Introduction
Diabetic nephropathy is the most common
microvascular complication of diabetes and the most common cause of
end-stage renal failure (1). With
the modern changes in dietary structure and life style, the
morbidity of diabetes has increased steadily. More than 10% of
people aged over 60 years suffer from type 2 diabetes (2), and ~20% of diabetic patients develop
diabetic nephropathy. Diabetic nephropathy is the second leading
cause of death in diabetic patients and deserves special clinical
attention (3).
Alprostadil is a drug that can effectively expand
renal blood vessels and directly act on glomerular arteries and
their smooth muscles after spasms caused by increased blood glucose
(4). Thus, alprostadil increases
renal blood flow, reduces the renal vascular resistance, and
glomerular capillary pressure (5),
improving renal blood supply and reducing the level of urine
protein (6). Alprostadil also
demonstrates activity inhibiting platelet aggregation,
anti-oxidation and stabilizing cell membranes. The treatment of
diabetic nephropathy has a long history, but a single application
of alprostadil fails to delay the pathological changes in renal
vascular endothelial cells; hence, alprostadil alone cannot
fundamentally prevent diabetic nephropathy. Calcium dobesilate can
effectively inhibit the synthesis of transforming growth factor
(TGF) and protein kinase C, thereby reducing blood viscosity and
vascular wall permeability. Moreover, calcium dobesilate has a
protective effect on the vascular endothelium, thus improving renal
function (7). In order to improve
the clinical outcomes and reverse the pathological process in
diabetic nephropathy, this study mainly discussed the therapeutic
value of alprostadil combined with calcium dobesilate for diabetic
nephropathy.
Subjects and methods
Subject data
We recruited 80 patients with diabetic nephropathy
in Weifang People's Hospital from January, 2015 to December, 2016.
Patients were comprehensively diagnosed via clinical manifestations
and biochemical tests, and they conformed to the diagnostic
criteria of diabetic nephropathy of the Chinese Medical Association
in 2010. Informed consents were signed by the patients and/or
guardians, and the study was approved by the Ethics Committee of
Weifang People's Hospital. Patients showing complications with
severe cardiopulmonary dysfunction, coagulation disorder, mental
disease, infection in other tissues and organs, secondary
hypertension, type 1 diabetes, and central nervous system disease
were excluded. According to a random number table, the patients
were divided into experimental (n=40) and control (n=40) groups.
The experimental group included 21 males and 19 females aged 49–75
years (63.5±2.7 years on average), with a duration of diabetes of
5–40 years (23.1±2.6 years on average) and a duration of diabetic
nephropathy of 2–12 years (6.1±1.1 years on average). The control
group contained 21 males and 19 females aged 49–75 years (63.3±2.6
years on average), with a duration of diabetes of 5–40 years
(23.0±2.5 years on average) and a duration of diabetic nephropathy
of 2–12 years (6.0±1.1 years on average). There were no significant
differences in gender, age, and duration of diabetes and diabetic
nephropathy between the two groups (p>0.05).
Methods
Patients underwent high-quality low-protein diabetic
diet intervention and subcutaneous injection of insulin to adjust
blood glucose, combined with antihypertensive and antiplatelet
drugs, and other comprehensive treatment. The control group was
treated with an intravenous injection of 10 µg alprostadil (NMPN
H10980024; Tide Pharmaceutical, Beijing, China) with 18 ml normal
saline once a day for 14 consecutive days as one treatment. The
next treatment was started two weeks later. The experimental group
was treated with alprostadil combined with calcium dobesilate
capsules (NMPN H20000711; Xi'an Lijun Pharmaceutical, Xi'an, China)
3 times a day (0.25 g/dose) for four consecutive weeks as one
treatment. Both groups were treated for 12 consecutive weeks as one
treatment cycle.
Experimental indexes
We compared time to remission of the clinical
symptom, changes in serum of small molecular protein level,
inflammatory cytokine level, and endocrine-related hormone level
between the two groups after intervention. We also compared changes
in fasting serum insulin (FINS), homeostasis model assessment of
insulin resistance (HOMA-IR) index, renal function between the two
groups. Finally, we examined the changes in brain derived
neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1)
levels in both groups before and after intervention.
