Introduction
As a common inflammatory disease, asthma is
characterized by reduced respiratory function and increased
infiltration of leukocytes, particularly eosinophils. This
inflammation usually leads to airway bronchoconstriction, increased
airway hyperresponsiveness (AHR) and mucus production (1). Controlling airway inflammation is
currently the primary strategy of asthma management (2). Synthetic glucocorticoids (GCs) are
widely used to treat chronic and acute inflammatory conditions
worldwide (3,4). Dexamethasone, as a typical GC, is the
standard treatment for multiple diseases, including asthma
(5), arthritis (6) and autoimmune disorders (7). However, the clinical use of
dexamethasone is limited by its side effects at high doses in
short-term treatment, or at low doses in long-term treatment
(8). Dexamethasone administration is
one of the most common causes of osteoporosis (9) and osteonecrosis of femoral head
(10). Therefore, a clinical
replacement for dexamethasone is urgently required in order to
treat chronic asthma without generating any adverse reactions.
Limethason is a derivative of dexamethasone
(10). It has previously been
demonstrated that limethason can effectively ameliorate arthritis,
macrophage-rich graft vs. host disease, arteriosclerosis and
macular edema (11–13). In certain chronic inflammatory
diseases, including Rheumatoid arthritis and hemophagocytic
syndrome, limethason is 2–5 times more potent than dexamethasone
phosphate against inflammation (14). As an ester prodrug of dexamethasone,
limethason has a markedly increased lipophilicity (15).
Although the efficacy of limethason has been
verified in multiple disorders, to the best of our knowledge, its
function in asthma has not yet been reported. In the present study,
the effects and adverse reactions of limethason were investigated
in an ovalbumin (OVA)-induced chronic asthma mouse model. The
present research could provide the basis for a new strategy of
chronic asthma therapy in the clinic.
Materials and methods
Animals
A total of 48 male BALB/c mice (weight, 20.27±1.09;
aged 6–8 weeks) were purchased from the Fourth Military Medical
University (Xi'an, China). Prior to the experiments, the mice were
kept under standard laboratory conditions (temperature, 23–25°C;
humidity, 40–60%; with access to a 12-h light/dark cycle) for 1
week. All mice were provided with water and standard chow ad
libitum. All animal protocols conformed to the guidelines of the
National Animal Welfare Law of China, and experiments were
performed in accordance with the Guidelines for the Care and Use of
Laboratory Animals (16). The study
protocols were approved by the Ethics Committee of Northwestern
Polyechnical University (Xi'an, China).
The model of chronic asthma
establishing
Mice were randomly divided into four groups (n=12 in
each group): A normal control (NC) group, an OVA group, a
dexamethasone and OVA (DEX + OVA) group and a limethason and OVA
(LIM + OVA) group. Except for the NC group, all mice were
sensitized with intraperitoneal injection of 10 µg OVA
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 100 µg
aluminium hydroxide (Sigma-Aldrich; Merck KGaA) in 0.2 ml saline at
days 0 and 14. Mice in the NC group were treated with 0.2 ml saline
in the same way. From day 15, the mice were challenged with 2.5%
(w/v) OVA (Sangon Biotech Co., Ltd., Shanghai, China) for 30 min
using a medical gas atomizer (KYWH-1006; Shenzhen Lijian Medical
Technology Co., Ltd., Shenzhen, China) after intraperitoneal
injecting 1 mg/kg dexamethasone sodium phosphate (China National
Medicines Co., Ltd., Shanghai, China) in the DEX + OVA group or 1.6
mg/kg limethason (MitsubishiPharma Co., Ltd., Guangzhou, China) in
the LIM + OVA group. This was performed three times per week for a
total of 9 weeks. The NC group was injected with 0.2 ml saline
instead of dexamethasone or limethason. Mice were sacrificed 8 days
after the last challenge (day 87) to characterize the effects of
limethason on the airways of chronic asthma animals. Hematoxylin
and eosin (H&E) staining and Periodic acid-Schiff (PAS)
staining were performed using 6 mice per each group. The remaining
6 mice in each group were used for bronchoalveolar lavage.
Measurement of AHR
AHR was estimated in unrestrained and conscious mice
by whole-body plethysmography (Buxco; Data Sciences International,
New Brighton, MN, USA) after the last aerosol challenge. Each mouse
was placed in a chamber, then exposed to aerosolized PBS, followed
by increasing concentrations of methacholine solutions
(Sigma-Aldrich; Merck KGaA): 6.25, 12.5, 25 and 50 mg/ml dissolved
in PBS. After adapting for 5 min, each exposure lasted for 3 min.
An index of airway obstruction was measured during the response
stage, which lasted 5 min. Finally, each mouse recovered for 3 min.
Response and recovery stage was repeated after each concentration
of methacholine. In the process of measuring AHR, some mice did not
respond well to the experiment and became eclamptic or showed signs
of difficulty in breathing, however mice did not succumb to the
conditions. Subsequently, data were not collected for some mice in
the NC (n=6), OVA (n=6), DEX + OVA (n=6) and LIM + OVA (n=6)
groups. However, at least five mice were provided in each
group.
Leukocyte count in bronchoalveolar
lavage fluid (BALF)
BALF analysis was performed in 6 mice in each group
after sacrifice. The esophagus of mice were cut with operating
scissors, ~2 mm incision was made, and cold PBS (4°C) was slowly
instilled into the lungs 0.5 ml at a time, for a total of three
times (1.5 ml). In the process of BAL, the operator failed to
recover the fluid which was instilled into the lungs of some mice.
However, the fluids from at least 5 mice per group were
successfully collected. Data were not collected from 1 mouse in the
NC, OVA and DEX + OVA group. The fluid was immediately centrifuged
at 4°C, 100 × g for 10 min. The total number of cells was counted
on ≥6 squares of a hemocytometer. Then, cell pellets were suspended
in 1 ml of PBS, and 100 µl of each solution was placed onto a
slide. After the slides were dried, cells were fixed in
paraformaldehyde for 20 min at 25°C and stained using Diff-Quik
stain reagents (Wuhan Goodbio Biotechnology Co., Ltd., China),
according to the manufacturer's protocol. Cells were counted
manually using a light microscope and a hemocytometer.
Histopathology of lung and bone
tissue, the index of organs/tissues were measured
Hematoxylin and eosin (H&E) staining and
periodic acid-Schiff (PAS) staining were conducted after mice were
sacrificed. Lung tissues were fixed in 4% (v/v) paraformaldehyde
for 24 h at 4°C before embedding in paraffin. Mouse femurs were
obtained after sacrifice and decalcified in 10% EDTA for 1 month
after being fixed in paraformaldehyde fixed for 24 h at 4°C.
