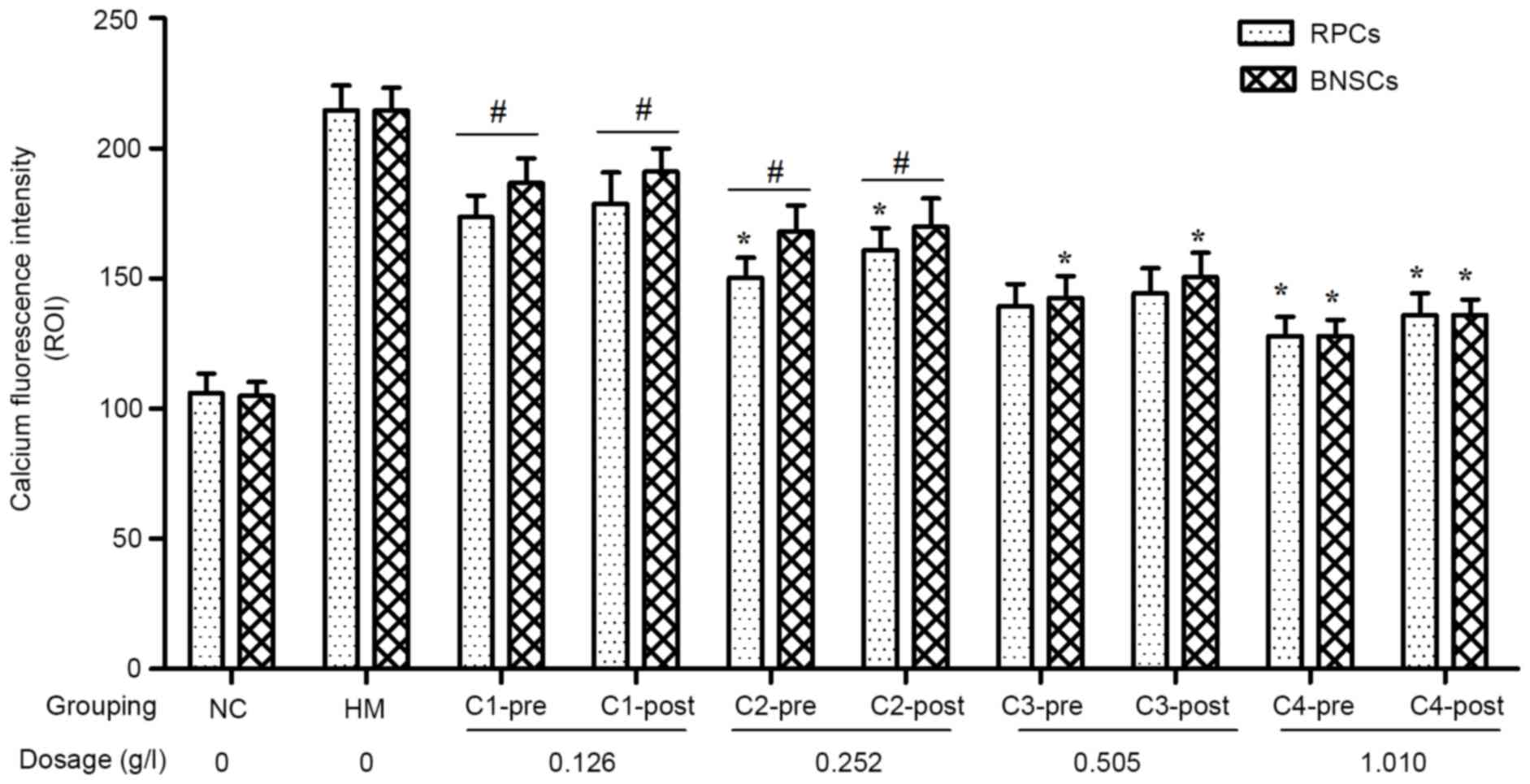
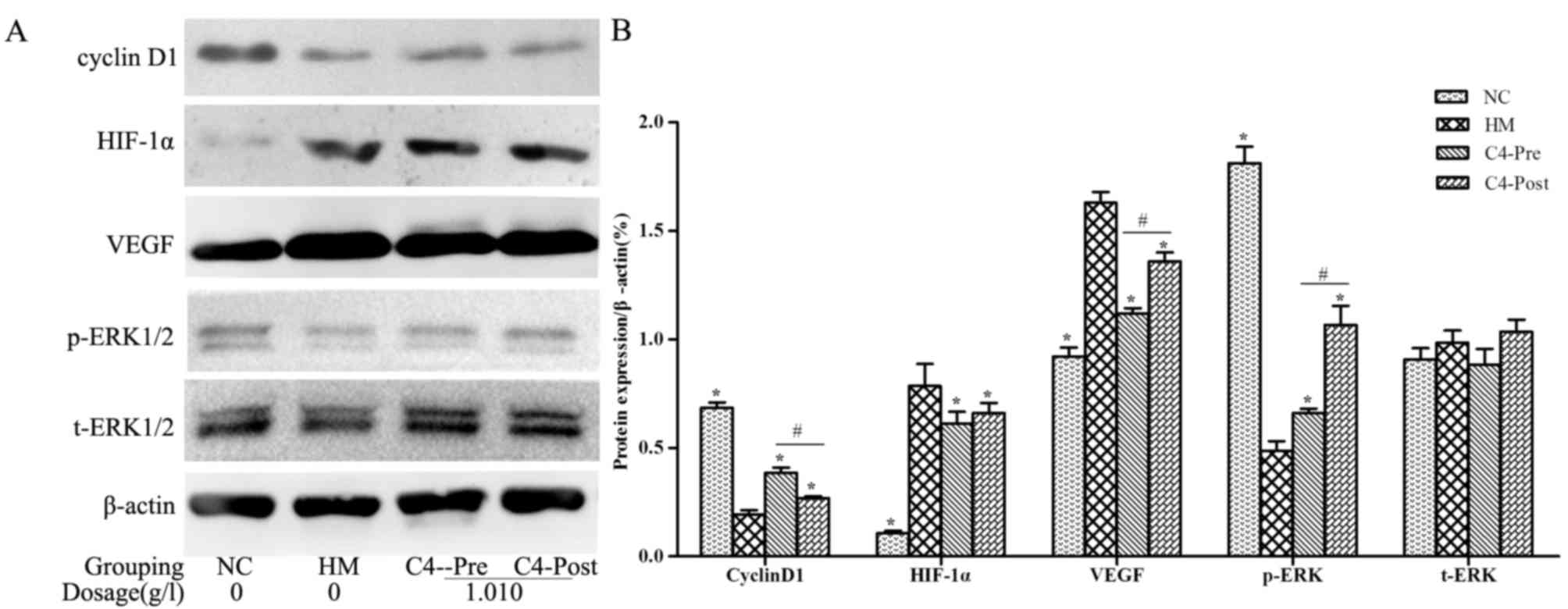
|
1
|
Garcia JM, Mendonça L, Brant R, Abud M,
Regatieri C and Diniz B: Stem cell therapy for retinal diseases.
World J Stem Cells. 7:160–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balmer J, Stanzel BV and Fischer MD: Stem
cell therapy for retinal diseases. Ophthalmologe. 112:728–737.
2015.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ng TK, Fortino VR, Pelaez D and Cheung HS:
Progress of mesenchymal stem cell therapy for neural and retinal
diseases. World J Stem Cells. 6:111–119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pellegrini G, De Luca M and Arsenijevic Y:
Towards therapeutic application of ocular stem cells. Semin Cell
Dev Biol. 18:805–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu D and Silva GA: Stem cell sources and
therapeutic approaches for central nervous system and neural
retinal disorders. Neurosurg Focus. 24:E112008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dunn-Thomas TE, Dobbs DL, Sakaguchi DS,
Young MJ, Honovar VG and Greenlee MH: Proteomic differentiation
between murine retinal and brain-derived progenitor cells. Stem
Cells Dev. 17:119–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu Z, Guan Y, Cui L, Song J, Gu J, Zhao H,
Xu L, Lu L, Jin Y and Xu GT: Transplantation of rat embryonic stem
cell-derived retinal progenitor cells preserves the retinal
structure and function in rat retinal degeneration. Stem Cell Res
Ther. 6:2192015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chacko DM, Das AV, Zhao X, James J,
Bhattacharya S and Ahmad I: Transplantation of ocular stem cells:
The role of injury in incorporation and differentiation of grafted
cells in the retina. Vision Res. 43:937–946. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Francis PJ, Wang S, Zhang Y, Brown A,
Hwang T, McFarland TJ, Jeffrey BG, Lu B, Wright L, Appukuttan B, et
al: Subretinal transplantation of forebrain progenitor cells in
nonhuman primates: Survival and intact retinal function. Invest
Ophthalmol Vis Sci. 50:3425–3431. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo Y, Saloupis P, Shaw SJ and Rickman DW:
Engraftment of adult neural progenitor cells transplanted to rat
retina injured by transient ischemia. Invest Ophthalmol Vis Sci.
