago, IL, USA) was used to analyze the
data. Quantitative data are shown as mean ± standard deviation, the
comparison among groups was shown by using single-factor ANOVA
analysis, while repeated measures analysis of variance was applied
in the intra-group. Qualitative data were compared with the number
of cases or percentage (%), χ2 test was used among
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of the rate of
apoptosis
In addition to the control group, the other groups
took longer to grow. The apoptotic rate decreased and at each
time-point of the moderate concentration and high concentration
groups was significantly lower than that of the model and low
concentration groups (P<0.05) (Table
I and Fig. 1).
 | Table I.Comparison of cell apoptosis rate
(%). |
Table I.
Comparison of cell apoptosis rate
(%).
| Groups | 1 day | 3 days | 7 days |
|---|
| Control |
0.3±0.1 |
0.2±0.1 |
0.2±0.1 |
| Model | 26.5±4.2 | 24.2±3.5 | 20.1±3.2 |
| Low
concentration | 21.4±4.0 | 20.2±3.6 | 15.5±3.2 |
| Moderate
concentration | 13.2±3.3 | 7.5±2.0 | 3.4±1.1 |
| High
concentraition | 13.3±3.4 | 7.6±2.2 | 3.5±1.3 |
| F-value | 7.524 | 9.623 | 12.522 |
| P-value | <0.001 | <0.001 | <0.001 |
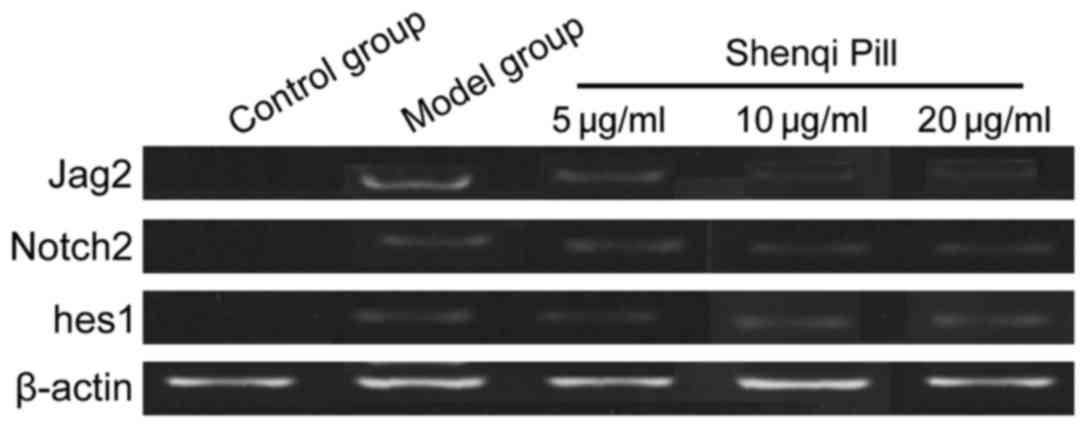
Comparison of mRNA Jag2/Notch2/hes1
expression levels
In addition to the control group, the other groups
were grown with extension of time. The mRNA Jag2/Notch2/hes1
expression levels decreased and the expression levels of the mRNA
Jag2/Notch2/hes1 of the concentration and high concentration group
at each time-point was significantly lower than that of the model
and low-dose groups, and the difference was significant (P<0.05)
(Table II and Fig. 2).
 | Table II.Comparison of mRNA Jag2/Notch2/hes1
expression levels. |
Table II.
Comparison of mRNA Jag2/Notch2/hes1
expression levels.