Sections of fixed paraffin lung tissues were cut to a thickness of
5 µm using the microtome, then stained with H&E at room
temperature for 28 min (Beyotime Institute of Biotechnology,
Shanghai, China). Lung tissues were also subjected to PAS reagent
at room temperature for 22 min (Wuhan Goodbio Biotechnology Co.,
Ltd.). The sections were visualized with a light microscope.
While lung tissues were obtained, liver, spleen,
kidney, gastrocnemius and brown adipose tissue were also extracted.
The weight of these organs and tissues was measured using an
electronic scale and calculated as follows: Organ index=organ
weight/body weight; Tissue index=tissue weight/body weight. In the
process of obtaining the organs and tissues, some of which were
incomplete due to human error. However, at least five mice were
provided in each group.
Analysis of bone mineral content (BMC)
and bone mineral density (BMD) using dual-energy X-ray
absorptiometry (DXA)
A DXA device (InAlyzer, MEDIKORS, Inc., Seongnam,
Korea) was used to measure the BMC and BMD of experimental mice on
day 78. The detection sensitivity of the DXA instrument was 0.001
g/cm2. A standard calibration block was used to
calibrate the DXA device before measurements were made, according
to the operator's manual. BMC and BMD measurements were taken after
mice from the four groups had been anesthetized with 1.5% sodium
pentobarbital (50 mg/kg, intraperitoneal injection; Kehao Biotech
Co., Ltd., Xi'an, China.). The BMC and BMD of the femora and total
body were obtained. The mean BMD at each site was recorded. In the
process of measuring BMC and BMD, The data were not included for
some mice in the NC group (n=4) and LIM + OVA group (n=1) as
unconscious movement during imaging resulted in inaccurate data.
Subsequently data was not collected in all mice. However, data was
collected in at least seven mice per group.
Statistical analysis
Statistical differences among groups were analyzed
by one-way analysis of variance, followed by Tukey's multiple
comparison test. Statistical analysis was performed using GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Data are
presented as the mean ± standard error of the mean. P<0.05 was
considered to indicate a statistically significant difference.
Results
Limethason decreases AHR
To evaluate the effects of limethason on airway
function in a murine chronic asthma model, AHR to methacholine was
examined at day 8 after the final OVA challenge using whole-body
plethysmography (Fig. 1A). With
increased doses of methacholine, enhanced pause (penh) values
increased to different degrees in the NC, OVA, DEX + OVA and LIM +
OVA groups (Fig. 1B). At a
concentration of 50 mg/ml, the OVA group increased 1.7-fold
(P<0.05) compared with the NC group. The penh value of DEX + OVA
was slightly lower compared with the OVA group, but the difference
was not observed to be significant. In the LIM + OVA group, the
penh values were similar to the NC group, and decreased by
34.3±5.14% compared with the DEX + OVA group (P<0.05).
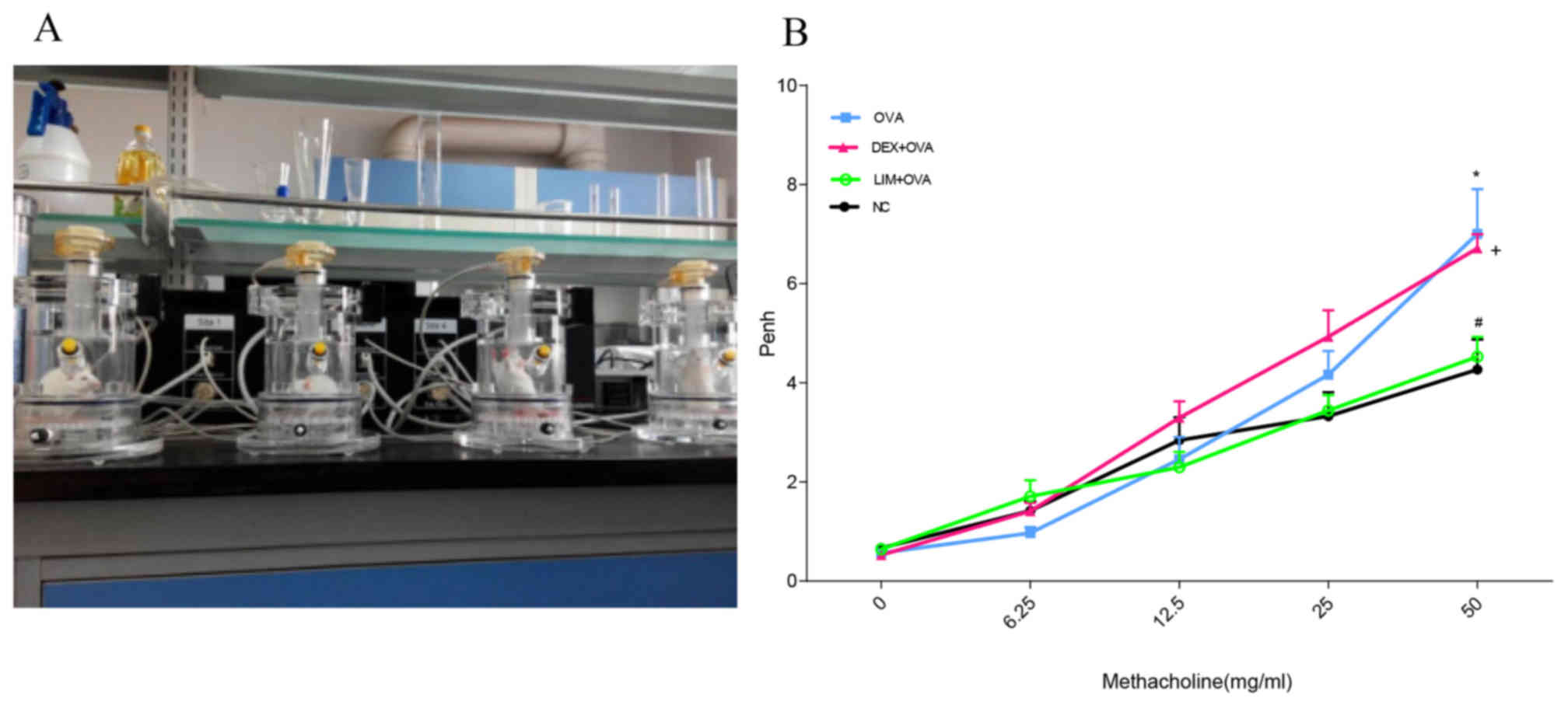 | Figure 1.Effect of limethason treatment on
AHR. (A) AHR measurement equipment. (B) AHR was measured at day 8
after the last OVA challenge, and all mice were administered with
methacholine (6.25–50 mg/ml). NC group, n=6; OVA group, n=6; DEX +
OVA group, n=6; LIM + OVA group, n=6. Data are expressed as the
mean ± standard error of the mean. *P<0.05 vs. NC;
#P<0.05 vs. OVA; +P<0.05 vs. LIM + OVA.