44:3194–3201. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishida A, Takahashi M, Tanihara H, Nakano
I, Takahashi JB, Mizoguchi A, Ide C and Honda Y: Incorporation and
differentiation of hippocampus-derived neural stem cells
transplanted in injured adult rat retina. Invest Ophthalmol Vis
Sci. 41:4268–4274. 2000.PubMed/NCBI
|
|
12
|
Chen YH and Wu LQ: Therapeutic effect of
compound anisodine for primary open angle glaucoma. Zhejiang Da Xue
Xue Bao Yi Xue Ban. 40:659–662. 2011.(In Chinese). PubMed/NCBI
|
|
13
|
Wang W, Huang Y, Zhang J, Jiang J and
Huang J: Efficacy of cytidine-5′-diphosp-bocholine combined with
compound anisodine in the treatment of early optic nerve contusion.
Eye science. 27:37–40. 2012.PubMed/NCBI
|
|
14
|
Wang Z: Observation on clinical
application of compound Anisodine for patients with optic atrophy
disease. Huli Yanjiu. 24:16572010.
|
|
15
|
Yi CM, Yu HY, Zhang L, Mfng W, Peng HY,
Liu LB and Yang CY: Clinical observation of compound anisodine
treating for ischemic ophthalmopathy. Inner Mongolia Med J. 1:011,
31–34. 2008.
|
|
16
|
Kelley MW, Turner JK and Reh TA:
Regulation of proliferation and photoreceptor differentiation in
fetal human retinal cell cultures. Invest Ophthalmol Vis Sci.
36:1280–1289. 1995.PubMed/NCBI
|
|
17
|
Clarke L and van der Kooy D: Low oxygen
enhances primitive and definitive neural stem cell colony formation
by inhibiting distinct cell death pathways. Stem Cells.
27:1879–1886. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baranov PY, Tucker BA and Young MJ:
Low-oxygen culture conditions extend the multipotent properties of
human retinal progenitor cells. Tissue Eng Part A. 20:1465–1475.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu DY and Cringle SJ: Oxygen distribution
in the mouse retina. Invest Ophthalmol Vis Sci. 47:1109–1112. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cringle SJ and Yu DY: Oxygen supply and
consumption in the retina: Implications for studies of retinopathy
of prematurity. Doc Ophthalmol. 120:99–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo Z, Shi F, Zhang L, Zhang H, Yang J, Li
B, Jia J and Wang X and Wang X: Critical role of L-type
voltage-dependent Ca2+ channels in neural progenitor cell
proliferation induced by hypoxia. Neurosci Lett. 478:156–160. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song C, Shen W and He Q: An experimental
study on compound anisodine III for softening scar of mouse skin
after burn. Zhonghua Yan Ke Za Zhi. 32:176–178. 1996.(In Chinese).
PubMed/NCBI
|
|
23
|
Zhu Y, Song C and Wang S: The changes of
hemodynamics in ocular trauma and treatment with compound
anisodine. Zhonghua Yan Ke Za Zhi. 32:110–113. 1996.(In Chinese).
PubMed/NCBI
|
|
24
|
Liu WD, Chen LL, Shen CY and Jiang LB:
Neuroprotective effect of compound anisodine in a mouse model with
chronic ocular hypertension. Chin Med J (Engl). 128:2652–2657.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ejtehadifar M, Shamsasenjan K,
Movassaghpour A, Akbarzadehlaleh P, Dehdilani N, Abbasi P,
Molaeipour Z and Saleh M: The effect of hypoxia on mesenchymal stem
cell biology. Adv Pharm Bull. 5:141–149. 2015. View Article : Google Scholar : PubMed/NCBI
|