Introduction
Significant damage may occur to the distal and
proximal ends of the sciatic nerve due to trapping, crushing or
stretching induced by accident or injury (1). Ineffective treatment of nerve injuries
may lead to partial or complete loss of sensory, as well as motor
and autonomic function (2). Loss of
productivity, socioeconomic and psychological problems may be
observed in individuals with nerve damage that results in
functional impairment (2,3).
Crush injuries may cause the total loss of motor and
sensory functions in the sciatic nerve (2,4).
Crush-type injuries in rats are an auxotrophic model frequently
used to investigate nerve regeneration following injury and are
characterized by the complete severing of the nerve axon and
myelin. Morphological, electrophysiological and functional effects
may differ depending on the degree, duration and characteristics of
the induced damage. Acute short-term compression results in nerve
ischemia, hypoxia, edema, an increase in vascular permeability and
the blockage of axoplasmic flow (5).
Following nerve injury, a process known as Wallerian degeneration
begins at the site of the axon distal to injury (4,5).
However, persistent crush adversely affects microcirculation.
Peripheral neural microcirculation, which serves an important role
in nerve regeneration, affects nerve injury and regeneration, the
blood-oxygen supply, neurotrophic effects, the maintenance of
neural conduction and axonal transport (4). Biochemical and functional changes may
occur under these conditions, including changes in the expression
of myelin proteins (5). Reperfusion
may occur and the subsequent decrease in pressure on nerve tissue
leads to the high-pressure redeposition of oxygen and nutrients and
the increased formation of free radicals, which leads to lipid
peroxidation (LPO) and tissue damage (3). The cumulative impact of ischemic and
mechanical processes in this type of nerve damage may be more
pronounced than their individual effects (3,6,7). Although peripheral nerves are capable
of undergoing regeneration following injury, this process and the
post-traumatic results are typically slow and weak. Peripheral
nerve trauma is therefore a significant cause of morbidity
(6). A large amount of research has
been conducted aiming to reduce the effects of peripheral nerve
damage; however, to the best of our knowledge, no effective
therapeutic strategies have been identified (6).
Quercetin (3,3′,4′,5,7-penta-hydroxy flavone) is a
ubiquitous plant flavonoid compound from the polyphenolic group
(8). It is found in many fruits,
vegetables and aromatic herbs, including onions, broccoli, green
tea, Ginkgo biloba and St. Johns-wort (Hypericum perforatum)
(8). Previous pharmacological
studies have reported that quercetin has powerful antioxidant,
antiangiogenic, anti-inflammatory, neuroprotective and
anti-apoptotic properties (8,9). Due to
its powerful antioxidant and anti-inflammatory activity, it has
been suggested that quercetin may prevent various diseases,
including diabetes, cancer and obesity (10). The effectiveness of quercetin at
treating ischemic injuries has also been identified; quercetin is
neuroprotective in cerebral ischemia, as it decreases matrix
metalloproteinase-9 levels and reduces free radical production
(10). Another study reported that
quercetin exhibited neuroprotective effects against
aluminum-induced cognitive impairments in rats (11).
The aim of the present study was to evaluate the
neuroprotective and antioxidant efficacy of quercetin in an
experimental rat model of nerve crush injury, using
histomorphometric and biochemical parameters to assess its
effects.
Materials and methods
Animals
A total of 48 male Sprague-Dawley rats (10–12 weeks
old, weighing 250–300 g) were used in the present study. Rats were
obtained from the Karadeniz Technical University Medical School
Experimental Research Center (Trabzon, Turkey). All animals were
housed in a controlled laboratory environment at room temperature
(22±2°C) in 50±10% humidity and under a 12 h light/dark cycle.
Standard lab chow and water were provided ad libitum throughout the
experiment. All animals received humane care according to the
criteria outlined in the Guide for the Care and Use of Laboratory
Animals published by the National Institutes of Health (12) and the present study was approved by
the Institutional Animal Ethical Committee of Karadeniz Technical
University (license no. 30.06.2015/4; 12.05.2016/272).
Study groups
The animals were assigned into one of the 8
following groups (n=6/group): A sham 7-day group (S-7), in which, a
surgical incision was made and closed on the first day of the
experiment and animals were sacrificed on day 7; a sham 28-day
group (S-28), in which a surgical incision was made and closed on
the first day of the experiment and animals were sacrificed on day
28; a quercetin 7-day group (Q-7), in which a previously utilized
dosage of 200 mg/kg quercetin (13)
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was dissolved in
0.5% dimethyl sulfoxide (DMSO; Merck KGaA) and administered
intragastrically by the oral route from the first day of the
experiment for 7 days. Rats were then sacrificed on day 7; a
quercetin 28-day group (Q-28), in which 200 mg/kg quercetin
dissolved in 0.5% DMSO was administered intragastrically by the
oral route for 7 days. Rats were sacrificed on day 28; a trauma
7-day group (T-7), in which the sciatic nerve was exposed by a
surgical incision and crush injury was induced. The incision was
closed and the rats were sacrificed on day 7; a trauma 28-day group
(T-28), in which the sciatic nerve was exposed by a surgical
incision and crush injury was induced. The incision was closed and
the rats were sacrificed on day 28; a trauma+quercetin therapy
7-day group (T+Q-7), in which the sciatic nerve was exposed by a
surgical incision and crush injury was induced. The incision was
closed and 200 mg/kg quercetin dissolved in 0.5% DMSO was
administered intragastrically by the oral route for 7 days. Rats
were sacrificed on day 7; a trauma+quercetin therapy 28-day group
(T+Q-28), in which the sciatic nerve was exposed by a surgical
incision and crush injury was induced. The incision was closed and
200 mg/kg quercetin dissolved in 0.5% DMSO was administered
intragastrically by the oral route for 7 days. Rats were sacrificed
on day 28.
Surgical procedures
All experimental procedures were performed with the
rats in a prone position under anesthesia with ketamine (90 mg/kg;
Ketalar® 50 mg/kg; Pfizer Inc., New York, NY, USA) and
xylazine (10 mg/kg; Rompun® 2%; Bayer, Pittsburgh, PA,
USA), which were administered intraperitoneally. The right lateral
femoral region was cleaned with an antiseptic solution and a
surgical incision was made using a scalpel. A unilateral muscular
incision was made from the greater trochanter to the mid-femur. For
the sham groups, the sciatic nerve was exposed and closed and no
further procedures were performed. For the trauma groups, the
sciatic nerve was exposed following muscle incision. Crush injury
was then induced by attaching a microclamp to the sciatic nerve for
1 min. The skin was then closed with a double layer of 4.0 silk
sutures to the subcutaneous tissue to prevent potential suture
opening and infection in the area of the incision during subsequent
stages of the experiment. Following the surgical procedure, rats
were left to heal for 7- and 28-days depending on the group they
had been assigned to. The time points of 7- and 28-days were
selected to analyze the effects of quercetin in a rat model of
sciatic nerve injury as degeneration in the myelin sheath becomes
apparent after 36–48 h and axon continuity is typically lost 48–96
h following injury when impulse transmission is impaired (14,15).
Schwann cells and macrophages work together during phagocytosis to
clean the wound site in a process that lasts between 1 week and
several months. Additionally, degenerated nerve remnants are
typically eliminated over 5–8 weeks (16). Therefore, 7- and 28-day intervals
were used to assess the early and late effects of the degeneration
process in the nerve crush injury model.
At the end of the experimental period, sciatic nerve
tissue was removed from the proximal and distal aspects and blood
serum specimens were placed into tubes containing EDTA without
anticoagulant for subsequent biochemical parameter analysis. Serum
samples were obtained following the centrifugation at 3,000 × g for
10 min at room temperature and then stored at −80°C prior to
further analysis. Extracted sciatic nerve tissues were divided into
two parts: Half was processed prior to histological analysis and
the other half was placed in an Eppendorf tube for biochemical
analyses and stored at −80°C.
Histopathological preparation and
evaluation of rat sciatic nerves
Osmium tetroxide (OsO4; abcr GmbH,
Karlsruhe, Germany) induces good fixation of myelinated axons at
the peripheral nerve and toluidine blue was used for staining, as
previously described (17). Briefly,
tissue samples taken 0.5 cm distally to the sciatic nerve injury
were fixed with 2.5% glutaraldehyde (Merck KGaA) in a 0.4 M PBS
solution (pH 7.4) for 4 h at 4°C. Post-fixation, tissue samples
were fixed in 1% OsO4 for 1 h at 4°C and subsequently
passed through an increasing series of alcohol prior to embedding
in epoxy resin. Tissue sections were cut into 0.5-µm-thick sections
using a Leica Reichert Ultracut R ultramicrotome (Leica
Microsystems GmbH, Wetzlar, Germany) and stained at 50–75°C for 30
sec with toluidine blue.