AHR, airway hyperresponsiveness; NC, normal control; OVA,
ovalbumin; LIM, limethason; DEX, dexamethasone; penh, enhanced
pause. |
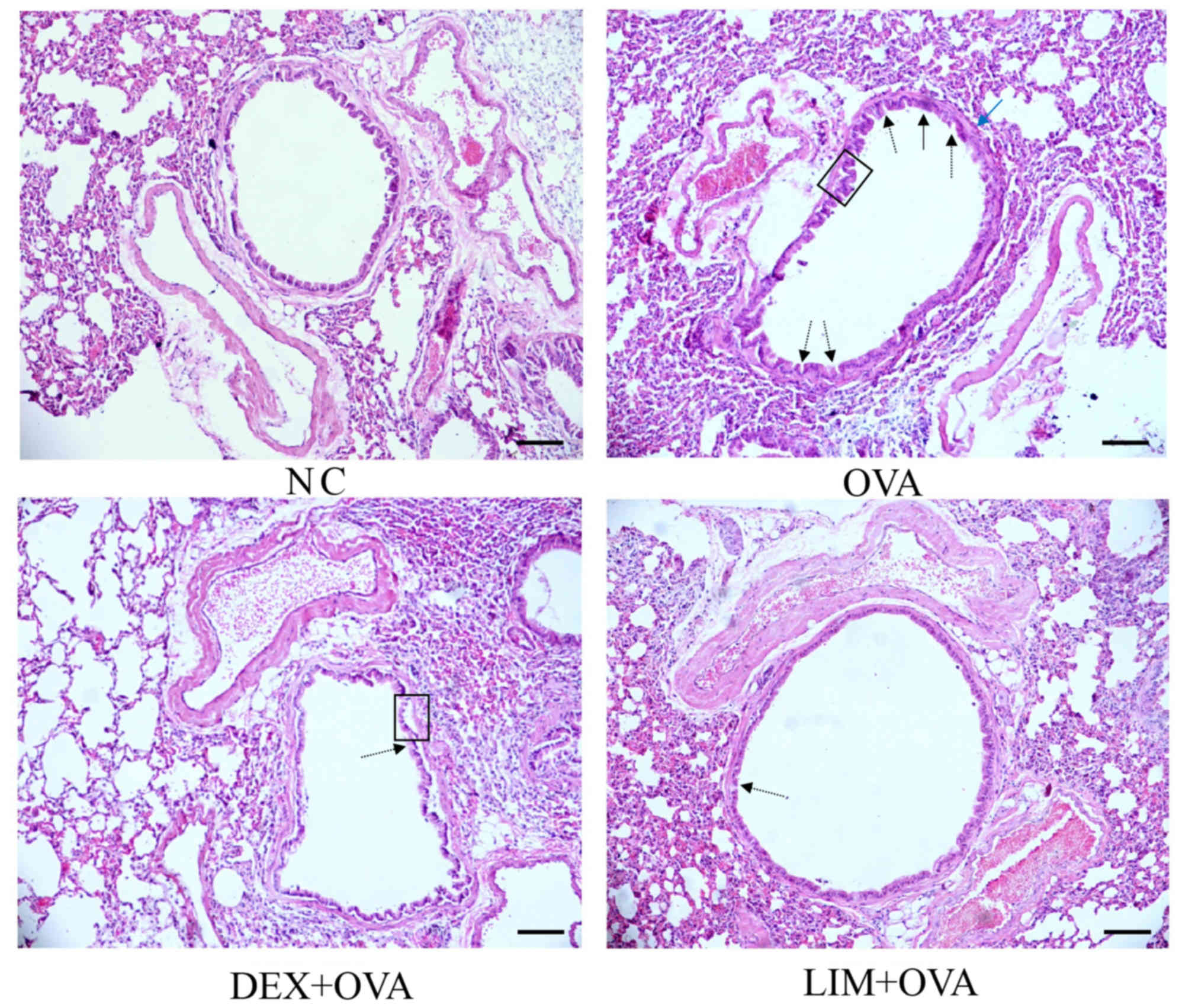
Limethason reduces inflammation in
lung tissue
To detect the anti-inflammatory effects of
limethason, lung tissues were collected after the last OVA
challenge. In the OVA group, leukocytes infiltrated into and around
the bronchiole and mucous membrane, epithelial cells detached from
the bronchiole, and the number of goblet cells increased compared
with the NC group. (Fig. 2).
Meanwhile, the airway smooth muscle became thicker. In the DEX +
OVA group, epithelial cells detached from the bronchiole, but
leukocytes had infiltrated less compared with the OVA group and
there was no obvious thickening of smooth muscle. In the LIM + OVA
group, infiltration of leukocytes was reduced compared with the OVA
group, and epithelial cells did not detach from the bronchiole.
However, there was no obvious thickening of smooth muscle.
Limethason reduces OVA-induced
eosinophilia in BALF
To evaluate the suppression of eosinophilia by
limethason, after the last OVA challenge, cells were classified and
counted as a percentage of total leukocytes in the BALF (Fig. 3). In the OVA group, a 5.8-fold
increase in the level of eosinophils was observed compared with the
NC group (P<0.001). Following limethason treatment, the
percentage of eosinophils decreased by 47.2±7.1% compared with the
OVA group (P<0.01). Following dexamethasone treatment the
percentage of eosinophils decreased by 39.3±13.23% compared with
the OVA group. The level of macrophages in the OVA group was
observed to be 45.2±5.9% lower compared with the NC group
(P<0.01). Following limethason treatment, the level of
macrophages increased by 50.7±15.1% compared with the OVA group.
The level of macrophages in the DEX + OVA group decreased by
32.6±8.7% compared with the NC group (P<0.05).
