Introduction
Ovarian cancer is one of the most common human
malignancies in females, resulting in a high rate of mortality
(1). Extensive efforts have been
made to improve treatment; however, the survival rate for patients
with chemoresistant ovarian cancer remains low (2–4). It has
been reported that certain oncogenes and tumor suppressors are
dysregulated and serve critical functions during the development
and progression of ovarian cancer, and some of these have been
suggested to be promising therapeutic targets or candidates
(5,6). Thus, investigations into the functions
of these genes appear to be beneficial for developing novel
strategies for the treatment of ovarian cancer.
MicroRNAs (miRs), a class of non-coding RNAs 18–25
nucleotides in length, inhibit the expression of their target genes
by binding to the 3′-untranslated regions (3′UTRs) of their target
mRNAs, inducing mRNA degradation or protein translation inhibition
(7,8). It has been well documented that miRs
are involved in the regulation of numerous cellular processes,
including cell survival, differentiation, proliferation and
motility (8–11). Additionally, numerous miRs have been
reported to be involved in the malignant phenotypes of cancer
cells, including cancer cell proliferation, migration, invasion and
chemoresistance, by negatively regulating the expression of
oncogenes or tumor suppressors (12–15).
miR-142-3p has been indicated to act as a tumor suppressor in
several common cancer types (16–18). For
instance, the downregulation of miR-142-3p may contribute to
thyroid follicular tumorigenesis by targeting ASH1 like histone
lysine methyltransferase and histone-lysine-N-methyltransferase 2A
(16). However, the underlying
mechanism of miR-142-3p in ovarian cancer is yet to be
investigated.
Sirtuin 1 (SIRT1) is a member of the sirtuin family
of proteins and functions as an intracellular regulatory protein
with mono-ADP-ribosyltransferase and deacetylase activity (19). Recently, Shuang et al
(20) reported that SIRT1
overexpression contributed to chemoresistance and poor prognosis in
serous epithelial ovarian cancer. In addition, Mvunta et al
(21) revealed that SIRT1 also
promoted ovarian cancer cell invasion. Therefore, SIRT1 functions
as an oncogene in ovarian cancer; however, the regulatory mechanism
of SIRT1 expression is largely unknown.
The present study aimed to investigate the
expression of miR-142-3p in ovarian cancer, as well as the
molecular mechanism of miR-142-3p underlying the proliferation and
chemoresistance of ovarian cancer cells.
Materials and methods
Tissue collection
The present study was approved by the ethics
committee of the First Affiliated Hospital of Xinxiang Medical
University (Weihui, China). Ovarian cancer tissues (n=58) and their
matched adjacent normal tissues were collected from 58 patients
with ovarian cancer from the First Affiliated Hospital of Xinxiang
Medical University between September 2014 and April 2016. The
patients were between 44 and 68 years old, with a mean age of 57.7
years. Written informed consent was obtained from all patients. No
patients received radiation therapy or chemotherapy prior to
surgical resection. The tissues were immediately snap-frozen in
liquid nitrogen following surgical removal and stored until use.
The clinical characteristics of patients, as determined using
tumor, node, metastasis staging are summarized in Table I (22). Patients were included in the present
study if they exhibited primary ovarian cancer and were excluded if
they had received radiation therapy or chemotherapy prior to
surgical resection. In addition, all patients involved in the
present study were categorized into a high miR-142-3p expression
group and a low miR-142-3p expression group, based on the mean
expression value (1.16) of miR-142-3p.
 | Table I.Association between miR-142-3p
expression and clinicopathological characteristics of patients with
ovarian cancer. |
Table I.
Association between miR-142-3p
expression and clinicopathological characteristics of patients with
ovarian cancer.
| Variable | Cases (n=58) | Low miR-142-3p
(n=32) | High miR-142-3p
(n=26) | P-value |
|---|
| Age, years |
|
|
| 0.594 |
| ≤55 | 22 | 11 | 11 |
|
|
>55 | 36 | 21 | 15 |
|
| Differentiation |
|
|
| 0.027a |
|
Well/moderately | 37 | 16 | 21 |
|
|
Poor | 21 | 16 | 5 |
|
| Lymph node
metastasis |
|
|
| 0.156 |
|
Present | 17 | 12 | 5 |
|
|
Absent | 41 | 20 | 21 |
|
| Clinical stage |
|
|
| 0.113 |
|
I–II | 33 | 15 | 18 |
|
|
III–IV | 25 | 17 | 8 |
|
Cell culture
Normal human ovarian epithelial cell line IOSE386,
human ovarian cancer cell lines (SKOV3, HOC1, HO-8910) and the
cisplatin-resistant ovarian cancer cell line SKOV3/DDP cells were
purchased from American Type Culture Collection (Manassas, VA,
USA). Ovarian cancer OVAC cells were purchased from Cell Bank of
Central South University (Changsha, China). The cell lines were
cultured in RPMI-1640 (Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) supplemented with 15% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in 5%
CO2.