Subsequently, tissue samples were obtained 0.5 cm
distally to the sciatic nerves. These were then fixed in 10%
formalin at room temperature for 4 days, processed using routine
histological procedures and embedded in paraffin. Sections 5
µm-thick were sliced from paraffin blocks using a fully automated
microtome (Leica Microsystems GmbH). Sections were stained with
Masson's Trichrome using the Masson Trichrome Stain
kit-methyl/aniline blue according to the manufacturer's protocol,
(Atom Scientific, Cheshire, UK) prior to morphological and
morphometric analyses. Morphometric analysis of the sciatic nerve
sections, the numbers of myelinated nerve fibers, the myelin sheath
thickness and nerve fiber diameter were measured in five distinct
areas from each section. All slides were photographed using an
Olympus BX 51 light microscope (Olympus Corporation, Tokyo, Japan)
at a magnification of ×100 and analyzed using Analysis 5 Research
software 2.8 (Olympus Soft Imaging Solutions GmbH, Münster,
Germany).
Terminal deoxynucleotidyl transferase
deoxyuridine triphosphate nick-end labeling (TUNEL) assay
A TUNEL assay was used to analyze apoptosis in the
sciatic nerve tissue specimens and DNA fragmentations were
identified in cells from each tissue. TUNEL staining of sections
was performed using an in situ cell death detection kit
(Roche Diagnostics GmbH, Mannheim, Germany) according to the
manufacturer's protocol. Apoptotic evaluation from the
TUNEL-stained slides was performed using a light microscope at a
magnification of ×400 in five random fields of view. Homogeneously
stained TUNEL (+) Schwann cells with no necrotic areas were defined
as apoptotic. Apoptotic and normal cells were recorded by counting
100 Schwann cells in five areas in each tissue at a magnification
of ×400. The apoptotic index (AI) was then calculated using the
formula AI=[TUNEL (+) cell number/total cell number] ×100.
Biochemical analyses and techniques
employed
Serum and sciatic nerve tissue malondialdehyde (MDA)
levels were measured using previously described methods (18,19). The
red color resulting from the reaction between thiobarbituric acid
and the lipid peroxidation product MDA was measured
spectrophotometrically and serum MDA levels were expressed as
nmol/ml. MDA levels in sciatic nerve tissue were determined using a
method based on calculating the absorbance of the color of the
complex formed by MDA with thiobarbituric acid in an acidic
environment at 532 nm using a VERSA max tunable microplate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA) and MDA levels were
expressed as nmol/g wet tissue.
Sciatic nerve tissue- and serum catalase (CAT)
levels were determined using a method based on measuring the
absorbance of the yellow complex formed by ammonium molybdate with
H2O2 at 405 nm as previously described
(20) and the results was expressed
as U/mg protein. Sciatic nerve tissue and serum superoxide
dismutase (SOD) activity was determined using the method described
by Sun et al (21). The
absorbance of the purple formazan molecule forming as a result of
the reduction of nitroblue tetrazolium by
O2.−s formed by the xanthine-xanthine oxidase
was measured at 560 nm using a VERSA max tunable microplate reader.
SOD activity was divided by the total protein level and that
expressed as U/mg protein. Glutathione (GSH) levels were determined
using the method described by Ellman (22). This method is based on derivatization
of GSH with 5,5′-dithiobis 2-nitrobenzoic acid (Sigma-Aldrich) and
the formation of a yellow complex, which was measured at 410 nm
using a VERSA max tunable microplate reader.
Statistical analysis
The data from the present study was analyzed using
SPSS software version 22 (IBM Corp., Armonk, NY, USA).
Compatibility with normal distribution was examined using the
Shapiro-Wilk test. The Kruskal-Wallis test was used to compare
multiple variables in independent groups not exhibiting normal
distribution and the Mann-Whitney U test was used to compare
two-way variables. The Friedman test was applied to compare more
than two variables in dependent groups not exhibiting normal
distribution and a post-hoc Wilcoxon test was utilized for the
comparison of two-way variables. Values obtained from variables
were expressed as the mean ± standard deviation and P<0.05 was
considered to indicate a statistically significant difference.
Results
Rat body weight
The body weight of the rats increased over time from
baseline during the 4-week study period in all groups (Table I), however there was no significant
variation in body weight among all groups.
 | Table I.Body weights of rats in all groups
throughout the experimentation period. |
Table I.
Body weights of rats in all groups
throughout the experimentation period.
|
| Body weight
(g) |
|---|
|
|
|
|---|
| Group | Day 0 | 1 week | 2 weeks | 3 weeks | 4 weeks |
|---|
| S-7 |
292.85±10.65 |
298.30±9.62 |
301.88±8.93 |
309.60±8.83 |
313.38±8.66 |
| S-28 |
294.06±9.21 |
299.34±8.78 |
304.22±8.99 |
311.24±10.45 |
313.67±8.57 |
| Q-7 |
292.80±10.93 |
297.49±11.28 |
299.16±9.53 |
306.83±9.30 |
313.16±7.33 |
| Q-28 |
295.47±13.47 |
299.76±13.16 |
305.05±13.05 |
314.16±11.08 |
317.66±9.75 |
| T-7 |
291.81±12.26 |
299.34±14.05 |
309.83±13.49 |
315.33±10.01 |
319.16±8.88 |
| T-28 |
292.76±12.02 |
298.23±11.31 |
306.33±10.91 |
311.50±11.20 |
315.66±8.73 |
| T+Q-7 |
296.97±14.55 |
301.20±12.97 |
308.50±15.48 |
311.33±14.30 |
313.33±13.07 |
| T+Q-28 |
296.42±12.64 |
300.26±12.69 |
308.16±9.76 |
311.33±10.40 |
315.66±9.13 |
Histopathological observations in
sciatic nerve tissue
Histopathological analysis of the sciatic nerve
tissues from all groups was performed using Masson's trichrome and
toluidine blue staining of semi-thin sections. Microscopic
examination of the sciatic nerve sections from groups S-7, S-28,
Q-7 and Q-28 revealed impairment of the myelin sheath in certain
sections; however the sciatic nerve maintained its normal
morphological structure (Figs. 1 and
2). Additional trauma images were
presented to clarify the damage caused by the trauma model
(Fig. 1; T-7-A, -B, -C; T-28-A, -B,
-C). In the T-7 group, it was observed that the nerve was
surrounded by epineurium (Fig. 2),
occasional fibrotic tissue (Fig. 2)
beneath the epineurium invaginating the perineurium and there was
occasional loss of integrity in the perineurium (Fig. 1). In the majority of myelinated
axons, the axon and myelin sheath were fully degenerated.
Furthermore, expansion in the axons, occasional invagination of the
myelin sheath into the axon, axonal swelling and severe compromise
of the normal concentric lamellar structure of the myelin sheath
were observed (Fig. 1). The presence
of mast cells and capillary vessels was also observed between the
myelinated axons (Fig. 2). These
results indicate that Wallerian degeneration occurred (Figs. 1 and 2). In the T-28 group, mild fibrotic tissue
growth was observed beneath the epineurium surrounding the nerve
(Fig. 2). In some myelinated axons,
the axon and myelin sheath were degenerated, the normal concentric
lamellar structure of the myelin sheath was impaired and the myelin
sheath was invaginated into the axon (Fig. 1). When the morphology of the T-28
group was compared with that of the T-7 group, the presence of
nerve fibers indicating that the regeneration process had begun was
observed in the T-28 group (Figs. 1
and 2). Light microscopic
examination of semi-thin sciatic nerve sections from the T+Q-7
group revealed that regeneration had begun in myelinated nerve
fibers and that the axon and myelin sheath structures were also
included within this process. In addition, in some myelinated nerve
fibers, the normal structure of the myelin sheath around the axon
was impaired, the myelin sheath and axon were degenerated and the
presence of phagocytic cells contiguous to these areas was
observed. The degeneration structures of nerve fibers intensively
observed in the T-7 group had begun to decrease in this group;
however, the regeneration process was still ongoing (Fig. 1). Microscopic examination of
semi-thin sciatic nerve sections from the T+Q-28 group revealed
visible myelinated nerve fiber regeneration compared with the T-28
group, regenerated axonal bundles progressing from the periphery
and occasional commencement of a return to normal structure in
terms of axonal structure and the myelin sheath. Compared with the
T+Q-7 group, the axonal structure and myelin sheath in the T+Q-28
group were significantly closer to normal and the application of
quercetin made a positive contribution to the regeneration process
(Figs. 1 and 2).