Evaluation methods
Elbow venous blood was drawn in the early morning
and immediately submitted for detection of relevant biochemical
indexes. The levels of serum small molecule proteins as
β2-microglobulin (β2-MG), cystatin C (CysC), and retinol binding
protein (RBP) were detected via enzyme-linked immunosorbent assay
(ELISA). All reagents were provided by Beijing Jingmei Biology
(Beijing, China). Tumor necrosis factor-α (TNF-α) and interleukin-6
(IL-6) were detected via double-antibody single-step sandwich
method. C-reactive protein (CRP) was detected via
immunoturbidimetric assay. 25-hydroxyvitamin D was detected via
unidimensional chromatography. Parathyroid hormone was detected via
ELISA. Angiotensin II was detected via reversed-phase
high-performance liquid chromatography (RP-HPLC). FINS was detected
via Beckman Access DXI 800 chemiluminescent analyzer. HOMA-IR index
= [fasting blood glucose (mmol/l) × FINS (mU/l)]/22.5. Renal
function tests included the determination of blood urea nitrogen
(urease - Nessler color-developing method), creatinine
(chemiluminescence method), and uric acid levels (ELISA). BDNF was
detected via ELISA. The normal reference range of IGF-1 in adults
is 80–360 ng/ml.
Statistical analysis
SPSS 13.0 (IBM Corp., New York, NY, USA) was used
for statistical processing. Measurement data were presented as mean
± standard deviation (mean ± SD). T-test was used for the
comparison of means between the two groups and Chi-square test was
used for the comparison of rates between the two groups. p<0.05
suggested that the difference was statistically significant.
Results
Time to clinical remission after
intervention
To determine the time to clinical remission, we
examined the symptoms of mental fatigue and weakness, limb edema,
soreness and swelling of waist and knee, cold limbs and limb
numbness and pain. The experimental group achieved remission of
each symptom faster than the control group and the differences were
statistically significant (p<0.05) (Table I).
 | Table I.Time to clinical remission after
intervention (day, mean ± SD). |
Table I.
Time to clinical remission after
intervention (day, mean ± SD).
| Group | Mental fatigue and
weakness | Limb edema | Soreness and swelling
of waist and knee | Cold limbs | Limb numbness and
pain |
|---|
| Experimental | 6.7±1.1 | 2.7±0.3 | 7.1±1.3 | 8.9±0.8 | 12.3±1.5 |
| Control | 12.4±1.6 | 5.5±0.6 | 10.3±1.5 | 12.3±1.5 | 20.1±1.9 |
| t-value | 18.567 | 26.399 | 10.196 | 12.649 | 20.379 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
Small molecular weight proteins in
blood after intervention
After intervention, we analyzed renal function by
measuring the levels of small molecular proteins in blood,
including β2-MG, CysC and RBP. The experimental group showed levels
within the normal levels for β2-MG, CysC and RBP, but the control
group still showed elevated levels of β2-MG and CysC (Table II). Comparing the values of the
proteins for the two groups, the experimental group exhibited lower
levels for all three proteins compared to those in the control
group. The differences were statistically significant (p<0.05)
(Table II).
 | Table II.Levels of small molecular proteins in
blood after intervention (µg/ml, mean ± SD). |
Table II.
Levels of small molecular proteins in
blood after intervention (µg/ml, mean ± SD).
| Group | β2-MG | CysC | RBP |
|---|
| Normal | 1.60–3.0 | 0.51–1.09 | 25–70 |
| Experimental | 2.11±0.2 | 0.81±0.12 | 25.31±0.26 |
| Control | 3.53±0.31 | 1.32±0.23 | 43.27±0.39 |
| t-value | 24.344 | 12.433 | 242.338 |
| P-value | <0.05 | <0.05 | <0.05 |
Levels of inflammatory cytokines after
intervention
After intervention, we examined the levels of the
inflammatory cytokines TNF-α, IL-6 and CRP to determine recovery
from the nephropathy. The experimental group showed slightly high
levels of TNF-α and normal levels of IL-6 and CRP (Table III). In contrast, the control
groups showed normal levels of CRP and elevated levels of TNF-α and
IL-6. Comparing the levels of cytokines in the two groups indicated
that they were all lower in the experimental groups compared to the
control group. The differences were statistically significant
(p<0.05) (Table III).
 | Table III.Levels of inflammatory cytokines after
intervention (mean ± SD). |
Table III.