|
| Jag2 | Notch2 | hes1 |
|---|
|
|
|
|
|
|---|
| Groups | 1 day | 3 days | 7 days | 1 day | 3 days | 7 days | 1 day | 3 days | 7 days |
|---|
| Control | 0.06±0.02 | 0.05±0.01 | 0.05±0.02 | 0.04±0.01 | 0.03±0.01 | 0.03±0.01 | 0.03±0.01 | 0.02±0.01 | 0.03±0.01 |
| Model | 0.42±0.06 | 0.33±0.04 | 0.28±0.04 | 0.36±0.04 | 0.30±0.03 | 0.26±0.03 | 0.34±0.03 | 0.30±0.03 | 0.25±0.03 |
| Low
concentration | 0.40±0.05 | 0.30±0.05 | 0.25±0.03 | 0.33±0.03 | 0.27±0.04 | 0.24±0.03 | 0.31±0.03 | 0.27±0.03 | 0.22±0.03 |
| Moderate
concentration | 0.32±0.04 | 0.26±0.03 | 0.19±0.03 | 0.26±0.03 | 0.20±0.02 | 0.13±0.02 | 0.25±0.03 | 0.18±0.03 | 0.12±0.03 |
| High
concentration | 0.33±0.04 | 0.28±0.04 | 0.21±0.04 | 0.27±0.03 | 0.21±0.03 | 0.15±0.02 | 0.25±0.03 | 0.20±0.03 | 0.13±0.03 |
| F-value | 6.529 | 6.967 | 7.421 | 7.201 | 7.424 | 7.625 | 6.938 | 6.857 | 7.203 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
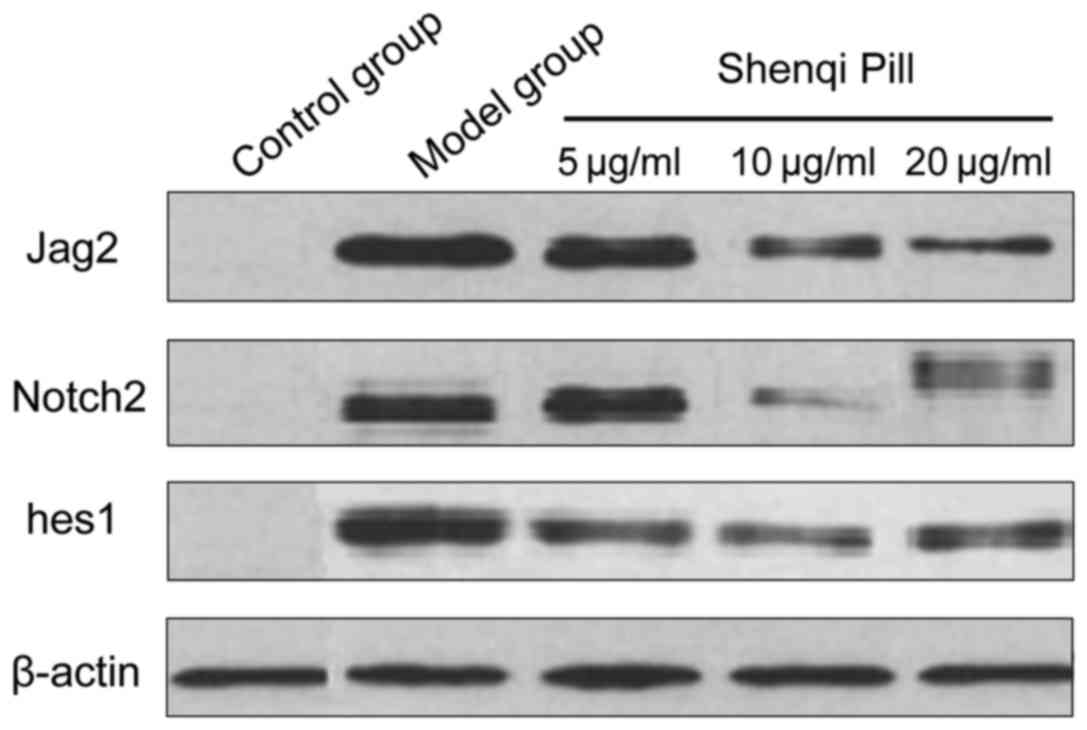
Comparison of Jag2/Notch2/hes1 protein
expression levels
In addition to the control group, the other groups
took longer to grow. The Jag2/Notch2/hes1 protein expression levels
decreased and the Jag2/Notch2/hes1 protein expression levels of
concentration and high concentration group at each time-point was
significantly lower than that of the model group and low-dose group
(P<0.05) (Table III and
Fig. 3).
 | Table III.Comparison of Jag2/Notch2/hes1 protein
expression levels. |
Table III.