Cell transfection
To study the function of miR-142-3p and SIRT1, SKOV3
and SKOV3/DDP cells were transfected with miR-142-3p mimic (cat.
no. B01001), scrambled miR control (cat. no. B01001; miR-NC),
negative control (NC) inhibitor (cat. no. B03001; anti-NC),
miR-142-3p inhibitor (cat. no. B03001; anti-miR-142-3p; all
obtained from Shanghai GenePharma Co., Ltd., Shanghai, China), or
co-transfected with miR-142-3p mimic and pcDNA3.1-SIRT1 plasmid
(Yearthbio, Changsha, China), or co-transfected with miR-142-3p
mimic and blank pcDNA3.1 vector using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from tissues and cell lines was extracted
using RNAiso plus (Takara Bio, Inc., Otsu, Japan). RNA was then
converted into cDNA on an ABI 7300 plus system (Thermo Fisher
Scientific, Inc.) using a PrimeScript® RT reagent kit
(Takara Bio, Inc.), according to the manufacturer's protocol.
RT-qPCR was conducted using a Mir-XTM miRNA qPCR SYBR®
kit (Takara Bio, Inc.) and SYBR Premix Ex Taq II (Takara Bio, Inc.)
on an ABI 7300 plus system. U6 was used as the internal reference
for miR-142-3p expression. GAPDH was used as internal reference for
mRNA expression. The SIRT1 primer sequences were:
5′-TAGCCTTGTCAGATAAGGAAGGA-3′ and 5′-ACAGCTTCACAGTCAACTTTGT-3′. The
GAPDH primer sequences were: 5′-CTGGGCTACACTGAGCACC-3′ and
5′-AAGTGGTCGTTGAGGGCAATG-3′. The primers for miR-142-3p (cat. no.
HmiRQP0186) and U6 (cat. no. HmiRQP9001) were purchased from
Guangzhou Fulengen Co., Ltd. (Guangzhou, China); sequences were not
supplied. The PCR reaction conditions were: 95°C for 3 min,
followed by 40 cycles of denaturation at 95°C for 15 sec and
annealing/elongation at 60°C for 30 sec. The relative expression
was analyzed using the 2−ΔΔCq method (23).
Drug sensitivity assay
The drug sensitivity of SKOV3/DDP cells was measured
using a Cell Counting kit-8 (CCK)-8 assay. SKOV3/DDP cells
(1×105 cells/ml) were seeded in 96-well plates and
cultured at 37°C for 24 h. Cells were treated with cisplatin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at various
concentrations (2, 4, 8, 16, 32, 64, 128, 256 and 512 µg/ml).
Following incubation at 37°C for 48 h, 10 µl CCK-8 reagent
(Sigma-Aldrich; Merck KGaA) was added into each well and then cells
were cultured at 37°C for 2 h. The absorbance of each sample was
measured at 450 nm using a plate reader (TECAN Infinite M200; Tecan
Group, Ltd., Männedorf, Switzerland).
Western blot analysis
Tissues and all cells lines used in this study were
lysed in cold radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Haimen, China). Protein concentration
was determined using a Bicinchoninic Acid Protein Assay kit
(Pierce; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols. The protein (50 µg per lane) was
separated via 12% SDS-PAGE (Pierce; Thermo Fisher Scientific, Inc.)
and then transferred onto a polyvinylidene difluoride (PVDF)
membrane (Thermo Fisher Scientific, Inc.). Following blocking in 5%
non-fat dried milk in PBS at room temperature for 3 h, the PVDF
membrane was incubated with rabbit anti-SIRT1 primary antibody
(1:100; ab32441; Abcam, Cambridge, MA, USA) or rabbit anti-GAPDH
primary antibody (1:100; cat. no. ab9485; Abcam) at room
temperature for 3 h. Following washing with PBS with Tween-20 for
10 min, the membrane was incubated with the horseradish peroxidase
conjugated goat anti-rabbit secondary antibody (1:5,000; cat. no.
ab205718; Abcam) at room temperature for 1 h. Results were
visualized using an enhanced chemiluminescence kit (Pierce; Thermo
Fisher Scientific, Inc.). Protein expression levels were analyzed
with Image-Pro Plus software 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA). GAPDH was used as the internal reference.
Cell proliferation analysis
SKOV3 cells (2×104) were seeded in
96-well plates, each well with 100 µl of fresh serum-free medium
with 0.5 g/l MTT (Sigma-Aldrich; Merck KGaA). This cell line was
selected as it demonstrated that the lowest expression of
miR-142-3p. Following incubation at 37°C for 0, 24, 48 and 72 h,
the medium was removed and 50 µl dimethyl sulfoxide (Sigma-Aldrich;
Merck KGaA) was added. Following incubation at 37°C for 10 min, the
absorbance of each sample was measured at 570 nm using a plate
reader (TECAN Infinite M200; Tecan Group, Ltd.).
Bioinformatics analysis
TargetScan version 7.1 (www.targetscan.org) was used to predict the target
genes of miR-142-3p, according to the manufacturer's protocol.
