Introduction
Septic shock is a serious clinical syndrome that
typically develops as a result of infection-induced sepsis
(1,2). Large-scale microorganism invasion will
cause a stress reaction in tissues and organs. Moderate stress
responses may enhance the defensive capacity of the body and
control the spread of infection (3);
however, prolonged exposure to pathogens can aggravate tissue/organ
damage, particularly following a stress reaction (4,5). During
septic shock, damaged tissues and organs stimulate the release of
large amounts of inflammatory mediators, including tumor necrosis
factor (TNF)-α and interleukin (IL)-6, from inflammatory cells,
which aggravates the inflammatory response (6). Septic shock is often accompanied by
organ dysfunction and tissue hypoperfusion. If it is not treated in
a timely manner, septic shock is able to induce acute circulatory
failure, resulting in patient mortality (7,8). An
epidemiological study suggested that the mortality rate of patients
with septic shock is high at >40% (9). With modern medicine, the prognosis of
patients with septic shock has improved significantly (10). However, treating septic shock is
dependent on improving the balance of the internal physiological
environment inside and outside of cells, to reduce inflammation and
prevent organ damage (11).
It has previously been reported that multiple organ
failure is the typical cause of mortality in patients with septic
shock and symptoms of the disease include low perfusion of organs
and an inability to maintain normal metabolic functions (12,13).
Cardiac systolic and diastolic functions are directly associated
with the prognosis of patients with septic shock (14,15). It
has previously been reported that endotoxins and inflammatory
factors in the peripheral blood of patients with septic shock are
the main causes of myocardial injury (16). Cardiomyocytes synthesize cytokines,
including TNF-α, aggravate myocardial injury, upregulate cytokine
receptor expression and participate in the pathogenesis of septic
shock (17). In view of the
important roles of cytokines in septic shock-induced myocardial
injury, researchers have attempted to use cytokine antagonists as a
treatment for patients with septic shock (18,19).
However, the effect of single cytokines is limited, as the
inflammatory response of septic shock is described as a cascade
(20,21). Therefore, improving our understanding
of the molecular mechanism of myocardial injury in septic shock may
be of great significance when developing novel treatments.
MicroRNA (miRNA or miR) is a class of endogenous,
highly conserved non-coding RNA molecules 18–22 nucleotides in
length (22). miRNAs inhibit the
translation of proteins by binding to the 3′-untranslated region
(UTR) of mRNAs (23). miRNAs are
stable in body fluids, making them suitable biomarkers for diseases
(24). Previous studies have
demonstrated that a variety of miRNAs are associated with the
myocardial injury repair, including miR-210 and miR-214 (25,26),
suggesting that miRNAs serve important roles in the diagnosis and
treatment of myocardial injury. miR-494-3p is a novel
cardiovascular and tumor-associated miRNA that serves important
roles in tumor proliferation, invasion and metastasis (27). It has been reported that miR-494-3p
is associated with the regulation of vascular injury and repair, as
well as regulation of the phosphatase and tensin homolog (PTEN),
phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin
(mTOR) signaling pathways (28).
PTEN is located on chromosome 10q23.3 and comprises 9 exons
(29). It encodes a protein 403
amino acids in length and has phosphatase activity (30). It has been reported that PTEN is able
to inhibit the development of tumors by antagonizing tyrosine
kinase and other phosphorylases responsible for angiogenesis, cell
survival and other biological processes (31). PTEN is also associated with the
PI3K/protein kinase B (AKT) signaling pathway (32). In the present study, the role of
miR-494-3p in myocardial injury in patients with septic shock was
investigated. The aim was to provide an experimental basis for the
diagnosis and treatment of septic shock-induced myocardial
injury.
Patients and methods
Patients
A total of 22 patients with sepsis and 17 patients
with septic shock were included in the present study. Patients were
excluded from the current study if they possessed any tumor,
diabetes, autoimmune diseases or a had a history of taking certain
medication, including thyroxine. All patients were treated at
Intensive Medicine Department, Linyi Central Hospital (Linyi,
China) between December 2016 and March 2017. Among the 39 patients
(22 with sepsis, 17 with septic shock), 29 were male and 10 were
female. The age range was 38–58 years. Among the 22 patients with
sepsis, 18 were male and 4 were female (age range, 38–53 years);
among the 17 patients with septic shock, 11 were male and 6 were
female (age range, 40–51 years). In addition, 20 healthy subjects
(10 male and 10 female; age range, 35–48 years) who underwent
physical examinations at the same hospital and time period were
included as a control group. Peripheral blood (5 ml) was collected
from all subjects at the start of the study. Samples were
centrifuged at a speed of 12,000 × g at 4°C for 10 min to separate
serum and were stored at −80°C. Clinical information and
pathological data of all patients were collected (data not shown).