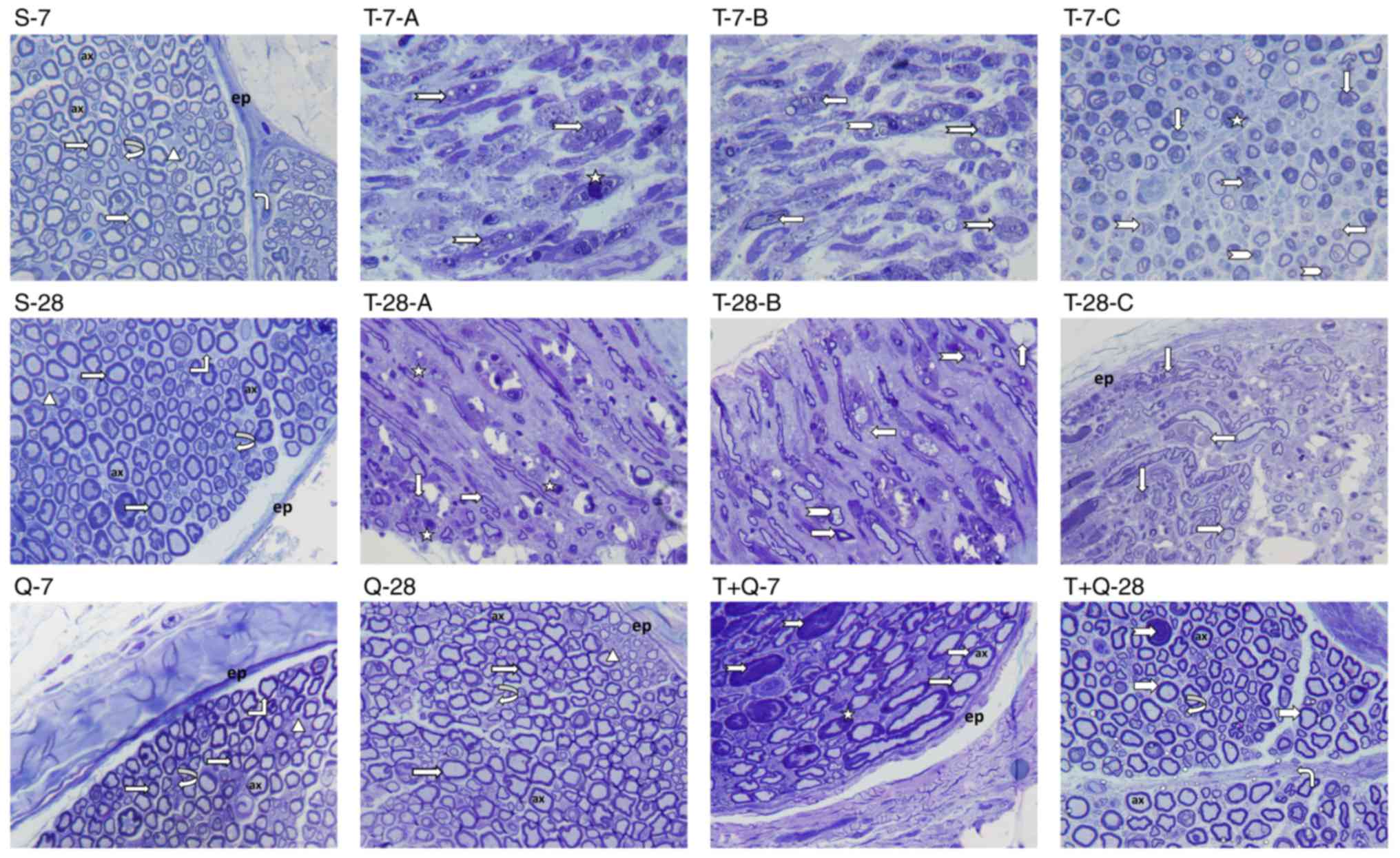 | Figure 1.Toluidine blue staining was performed
on sciatic nerve tissue sections and representative images of the
results from each group are presented. Notable features are
indicated on the figure as follows: Epineurium (ep), perineurium
(twisted arrow), endoneurium (upward-twisted arrow), axon (ax),
myelinated nerve fiber (right arrow), few myelinated nerve fibers
(left-twisted arrow), unmyelinated nerve fiber (arrowhead), myelin
sheath and axon degeneration (right notched arrow), axonal swelling
(star), axon dilatation (upward-arrow), myelin sheath invaginated
into the axon (downward-arrow), disorganization in the concentric
lamellar structure of the myelin sheath (left arrow), separation in
the concentric lamellae of the myelin sheath (angled double
separator). Magnification, ×100. S-7, sham 7 day group; S-28, sham
28 day group; Q-7, quercetin 7 day group; Q-28, quercetin 28 day
group; T-7-A, -B and -C; trauma 7 day group images -A, -B and -C;
T-28-A, -B and -C, trauma 28 day group images -A, -B and -C; T+Q-7,
trauma+quercetin 7 day group; T+Q-28, trauma+quercetin 28 day
group. |
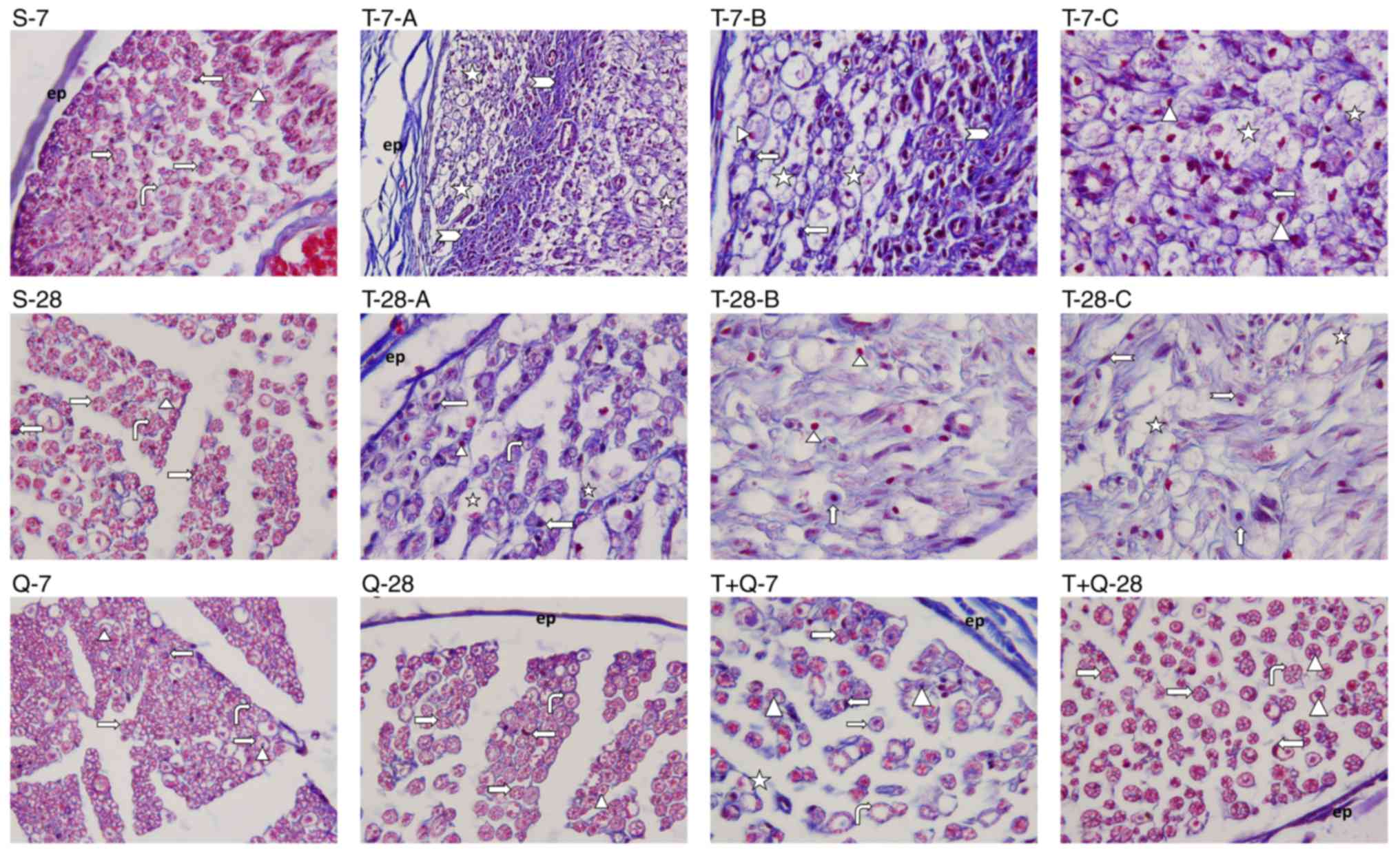 | Figure 2.Masson's trichrome staining was
performed on sciatic nerve tissue sections and representative
images of the results from each group are presented. Notable
features are indicated on the figure as follows: Myelinated nerve
fiber (right arrow), axon (arrow head), Schwann cell nucleus (left
arrow), epineurium (ep), endoneurium (twisted arrow), mast cell
(upward arrow), fibroblast (right notched arrow), fibrocytes (left
notched arrow), axon and myelin sheath degeneration (star), axonal
swelling (star), fibrotic tissue (angled double separator
Magnification, ×100. S-7, sham 7 day group; S-28, sham 28 day
group; Q-7, quercetin 7 day group; Q-28, quercetin 28 day group;
T-7-A, -B and -C; trauma 7 day group images -A, -B and -C; T-28-A,
-B and -C, trauma 28 day group images -A, -B and -C; T+Q-7,
trauma+quercetin 7 day group; T+Q-28, trauma+quercetin 28 day
group. |
Morphometric observations
Morphometric results concerning myelin sheath
thickness, nerve fiber diameter and the number of myelinated nerve
fibers in sciatic nerve tissues from the groups are presented in
Table II. The myelin sheath