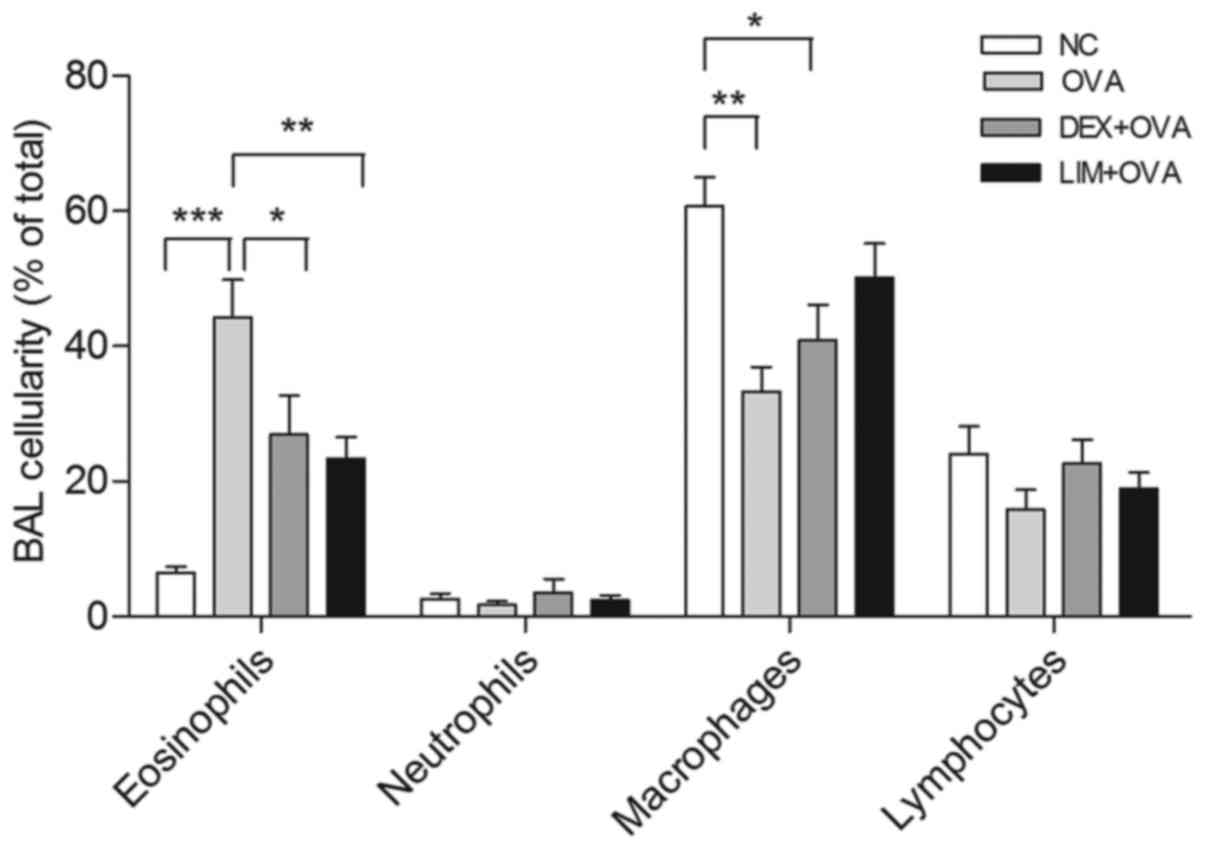 | Figure 3.Effect of limethason on inflammatory
cell response. Lymphocytes, macrophages, neutrophils and
eosinophils were counted in BAL fluid analysis. NC group, n=5; OVA
group, n=5; DEX + OVA group, n=5, LIM + OVA group, n=6. Data are
presented as the mean ± standard error of the mean. *P<0.05,
**P<0.01, ***P<0.001. BAL, bronchoalveolar lavage; NC, normal
control; OVA, ovalbumin; LIM, limethason; DEX, dexamethasone. |
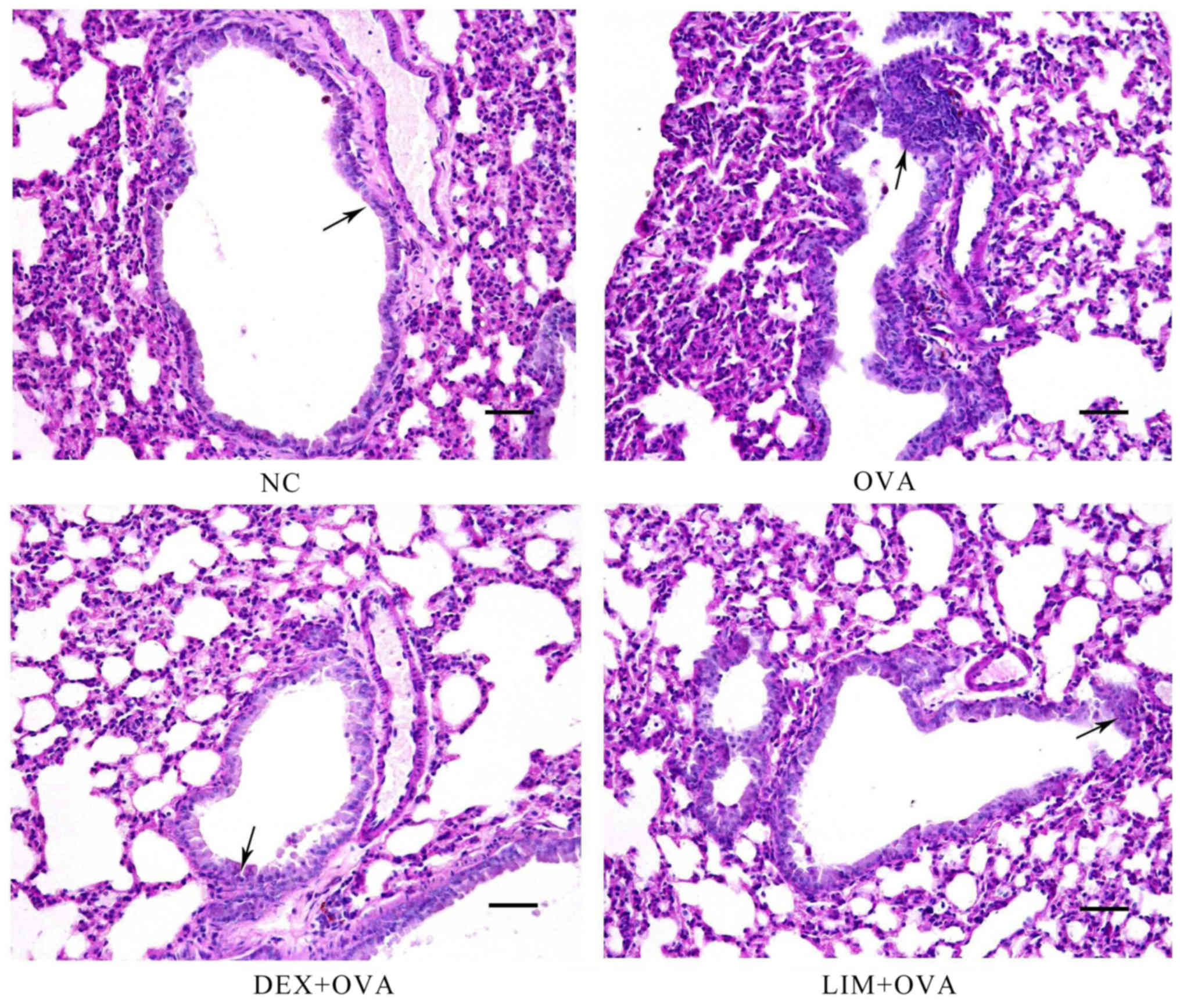
Limethason suppresses mucus
overproduction
One of the key characteristics of airway remodeling
is mucus hypersecretion. PAS staining was performed to assess the
quantity of mucus in lung tissue. In the OVA group, abundant mucus
was observed, indicated by increased violet color in the bronchial
airways, in comparison with the NC group. However, mucus content
was markedly decreased in the DEX + OVA and LIM + OVA groups, with
the largest decrease in the LIM + OVA group. These results
suggested that limethason reduces mucus hypersecretion that occurs
in the process of airway remodeling (Fig. 4).