All experimental procedures were approved by the Ethics Committee
of Linyi Central Hospital. Written informed consent was obtained
from all patients or their families.
Cells
Rat cardiomyocytes (M6200; ScienCell Research
Laboratories, Inc., San Diego, CA, USA) were divided into the
negative control (NC) group, sepsis serum group and septic shock
serum group and cultured in 24-well plates (1×105/well)
containing Cardiac Myocyte Medium (CMM; cat. no. 6101; ScienCell
Research Laboratories, Inc.) at 37°C in an atmosphere containing 5%
CO2. When cells reached 70–90% confluence, the medium
was replaced by a mixture of 250 µl sepsis patient serum or septic
shock patient serum and 250 µl fresh CMM medium and incubated at
37°C in an atmosphere containing 5% CO2 for 24 h. Cells
in the control group were treated with a mixture of 250 µl serum
from healthy subjects and 250 µl fresh CMM medium for the same
duration.
At 1 day prior to transfection, 2×105
purchased cardiomyocytes were seeded in 24-well plates containing
serum-free CMM medium. When cells reached 70% confluence,
transfection was performed. In the first vial, 1.5 µl miR-494-3p
mimics (5′-TGAAACATACACGGGAAACCTC-3′; 20 pmol/µl; HanBio
Biotechnology Co., Ltd., Shanghai, China) or miR-NC (cat. no.
miR01201-1-5; 20 pmol/µl; HanBio Biotechnology Co., Ltd.) was mixed
with 50 µl Opti Mem medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). In the second vial, 1 µl
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.) was
mixed with 50 µl Opti Mem medium. Following incubation at room
temperature for 5 min, the two vials were combined and incubated at
room temperature for 20 min. Cells were subsequently treated with
this mixture at 37°C and 5% CO2 for 6 h, following which
the medium was replaced with CMM medium containing 10% fetal bovine
serum (Thermo Fisher Scientific, Inc.). For further assays, cells
were then incubated at 37°C in an atmosphere containing 5%
CO2 for 48 h prior to collection via digestion with
trypsin and centrifugation at 1,000 × g at room temperature for 5
min.
At 1 day prior to transfection, 2×105
M6200 cardiomyocytes were seeded into 24-well plates containing
serum-free Cardiac Myocyte Medium (CMM; cat. no. 6101; ScienCell
Research Laboratories, Inc.). When cells reached 70% confluence,
transfection was performed. In the first vial, 1.5 µl siR-PTEN
(5′-AACCCACCACAGCUAGAACTT-3′) or siR-NC
(5′-TTCTCCGAACGTGTCACGTTT-3′; 20 pmol/µl; HanBio Biotechnology Co.,
Ltd., Shanghai, China) was mixed with 50 µl Opti Mem medium (Thermo
Fisher Scientific, Inc.), respectively. In the second vial, 1 µl
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.) was
mixed with 50 µl Opti Mem medium. Following incubation for 5 min,
the two vials were combined and incubated at room temperature for
20 min. Cells were subsequently incubated for 6 h, following which
the medium was replaced with CMM containing 10% fetal bovine serum.
Cells were then incubated at 37°C in an atmosphere containing 5%
CO2 for 48 h prior to collection for further assays.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Peripheral blood (250 µl) was lysed with 750 µl
TRIzol LS isolation reagent (Thermo Fisher Scientific, Inc.). Total
RNA was extracted using the phenol chloroform method (33). The purity of RNA was determined by
A260/A280 using UV-spectrophotometry (Nanodrop ND2000; Thermo
Fisher Scientific, Inc.). cDNA was obtained by RT at 42°C for 30
min using the PrimeScript RT Regent kit (Takara Biotechnology Co.,
Ltd., Dalian, China) according to the manufacturer's protocol and
stored at −20°C. PCR was performed using the SYBR PrimeScript
RT-PCR Kit (Takara Biotechnology Co., Ltd.), with U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′ as
an internal reference. The reaction system (20 µl) comprised
RT-qPCR-mix (10 µl), upstream primer (0.5 µl) (miR-494-3p,
5′-TGAAACATACACGGGAAACCTC-3′; U6, 5′-CTCGCTTCGGCAGCACA-3′),
downstream universal primer (0.5 µl; provided with the kit), cDNA
(2 µl) and ddH2O (7 µl). Thermocycling conditions were
as follows: Initial denaturation at 95°C for 10 min followed by 40
cycles of 95°C for 1 min and 60°C for 30 sec (StepOnePlus Real-Time
PCR System; Thermo Fisher Scientific, Inc.). The 2−ΔΔCq
method (34) was used to calculate
the relative expression of miR-494-3p against the internal
reference. Each sample was tested in triplicate.