Levels of inflammatory cytokines after
intervention (mean ± SD).
| Group | TNF-α (ng/l) | IL-6 (ng/l) | CRP (mg/l) |
|---|
| Normal | 5–100 | 56.4–150.3 | ≤10 |
| Experimental | 105.3±5.6 | 79.6±3.4 | 6.3±0.1 |
| Control group | 258.1±11.5 | 125.3±5.1 | 11.3 ±1.6 |
| t-value | 75.552 | 47.155 | 19.726 |
| P-value | <0.05 | <0.05 | <0.05 |
Levels of endocrine hormones after
intervention
After intervention, we examined the levels of
25-hydroxyvitamin D, parathyroid hormone, and angiotensin II. The
experimental group showed normal levels of 25-hydroxyvitamin D, low
levels of parathyroid hormone, and high levels of angiotensin II
(Table IV). The control group
exhibited low levels of of 25-hydroxyvitamin D and parathyroid
hormone, and very high levels of angiotensin II. The differences
were statistically significant (p<0.05) (Table IV).
 | Table IV.Levels of endocrine-related hormones
after intervention (mean ± SD). |
Table IV.
Levels of endocrine-related hormones
after intervention (mean ± SD).
| Group | 25-hydroxy-vitamin D
(nmol/l) | Parathyroid hormone
(ng/l) | Angiotensin II
(ng/l) |
|---|
| Normal | ≥50 | 170–400 | 10–30 |
| Experimental | 65.33±3.21 | 158.61±13.63 | 36.58±1.70 |
| Control | 41.19±2.10 | 85.36±9.54 | 45.23±2.91 |
| t-value | 39.802 | 27.846 | 16.233 |
| P-value | <0.05 | <0.05 | <0.05 |
FINS and HOMA-IR indexes after
intervention
FINS was within normal range in both experimental
and control groups (Table V). But
FINS was significantly higher in the experimental group than that
in the control group. The HOMA-IR index in experimental group was
lower than that in control group (Table
V).
 | Table V.FINS and HOMA-IR indexes after
intervention (mean ± SD). |
Table V.
FINS and HOMA-IR indexes after
intervention (mean ± SD).
| Group | FINS (mU/l) | HOMA-IR |
|---|
| Normal | 3.0–24.9 | 1 |
| Experimental | 4.26±1.20 | 0.85±0.17 |
| Control | 8.35±1.61 | 1.88±0.91 |
| t-value | 12.882 | 7.037 |
| P-value | <0.05 | <0.05 |
Renal functions after
intervention
Next, we examined renal function indexes by
analyzing blood urea nitrogen, creatinine and uric acid. The levels
of urea nitrogen, creatinine, and uric acid in blood were elevated
in both the experimental and control groups. However, the levels
were lower in the experimental groups than those in control group.
The differences were statistically significant (p<0.05)
(Table VI).
 | Table VI.Renal function after intervention
(mean ± SD). |
Table VI.
Renal function after intervention
(mean ± SD).