Dual luciferase reporter assay
The wild type (WT) or mutant type (MT) of SIRT1
3′UTR was inserted into the multiple cloning site of the
psiCHECK™2 vector (Promega Corporation, Madison, WI,
USA). SKOV3 cells were co-transfected with 100 ng WT-SIRT1-3′UTR or
MT-SIRT1-3′UTR plasmid, and 100 nM miR-142-3p mimic or miR-NC using
Lipofectamine® 2000, according to the manufacturer's
protocols. Following transfection for 48 h, the Renilla
luciferase activity and firefly luciferase activity were determined
using a Dual-Luciferase Reporter Assay system (Promega
Corporation), according to the manufacturer's protocol. Firefly
luciferase activity was normalized to Renilla luciferase
activity.
Statistical analysis
All data in the present study are expressed as the
mean ± standard deviation. Statistical analysis was conducted using
SPSS 19.0 (IBM Corp., Armonk, NY, USA). The difference between two
groups was analyzed using Student's t-test and differences among
>2 groups were analyzed using one-way analysis of variance,
followed by a post hoc Turkey's post hot test. The association
between miR-142-3p expression and clinicopathological
characteristics of patients with ovarian cancer was analyzed using
the Chi-square test. Pearson correlation analysis was conducted for
the correlation between miR-142-3p and SIRT1 mRNA expression in
ovarian cancer tissues. P<0.05 was considered to indicate a
statistically significant difference. All analyses were performed
in triplicate.
Results
Downregulation of miR-142-3p in
ovarian cancer is associated with poor differentiation
Firstly, RT-qPCR data revealed that miR-142-3p
expression levels were significantly reduced in ovarian cancer
tissues compared with in adjacent tissues (Fig. 1A). To confirm these findings, the
expression of miR-142-3p in several common ovarian cancer cell
lines was investigated. As demonstrated in Fig. 1B, the expression levels of miR-142-3p
were significantly lower in ovarian cancer cell lines compared with
in the normal human ovarian epithelial cell line IOSE386 (Fig. 1B). Thus, miR-142-3p is downregulated
in ovarian cancer. In addition, patients were categorized into a
high miR-142-3p expression group and low miR-142-3p expression
group, based on the mean expression value of miR-142-3p. Further
investigation revealed that decreased expression levels of
miR-142-3p were significantly associated with poor differentiation
(Table I).
miR-142-3p suppresses the
proliferation and chemoresistance of ovarian cancer cells
The effect of miR-142-3p on the proliferation of
ovarian cancer cells was investigated. SKOV3 cells were transfected
with miR-142-3p mimic to upregulate its expression. Transfection
with miR-142-3p mimic revealed a significant increase in miR-142-3p
expression levels in SKOV3 cells compared with in the control
group. However, transfection with miR-NC did not affect the
expression of miR-142-3p in SKOV3 cells (Fig. 2A). As the transfection with miR-NC
did not affect miR-142-3p expression when compared with the control
group, the proliferation of SKOV3 cells in the control group was
not assessed. An MTT assay was then used to analyze cell
proliferation, which demonstrated that the proliferation of SKOV3
cells was significantly reduced within the miR-142-3p-transfected
group at 72 h compared with in cells transfected with miR-NC
(Fig. 2B). Thus, miR-142-3p may
serve a suppressive role in ovarian cancer cell proliferation.
 | Figure 2.Effect of miR-142-3p on ovarian cancer
cell proliferation and chemoresistance. SKOV3 cells were
transfected with miR-142-3p mimic or miR-NC, respectively, and
non-transfected SKOV3 cells were used as the control group.
Post-transfection, (A) RT-qPCR was used to determine miR-142-3p
expression levels. **P<0.01 vs. Control. (B) An MTT assay was
conducted to examine cell proliferation. **P<0.01 vs. miR-NC.
Subsequently, SKOV3/DDP cells were transfected with miR-142-3p
mimic or miR-NC, respectively, and non-transfected SKOV3/DDP cells
were used as the control group. Post-transfection, (C) RT-qPCR was
used to determine the miR-142-3p levels. **P<0.01 vs. Control.
(D) An MTT assay was conducted to examine cell proliferation.
**P<0.01 vs. miR-NC. miR, microRNA; NC, negative control
(scrambled); RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; SKOV3/DDP, cisplatin-resistant SKOV3 cells. |
The role of miR-142-3p in chemoresistance of ovarian
cancer cells was investigated. Cisplatin-resistant SKOV3/DDP cells
were transfected with miR-142-3p mimic or miR-NC, respectively.
Following transfection, the miR-142-3p levels were significantly
increased in the miR-142-3p group compared with in the control
group; transfection with miR-NC demonstrated no effect on the
expression of miR-142-3p in SKOV3/DDP cells (Fig. 2C). As the transfection with miR-NC
did not affect miR-142-3p expression when compared with the control
group, the survival rate of SKOV3/DDP cells in the control group
was not assessed. A CCK-8 assay was conducted to assess the
survival rate of cells treated with various concentrations of
cisplatin. Under the same concentration of cisplatin (from 4, 8,
16, 32 and 64 µg/ml), the survival rate of SKOV3/DDP cells was
significantly reduced in the miR-142-3p group compared with in the
miR-NC group (Fig. 2D). These
findings suggested that miR-142-3p may promote the sensitivity of
SKOV3/DDP cells to cisplatin.