Cell Counting kit (CCK)-8 assay for
cell proliferation
Cells (serum treatment groups, miRNA transfection
groups and interference groups) were seeded at a density of 3,000
cells/well in 96-well plates and cultured in CMM at 37°C in an
atmosphere containing 5% CO2. At 0, 24, 48 and 72 h, 20
µl CCK-8 (5 g/l; Beyotime Institute of Biotechnology, Haimen,
China) was added to the cells prior to incubation at 37°C and 5%
CO2 for 2 h. Subsequently, 150 µl CCK-8 reaction
solution was added and cells were incubated at 37°C for 2 h. The
absorbance of each well was measured at 490 nm to construct cell
proliferation curves. Each group was tested in triplicate.
Flow cytometry
Cells (1×106) from each group were washed
twice with precooled PBS and subjected to flow cytometry using a
Cell Cycle Assay kit (BD Biosciences, Franklin Lakes, NJ, USA)
according to the manufacturer's protocol. Cell cycle distribution
was analyzed using ModFit software (version 5.0; Verity Software
House, Topsham, ME, USA).
Following treatment with sera from healthy subjects,
patients with sepsis or patients with septic shock for 24 h, cells
(1×106) from each group were washed with precooled PBS
twice and subjected to flow cytometry using an Annexin V
fluorescein (FITC) Apoptosis Detection kit I (BD Biosciences;
included PI staining materials) according to the manufacturers
protocol. Cells with Annexin V-positive staining were early
apoptotic cells, those with propidium iodide (PI)-positive values
were necrotic and double-positive values were cells in late
apoptosis. ModFit software (version 5.0; Verity Software House,
Inc., Topsham, ME, USA) was used for the analysis of results.
Lactate dehydrogenase (LDH) assay
Cells were seeded in 24-well plates at a density of
2×105 cells/well and incubated with serum from healthy subjects,
patients with sepsis or patients with septic shock at 37°C for 24
h. Then, medium was replaced with fresh CMM medium, followed by
incubation at 37°C for 6 h. The supernatant was collected by
centrifugation at 12,000 × g and 4°C for 10 min. A total of 120 µl
supernatant was used for the LDH assay according to the
manufacturer's protocol (cat. no. C0016; Beyotime Institute of
Biotechnology). Absorbance was measured at 490 nm by a reader
(SpectraMax; Molecular Devices, LLC, Sunnyvale, CA, USA).
ELISA
LDH, TNF-α (cat. no. ab208348) and IL-6 (cat. no.
ab100712) ELISA kits (Abcam, Cambridge, UK) were used to measure
serum LDH, TNF-α and IL-6. Standards (50 µl) and samples (10 µl
serum and 40 µl diluent, provided by the kits) were added to
predefined microplate wells, while the blank well was left empty.
Horseradish peroxidase (HRP)-labeled conjugates (100 µl; provided
by the kit) were added to wells prior to sealing for incubation at
37°C for 1 h. Plates were washed five times, following which
substrates A (50 µl) and B (50 µl) were added into each well.
Following incubation at 37°C for 15 min, stop solution (50 µl) was
added and the absorbance of each well was measured at 450 nm. Each
sample was tested in triplicate.
Western blot analysis
Cells (1×106) from each group were
trypsinized and collected via centrifugation at a speed of 1,000 ×
g for 5 min at room temperature. Precooled radioimmunoprecipitation
assay buffer (1,000 µl; Beyotime Institute of Biotechnology) was
added to the samples. Phenylmethane sulfonyl fluoride (1 mM) was
used as a protease inhibitor. Following lysis for 30 min on ice,
the mixture was centrifuged at 10,000 × g and 4°C for 10 min.
Proteins were quantified using a bicinchoninic acid protein
concentration determination kit (RTP7102; Real-Times Beijing
Biotechnology Co., Ltd., Beijing, China). Protein samples were
mixed with 5X SDS loading buffer (Beyotime Institute of
Biotechnology) prior to denaturation in boiling water for 10 min.
Samples (20 µg) were separated using 10% SDS-PAGE gels. Resolved
proteins were transferred to polyvinylidene difluoride membranes on
ice (100 V; 1 h) and blocked with 5% skimmed milk at room
temperature for 1 h. Membranes were subsequently incubated with
rabbit anti-human PTEN (1:1,000; cat. no. ab32199), rabbit
anti-human IL-6 (1:1,000; cat. no. ab6672), rabbit anti-human TNF-α
(1:800; cat. no. ab6671) and mouse anti-human GAPDH (1:4,000; cat.
no. ab8245; all Abcam) polyclonal primary antibodies at 4°C
overnight. Following extensive washing with PBS with Tween 20
(PBST) five times for 5 min, membranes were incubated with goat
anti-rabbit (1:4,000; cat. no. A0208) or goat anti-mouse (1:4,000;
cat. no. A0216; all Abcam) HRP-conjugated secondary antibodies for
1 h at room temperature. Membranes were washed with PBST five times
for 5 min and developed using an enhanced chemiluminescence
detection kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for
imaging. Image lab v3.0 software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to acquire and analyze imaging signals.