| Group | Blood urea nitrogen
(mmol/l) | Creatinine
(µmol/l) | Uric acid
(mmol/l) |
|---|
| Normal | 3.2–6.1 | 88.4–159.1 | 89–417 |
| Experimental | 7.83±0.35 | 215.63±35.13 | 9.12±1.0 |
| Control | 10.31±2.40 | 359.61±48.21 | 11.20±0.9 |
| t-value | 6.467 | 15.265 | 9.778 |
| P-value | <0.05 | <0.05 | <0.05 |
Levels of growth factors before and
after intervention
Lastly, we examined the levels of two growth
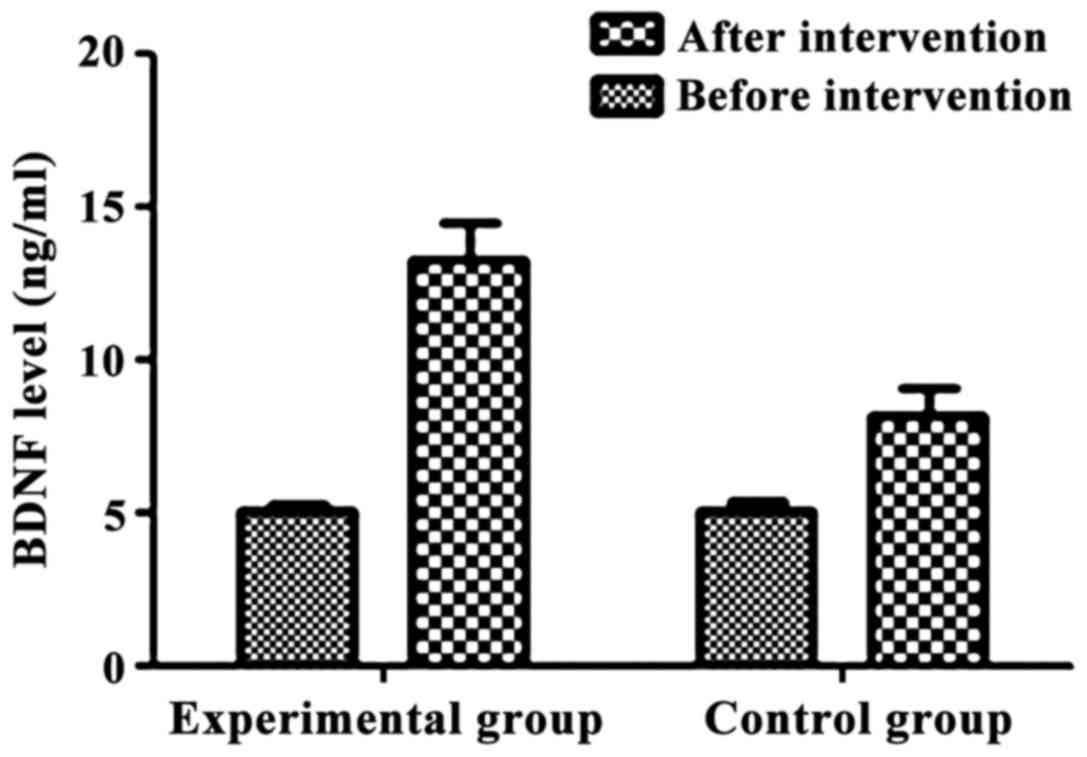
factors, BDNF and IGF-1, before and after the intervention. BDNF
was low in both groups before intervention and there was no
statistically significant difference between the two groups
(Table VII). BDNF levels were
significantly higher in both groups after intervention compared to
those before the intervention. The levels of BDNF in the
experimental group were higher than that in control group after
intervention The differences were statistically significant
(p<0.05) (Table VII and
Fig. 1).
 | Table VII.BDNF levels before and after
intervention (ng/ml, mean ± SD). |
Table VII.
BDNF levels before and after
intervention (ng/ml, mean ± SD).
| Group | Before
intervention | After
intervention | t-value | P-value |
|---|
| Normal | 6.15–14 |
|
|
|
| Experimental | 5.1±0.2 | 13.31±1.25 | 41.018 | 0.000 |
| Control | 5.1±0.3 | 8.21±0.93 | 20.129 | 0.000 |
| t-value | 0.000 | 20.703 | – | – |
| P-value | 1.000 | <0.05 | – | – |
Similarly, IGF-1 was low in both groups before
intervention and there was the differences were not statistically
significant (p>0.05) in the IGF-1 level between the two groups
before the intervention (Table
VIII). IGF-1 levels were significantly elevated in both groups
after the intervention than those before intervention. The
differences were statistically significant (p<0.05) (Table VIII and Fig. 2).
 | Table VIII.IGF-1 levels before and after
intervention (ng/ml, mean ± SD). |
Table VIII.
IGF-1 levels before and after
intervention (ng/ml, mean ± SD).
| Group | Before
intervention | After
intervention | t-value | P-value |
|---|
| Experimental | 93.1±3.3 | 342.6±12.5 | 122.056 | 0.000 |
| Control | 93.2±3.2 | 211.2±10.6 | 67.401 | 0.000 |
| t-value | 0.138 | 50.707 | – | – |
| P-value | 0.891 | <0.05 | – | – |
Discussion
Diabetic nephropathy is one of the most common
systemic complications in diabetic patients. This problem is caused
mainly by glomerular sclerosis due to long-term chronic
hyperglycemia (8). The pathogenesis
of diabetic nephropathy is complex and most scholars believe that
it is mainly caused by renal hypertrophy, thickening of the tubular
and glomerular basement membrane due to glomerular hyperperfusion,
and increased filtration rate (9).