SIRT1 is a direct target gene of
miR-142-3p in ovarian cancer cells
Based on the bioinformatics analysis data, SIRT1 was
reported to be a putative target gene of miR-142-3p (Fig. 3A). To confirm this prediction,
luciferase vectors containing WT or MT SIRT1 3′-UTR were employed
(Fig. 3B). Luciferase reporter assay
data indicated that luciferase activity was significantly reduced
in SKOV3 and SKOV3/DDP cells co-transfected with the WT-SIRT1-3′UTR
plasmid and miR-142-3p mimic, but unaltered in SKOV3 cells
co-transfected with the MT-SIRT1-3′UTR plasmid and miR-142-3p
mimic, when compared with the control group (Fig. 3C and D). Therefore, miR-142-3p may
bind to the 3′UTR of SIRT1 mRNA in ovarian cancer SKOV3 and
SKOV3/DDP cells.
In the present study, the effect of miR-142-3p on
the expression of SIRT1 in ovarian cancer cells was analyzed. As
presented in Fig. 4A and B, the mRNA
and protein expression levels of SIRT1 in SKOV3 and SKOV3/DDP cells
were significantly reduced in the miR-142-3p group compared with in
the miR-NC group. To further confirm these data, SKOV3 and
SKOV3/DDP cells were transfected with miR-142-3p inhibitor or NC
inhibitor. Following transfection, miR-142-3p expression levels
were significantly decreased in the miR-142-3p inhibitor group
compared with in the control group; however, transfection with NC
inhibitor did not markedly affect miR-142-3p expression in SKOV3
and SKOV3/DDP cells (Fig. 4C).
Further investigation demonstrated that the mRNA and protein
expression levels of SIRT1 were significantly higher in the
anti-miR-142-3p group compared with in the anti-NC group (Fig. 4D and E). Collectively, these findings
indicated that miR-142-3p may inhibit SIRT1 expression by directly
binding to the 3′UTR of SIRT1 mRNA in ovarian cancer cells.
 | Figure 4.Effects of miR-142-3p on SIRT1
expression. (A) RT-qPCR and (B) western blotting were used to
detect the mRNA and protein expression levels of SIRT1 in SKOV3 and
SKOV3/DDP cells transfected with miR-142-3p mimic or miR-NC,
respectively. **P<0.01 vs. miR-NC. Subsequently, SKOV3 and
SKOV3/DDP cells were transfected with anti-miR-142-3p or anti-NC,
respectively. Non-transfected cells were used as the control group.
(C) Post-transfection, RT-qPCR was used to examine the miR-142-3p
expression. **P<0.01 vs. Control. (D) RT-qPCR and (E) western
blotting were used to detect the mRNA and protein expression levels
of SIRT1. **P<0.01 vs. anti-NC. miR, microRNA; miR-NC, negative