Relative PTEN expression was measured against GAPDH. Each test was
performed in triplicate.
Dual luciferase reporter assay
Based on the results of a Targetscan search (v7.1;
http://www.targetscan.org), wild-type (WT) and
mutant seed regions of miR-494-3p in the 3′-UTR of the PTEN gene
were chemically synthesized in vitro, with SpeI and
HindIII restriction sites and cloned into pMIR-REPORT
luciferase reporter plasmids (Beyotime Institute of Biotechnology).
Plasmids (0.5 µg; 20 pmol/µl) with WT or mutant 3′-UTR DNA
sequences were co-transfected with miR-494-3p mimics (20 pmol/µl)
into 293T cells (Type Culture Collection of the Chinese Academy of
Sciences, Shanghai, China) using Lipofectamine® 3000
(Thermo Fisher Scientific, Inc.). Following cultivation for 24 h,
cells were lysed using a dual luciferase reporter assay kit
(Promega Corporation, Madison, WI, USA) according to the
manufacturer's protocol and the fluorescence intensity was measured
using a GloMax 20/20 luminometer (Promega Corporation).
Renilla fluorescence activity was used as internal
reference. Each test was performed in triplicate.
Statistical analysis
Results were analyzed using SPSS 17.0 statistical
software (SPSS, Inc., Chicago, IL, USA). Data are expressed as the
mean ± standard deviation. Multiple group comparisons were analyzed
using one-way analysis of variance followed by Student Newman-Keuls
post-hoc test. Spearman's correlation analysis was performed to
evaluate the correlation between miR-494-3p and LDH levels.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Reduced miR-494-3p expression in
peripheral blood is correlated with myocardial damage in patients
with septic shock
RT-qPCR results revealed that miR-494-3p levels were
significantly decreased in patients with sepsis and patients with
septic shock compared with healthy subjects (P<0.05) (Fig. 1A). In addition, miR-494-3p levels
were significantly decreased in patients with septic shock compared
with patients with sepsis (P<0.05) (Fig. 1A). ELISA was performed to measure
serum LDH and the data suggested a correlation between miR-494-3p
and LDH in patients with sepsis (correlation coefficient, 0.590;
P<0.05) (Fig. 1B) and in patients
with septic shock (correlation coefficient, 0.729; P<0.05)
(Fig. 1C). The results suggest that
reduced miR-494-3p expression is associated with myocardial damage
in patients with septic shock.
Serum from patients with septic shock
downregulates miR-494-3p expression in rat cardiomyocytes
RT-qPCR results revealed that miR-494-3p was
significantly decreased in rat cardiomyocytes incubated with serum
from patients with sepsis or patients with septic shock were
compared with those incubated with serum from healthy subjects
(P<0.05) (Fig. 2A). No
significant differences were observed in miR-494-3p expression
between rat cardiomyocytes incubated with serum from patients with
sepsis or serum from patients with septic shock (P>0.05)
(Fig. 2A). Furthermore, the
absorbance of rat cardiomyocytes incubated with serum from patients
with septic shock or patients with sepsis for 48 h or 72 h was
significantly decreased compared with the control group (P<0.05)
(Fig. 2B). Cell cycle analysis
demonstrated that the percentage of cells in G1 phase
was significantly increased in the sepsis serum and septic shock
serum groups compared with the control group (P<0.05) (Fig. 2C), while there was no significant
difference between the sepsis serum and the septic shock serum
groups (P>0.05) (Fig. 2C). The
apoptosis rate of rat cardiomyocytes in the septic shock serum
group was significantly increased compared with the sepsis serum
and control groups (P<0.05) (Fig.