If patients with diabetic nephropathy are not treated timely, they
develop renal failure and even uremia, threatening their lives.
Therefore, special clinical attention should be paid to patients
with diabetic nephropathy (10).
In this study, patients with diabetic nephropathy
received alprostadil alone or alprostadil combined with calcium
dobesilate as symptomatic treatment. The remission time of clinical
symptoms, such as mental fatigue and weakness, limb edema, soreness
and swelling of waist and knee, cold limbs and limb numbness and
pain, was significantly shorter in the experimental group than that
in the control group, suggesting that the combined treatment can
more efficiently relieve the clinical symptoms. In addition, the
levels of β2-MG, CysC and RBP in serum were significantly lower in
the experimental group than those in the control group, suggesting
that the application of the combined treatment significance
improves the renal filtering of small proteins. Moreover, the
levels of TNF-α, IL-6, CRP and angiotensin II were significantly
lower in the experimental group than those in control group. In
contrast, the levels of 25-hydroxyvitamin D and parathyroid hormone
were significantly higher in the experimental group than those in
the control group. Thus, application of alprostadil combined with
calcium dobesilate alleviates the inflammatory response and
improves the levels of endocrine hormones. Additionally, FINS was
significantly higher and HOMA-IR index was lower in experimental
group than that in control group. Finally, the levels of BDNF and
IGF-1 were higher in the experimental group than those in the
control group after intervention, suggesting that the combined
treatment increases insulin level, reduce insulin resistance, and
regulate the levels of BDNF and IGF-1.
The application of alprostadil combined with calcium
dobesilate in patients with diabetic nephropathy can more
efficiently reduce the microvascular wall permeability, decrease
the whole blood viscosity, and inhibit the platelet aggregation
(11–13). These effects significantly alleviate
the clinical symptoms, reducing small molecule proteins from blood,
and improving renal functions (14).
Moreover, calcium dobesilate can also reduce the vascular
endothelial injury and inhibit apoptosis, restoring the filtration
of small proteins, such as β2-MG, CysC and RBP. In addition,
calcium dobesilate eliminates oxygen (hydroxyl) radicals and
inhibits the inflammatory response (15), reducing the levels of inflammatory
cytokines. Moreover, calcium dobesilate can effectively stabilize
capillary endothelial cells, reduce endothelial cell rupture
(16), improve lymphatic return and
other functions (17), inhibit
secretion and synthesis of aldose reductase (18), and decrease the levels of sorbitol
(19,20), effectively regulating the levels of
BDNF and IGF-1.
In conclusion, treatment with alprostadil and
calcium dobesilate in patients with diabetic nephropathy
effectively relieves clinical symptoms, improves the renal
functions, reduces the levels of small molecule proteins in blood,
alleviates the inflammatory response, and regulates the levels of
BDNF and IGF-1.
References
|
1
|
Hung CC, Lin HY, Hwang DY, Kuo IC, Chiu
YW, Lim LM, Hwang SJ and Chen HC: Diabetic retinopathy and clinical
parameters favoring the presence of diabetic nephropathy could
predict renal outcome in patients with diabetic kidney disease. Sci
Rep. 7:12362017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Das F, Ghosh-Choudhury N, Venkatesan B,
Kasinath BS and Choudhury Ghosh G: PDGF receptor-β uses Akt/mTORC1
signaling node to promote high glucose-induced renal proximal
tubular cell collagen I (α2) expression. Am J Physiol Renal
Physiol. 313:F291–F307. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen YL, Qiao YC, Xu Y, Ling W, Pan YH,
Huang YC, Geng LJ, Zhao HL and Zhang XX: Serum TNF-α concentrations
in type 2 diabetes mellitus patients and diabetic nephropathy
patients: A systematic review and meta-analysis. Immunol Lett.