control (scramble) miR; anti-miR-142-3p, miR-142-3p inhibitor;
anti-NC, negative control inhibitor; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; SIRT1,
sirtuin 1. |
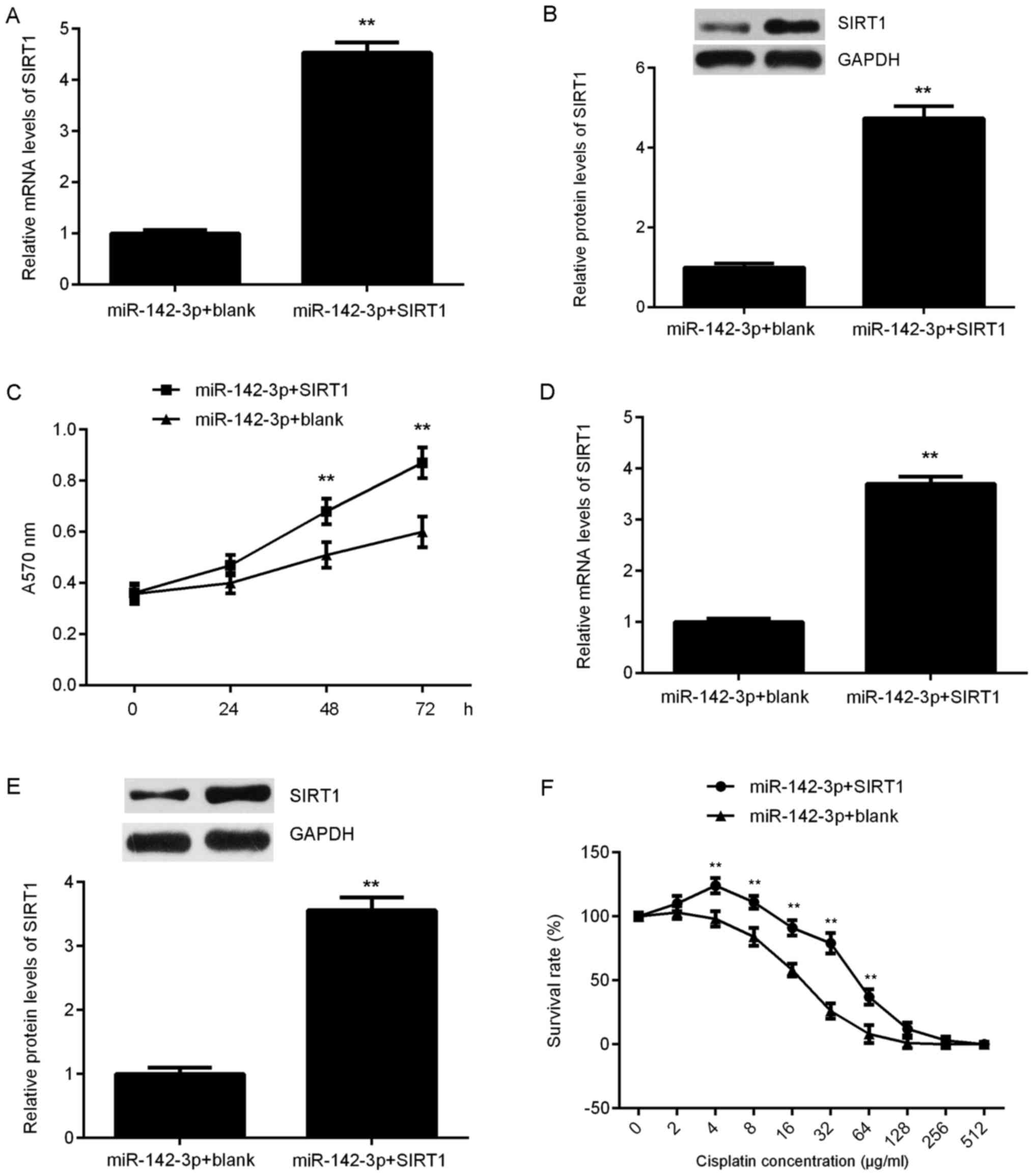
SIRT1 rescues the miR-142-3p-mediated
suppression of proliferation and chemoresistance of ovarian cancer
cells
Based on the aforementioned findings, it was
suggested that SIRT1 may be involved in miR-142-3p-mediated
suppression of ovarian cancer cell proliferation and
chemoresistance. To clarify this speculation,
miR-142-3p-overexpressing SKOV3 cells were transfected with an
SIRT1-expression plasmid to upregulate its expression, or a blank
vector as the control. Following transfection, the mRNA and protein
levels of SIRT1 were significantly increased in the miR-142-3p +
SIRT1 group compared with in the miR-142-3p + blank group (Fig. 5A and B). MTT assay data further
revealed that the proliferation of SKOV3 cells were significantly
increased in the miR-142-3p + SIRT1 group compared with in the
miR-142-3p + blank group at 48 and 72 h (Fig. 5C), indicating that SIRT1 may have
rescued the suppressive effect of miR-142-3p on SKOV3 cell
proliferation. Subsequently, miR-142-3p-overexpressing SKOV3/DDP
cells were also transfected with an SIRT1-expression plasmid, and
the mRNA and protein levels of SIRT1 were significantly upregulated
following transfection (Fig. 5D and
E). A CCK-8 assay was then conducted to assess the survival
rates of SKOV3/DDP cells treated with cisplatin at various
concentrations. Under the same concentrations of cisplatin, the
survival rate was significantly higher at 4, 8, 16, 32 and 64 µg/ml
in the miR-142-3p + SIRT1 group compared with in the miR-142-3p +
blank group (Fig. 5F). Thus, SIRT1
rescued the miR-142-3p-mediated suppression of chemoresistance of
SKOV3/DDP cells.
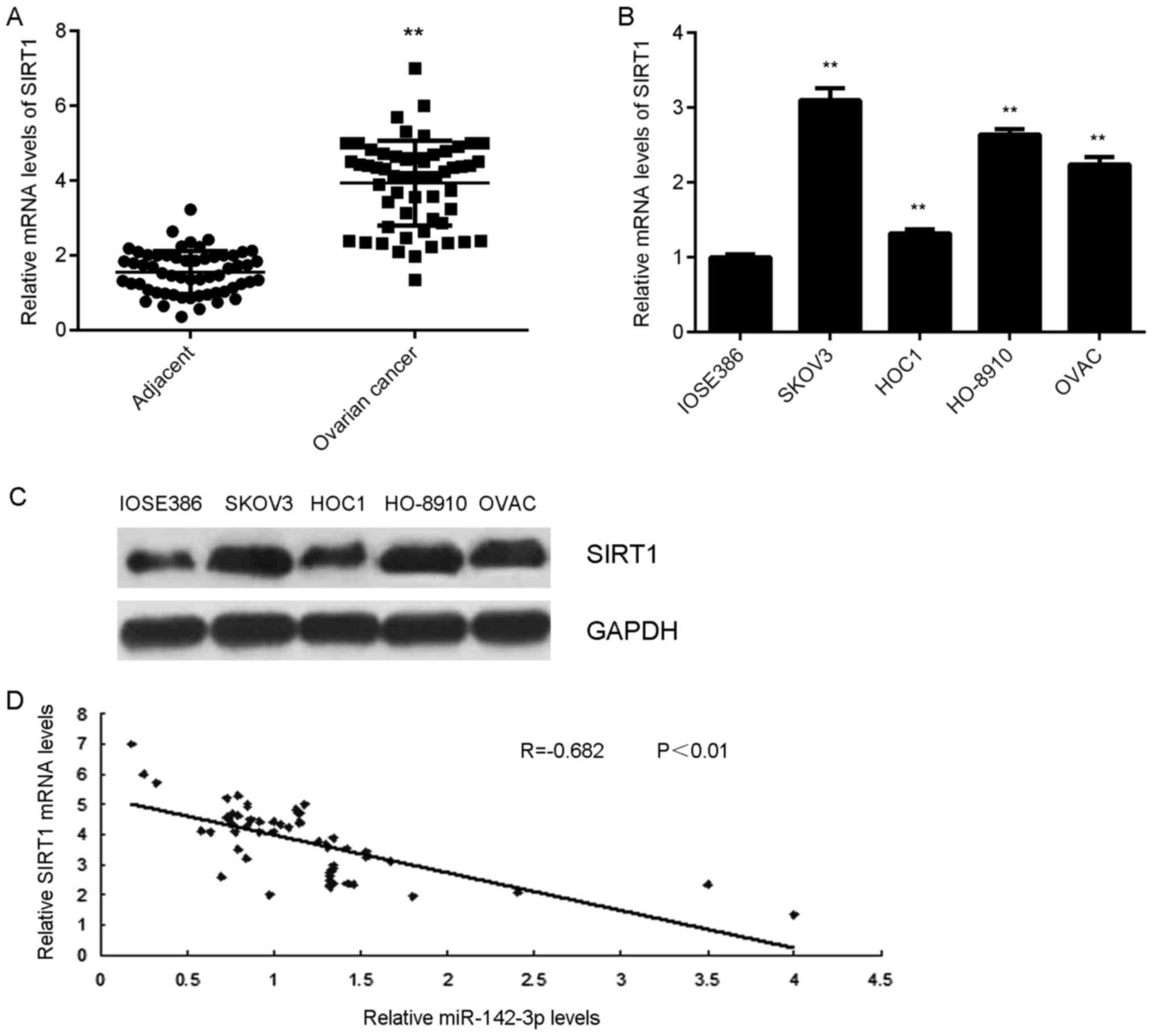
SIRT1 is highly upregulated in ovarian
cancer
The expression of SIRT1 in ovarian cancer tissues
and cell lines was detected. The results indicated that SIRT1 mRNA
expression levels were significantly higher in ovarian cancer
tissues compared with in adjacent tissues (Fig. 6A). Additionally, the mRNA and protein
expression levels of SIRT1 were significantly upregulated in
ovarian cancer cell lines compared with in normal ovarian
epithelial cells (Fig. 6B and C). In
addition, a negative correlation between SIRT1 and miR-142-3p
expression in ovarian cancer tissues was observed (Fig. 6D). These findings suggested that
downregulation of miR-142-3p may contribute to the upregulation of