2D), while apoptosis was significantly increased in the sepsis
serum group compared with the control group (P<0.05) (Fig. 2D). The results indicated that serum
from patients with septic shock downregulated miR-494-3p expression
in rat cardiomyocytes.
miR-494-3p overexpression inhibits rat
cardiomyocyte injury induced by serum from septic shock
patients
To further investigate the effect of miR-494-3p on
serum-induced cardiomyocyte injury, rat cardiomyocytes that were
treated with serum from patients with septic shock were transfected
with miR-NC and miR-494-3p sequences, using untreated
cardiomyocytes as blank control. RT-qPCR revealed that miR-494-3p
expression was significantly increased in cells transfected with
miR-494-3p compared with the miR-NC group (P<0.05) (Fig. 3A). CCK-8 assays revealed that the
absorbance of septic shock serum-treated rat cardiomyocytes
transfected with miR-494-3p was significantly increased compared
with serum-treated cells in the miR-NC group at 48 and 72 h
(P<0.05) (Fig. 3B). In addition,
the percentage of cells in the G1 phase was
significantly decreased in the miR-494-3p-transfected serum-treated
group compared with the miR-NC-transfected serum-treated group
(P<0.05) (Fig. 3C). The number of
apoptotic cells in the miR-494-3p + serum group was significantly
reduced compared with the miR-NC + serum group (P<0.05)
(Fig. 3D). These results suggest
that miR-494-3p overexpression protects rat cardiomyocytes against
injury induced by sera from patients with septic shock.
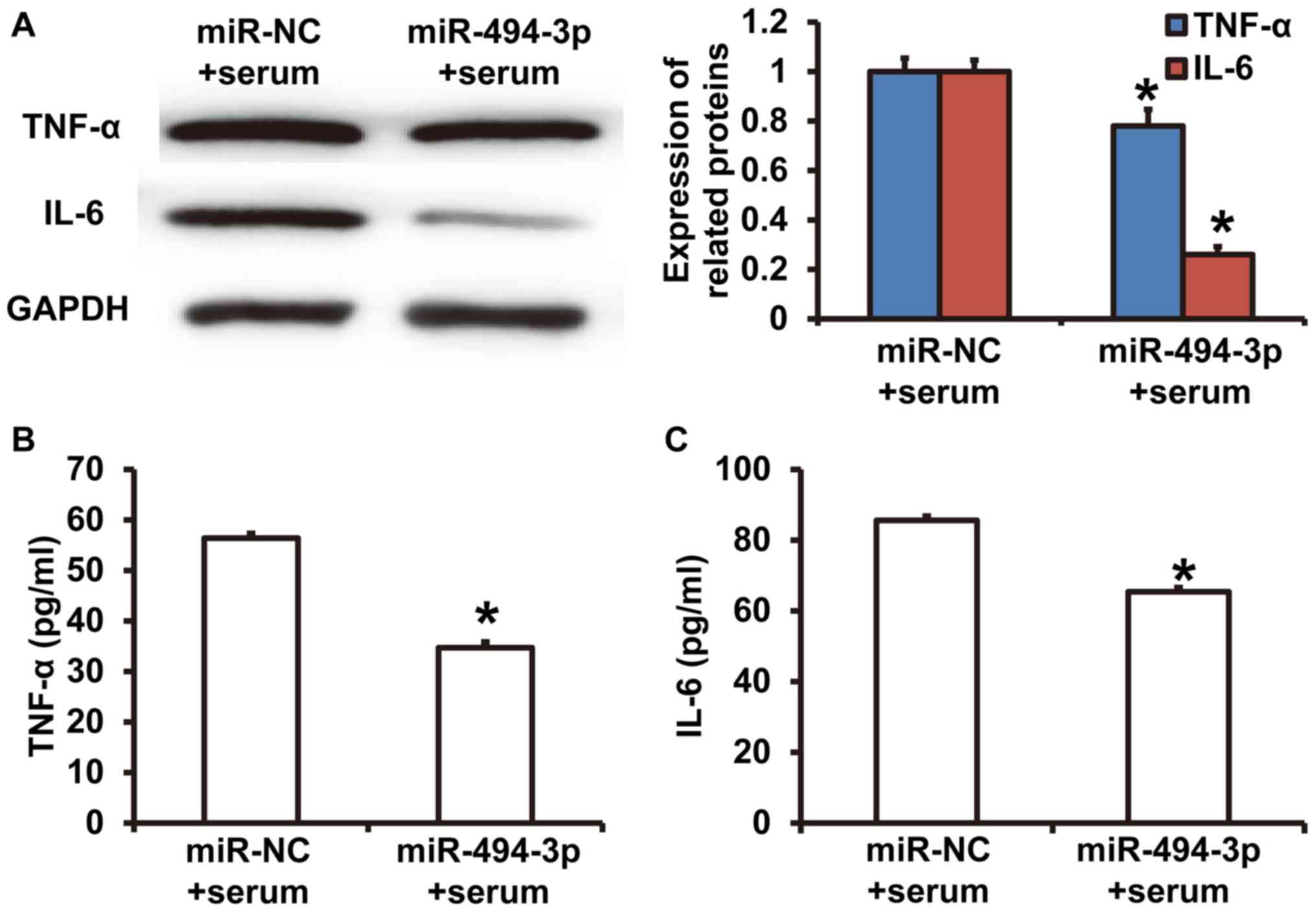
miR-494-3p expression reduces the
synthesis and release of TNF-α and IL-6 in rat cardiomyocytes
Western blotting revealed that TNF-α and IL-6
expression was significantly decreased in cardiomyocytes in the
miR-494-3p + serum group compared with the miR-NC + serum group
(P<0.05) (Fig. 4A). ELISA results
revealed that TNF-α and IL-6 concentrations in culture supernatants
from cardiomyocytes in the miR-494-3p + serum group were
significantly decreased compared with the miR-NC + serum group
(P<0.05) (Fig. 4B and C). The
results indicated that miR-494-3p expression reduced the synthesis
and release of TNF-α and IL-6 from rat cardiomyocytes.