Limethason exerts little influence on
the morphology of femoral head, BMC or BMD
H&E staining of the femur was performed in order
to evaluate the impact of limethason on bone tissue. BMC and BMD
were tested by DXA 1 week before mice were sacrificed. In the NC
group, trabecular bone was arranged neatly and completely with
normal thickness. The bone lacuna of the femoral head was
predominantly filled, while staining of cell nuclei was
homogeneous. There were no notable differences in the OVA, DEX +
OVA or LIM + OVA groups compared with the NC group (Fig. 5A). The BMC in the OVA, DEX + OVA and
LIM + OVA groups was similar to the NC group (Fig. 5B). For total BMD, the LIM + OVA and
DEX + OVA group were not significantly different from the NC group
(Fig. 5C). However, BMD in the OVA
group was significantly lower compared with the NC group
(P<0.01). These results suggested that limethason has a low risk
of causing osteonecrosis and bone loss.
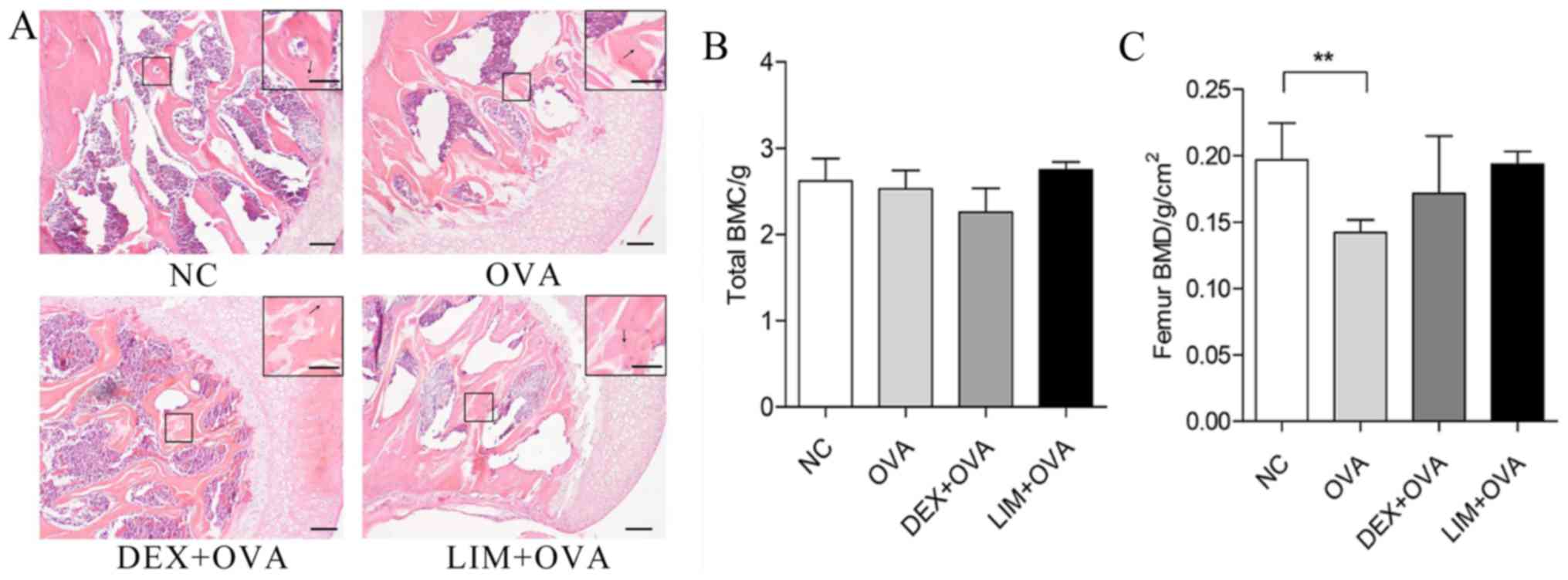 | Figure 5.Effect of limethason on bone tissue
in an OVA-induced chronic asthma mice model. (A) Hematoxylin and
eosin staining for head of femur sections. Scale bar=100 µm. Arrows
represent osteocytes in bone lacuna. (B) Whole-body BMC. NC group,
n=8; OVA group, n=7; DEX + OVA group, n=12; LIM + OVA group, n=10.
(C) Femur BMD. NC group, n=8; OVA group, n=12; DEX + OVA group,
n=12; LIM + OVA group, n=11. Data are presented as the mean ±
standard error of the mean. **P<0.01. BMC, bone mineral content;
BMD, bone mineral density; NC, normal control; OVA, ovalbumin; LIM,
limethason; DEX, dexamethasone. |
Limethason has no adverse impact on
liver, spleen, kidney, gastrocnemius or brown adipose tissue
To assess whether limethason has side effects on
organs and tissues in the process of treatment, all organs and
tissues were collectesd after the last OVA challenge. The weight of
liver, spleen, kidney, gastrocnemius and brown adipose tissue of
mice were measured. No significant differences in the organ index
of kidney, spleen or liver were identified between the NC, OVA, DEX
+ OVA or LIM + OVA groups (Fig.
6A-C). There were also no significant differences in
gastrocnemius or brown adipose tissue between the four groups
(Fig. 6D and E).