Comparison of Jag2/Notch2/hes1 protein
expression levels.
| Groups | Jag2 | Notch2 | hes1 |
|---|
|
|
|
|
|
|---|
|
| 1 day | 3 days | 7 days | 1 day | 3 days | 7 days | 1 day | 3 days | 7 days |
|---|
| Control | 0.06±0.02 | 0.05±0.01 | 0.05±0.02 | 0.04±0.01 | 0.03±0.01 | 0.03±0.01 | 0.03±0.01 | 0.02±0.01 | 0.03±0.01 |
| Model | 0.48±0.07 | 0.41±0.06 | 0.36±0.06 | 0.39±0.05 | 0.32±0.04 | 0.28±0.04 | 0.36±0.04 | 0.32±0.04 | 0.27±0.03 |
| Low
concentration | 0.44±0.05 | 0.39±0.06 | 0.33±0.05 | 0.35±0.04 | 0.30±0.04 | 0.26±0.05 | 0.33±0.04 | 0.29±0.03 | 0.24±0.04 |
| Moderate
concentration | 0.35±0.04 | 0.28±0.05 | 0.20±0.04 | 0.28±0.04 | 0.22±0.03 | 0.15±0.03 | 0.26±0.04 | 0.19±0.04 | 0.15±0.03 |
| High
concentration | 0.36±0.04 | 0.29±0.06 | 0.21±0.05 | 0.29±0.04 | 0.21±0.05 | 0.16±0.03 | 0.27±0.05 | 0.20±0.03 | 0.15±0.04 |
| F-value | 7.231 | 7.345 | 7.626 | 7.528 | 7.717 | 7.962 | 8.231 | 8.465 | 8.721 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Discussion
Notch signaling pathway is a major signaling
pathway, which mediates the direct contact between the cells and
also plays an important role in the regulation of cell growth,
differentiation, tissue regeneration and the stability of
intracellular environment. The Notch family consists of a group of
highly conserved proteins including four cognate receptor Notch
l-4, 5 types of homolog ligand Delta-like 1, 3 and 4, and Jagged 1
and 2, and two types of downstream target genes: The Hes and Hey
families. When tissue is subjected to hypoxia and drug damage, the
activation of ligand expression would increase, which can result in
the series of proteolytic cleavage of Notch receptors via the Notch
receptor binding through cell to cell contact, and then release the
Notch intracellular domain of activity; the Notch intracellular
domain activity is transferred to the cell nucleus and combined
with the transcription factor binding, starting the transcription
of the target gene, regulating and controlling the cell
proliferation (7,8).
The Jinguishenqi pill is composed of 8 g
Rehmannia, jujube peel, 4 g Chinese yam, 3 g each of
Alisma, paeonol, Poria cocos and Cassia twig,
and 1 g aconite. The original description: ‘Rehmannia is
sweet-warm, enriching yin and nourishing the kidney as the main
drug, supplemented by acid tepid dogwood, nourishing liver and
kidney, protecting vital essence with astringency; yam was sweet
taste and neutral nature, good at strengthening spleen and
tonifying kidney; plus a small amount of aconite and cinnamon which
could warm kidney and enhance Yangqi, fill Mingmen real fire,
conduct the fire back to its origin; accompanied with plantain
regulating the waterways, draining water evil in renal; tuckahoe
tonifying spleen, clearing damp and promoting diuresis; cortex
mouton clearing liver fire’, in various drug combinations (9,10). The
study indicated that in addition to the control group, each group
grew for a longer time. The levels of apoptosis rate,
Jag2/Notch2/hes1 mRNA and protein expression decreased and the
levels of apoptosis, Jag2/Notch2/hes1 mRNA and protein expression
of concentration and high concentration group were obviously lower
than those of the model group and low dose group at each
time-point, the difference was statistically significant.