PTEN knockdown alleviates injuries of
rat cardiomyocytes that are induced by septic shock serum
To identify the target gene of miR-494-3p,
TargetScan was used. It was revealed that PTEN was a target gene
for miR-494-3p (Fig. 5A). To detect
of PTEN expression, western blotting was performed. The results
demonstrated that the greyscale value of PTEN from rat
cardiomyocytes transfected with miR-494-3p was significantly lower
compared with the in miR-NC group (P<0.05) (Fig. 5B). Western blotting revealed that
PTEN expression was significantly decreased in cardiomyocytes
treated with siR-PTEN compared with the siR-NC group (P<0.05)
(Fig. 5C). CCK-8 assays revealed
that the absorbance of cardiomyocytes was significantly increased
in the siR-PTEN group compared with the siR-NC group at 48 and 72 h
(P<0.05) (Fig. 5D), reaching a
level similar to cardiomyocytes cultured under normal conditions
(control) (P>0.05) (Fig. 5D).
Cell cycle analysis suggested that the percentage of cells in
G1 phase in the siR-PTEN group was significantly
decreased compared with the siR-NC group (P<0.05) (Fig. 5E). Flow cytometry demonstrated that
siR-PTEN treatment significantly decreased the apoptotic rate
compared with the siR-NC group (P<0.05) (Fig. 5F). LDH assay results revealed that
LDH levels were significantly decreased in the culture supernatant
from cardiomyocytes in the siR-PTEN group compared with the siR-NC
group (P<0.05) (Fig. 5G). The LDH
level in control group was too low and so was excluded from the
figure. The results suggested that PTEN knockdown alleviated septic
shock serum-induced injuries in rat cardiomyocytes.
 | Figure 5.Effect of PTEN on rat cardiomyocyte
injury induced by septic shock serum. (A) Bioinformatics prediction
of binding sites between miR-494-3p and PTEN mRNA. Western blots of
PTEN in rat cardiomyocytes transfected with (B) miR-NC, miR-494-3p
mimics, (C) siR-NC or siR-PTEN. Effect of PTEN overexpression on
(D) proliferation, (E) cell cycle distribution, (F) apoptosis and
(G) LDH expression. *P<0.05 vs. siR-NC group. Control,
cardiomyocytes cultured under normal conditions; miR, microRNA; NC,
negative control; siR, short interfering RNA; PTEN, phosphatase and
tensin homolog; LDH, lactate dehydrogenase. |
PTEN triggers TNF-α and IL-6 release
from rat cardiomyocytes treated with septic shock serum
To investigate how PTEN affects the release of
cytokines, ELISA was performed. The results demonstrated that TNF-α
and IL-6 levels were significantly decreased in the culture
supernatant of the siR-PTEN group compared with the siR-NC group
(P<0.05) (Fig. 6A and B). The
results indicate that PTEN promotes the release of TNF-α and IL-6
from rat cardiomyocytes treated with septic shock serum.