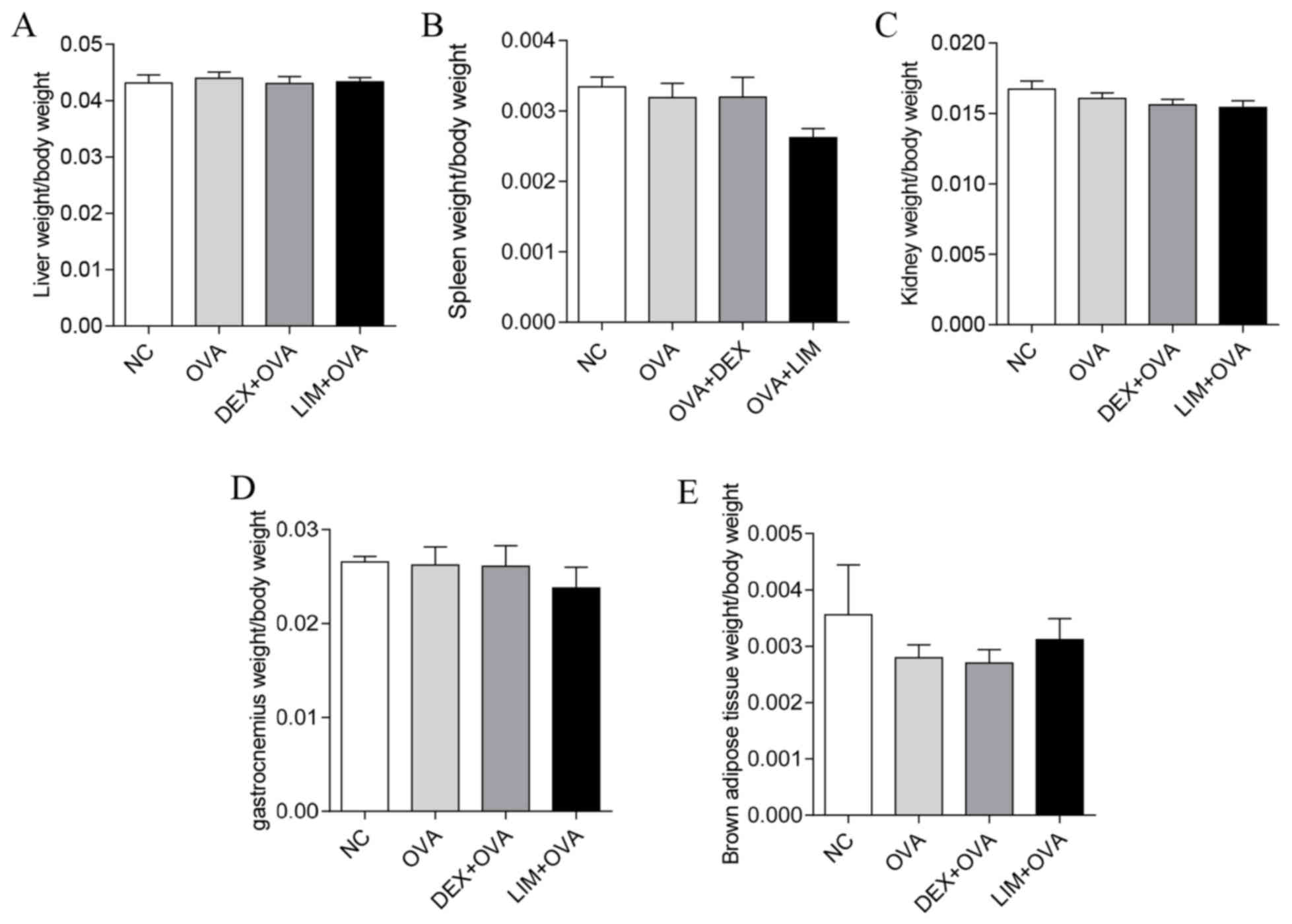 | Figure 6.Side effects of limethason on other
organs and tissues of mice. The organ/tissue indexes were
calculated as followed: Organ or tissue weight/body weight. Data
are presented as the mean ± standard error of the mean. (A and C)
Liver and kidney indexes: NC group, n=7; OVA group, n=11; DEX + OVA
group, n=10; LIM + OVA group, n=11. (B) Spleen index: NC group,
n=7; OVA group, n=11; DEX + OVA group, n=10; LIM + OVA group, n=11.
(D) Gastrocnemius index: NC group, n=7; OVA group, n=10; DEX + OVA,
n=10; LIM + OVA, n=11. (E) Brown adipose tissue index: NC group,
n=7; OVA group, n=10; DEX + OVA, n=10; LIM + OVA, n=11. NC, normal
control; OVA, ovalbumin; LIM, limethason; DEX, dexamethasone. |
Discussion
As an effective anti-inflammatory drug, limethason
is used for treating multiple disorders, including macrophage
activation syndrome, hemophagocytic lymphohistiocytosis and
idiopathic pulmonary hemosiderosis (15,17,18). In
addition, Hoshi et al (19)
demonstrated that this drug has a higher treatment rate of
rheumatoid arthritis with lower risk of side effects compared with
dexamethasone. To the best of our knowledge, the present study is
the first to investigate the effects of limethason on chronic
asthma and its side effects. An OVA-induced mouse model of chronic
asthma was established using the classical method (20), and dexamethasone was used as a
positive control.
AHR is a feature of asthma, which is a chronic
disease of the lower respiratory tract (21). In most situations, AHR is associated
with airway inflammation, and is the gold standard measurement of
bronchial constriction in asthma (22). Penh is an index of air constriction
that reflects the level of AHR; the higher the penh value, greater
the extent of AHR (23,24). A notable finding in the present study
was that limethason effectively inhibits AHR, and was more
effective compared with dexamethasone. This result suggested that
limethason is a potential bronchodilator for chronic asthma
intervention.
To evaluate the suppressive function of limethason
on leukocyte infiltration in the lungs of OVA-induced chronic
asthma mice, H&E staining was used in lung tissue sections. The
results indicated that limethason notably prevented inflammatory
cell infiltration, and was more effective compared with
dexamethasone. It is generally accepted that eosinophils are the
primary leukocyte to infiltrate in OVA-induced mice (25). Thus, in the present study, the
percentage of different leukocyte types was measured in BALF. The
data indicated that limethason was more effective at suppressing
eosinophils compared with dexamethasone. With regards to the
quantity of leukocytes, macrophages and lymphocytes were the
prominent types in the BALF of control mice, whereas eosinophils
and neutrophils were present at lower levels. These results were
consistent with Lei et al (26). Eosinophils are associated with airway
remodeling and required for pulmonary mucus accumulation in asthma
(27,28). To further investigate the effect of
limethason on eosinophils, PAS staining was used to examine mucus
secretion in the lungs. The results indicated that limethason
markedly inhibited mucus secretion. Mucus secretion increases
permeability of the capillary wall, which leads to the serous fluid
being exuded. Phlegm is also closely associated with the function
of the lung. Therefore, it was proposed that limethason decreases
mucus secretion by recovering pulmonary function (29,30).