thickness and myelinated nerve fiber numbers in the T-7 and T-28
groups were significantly decreased compared with the S-7 and S-28
groups, respectively (all P<0.05). No significant differences in
morphology were observed between the T-7 and T+Q7 groups. However,
the T+Q-28 group exhibited a significantly increased number of
myelinated nerve fibers and significantly increased nerve fiber
diameters compared with the T-28 group (P<0.05). Furthermore,
the T+Q-28 group had a significantly increased number of myelinated
nerve fibers and nerve fiber diameter compared with the T-28 and
T+Q-7 groups (all P<0.05).
 | Table II.Morphometric findings in the
experimental groups. |
Table II.
Morphometric findings in the
experimental groups.
| Group | Myelin sheath
thickness (µm) | Nerve fiber
diameter (µm) | Number of
myelinated nerve fibers | Apoptotic index
(%) |
|---|
| S-7 |
2.66±0.28 |
7.71±0.81 |
75.33±7.31 |
19.28±6.65 |
| S-28 |
2.22±0.18 |
7.95±0.55 |
89.83±11.53 |
27.69±3.75 |
| Q-7 |
2.63±0.28 |
7.88±0.81 |
78.00±11.38 |
20.64±6.96 |
| Q-28 |
2.68±0.19 |
7.19±0.70 |
76.00±8.50 |
26.68±4.77 |
| T-7 |
0.92±0.22a |
4.08±0.83a |
51.66±5.08a |
71.64±10.33a |
| T-28 |
1.13±0.30b |
4.06±0.85b |
61.66±5.92b |
53.37±9.41b |
| T+Q-7 |
1.01±0.38 |
4.37±0.35 |
52.66±3.20 |
43.91±7.51c |
| T+Q-28 |
1.46±0.05 |
6.05±0.30d,e |
73.50±12.67d,e |
38.14±4.43d |
Immunohistochemical findings
AIs of the sciatic nerve tissues were determined by
a TUNEL assay (Fig. 3) and the
results are presented in Table II.
AI values in Schwann cells from the sciatic nerve tissue were
significantly increased in the T-7 and T-28 groups compared with
the S-7 and S-28 groups, respectively (P<0.05). In addition, in
terms of AI, no differences were observed between the T-7 and T-28
groups and the S-7 and S-28 groups. The AI in the T+Q-7 group was
significantly lower than in the T-7 group (P<0.05).
Additionally, the AI was significantly decreased in the T+Q-28
group compared with the T-28 group (P<0.05). No significant
differences in the AI were observed between the T+Q-28 and T+Q-7
groups.
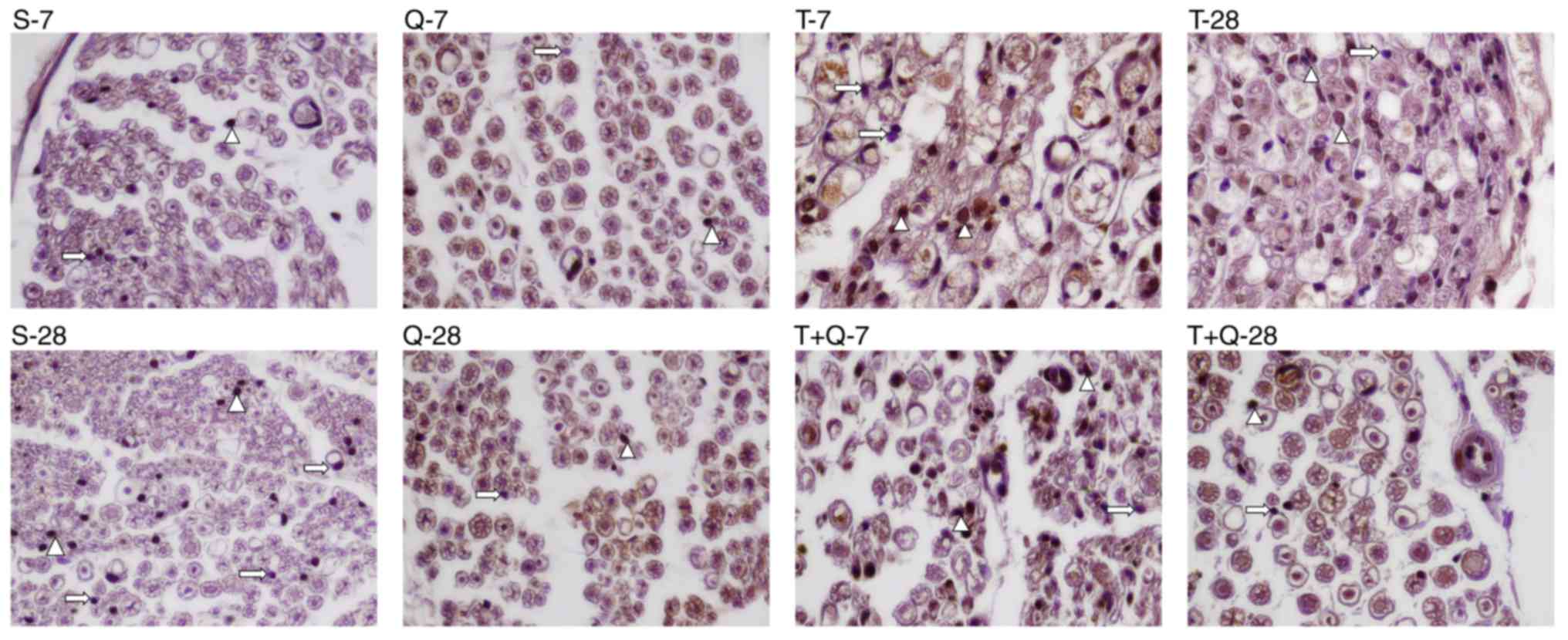 | Figure 3.Terminal deoxynucleotidyl transferase
deoxyurıdıne triphosphate nick-end labeling assays were performed
on Schwann cells to determine their apoptotic index values.