SIRT1 in ovarian cancer.
Discussion
The regulatory mechanism of miR-142-3p underlying
ovarian cancer progression and chemoresistance is largely unclear.
In the present study, miR-142-3p expression levels were
significantly lower in ovarian cancer tissues and cell lines, when
compared with those in adjacent tissues and normal human ovarian
epithelial cell line IOSE386, respectively. The reduced expression
of miR-142-3p was significantly associated with advanced
malignancy. Ectopic expression of miR-142-3p significantly
inhibited the proliferation of ovarian cancer cells and increased
the sensitivity of SKOV3/DDP cells to cisplatin. SIRT1 was
identified as a target gene of miR-142-3p and its expression was
negatively regulated by miR-142-3p in ovarian cancer cells. Further
investigation demonstrated that SIRT1 rescued the suppressive
effects of miR-142-3p on the proliferation and chemoresistance of
ovarian cancer cells. In addition, SIRT1 was significantly
upregulated in ovarian cancer; a negative correlation between the
expression levels of SIRT1 and miR-142-3p in ovarian cancer tissues
was observed.
miR-142-3p has been reported to serve a suppressive
role in numerous common malignances (24). For instance, miR-142-3p inhibits the
invasion of breast cancer cells by targeting Wiskott-Aldrich
syndrome like, integrin aV and additional cytoskeletal elements
(24). Furthermore, miR-142-3p may
function as a potential tumor suppressor by directly targeting high
mobility group box 1 in non-small-cell lung carcinoma (25). Recently, Wu et al (26) reported that the levels of serum
miR-142-3p were lower in high-pathological grade of ovarian cancer
than in low grade of ovarian cancer. These findings suggested that
aberrant expression of miR-142-3p may be involved in the
progression of ovarian cancer; miR-142-3p may serve as a serum
biomarker to distinguish ovarian cancer of various grades. However,
the expression levels of miR-142-3p in ovarian cancer tissues have
not been previously been studied. In the present study, it was
reported that miR-142-3p was significantly downregulated in ovarian
cancer tissues and cell lines, and the reduced expression of
miR-142-3p was associated with poor differentiation in ovarian
cancer, suggesting that its downregulation may contribute to the
malignant progression of ovarian cancer. Further investigation
revealed that restoration of miR-142-3p expression significantly
inhibited the proliferation of SKOV3 cells.