The key ingredient of limethason is dexamethasone,
so the same dose of dexamethasone was used in the DEX + OVA and LIM
+ OVA groups. It has been established that GCs exhibit harmful
effects on bone, muscle and cartilage at histological doses
(31). In particular, the excessive
use of GCs results in bone loss (32). GCs are the most common cause of
secondary osteoporosis and a major reason for non-traumatic
osteonecrosis (33). Between 9 and
40% of patients suffer from osteonecrosis after receiving long-term
GC therapy (34). Osteoporosis is a
systemic skeletal disorder, which is characterized by low BMD and
deteriorating bone micro-architecture (35). In order to survey the side effects of
limethason on bone tissue in the present study, H&E staining of
the femur was performed. No notable differences were identified
between the groups. Liu et al (36) used dexamethasone up to 2.66 mg/kg/day
for 6 weeks to develop a mouse model of dexamethasone-induced
osteonecrosis. Yoon et al (37) used 5 mg/kg prednisolone to establish
a GC-induced osteonecrosis model in rats. However, the dose of
dexamethasone in the present study was lower than in these previous
studies, which may be the reason that no difference in bone
micro-architecture was observed between the groups. However, a
novel finding in the present research was that BMD was decreased in
the OVA group. It has been verified that asthma has a association
with BMD, and asthma is a well-known risk factor to osteoporosis
(38,39).
It is well known that organ weight is a sensitive
indicator of drug toxicity. Organ weight and organ index are key
characteristics that indicate the health of organisms. To detect
the effect of limethason on organs and tissues, organ and tissue
indexes were measured in the present study. However, no significant
differences were observed between the groups. These results
indicated that limethason did not damage the liver, spleen, kidney,
gastrocnemius or brown adipose tissue. Notably, dexamethasone did
not seem to cause any damage either. This is a limitation in this
study. The present results for gastrocnemius and brown adipose
tissue were inconsistent with the findings of previous studies
(40,41). This may be because the duration of
the experiment was not long enough. Although it has been reported
that dexamethasone induces skeletal muscle atrophy (42), overuse of GC is also associated with
human obesity (43). The present
results for gastrocnemius and brown adipose tissue were
inconsistent with the findings of previous studies. This may be
because the duration of the experiment was not long enough to
observe marked differences between groups.
In conclusion, the present study confirmed that
limethason suppresses inflammation in an OVA-induced chronic asthma
murine model, and briefly evaluated adverse reactions to
limethason. The present results indicated that limethason
effectively inhibits AHR, leukocyte (particularly eosinophil)
infiltration and mucus hypersecretion, but does not cause side
effects on the head of femur, liver, spleen, kidney, gastrocnemius
or brown adipose tissue. These results indicate that limethason may
be more effective for treating chronic asthma compared with
dexamethasone, with no obvious side effects on tissues or organs
during treatment. The present study provides a novel approach for
treating chronic asthma, and limethason could potentially be used
instead of dexamethasone for treating chronic asthma. The
underlying molecular mechanism of limethason could be investigated
in future studies.
Acknowledgements
This study was supported by the Fundamental Research
Funds for the Central Universities (grant no. lzujbky-2015-188) and
the National Natural Science Foundation of China (grant no.
81601913).
References
|
1
|
Djukanović R, Roche WR, Wilson JW, Beasley
CR, Twentyman OP, Howarth RH and Holgate ST: Mucosal inflammation
in asthma. Am Rev Respir Dis. 142:434–457. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee M, Kim S, Kwon OK, Oh SR, Lee HK and
Ahn K: Anti-inflammatory and anti-asthmatic effects of resveratrol,
a polyphenolic stilbene, in a mouse model of allergic asthma. Int
Immunopharmacol. 9:418–424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barnes PJ: Glucocorticosteroids: Current
and future directions. Br J Pharmacol. 163:29–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barnes PJ: Mechanisms and resistance in
glucocorticoid control of inflammation. J Steroid Biochem Mol Biol.
120:76–85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parikh K, Hall M, Mittal V, Montalbano A,
Gold J, Mahant S, Wilson KM and Shah SS: Comparative effectiveness
of dexamethasone versus prednisone in children hospitalized with
asthma. J Pediatr. 167:639–44 e14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Vollenhoven RF: Treatment of
rheumatoid arthritis: State of the art 2009. Nat Rev Rheumatol.
5:531–541. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peine KJ, Guerau-de-Arellano M, Lee P,
Kanthamneni N, Severin M, Probst GD, Peng H, Yang Y, Vangundy Z,
Papenfuss TL, et al: Treatment of experimental autoimmune
encephalomyelitis by codelivery of disease associated Peptide and
dexamethasone in acetalated dextran microparticles. Mol Pharm.
11:828–835. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang X, Zhao M, Wang Y, Zhu H, Zhao S, Wu
J, Song Y and Peng S: RGD (F/S/V)-Dex: Towards the development of
novel, effective and safe glucocorticoids. Drug Des Devel Ther.
10:1059–1076. 2016.PubMed/NCBI
|
|
9
|
Ren H, Liang D, Jiang X, Tang J, Cui J,
Wei Q, Zhang S, Yao Z, Shen G and Lin S: Variance of spinal
osteoporosis induced by dexamethasone and methylprednisolone and
its associated mechanism. Steroids. 102:65–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Daull P, Paterson CA, Kuppermann BD and
Garrigue JS: A preliminary evaluation of dexamethasone palmitate
emulsion: A novel intravitreal sustained delivery of corticosteroid
for treatment of macular edema. J Ocul Pharmacol Ther. 29:258–269.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anderson R, Franch A, Castell M,
Perez-Cano FJ, Bräuer R, Pohlers D, Gajda M, Siskos AP, Katsila T,
Tamvakopoulos C, et al: Liposomal encapsulation enhances and
prolongs the anti-inflammatory effects of water-soluble
dexamethasone phosphate in experimental adjuvant arthritis.
Arthritis Res Ther. 12:R1472010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nishiwaki S, Nakayama T, Murata M, Nishida
T, Terakura S, Saito S, Kato T, Mizuno H, Imahashi N, Seto A, et
al: Dexamethasone palmitate ameliorates macrophages-rich
graft-versus-host disease by inhibiting macrophage functions. PLoS
One. 9:e962522014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chono S, Tauchi Y and Morimoto K: Aortic
drug delivery of dexamethasone palmitate incorporated into lipid
microspheres and its antiatherosclerotic effect in atherogenic
mice. J Drug Target. 13:407–414. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Y, Li H, Gao K, Liu M, Sun Y, Yan T,
Fawcett JP, Cui Y and Gu J: Simultaneous quantitation of
dexamethasone palmitate and dexamethasone in human plasma by liquid
chromatography/tandem mass spectrometry. J Chromatogr B Analyt
Technol Biomed Life Sci. 862:119–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Doi T, Ohga S, Ishimura M, Takada H, Ishii
K, Ihara K, Nagai H and Hara T: Long-term liposteroid therapy for
idiopathic pulmonary hemosiderosis. Eur J Pediatr. 172:1475–1481.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Janet C: Garber: Guide for The Care
Laboratory Animals. 8th. The National Academics press; Washington,
DC: pp. 1-175.c142011
|
|
17
|
Otsubo K, Fukumura A, Hirayama M, Morimoto
T, Kato M and Mochizuki H: Hemophagocytic lymphohistiocytosis
caused by systemic herpes simplex virus type 1 infection:
Successful treatment with dexamethasone palmitate. Pediatr Int.