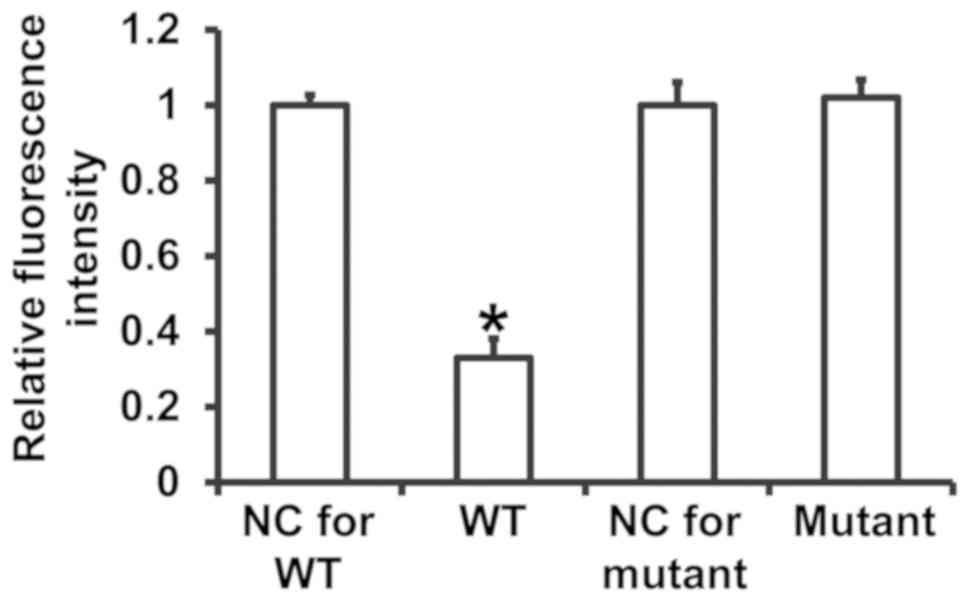
miR-494-3p binds to the 3′-UTR of PTEN
mRNA to regulate its expression
To assess the interaction between miR-494-3p and the
3′-UTR of PTEN mRNA, dual luciferase reporter assays were
performed. The fluorescence value of cells cotransfected with
miR-494-3p mimics and pMIR-REPORT-WT luciferase reporter plasmids
was significantly decreased compared with the negative control
group for WT (P<0.05) (Fig. 7).
In contrast, the fluorescence value of cells cotransfected with
miR-494-3p mimics and pMIR-REPORT-mutant luciferase reporter
plasmid was not significantly different compared with the negative
control group for mutant (P>0.05) (Fig. 7). The results suggest that miR-494-3p
binds to the 3′-UTR of PTEN mRNA to regulate expression.
Discussion
Septic shock is a common acute disease that occurs
secondary to sepsis and is typically caused by microbial infection
(35). Patients with septic shock
often suffer from multiple organ damage, while decreased systolic
and diastolic capacity resulting from myocardial cell injury can
lead to microcirculatory perfusion disorder, accelerate the
multiple organ failure and seriously affect the prognosis of the
patients (36). The mechanism of
myocardial injury in patients with septic shock is not fully
understood, although it has been suggested that this process mainly
includes myocardial cell proliferation inhibition, as well as
increased apoptosis and inflammation (37). miRNAs are an important class of
posttranscriptional regulatory factors that participate in numerous
pathophysiological processes (38).
Septic shock results in a type of hypoperfusion-induced ischemia
reperfusion injury regulated by a variety of miRNAs (39). miR-142-3p inhibits
hypoxia/reoxygenation-induced myocardial cell injury by targeting
the high mobility group box 1 gene (39). In addition, miR-378 protects
intestinal tissues by inhibiting the apoptosis of intestinal
epithelial cells and resisting ischemia reperfusion injury
(40). miR-494-3p is a novel miRNA
that serves important roles in the occurrence and development of
tumors (41). It has been reported
that miR-494-3p inhibits hepatic ischemia-reperfusion injury by
activating the PI3K/AKT signaling pathway (42). In the present study, it was evaluated
whether miR-494-3p serves a role in septic shock-induced myocardial
injury. The results revealed that miR-494-3p expression was reduced
in patients with septic shock and was negatively correlated with
LDH levels, a marker of myocardial injury. This suggests that
miR-494-3p may be associated with myocardial injury. At the
cellular level, incubation with sera from patients with septic
shock downregulated miR-494-3p expression in rat cardiomyocytes and
promoted the release of LDH into cell culture supernatants.
Functional experiments demonstrated that sera from patients with
septic shock inhibited the proliferation of rat cardiomyocytes and
promoted apoptosis and inflammation in rat cardiomyocytes.
Furthermore, serum from patients with septic shock stimulated the
synthesis and release of TNF-α and IL-6 from rat cardiomyocytes.
Rescue experiments revealed that miR-493-3p overexpression
ameliorates the effects of septic shock serum treatment on
proliferation and apoptosis in rat cardiomyocytes. miR-494-3p
overexpression also stimulated the release of LDH, TNF-α and IL-6
into the culture supernatants of rat cardiomyocytes. These results
suggest that miR-493-3p has a protective effect on
cardiomyocytes.
In the present study, bioinformatics analysis
suggested that miR-494-3 may target and regulate PTEN. It has been
reported that miR-494-3p serves a role in regulating PTEN
expression in tumors and ischemia-reperfusion injury (43). The results presented in the current
study suggest that miR-494-3p downregulates PTEN protein
expression. Additionally, dual luciferase reporter assays confirmed
that miR-494-3p directly binds to the 3′-UTR of PTEN.