During chemotherapy, ovarian cancer cells are prone
to drug resistance, which is associated with cancer recurrence and
mortality (2). Thus, investigation
into the molecular mechanism underlying chemoresistance in ovarian
cancer is urgently required for identifying novel and effective
therapeutic targets. In the present study, the overexpression of
miR-142-3p significantly inhibited the resistance of
cisplatin-resistant SKOV3/DDP cells. Similarly, miR-142-3p was
previously reported to improve the drug sensitivity of acute
myelogenous leukemia and NSCLC (27,28).
Subsequently, bioinformatics analysis and a
luciferase reporter assay were performed to study the potential
targets of miR-142-3p in ovarian cancer cells. The data of the
present study indicated that SIRT1 may be a direct target gene of
miR-142-3p, and its expression was negatively mediated by
miR-142-3p in SKOV3 and SKOV3/DDP cells. SIRT1 has been
demonstrated to regulate various cellular functions including DNA
repair, cell survival and metabolism via the deacetylation of
target proteins such as histone and p53 (29). Recently, Asaka et al (29) reported that SIRT1 promoted the growth
and cisplatin resistance of endometrial carcinoma cells. Previous
studies have demonstrated that the expression levels of SIRT1 were
increased in cisplatin-resistant ovarian cancer tissues compared
with in cisplatin-sensitive ovarian cancer tissues; SIRT1
significantly enhanced the proliferation, chemoresistance and
aggressiveness of ovarian cancer cells by upregulating numerous
antioxidant pathways to inhibit oxidative stress (21,30).
Therefore, the results of the present study suggested that the
suppressive effects of miR-142-3p on the proliferation and
chemoresistance of ovarian cancer cells may have occurred via the
inhibition of SIRT1 expression.
SIRT1 was previously reported to be significantly
upregulated in malignant ovarian epithelial tumors compared with in
benign and borderline epithelial tumors (30). Similarly, SIRT1 expression levels
were upregulated in ovarian cancer tissues and cell lines compared
with normal ovarian tissues and cells in the present study; a
negative correlation between SIRT1 and miR-142-3p expression levels
in ovarian cancer tissues was also observed. This suggested that
upregulation of SIRT1 may be due to the decreased expression of
miR-142-3p.
In conclusion, miR-142-3p, which is significantly
downregulated in ovarian cancer, may serve a suppressive role in
the proliferation and chemoresistance of ovarian cancer cells, at
least partially via the direct targeting of SIRT1. Therefore, the
findings of the present study suggest that miR-142-3p may be a
promising therapeutic candidate for the treatment of ovarian
cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JG performed statistical analysis, and wrote and
submitted the manuscript. ZD designed the study and revised the
manuscript. NW, XL, YX, YC and SL performed cellular and molecular
experiments.
Ethics approval and consent to
participate
This study was approved by the First Affiliated
Hospital of Xinxiang Medical University (Weihui, China). Written
informed consent was obtained from all patients.
Consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun Y, Liu JH, Jin L, Sui YX, Han LL and
Huang Y: Effect of autophagy-related beclin1 on sensitivity of
cisplatin-resistant ovarian cancer cells to chemotherapeutic
agents. Asian Pac J Cancer Prev. 16:2785–2791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu PN, Yan MD, Lai HC, Huang RL, Chou YC,
Lin WC, Yeh LT and Lin YW: Downregulation of miR-29 contributes to
cisplatin resistance of ovarian cancer cells. Int J Cancer.
134:542–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun Y, Liu JH, Jin L, Sui YX, Lai L and
Yang Y: Inhibition of Beclin 1 expression enhances
cisplatin-induced apoptosis through a mitochondrial-dependent
pathway in human ovarian cancer SKOV3/DDP cells. Oncol Res.
21:261–269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miao Y, Lu M, Yan Q, Li S and Feng Y:
Inhibition of proliferation, migration, and invasion by knockdown
of pyruvate kinase-M2 (PKM2) in ovarian cancer SKOV3 and OVCAR3
cells. Oncol Res. 24:463–475. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Teng Y, Zuo X, Hou M, Zhang Y, Li C, Luo W
and Li X: A Double-negative feedback interaction between
MicroRNA-29b and DNMT3A/3B contributes to ovarian cancer
progression. Cell Physiol Biochem. 39:2341–2352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang Z, Zhang Y, Cao R, Li L, Zhong K,
Chen Q and Xiao J: miR-5195-3p inhibits proliferation and invasion
of human bladder cancer cells by directly targeting oncogene KLF5.
Oncol Res. 25:1081–1087. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X and Chen J: MiR-3188 regulates cell
proliferation, apoptosis, and migration in breast cancer by
targeting TUSC5 and regulating the p38 MAPK signaling pathway.