58:390–393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakagishi Y, Shimizu M, Kasai K, Miyoshi M
and Yachie A: Successful therapy of macrophage activation syndrome
with dexamethasone palmitate. Mod Rheumatol. 26:617–620.
2016.PubMed/NCBI
|
|
19
|
Hoshi K, Mizushima Y, Shiokawa Y, Kageyama
T, Honma M, Kashiwazaki S, Shichikawa K, Tsunematsu T and Kaneko K:
Double-blind study with liposteroid in rheumatoid arthritis. Exp
Clin Res. 11:621–626. 1985.
|
|
20
|
Jain VV, Kitagaki K, Businga T, Hussain I,
George C, O'shaughnessy P and Kline JN: CpG-oligodeoxynucleotides
inhibit airway remodeling in a murine model of chronic asthma. J
Allergy Clin Immunol. 110:867–872. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Freishtat RJ, Nagaraju K, Jusko W and
Hoffman EP: Glucocorticoid efficacy in asthma: Is improved tissue
remodeling upstream of anti-inflammation. J Investig Med. 58:19–22.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang Z, Xu Y, Wen X, Nie H, Hu T, Yang X,
Chu X, Yang J, Deng X and He J: Rosmarinic acid attenuates airway
inflammation and hyperresponsiveness in a murine model of asthma.
Molecules. 21:pii: E7692016. View Article : Google Scholar
|
|
23
|
Deng Y, Chen W, Zang N, Li S, Luo Y, Ni K,
Wang L, Xie X, Liu W, Yang X, et al: The antiasthma effect of
neonatal BCG vaccination does not depend on the Th17/Th1 but
IL-17/IFN-γ balance in a BALB/c mouse asthma model. J Clin Immunol.
31:419–429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang CQ, Li W, Wu B, Chen WM, Chen LH, Mo
GW, Zhang QF, Gong L, Li J, Zhang HC, et al: Pheretima aspergillum
decoction suppresses inflammation and relieves asthma in a mouse
model of bronchial asthma by NF-κB inhibition. J Ethnopharmacol.
189:22–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim SG and Lee E, Park NY, Park HH, Jeong
KT, Kim KJ, Lee YJ, Jin M and Lee E: Britanin attenuates
ovalbumin-induced airway inflammation in a murine asthma model.
Arch Pharm Res. 39:1006–1012. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lei W, Zeng DX, Zhu CH, Liu GQ, Zhang XQ,
Wang CG, Wang Q and Huang JA: The upregulated expression of
OX40/OX40L and their promotion of T cells proliferation in the
murine model of asthma. J Thorac Dis. 6:979–987. 2014.PubMed/NCBI
|
|
27
|
Lee JJ, Dimina D, Macias MP, Ochkur SI,
McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA,
et al: Defining a link with asthma in mice congenitally deficient
in eosinophils. Science. 305:1773–1776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Humbles AA, Lloyd CM, McMillan SJ, Friend
DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH and
Gerard C: A critical role for eosinophils in allergic airways
remodeling. Science. 305:1776–1779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao YT, Zhang XG, Bai L, Li LQ and Yu JE:
Effects of Chinese herbal medicine Pingchuan Formula on airway
inflammation, interferon-γ and interleukin-4 in mice with asthma.
Zhong Xi Yi Jie He Xue Bao. 10:807–813. 2012.(In Chinese).
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu F, Yu J, Bai L, Xue Z and Zhang X:
Pingchuan formula improves asthma via restoration of the Th17/Treg
balance in a mouse model. BMC Complement Altern Med. 15:2342015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hardy R and Cooper MS:
Glucocorticoid-induced osteoporosis-a disorder of mesenchymal
stromal cells? Front Endocrinol (Lausanne). 2:242011.PubMed/NCBI
|
|
32
|
Zhu FB, Wang JY, Zhang YL, Quan RF, Yue
ZS, Zeng LR, Zheng WJ, Hou Q, Yan SG and Hu YG: Curculigoside
regulates proliferation, differentiation, and pro-inflammatory
cytokines levels in dexamethasone-induced rat calvarial
osteoblasts. Int J Clin Exp Med. 8:123372015.PubMed/NCBI
|
|
33
|
Weinstein RS: Glucocorticoid-induced
osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am.
41:595–611. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weinstein RS: Glucocorticoid-induced
osteonecrosis. Endocrine. 41:183–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bilezikian JP: Combination anabolic and
antiresorptive therapy for osteoporosis: Opening the anabolic
window. Curr Osteoporos Rep. 6:24–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu C, Janke LJ, Kawedia JD, Ramsey LB,
Cai X, Mattano LA Jr, Boyd KL, Funk AJ and Relling MV: Asparaginase
potentiates glucocorticoid-induced osteonecrosis in a mouse model.
PLoS One. 11:e01514332016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoon HY, Cho YS, Jin Q, Kim HG, Woo ER and
Chung YS: Effects of ethyl acetate extract of poncirus trifoliata
fruit for glucocorticoid-induced osteoporosis. Biomol Ther (Seoul).
20:89–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee DW and Choi EY: A comparative study of
bone mineral density among patients with obstructive lung diseases
in Korea. Int J Tuberc Lung Dis. 19:1246–1251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jung JW, Kang HR, Kim JY, Lee SH, Kim SS
and Cho SH: Are asthmatic patients prone to bone loss? Ann Allergy
Asthma Immunol. 112:426–431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim JW, Ku SK, Han MH, Kim KY, Kim SG, Kim
GY, Hwang HJ, Kim BW, Kim CM and Choi YH: The administration of
Fructus Schisandrae attenuates dexamethasone-induced muscle atrophy
in mice. Int J Mol Med. 36:29–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lv Y, Yu J, Sheng Y, Huang M, Kong X, Di
W, Liu J, Zhou H, Liang H and Ding G: Glucocorticoids suppressing
the browning of adipose tissue via miR-19b in male mice.
Endocrinology. Oct 24–2017.(Epub ahead of print).
|
|
42
|
Massaccesi L, Goi G, Tringali C, Barassi
A, Venerando B and Papini N: Dexamethasone-induced skeletal muscle
atrophy increases O-GlcNAcylation in C2C12 cells. J Cell Biochem.
117:1833–1842. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mammi CM, Marzolla V, Armani A, Feraco A,
Antelmi A, Maslak E, Chlopicki S, Cinti F, Hunt H, Fabbri A and
Caprio M: A novel combined glucocorticoid-mineralocorticoid
receptor selective modulator markedly prevents weight gain and fat
mass expansion in mice fed a high-fat diet. Int J Obes (Lond).
40:964–972. 2016. View Article : Google Scholar : PubMed/NCBI
|