Representative images from each group are displayed and notable
features are indicated on the figure as follows: Apoptotic Schwann
cell nucleus (arrow head), normal Schwann cell nucleus (right
arrow). Magnification, ×100. S-7, sham 7 day group; S-28, sham 28
day group; Q-7, quercetin 7 day group; Q-28, quercetin 28 day
group; T-7-A, -B and -C; trauma 7 day group images -A, -B and -C;
T-28-A, -B and -C, trauma 28 day group images -A, -B and -C; T+Q-7,
trauma+quercetin 7 day group; T+Q-28, trauma+quercetin 28 day
group. |
Biochemical parameter findings
Levels of MDA, SOD, CAT and GSH in the serum and
tissue of all groups were measured and the results are presented in
Table III. There was an increase
in tissue MDA in the T-7 group compared with the S-7 group; however
it was not significant. Serum MDA levels were significantly lower
in the T-28 group compared with the S-7 group (P<0.05) and
tissue MDA levels were significantly lower in the T+Q-7 group
compared with the T-7 group (P<0.05). A significant increase in
serum SOD activity in the sciatic nerve was observed in the T+Q-28
group compared with the T+Q-7 group (P<0.05). Additionally, a
significant decrease was observed in serum CAT activity in the T-28
group compared with the S-7 group (P<0.05). However, a
significant increase was observed in serum CAT activity in the
T+Q-7 group compared with the T-7 group (P<0.05). Serum GSH
activity was significantly lower in the S-28 group compared with
the S-7 group (P<0.05), however no statistically significant
differences in GSH activity were observed among the other
groups.
 | Table III.Biochemical findings for the rat
sciatic nerve. |
Table III.
Biochemical findings for the rat
sciatic nerve.
|
| MDA | SOD | CAT | GSH |
|---|
|
|
|
|
|
|
|---|
| Groups | Tissue
(nmol/mgr) | Serum (nM) | Tissue (U/g) | Serum (nM) | Tissue (kU/mg) | Serum (kU/l) | Tissue (mM/gr) | Serum (mM/l) |
|---|
| S-7 |
5.34±1.18 |
0.28±0.07 |
1,181.54±427.90 |
0.45±0.32 |
0.96±0.21 |
1,131.04±330.50 |
5.70±1.32 |
0.02±0.007 |
| S-28 |
3.52±1.11 |
0.12±0.01 |
1,432.13±266.67 |
0.85±0.31 |
1.09±0.17 |
661.32±146.13 |
10.45±2.31 |
0.02±0.002a |
| Q-7 |
4.29±1.77 |
0.73±0.24 |
1,127.72±48.72 |
0.39±0.15 |
1.08±0.19 |
902.44±229.16 |
5.61±2.22 |
0.01±0.008 |
| Q-28 |
1.62±0.91 |
0.29±0.19 |
3,169.99±1,011.12 |
1.37±0.31 |
1.33±0.30 |
561.10±155.72 |
6.96±2.92 |
0.02±0.002 |
| T-7 |
6.82±2.81 |
0.16±0.08 |
2,226.54±976.69 |
0.67±0.33 |
1.02±0.26 |
740.19±41.45 |
4.99±2.11 |
0.02±0.007 |
| T-28 |
4.81±2.16 |
0.06±0.03a |
3,290.18±2,122.73 |
2.04±0.75 |
1.09±0.19 |
465.29±199.84a |
7.74±1.49 |
0.02±0.003 |
| T+Q-7 |
1.71±0.79b |
0.39±0.16 |
1,897.45±534.12 |
0.27±0.25 |
1.16±0.14 |
992.43±113.96b |
6.54±1.63 |
0.02±0.003 |
| T+Q-28 |
1.78±0.58 |
0.40±0.14 |
2,448.04±1,151.05 |
1.04±0.12c |
1.30±0.30 |
990.89±227.36 |
6.29±1.62 |
0.02±0.003 |
Discussion
Peripheral nerves may be exposed to various types of
damage, including crush, compression and tension injuries (2). Major functional deficits occur when
severed axons are unable to re-establish continuity with the distal
aspect due to impairment of motor neurons, a surrounding
environment hostile to Schwann cell survival and insufficient nerve
regeneration capacity (23).
Peripheral nerve trauma is therefore a substantial health problem
and is often difficult for clinicians to treat (23).
In addition to mechanical injury, nerve damage
induced by clamps induces ischemia (7). One of the principal causes of
functional loss following nerve compression is mechanical tissue
crush occurring in the nerve (7).
The crush type nerve injury model is the most widely used model for
research into the cellular and molecular mechanisms of the
peripheral nerve (4,24). In the present study, a rat model was
modified from a previous study (3)
and utilized to investigate damage arising from sciatic nerve crush
trauma.
The body weights of all animals in the present study
were assessed throughout the experiment and significant increases
were observed in all groups over the 4 weeks compared with
baseline. However, no significant differences were observed among
the groups. In a previous study on sciatic nerve damage in mice and
rats, Vogelaar et al (25)
observed that mice did not make full use of damaged claws until day
28 postoperatively and rats did not do so until day 70. This
suggests that the movement of animals is reduced due to an
immobilization effect resulting from trauma and that the weight
gain observed in the present study may be associated with this
immobility.
Unlike the central nervous system, peripheral nerves
may initiate a regeneration process at the injury site. Axonotmesis
may be observed following crush injuries to nerves and Wallerian
degeneration may occur distal to the injury in addition to axonal
destruction, despite the integrity of the endothelial sheath being
preserved. Although the functional outcome may be satisfactory due
to spontaneous regeneration of the distal nerve stump, failure may
also be observed due to nerve cell death and end organ atrophy
(26,27). Additionally, wounded tissue above the
injury may mechanically block axonal extension and have an adverse
impact on the healing process. Wallerian degeneration leads to the
accumulation of macrophages and other white blood cells that assist
with the removal of damaged axons, myelin sheath proteins and
lipids in the damaged area. Furthermore, Schwann cells divide
rapidly and produce dedifferentiated daughter cells that help
remove components of the myelin and subsequently serve an active
role in nerve regeneration by providing pathways for axon renewal
(26–28). Peripheral nervous system regeneration
may not always result in a full functional recovery; Galtrey and
Fawcett (29) reported that axonal
regeneration lasted ~56 days (2–3.5 mm/day) and that functional
recovery occurred in 21–42 days. To be considered a fully
functional recovery, axon diameter and myelin sheath thickness must
return to normal values.
A rat model of crush type sciatic nerve damage was
established in the present study and morphological evaluation was
performed in the early (7 days) and late periods (28 days). Diffuse
degeneration was observed in the axons and myelin sheath in the
sciatic nerves of rats in the T-7 group. The normal concentric
lamellar structure of the myelin sheath was compromised and myelin
sheath lamellae were separated from one another and fragmented.
Axonal swelling was particularly evident and certain axons were
completely degenerated. These results are compatible with the
results of previous studies investigating Wallerian degeneration
(30,31). Furthermore, in sciatic nerve sections
from rats in the T-28 group, the axon and myelin sheath in certain
axons had degenerated and the normal concentric lamellar structure
of the myelin sheath was impaired; however in addition to this
degeneration, the regeneration process had begun and nerve fibers
in which myelin production had commenced were observed. Significant
differences were observed between the trauma groups and the sham
group in terms of the myelin sheath thickness, nerve fiber diameter
and the numbers of myelinated nerve fibers. Previous studies have
also reported histological changes, including in numbers of
myelinated nerve fiber numbers and diameters of myelinated nerve
fibers, following sciatic nerve damage (30–32).
Yüce et al (2) examined the
nerve tissues from rats exposed to crush type nerve damage using
electron microscopy and reported the disorganization of myelin, the
loss of myelinated fibers and the absence of myelin ovoids and
cytoskeleton by the end of week 1. By the end of the week 4, the
formation of several thin, myelinated nerve fibers had been
reported and the presence of non-myelinated axons was also
observed. Additionally, decreases in myelin sheath thickness, nerve
fiber diameter and axon diameter were reported following light
microscopic morphometric examination of tissues. This process
demonstrates the stages of regeneration following nerve
degeneration and also supports the results of the present
study.
Noorafshan et al (33) reported a decrease of 30% in the
proximal nerve and 36% in the distal nerve in terms of the mean
myelinated nerve fiber diameter on day 28 following crush nerve
damage. They also reported losses of 48% in the proximal aspect of
the nerve and 56% in the distal aspect regarding the total numbers
of myelinated nerve fibers. Another previous study reported
findings compatible with Wallerian injury on days 7 and 28
following nerve damage (34). In the
present study, the morphological injury findings that developed
following crush type nerve damage were compatible with previous
studies and may serve as a reference for further studies concerning
the process and stages of regeneration.