186:52–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eissa S, Matboli M and Bekhet MM: Clinical
verification of a novel urinary microRNA panal: 133b, −342 and −30
as biomarkers for diabetic nephropathy identified by bioinformatics
analysis. Biomed Pharmacother. 83:92–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gnudi L, Coward RJ and Long DA: Diabetic
nephropathy: Perspective on novel molecular mechanisms. Trends
Endocrinol Metab. 27:820–830. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
John S: Complication in diabetic
nephropathy. Diabetes Metab Syndr. 10:247–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yun KJ, Kim HJ, Kim MK, Kwon HS, Baek KH,
Roh YJ and Song KH: Risk factors for the development and
progression of diabetic kidney disease in patients with type 2
diabetes mellitus and advanced diabetic retinopathy. Diabetes Metab
J. 40:473–481. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kubota M, Watanabe R, Yamaguchi M,
Hosojima M, Saito A, Fujii M, Fujimura S and Kadowaki M: Rice
endosperm protein slows progression of fatty liver and diabetic
nephropathy in Zucker diabetic fatty rats. Br J Nutr.
116:1326–1335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Rasheed NM, Al-Rasheed NM, Al-Amin MA,
Hasan IH, Al-Ajmi HN, Mohammad RA and Attia HA: Fenofibrate
attenuates diabetic nephropathy in experimental diabetic rat's
model via suppression of augmented TGF-β1/Smad3 signaling pathway.
Arch Physiol Biochem. 122:186–194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jose MJ, Varkey V, Chandni R, Zubaida PA
and Maliekkal J: The role of smoking as a modifiable risk factor in
diabetic nephropathy. J Assoc Physicians India. 64:34–38.
2016.PubMed/NCBI
|
|
11
|
Liljedahl L, Pedersen MH, Norlin J,
McGuire JN and James P: N-glycosylation proteome enrichment
analysis in kidney reveals differences between diabetic mouse
models. Clin Proteomics. 13:22–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang M, Yao D, Wang S, Yan Q and Lu W:
Long non-coding RNA ENSMUST00000147869 protects mesangial cells
from proliferation and fibrosis induced by diabetic nephropathy.
Endocrine. 54:81–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma R, Chaudhari S and Li W: Canonical
transient receptor potential 6 channel: A new target of reactive
oxygen species in renal physiology and pathology. Antioxid Redox
Signal. 25:732–748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hanefeld M, Appelt D, Engelmann K, Sandner
D, Bornstein SR, Ganz X, Henkel E, Haase R and Birkenfeld AL: Serum
and plasma levels of vascular endothelial growth factors in
relation to quality of glucose control, biomarkers of inflammation,
and diabetic nephropathy. Horm Metab Res. 48:6202016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ge J, Miao JJ, Sun XY and Yu JY: Huangkui
capsule, an extract from Abelmoschus manihot (L.) medic,
improves diabetic nephropathy via activating peroxisome
proliferator-activated receptor (PPAR)-α/γ and attenuating
endoplasmic reticulum stress in rats. J Ethnopharmacol.
189:238–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang YS, Lee MH, Song HK, Kim JE, Ghee JY,
Cha JJ, Lee JE, Kim HW, Han JY and Cha DR: Chronic administration
of visfatin ameliorated diabetic nephropathy in type 2 diabetic
mice. Kidney Blood Press Res. 41:311–324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
You YK, Huang XR, Chen HY, Lyu XF, Liu HF
and Lan HY: C-reactive protein promotes diabetic kidney disease in
db/db Mice via the CD32b-Smad3-mTOR signaling Pathway. Sci Rep.
6:267402016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji ZZ and Xu YC: Melatonin protects
podocytes from angiotensin II-induced injury in an in vitro
diabetic nephropathy model. Mol Med Rep. 14:920–926. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu Y, Tang L, Li Y and He Q: High
glucose-induced fibronectin upregulation in cultured mesangial
cells involves caveolin-1-dependent RhoA-GTP activation via Src
kinase. Mol Med Rep. 14:963–968. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Misra A and Shrivastava U: Obstructive
sleep apnea and diabetic nephropathy. Diabetes Technol Ther.
18:405–407. 2016. View Article : Google Scholar : PubMed/NCBI
|