Oncol Res. May 26–2017.(Epub ahead of print). View Article : Google Scholar
|
|
12
|
Zhou Y, Yang C, Wang K, Liu X and Liu Q:
MicroRNA-33b inhibits the proliferation and migration of
osteosarcoma cells via targeting hypoxia-inducible factor-1α. Oncol
Res. 25:397–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang M, Zhai X, Ge T, Yang C and Lou G:
MiR-181a-5p Promotes proliferation and invasion, and inhibits
apoptosis of cervical cancer cells via regulating inositol
polyphosphate-5-phosphatase A (INPP5A). Oncol Res. Jun
23–2017.(Epub ahead of print). View Article : Google Scholar
|
|
14
|
Wang C, Zhou B, Liu M, Liu Y and Gao R:
miR-126-5p restoration promotes cell apoptosis in cervical cancer
by targeting Bcl2l2. Oncol Res. 25:463–470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Xiang Z, Liu Y, Xu B and Tang J:
MicroRNA-133b inhibits proliferation, cellular migration, and
invasion via targeting LASP1 in hepatocarcinoma cells. Oncol Res.
25:1269–1282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Colamaio M, Puca F, Ragozzino E, Gemei M,
Decaussin-Petrucci M, Aiello C, Bastos AU, Federico A, Chiappetta
G, Del Vecchio L, et al: miR-142-3p down-regulation contributes to
thyroid follicular tumorigenesis by targeting ASH1L and MLL1. J
Clin Endocrinol Metab. 100:E59–E69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghanbari R, Mosakhani N, Asadi J, Nouraee
N, Mowla SJ, Yazdani Y, Mohamadkhani A, Poustchi H, Knuutila S and
Malekzadeh R: Downregulation of plasma MiR-142-3p and MiR-26a-5p in
patients with colorectal carcinoma. Iran J Cancer Prev.
8:e23292015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Shan WF, Jin TT, Wu GQ, Xiong XX,
Jin HY and Zhu SM: Propofol exerts anti-hepatocellular carcinoma by
microvesicle-mediated transfer of miR-142-3p from macrophage to
cancer cells. J Transl Med. 12:2792014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ramis MR, Esteban S, Miralles A, Tan DX
and Reiter RJ: Caloric restriction, resveratrol and melatonin: Role
of SIRT1 and implications for aging and related-diseases. Mech
Ageing Dev. 146–148:28–41. 2015. View Article : Google Scholar
|
|
20
|
Shuang T, Wang M, Zhou Y and Shi C:
Over-expression of Sirt1 contributes to chemoresistance and
indicates poor prognosis in serous epithelial ovarian cancer (EOC).
Med Oncol. 32:2602015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mvunta DH, Miyamoto T, Asaka R, Yamada Y,
Ando H, Higuchi S, Ida K, Kashima H and Shiozawa T: SIRT1 regulates
the chemoresistance and invasiveness of ovarian carcinoma cells.
Transl Oncol. 10:621–631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Javadi S, Ganeshan DM, Qayyum A, Iyer RB
and Bhosale P: Ovarian cancer, the revised FIGO staging system, and
the role of imaging. AJR Am J Roentgenol. 206:1351–1360. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwickert A, Weghake E, Brüggemann K,
Engbers A, Brinkmann BF, Kemper B, Seggewiß J, Stock C, Ebnet K,
Kiesel L, et al: microRNA miR-142-3p inhibits breast cancer cell
invasiveness by synchronous targeting of WASL, integrin alpha V,
and additional cytoskeletal elements. PLoS One. 10:e01439932015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao P and Liu WL: MiR-142-3p functions as
a potential tumor suppressor directly targeting HMGB1 in
non-small-cell lung carcinoma. Int J Clin Exp Pathol.
8:10800–10807. 2015.PubMed/NCBI
|
|
26
|
Wu X, Zhi X, Liu M, Xie J and Zhao S:
[Elevated levels of dendritic cell-correlated miRNAs in ascites and
sera of patients with ovarian cancer]. Xi Bao Yu Fen Zi Mian Yi Xue
Za Zhi. 31:383–386. 2015.PubMed/NCBI
|
|
27
|
Zhang Y, Liu Y and Xu X: Upregulation of
miR-142-3p improves drug sensitivity of acute myelogenous leukemia
through reducing P-glycoprotein and repressing autophagy by
targeting HMGB1. Transl Oncol. 10:410–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Zhou X, Qiao J and Bao A:
MiR-142-3p overexpression increases chemo-sensitivity of NSCLC by
inhibiting HMGB1-mediated autophagy. Cell Physiol Biochem.
41:1370–1382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Asaka R, Miyamoto T, Yamada Y, Ando H,
Mvunta DH, Kobara H and Shiozawa T: Sirtuin 1 promotes the growth
and cisplatin resistance of endometrial carcinoma cells: A novel
therapeutic target. Lab Invest. 95:1363–1373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li D, Wu QJ, Bi FF, Chen SL, Zhou YM, Zhao
Y and Yang Q: Effect of the BRCA1-SIRT1-EGFR axis on cisplatin
sensitivity in ovarian cancer. Am J Transl Res. 8:1601–1608.
2016.PubMed/NCBI
|