Impairment of the vasa nervorum also occurs
following sciatic nerve crush injury and if compressive ischemia is
maintained over a sufficient period of time, blood flow to the
nerve may not be restored (35). A
number of different molecules, including endogenous chemical
mediators, may be involved in the underlying pathology of sciatic
nerve crush injury, in addition to ischemia, free radical
production (35) and apoptosis
(36,37). Cellular damage induced by oxidative
stress may trigger apoptosis. Free oxygen radicals (ROS) may
trigger apoptotic or necrotic cell death as they exhibit lipid
peroxidation (LPO) in catalytic reactions (38). ROS are regarded as an important
source of LPOs and cause oxidative stress by triggering changes in
a series of antioxidant activities (39,40).
Under normal conditions, cells are protected against oxidative
damage by several mechanisms and antioxidant molecules (41). The antioxidant enzymes involved are
SOD, CAT and glutathione peroxidase, while the cellular antioxidant
defense mechanisms are low molecular weight GSH and vitamins C and
E (41,42). SOD converts superoxide anion radicals
into hydrogen peroxide (H2O2), which is
broken down by CAT in the peroxisomes and GSH peroxidase in the
cytosol and mitochondria to form oxygen and water (43). GSH has been reported to be the
primary functioning defense system as a scavenger and co-factor in
metabolic detoxification of ROS against oxidative damage (38).
If damage occurs in a peripheral nerve, a response
involving a series of biochemical changes is triggered. Serious
injury may lead to neuronal edema, intensive neutrophil
infiltration and apoptosis. Increased neutrophil infiltration
causes an increase in myeloperoxidase activity and tissue MDA and
LPO levels. LPO may directly impair membrane functions and
indirectly harm cellular components (3). A significant increase has been observed
in LPOs following injury (3). Hall
and Braughler (44) reported peak
LPO levels at 1, 2 and 48 h following spinal cord trauma. Oxidative
stress is regarded as one of the main causes of nerve damage
following injury. However, little is known about changes in
antioxidant mRNA expression and changes following peripheral nerve
injury and regeneration (45). In
the present study, a small increase in the levels of tissue MDA was
observed in the S-7 group compared with the T-7 group. However,
serum MDA was significantly lower in the T-28 group compared with
the S-7 group. Tissue MDA levels were also significantly decreased
in the T+Q-7 group compared with the T-7 group. There was a
significant increase in serum SOD activity in the T+Q-28 group
compared with the T+Q-7 group. Although no significant differences
were observed between the trauma and sham groups, there was a
notable decrease in MDA levels in the groups receiving quercetin.
Several previous studies of sciatic nerve injury have reported an
increase in MDA, a LPO marker (3,46,47),
however the results of the present study are not fully aligned with
this. Furthermore, previous studies have reported that damage
occurs due to ischemic and mechanical effects in crush type
injuries in the sciatic nerve (2,3).
However, the severe histopathological injury observed in the
present study was considered to be due more to a mechanical effect
rather than ischemia and when combined with mild ischemic injury,
caused increased morphological damage.
Mechanical, ischemic and inflammatory processes are
initiated by nerve damage (2) and
there are various treatments available to reduce their impact on
nerve function. The effects of various treatment methods on nerve
regeneration have been assessed in a number of studies and
promising developments have been reported (48,49).
However, sciatic nerve damage is still clinically significant and
the mechanisms involved are not fully understood. Sciatic nerve
damage may have a negative impact on the patient's quality of life.
The majority of the positive effects of quercetin in the prevention
of several chronic diseases have been attributed to its antioxidant
activity (50). Quercetin acts as an
antioxidant by directly scavenging free radicals and also exhibits
an indirect effect by binding to iron and copper and inhibiting the
release of H2O2 and LPO caused by transition
metals (51). In addition to its
antioxidant activity, it has also been reported to possess a number
of other essential functions, including anticarcinogenic,
anti-ischemic, anti-inflammatory and antiallergenic properties
(52). In addition to its other
biological benefits, quercetin has been reported to contribute to
the protection of neuronal cells against oxidative stress-induced
neurotoxicity (53). Chen et
al (54) described the effects
of quercetin on sciatic nerve-crush injuries and the results
suggested that quercetin accelerated the functional recovery of
mice by upregulating neuronal intrinsic growth capacity and
postponing distal atrophy.
Previous studies investigating the effects of
quercetin on levels of antioxidant enzymes have reported that SOD
ameliorates oxidative stress in the brain and that quercetin
administered for 3 weeks significantly increased SOD levels in rats
(55) and significantly reduced
lipid peroxide levels (56). The
neuroprotective mechanism of quercetin in cerebral ischemia causes
a decrease in metalloproteinase-9 and reduces free radical
production (11). Although the
neuroprotective effects of quercetin on the central nervous system
have been evaluated, to the best of our knowledge no previous
studies have investigated its effect on peripheral nervous system
damage. In the present study, a significant decrease in tissue MDA
levels was observed in the T+Q-7 group compared with the T-7 group.
No significant differences were observed in myelin sheath thickness
or nerve fiber diameters between the T-7 group and the T+Q-7 group.
However, significant increases were observed in the myelinated
nerve fiber numbers and nerve fiber diameters in the T+Q-28 group
compared with the T-7 group. Significant increases were also
observed in nerve fiber diameters and myelinated nerve fiber
numbers in the T+Q-28 group compared with the T+Q-7 group. Although
the stages of normal regeneration had begun in sections from the
T-28 group, marked axonal and myelin injury were still present. A
marked degree of axon-myelin regeneration was also observed in the
T+Q-28 group and the nerve tissue morphology was almost as normal
as that in the T+Q-7 group rats. These results suggest that
quercetin notably shortened the healing period in a rat model of
crush type nerve damage and positively contributed to early healing
and productivity, partly via its antioxidant effects. However, the
lack of association between the biochemical and morphological
findings in the present study indicates that morphological damage
may arise as a result of a direct mechanical effect extraneous to
the nerve damage.
Schwann cells serve a major role in Wallerian
degeneration. They become active during the initial 24 h following
injury and undergo nuclear and cytoplasmic widening and an increase
in their mitotic rate (14,15). The primary role of the Schwann cell
is to assist with the removal of degenerated axonal and myelin
debris and their subsequent transfer to macrophages. Schwann cells
and macrophages work together in phagocytosis and cleaning of the
wound site in a process lasting between 1 week and several months
(14–16). The incidence of apoptotic cell death
in dorsal root ganglia neurons following axonotmesis is 20–50%
(16). Quercetin induces apoptosis,
prevents tumor development, increases membrane fluidity, inhibits
phospholipase A2 and protein kinases and protects the erythrocyte
membrane against oxidative damage in mice (57). Bcl-2-associated × protein expression,
caspase-3 and caspase-9 activation and levels of p53 mRNA, a
protein that regulates the cell cycle, increased significantly in a
dose-dependent manner in neuroblastoma cells taken from mice
administered with quercetin (58).
Gholami et al (59) reported
that quercetin may be beneficial in the treatment of sciatic
ischemic/reperfusion injury due to its anti-apoptotic and
anti-inflammatory effects and its ability to reduce the expression
of nuclear factor-κB. In the present study, the AI in Schwann cells
following sciatic nerve damage was significantly increased the T-7
and T-28 groups compared with the S-7 and S-28 groups,
respectively. A significant decrease in AI was observed in the
T+Q-7 group compared with the T-7 group and AI also significantly
decreased in the T+Q-28 group compared with the T-28 group.
According to these results, quercetin demonstrated a positive
effect on crushing type sciatic nerve damage, exhibiting
anti-apoptotic effects. These effects indicate that quercetin
treatment may aid the regeneration of sciatic nerves.
Recovery rates of patients with peripheral nerve
injury who undergo conventional surgical and medical treatments
have been disappointing (60,61).
Previous studies have reported that natural medicine may stimulate
nerve growth factor expression following nerve injury and promote
peripheral nerve regeneration and functional recovery (60–63).