Glomerular apoptosis rate reached the peak when acute kidney injury
occurred, and then decreased gradually. Jag2/Notch2/hes1 mRNA and
protein expression levels were also decreased, these changes
suggested that early intervention of acute kidney injury could
reduce the extent of tissue damage, and be conducive to
regeneration of tissue (11). Shenqi
Pill could relieve the damage of renal tubular epithelial cells by
inhibiting the activation and expression of Jag2/Notch2/hes1
signaling pathway. The different concentrations had different
effects, low concentration slightly inhibited Jag2/Notch2/hes1 mRNA
and protein expression, a suitable concentration such as 10–20 g/ml
could play a better supporting effect. The specific reasons for not
getting improved effects by the increase of the concentration
should be further analyzed.
The present study has confirmed the inhibitory
effects of Shenqi Pill on Jag2/Notch2/hes1 signaling pathway by
experiments in vitro. As the Shenqi pill is a compound drug,
it was not possible to determine the specific components which
played a major role. Application effects can be further explored in
in vivo animal experiments or clinical studies.
References
|
1
|
Gao Y, Zeng Z, Li T, Xu S, Wang X, Chen Z
and Lin C: Polydatin inhibits mitochondrial dysfunction in the
renal tubular epithelial cells of a rat model of sepsis-induced
acute kidney injury. Anesth Analg. 121:1251–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li K, Han Q, Yan X, Liao L and Zhao RC:
Not a process of simple vicariousness, the differentiation of human
adipose-derived mesenchymal stem cells to renal tubular epithelial
cells plays an important role in acute kidney injury repairing.
Stem Cells Dev. 19:1267–1275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng HT and Kopan R: The role of Notch
signaling in specification of podocyte and proximal tubules within
the developing mouse kidney. Kidney Int. 68:1951–1952. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang R, Zhou Q, Veeraragoo P, Yu H and
Xiao Z: Notch2/Hes-1 pathway plays an important role in renal
ischemia and reperfusion injury-associated inflammation and
apoptosis and the γ-secretase inhibitor DAPT has a nephroprotective
effect. Ren Fail. 33:207–216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nan Y, Zhou X, Liu Q, Zhang A, Guan Y, Lin
S, Kong L, Han Y and Wang X: Serum metabolomics strategy for
understanding pharmacological effects of ShenQi pill acting on
kidney yang deficiency syndrome. J Chromatogr B Analyt Technol
Biomed Life Sci. 19:102–103. 2015.
|
|
6
|
Xiong X, Wang P, Li X and Zhang Y: Shenqi
pill, a traditional Chinese herbal formula, for the treatment of
hypertension: a systematic review. Complement Ther Med. 23:484–493.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kramer J, Schwanbeck R, Pagel H, Cakiroglu
F, Rohwedel J and Just U: Inhibition of Notch signaling ameliorates
acute kidney failure and downregulates platelet-derived growth
factor receptor β in the mouse model. Cells Tissues Organs.
201:109–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Juillerat-Jeanneret L, Flohr A, Schneider
M, Walter I, Wyss JC, Kumar R, Golshayan D and Aebi JD: Targeted
γ-secretase inhibition to control the Notch pathway in renal
diseases. J Med Chem. 58:8097–8109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Long YL and Li ZM: Effect of jingui shenqi
pill and its disassembled recipes on ovarian functions in shen yang
deficiency female rats. Zhongguo Zhong Xi Yi Jie He Za Zhi.
33:967–971. 2013.(In Chinese). PubMed/NCBI
|
|
10
|
Xu CP, Zhu QJ, Song J, Li Z and Zhang D:
Acceleration of Jingui Shenqi Pill on the testis telomerase
activity in mice of Shen-yang deficiency. Zhongguo Zhong Xi Yi Jie
He Za Zhi. 33:252–255. 2013.(In Chinese). PubMed/NCBI
|
|
11
|
Sörensen-Zender I, Rong S, Susnik N,
Zender S, Pennekamp P, Melk A, Haller H and Schmitt R: Renal
tubular Notch signaling triggers a prosenescent state after acute
kidney injury. Am J Physiol Renal Physiol. 306:F907–F915. 2014.
View Article : Google Scholar : PubMed/NCBI
|