These results indicate that natural medicine may be a novel method
of promoting the repair of peripheral nerve injuries. Polymerase
chain reaction (PCR) is a common laboratory technique used to make
many copies of a specific DNA region in vitro (64). Klasan et al (65) reported that the Reg3 G gene serves a
major role in communication between injured axons and muscles and
may serve a significant role in skeletal muscle and peripheral
nerve regeneration. Al-Jumaily et al (66) examined the expression of dorsal root
ganglion (DRG) of members of three families of genes, which have
been demonstrated to induce the I Cl (Ca) current following injury
and revealed that that mBest1 and Tweety2 were the best candidates
to serve a role in the injury-induced I Cl (Ca) in DRG neurons
(66). Further studies are required
to fully elucidate the process of sciatic nerve
degeneration-regeneration. In addition, the use of PCR may be
beneficial to indicate the presence of relevant molecules within
the tissue.
In conclusion, the administration of quercetin 7 and
28 days following nerve damage exhibited an anti-apoptotic effect
and a positive impact on nerve regeneration in the present study.
Additionally, quercetin intake may shorten the Wallerian
degeneration that occurs following crush-type nerve injury,
accelerate myelination and partially reduce oxidative stress.
Further studies are required to assess different dosages of
quercetin and different application periods to determine its use as
a novel treatment option within a clinical setting.
Acknowledgements
This study was supported by the Scientific
Researches Fund of Karadeniz Technical University (grant no.
TDK-2015-5331).
References
|
1
|
Wang W, Li D, Li Q, Wang L, Bai G, Yang T,
Li Q, Zhu Z and Sun H: Erythropoietin promotes peripheral nerve
regeneration in rats by upregulating expression of insulin-like
growth factor-1. Arch Med Sci. 11:433–437. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yüce S, Cemal Gökçe E, Işkdemir A, Koç ER,
Cemil DB, Gökçe A and Sargon MF: An experimental comparison of the
effects of propolis, curcumin, and methylprednisolone on crush
injuries of the sciatic nerve. Ann Plast Surg. 74:684–692. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yildirim AE, Dalgic A, Divanlioglu D,
Akdag R, Cetinalp NE, Alagoz F, Helvacioglu F, Take G, Guvenc Y,
Koksal I and Belen AD: Biochemical and histopathological effects of
catechin on experimental peripheral nerve injuries. Turk Neurosurg.
25:453–460. 2015.PubMed/NCBI
|
|
4
|
Gao Y, Weng C and Wang X: Changes in nerve
microcirculation following peripheral nerve compression. Neural
Regen Res. 8:1041–1047. 2013.PubMed/NCBI
|
|
5
|
Roglio I, Bianchi R, Gotti S, Scurati S,
Giatti S, Pesaresi M, Caruso D, Panzica GC and Melcangi RC:
Neuroprotective effects of dihydroprogesterone and progesterone in
an experimental model of nevre crush injury. Neuroscience.
155:673–685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beck-Broichsitter BE, Lamia A, Geuna S,
Fregnan F, Smeets R, Becker ST and Sinis N: Does pulsed magnetic
field therapy influence nerve regeneration in the median nerve
model of the rat? Biomed Res Int 2014. 4017602014.
|
|
7
|
Schmelzer JD, Zochodne DW and Low PA:
Ischemic and reperfusion injury of rat peripheral nerve. Proc Natl
Acad Sci USA. 86:pp. 1639–1642. 1989; View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Waseem M and Parvez S: Neuroprotective
activities of curcumin and Quercetin with potential relevance to
mitochondrial dysfunction induced by oxaliplatin. Protoplasma.
253:417–430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arikan S, Ersan I, Karaca T, Kara S,
Gencer B, Karaboga I and Hasan Ali T: Quercetin protects the retina
by reducing apoptosis due to ischemia-reperfusion injury in a rat
model. Arq Bras Oftalmol. 78:100–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao RQ, Qi DS, Yu HL, Liu J, Yang LH and
Wu XX: Quercetin attenuates cell apoptosis in focal cerebral
ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling
pathway. Neurochem Res. 37:2777–2786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharma DR, Wani WY, Sunkaria A, Kandimalla
RJ, Verma D, Cameotra SS and Gill KD: Quercetin protects against
chronic aluminum-induced oxidative stress and ensuing biochemical,
cholinergic, and neurobehavioral impairments in rats. Neurotox Res.
23:336–357. 2013.PubMed/NCBI
|
|
12
|
Guide for the Care and Use of Laboratory
Animals, . National Research Council (US): Committee for the Update
of the Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press (US); Washington, DC: 2011
|
|
13
|
Ferreira PE, Lopes CR, Alves AM, Alves ÉP,
Linden DR, Zanoni JN and Buttow NC: Diabetic neuropathy: An
evaluation of the use of quercetin in the cecum of rats. World J
Gastroenterol. 19:6416–6426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirata K and Kawabuchi M: Myelin
phagocytosis by macrophages and nonmacrophages during wallerian
degeneration. Microsc Res Tech. 57:541–547. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mirajullah M and Shen X: Schwann cells:
Leader of nervenkitt. J Ayub Med Coll Abbottabad. 14:30–33.
2002.PubMed/NCBI
|
|
16
|
Burnett MG and Zager EL: Pathophysiology
of peripheral nerve injury: A brief review. Neurosurg Focus.
16:E12004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Scipio F, Raimondo S, Tos P and Geuna
S: A simple protocol for paraffin-embedded myelin sheath staining
with osmium tetroxide for light microscope observation. Microsc Res
Tech. 71:497–502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yagi K: Lipid peroxides related radicals
in clinical medicine free radicals in diagnostic medicine. Adv Exp
Med Biol. 366:1–15. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mihara M and Uchiyama M: Determination of
malonaldehyde precursor in tissues by thiobarbituric acid test.
Anal Biochem. 86:271–278. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Góth L: A simple method for determination
of serum catalase activity and revision of reference range. Clin
Chim Acta. 196:143–151. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Y, Oberley LW and Li Y: A simple
method for clinical assay of superoxide dismutase. Clin Chem.
34:497–500. 1988.PubMed/NCBI
|
|
22
|
Ellman GL: Tissue sulphydryl groups. Arch
Biochem Biophys. 82:70–77. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karşıdağ S, Özcan A, Sahin S, Karşidağ S,
Kabukçuoğlu F, Uğurlu K and Baş L: Electrophysiologic and
histopathologic evaluation of peripheral nerve regeneration at
different nerve segments and with different repair techniques. Acta
Orthop Traumatol Turc. 42:278–283. 2008.(In Turkish). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tos P, Ronchi G, Papalia I, Sallen V,
Legagneux J, Geuna S and Giacobini-Robecchi MG: Chapter 4: Methods
and protocols in peripheral nevre regeneration experimental
research: Part I-experimental models. Int Rev Neurobiol. 87:47–79.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vogelaar CF, Vrinten DH, Hoekman MF,
Brakkee JH, Burbach JP and Hamers FP: Sciatic nerve regeneration in
mice and rats: Recovery of sensory innervation is followed by a
slowly retreating neuropathic pain-like syndrome. Brain Res.
1027:67–72. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Glenn TD and Talbot WS: Signals regulating
myelination in peripheral nerves and the Schwann cell response to
injury. Curr Opin Neurobiol. 23:1041–1048. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li R, Wu J, Lin Z, Nangle MR, Li Y, Cai P,
Liu D, Ye L, Xiao Z, He C, et al: Single injection of a novel nerve
growth factor coacervate improves structural and functional
regeneration after sciatic nerve injury in adult rats. Exp Neurol.
288:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Shan Q, Meng Y, Pan J and Yi S:
Mrpl10 and Tbp are suitable reference genes for peripheral nerve
crush injury. Int J Mol Sci. 18:pii: E2632017. View Article : Google Scholar
|
|
29
|
Galtrey CM and Fawcett JW: The role of
chondroitin sulfate proteoglycans in regeneration and plasticity in
the central nervous system. Brain Res Rev. 54:1–18. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin ZS, Zhang H, Bo W and Gao W:
Erythropoietin promotes functional recovery and enhances nerve
regeneration after peripheral nerve injury in rats. AJNR Am J
Neuroradiol. 31:509–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gigo-Benato D, Russo TL, Tanaka EH, Assis
L, Salvini TF and Parizotto NA: Effects of 660 and 780 nm low-level
laser therapy on neuromuscular recovery after crush injury in rat
sciatic nerve. Lasers Surg Med. 42:673–682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gjurasin M, Miklic P, Zupancic B, Perovic
D, Zarkovic K, Brcic L, Kolenc D, Radic B, Seiwerth S and Sikiric
P: Peptide therapy with Pentadecapeptide BPC 157 in traumatic nerve
injury. Regul Pept. 160:33–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Noorafshan A, Omidi A and Karbalay-Doust
S: Curcumin protects the dorsal root ganglion and sciatic nerve
after crush in rat. Pathol Res Pract. 207:577–582. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang X, Ma J, Wei Q, Feng X, Qiao L, Liu
L, Zhang B and Yu W: Effect of frankincense extract on nerve
recovery in the rat sciatic nerve damage model. Evid Based
Complement Alternat Med. 2016:36172162016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Morani AS and Bodhankar SL:
Neuroprotective effect of vitamin E acetate in models of
mononeuropathy in rats. Neuroanatomy. 7:33–37. 2008.
|
|
36
|
Smith D, Tweed C, Fernyhough P and Glazner
GW: Nuclear factor-kappaB activation in axons and Schwann cells in
experimental sciatic nevre injury and its role in modulating axon
regeneration: Studies with etanercept. J Neuropathol Exp Neurol.
68:691–700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maeda T, Kiguchi N, Kobayashi Y, Ozaki M
and Kishioka S: Pioglitazone attenuates tactile allodynia and
thermal hyperalgesia in mice subjected to peripheral nerve injury.
J Pharmacol Sci. 108:341–347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Goraca A, Ciejka E and Piechota A: Effects
of extremely low frequency magnetic field on the parameters of
oxidative stress in heart. J Physiol Pharmacol. 61:333–338.
2010.PubMed/NCBI
|
|
39
|
Ragy MM: Effect of exposure and withdrawal
of 900-MHz electromagnetic waves on brain, kidney and liver
oxidative stress and some biochemical parameters in male rats.
Electromagn Biol Med. 34:279–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yurekli AI, Ozkan M, Kalkan T, Saybasili
H, Tuncel H, Atukeren P, Gumustas K and Seker S: GSM base station
electromagnetic radiation and oxidative stress in rats. Electromagn
Biol Med. 25:177–188. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tkalec M, Stambuk A, Srut M, Malarić K and
Klobučar GI: Oxidative and genotoxic effects of 900 MHz
electromagnetic fields in the earthworm Eisenia fetida. Ecotoxicol
Environ Saf. 90:7–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meral I, Mert H, Mert N, Deger Y, Yoruk I,
Yetkin A and Keskin S: Effects of 900-MHz electromagnetic field
emitted from cellular phone on brain oxidative stres and some
vitamin levels of guinea pigs. Brain Res. 1169:120–124. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Celino FT, Yamaguchi S, Miura C, Ohta T,
Tozawa Y, Iwai T and Miura T: Tolerance of spermatogonia to
oxidative stress is due to high levels of Zn and Cu/Zn superoxide
dismutase. PLoS One. 6:e169382011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hall ED and Braughler JM: Effects of
intravenous methylprednisolone on spinal cord lipid peroxidation
and Na+ + K+)-ATPase activity. Dose-response analysis during 1st
hour after contusion injury in the cat. J Neurosurg. 57:247–253.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lanza C, Raimondo S, Vergani L, Catena N,
Sénès F, Tos P and Geuna S: Expression of antioxidant molecules
after peripheral nerve injury and regeneration. J Neurosci Res.
90:842–848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ozturk O, Tezcan AH, Adali Y, Yıldırım CH,
Aksoy O, Yagmurdur H and Bilge A: Effect of ozone and
methylprednisolone treatment following crush type sciatic nerve
injury. Acta Cir Bras. 31:730–735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gutteridge JM: Lipid peroxidation and
antioxidants as biomarkers of tissue damage. Clin Chem.
41:1819–1828. 1995.PubMed/NCBI
|
|
48
|
Galloway EB III, Jensen RL, Dailey AT,
Thompson BG and Shelton C: Role of topical steroids in reducing
dysfunction after nevre injury. Laryngoscope. 110:1907–1910. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee M, Doolabh VB, Mackinnon SE and Jost
S: FK506 promotes functional recovery in crushed rat sciatic nerve.
Muscle Nerve. 23:633–640. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Halliwell B and Gutteridge JM: Role of
free radicals and catalytic metal ions in human disease: An
overview. Methods Enzymol. 186:1–85. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
da Silva EL, Piskula MK, Yamamoto N, Moon
JH and Terao J: Quecetin metabolites inhibit copper ion-induced
lipid peroxidation in rat plasma. FEBS Lett. 430:405–408. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Elik M, Serdaroğlu G and Özkan R:
Mİrisetin ve kuersetin bileşiklerinin antioksidan etkinliklerinin
dft yöntemiyle incelenmesi. CÜ Fen Bil Dergisi. 28:53–65. 2007.
|
|
53
|
Heo HJ and Lee CY: Protective effects of
quercetin and vitamin C against oxidative stress-induced
neurodegeneration. J Agric Food Chem. 52:7514–7517. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen MM, Qin J, Chen SJ, Yao LM, Zhang LY,
Yin ZQ and Liao H: Quercetin promotes motor and sensory function
recovery following sciatic nerve-crush injury in C57BL/6J mice. J
Nutr Biochem. 46:57–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hu QH, Wang C, Li JM, Zhang DM and Kong
LD: Allopurinol, rutin, and Quercetin attenuate hyperuricemia and
renal dysfunction in rats ınduced by fructose ıntake: Renal
organicion transporter ınvolvement. AM J Physiol Renal Physiol.
297:F1080–F1091. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Raju TA, Lakshmi AN, Anand T, Rao LV and
Sharma G: Protective effects of Quercetin during ınfluenza
virus-ınduced oxidative stres. Asia Pac J Clin Nutr. 9:314–317.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pawlikowska-Pawlega B, Gruszecki WI,
Misiak LE and Gawron A: The study of the quercetin action on human
erythrocyte membranes. Biochem Pharmacol. 66:605–612. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sugantha Priya E, Selvakumar K, Bavithra
S, Elumalai P, Arunkumar R, Raja Singh P, Brindha Mercy A and
Arunakaran J: Anti-cancer activity of quercetin in neuroblastoma:
An in vitro approach. Neurol Sci. 35:163–170. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gholami M, Khayat ZK, Anbari K, Obidavi Z,
Varzi A, Boroujeni MB, Alipour M, Niapoor A and Gharravi AM:
Quercetin ameliorates peripheral nerve ischemia-reperfusion injury
through the NF-kappa B pathway. Anat Sci Int. 92:330–337. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yu J, Zhang Y, Sun S, Shen J, Qiu J, Yin
X, Yin H and Jiang S: Inhibitory effects of astragaloside IV on
diabetic peripheral neuropathy in rats. Can J Physiol Pharmacol.
84:579–587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cheng CY, Yao CH, Liu BS, Liu CJ, Chen GW
and Chen YS: The role of astragaloside in regeneration of the
peripheral nerve system. J Biomed Mater Res A. 76:463–469. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang X and Chen J: The mechanism of
astragaloside IV promoting sciatic nerve regeneration. Neural Regen
Res. 8:2256–2265. 2013.PubMed/NCBI
|
|
63
|
Tohda C, Tamura T, Matsuyama S and Komatsu
K: Promotion of axonal maturation and prevention of memory loss in
mice by extracts of Astragalus mongholicus. Br J Pharmacol.
149:532–541. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Pilotte N, Papaiakovou M, Grant JR,
Bierwert LA, Llewellyn S, McCarthy JS and Williams SA: Improved
PCR-based detection of soil transmitted helminth infections using a
next-generation sequencing approach to assay design. PLoS Negl Trop
Dis. 10:e00045782016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Klasan GS, Ivanac D, Erzen DJ, Picard A,
Takasawa S, Peharec S, Arbanas J, Girotto D and Jerkovic R: Reg3G
gene expression in regenerating skeletal muscle and corresponding
nerve. Muscle Nerve. 49:61–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Al-Jumaily M, Kozlenkov A, Mechaly I,
Fichard A, Matha V, Scamps F, Valmier J and Carroll P: Expression
of three distinct families of calcium-activated chloride channel
genes in the mouse dorsal root ganglion. Neurosci Bull. 23:293–299.
2007. View Article : Google Scholar : PubMed/NCBI
|

