In conclusion, the present study revealed that
miR-494-3p downregulation in the peripheral blood of patients with
septic shock is correlated with myocardial injury. miR-494-3p
promotes proliferation and inhibits apoptosis in cardiomyocytes, as
well as stimulating the synthesis and release of cytokines.
miR-494-4p overexpression alleviates myocardial cell injury and may
be used as a potential diagnostic and therapeutic target. The small
sample size used in the present study is a limitation, and future
studies should be performed to confirm these findings.
Acknowledgements
The authors would like to thank President Zhigang
Zhang and Director Wenhong Peng of the Linyi Central Hospital for
their support and instructions.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PW and JL designed the current study. PW and LK
performed experiments. PW, LK and JL analyzed the data. All authors
collaborated to interpret results and develop the manuscript. The
final version of the manuscript was read and approved by all
authors, and each author believes that the manuscript represents
honest work.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Ethics Committee of Linyi Central Hospital. Written informed
consent was obtained from all patients or their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Y, Wan W, Fang F, Guo L, Zhao Y, Zhang
X and Huang F: Clinical relevance of peroxisome
proliferator-activated receptor-gamma gene polymorphisms with
sepsis. J Clin Lab Anal. 32:e223402018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou X, Ding B, Ye Y, Tang G and Zhang Z:
An appropriate mean arterial pressure (MAP) does not always mean
hemodynamic stability in septic shock patients. J Crit Care.
43:397–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Santos-Junior MN, Rezende IS, Souza CLS,
Barbosa MS, Campos GB, Brito LF, Queiroz ÉC, Barbosa EN, Teixeira
MM, Da Silva LO, et al: Ureaplasma diversum and its
membrane-associated lipoproteins activate inflammatory genes
through the NF-κB pathway via toll-like receptor 4. Front
Microbiol. 9:15382018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zasada M, Lenart M, Rutkowska-Zapala M,
Stec M, Durlak W, Grudzień A, Krzeczkowska A, Mól N, Pilch M,
Siedlar M and Kwinta P: Analysis of PD-1 expression in the monocyte
subsets from non-septic and septic preterm neonates. PLoS One.
12:e01868192017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu LY and Xu D: Changes in blood oxygen
metabolism indices and their clinical significance in children with
septic shock. Zhongguo Dang Dai Er Ke Za Zhi. 19:1124–1128.
2017.(In Chinese). PubMed/NCBI
|
|
6
|
Dargent A, Nguyen M, Fournel I, Bourredjem
A, Charles PE and Quenot JP; EPISS study group, : Vasopressor
cumulative dose requirement and risk of early death during septic
shock: An analysis from the episs cohort. Shock. 49:625–630. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharawy N, Mahrous R, Whynot S, George R
and Lehmann C: Clinical relevance of early sublingual
microcirculation monitoring in septic shock patients. Clin
Hemorheol Microcirc. 68:347–359. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park JS, Choi HS, Yim SY and Lee SM: Heme
oxygenase-1 protects the liver from septic injury by modulating
tLR4-mediated mitochondrial quality control in mice. Shock.
50:209–218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding W and Jiang L: The Effects of
afebrile characteristic on patients with suspected septic shock:
Several facts need to be noticed. Crit Care Med. 45:e11912017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim YC, Song JE, Kim EJ, Choi H, Jeong WY,
Jung IY, Jeong SJ, Ku NS, Choi JY, Song YG and Kim JM: A simple
scoring system using the red blood cell distribution width, delta
neutrophil index, and platelet count to predict mortality in
patients with severe sepsis and septic shock. J Intensive Care Med.
1:8850666187874482018.
|
|
11
|
Vazquez-Guillamet MC, Vazquez R, Micek ST
and Kollef MH: Predicting resistance to piperacillin-tazobactam,
cefepime and meropenem in septic patients with bloodstream
infection due to gram-negative bacteria. Clin Infect Dis.
65:1607–1614. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Erwin BL, Denaburg MA, Barker AB, McArdle
PJ, Windham ST and Morgan CJ: Evaluation of vasopressin for septic
shock in patients on chronic renin-angiotensin-aldosterone system
inhibitors. Crit Care Med. 45:e1226–e1232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hyvernat H, Doyen D, Barel R, Kaidomar M,
Goubaux B, Pradier C, Panaïa-Ferrari P, Dellamonica J and Bernardin
G: Is inappropriate response to cosyntropin stimulation test an
indication of corticosteroid resistance in septic shock? Shock.
498:543–550. 2018. View Article : Google Scholar
|
|
14
|
Makara MA, Hoang KV, Ganesan LP, Crouser
ED, Gunn JS, Turner J, Schlesinger LS, Mohler PJ and Rajaram MV:
Cardiac electrical and structural changes during bacterial
infection: An instructive model to study cardiac dysfunction in
sepsis. J Am Heart Assoc. 5:e0038202016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martin L, Peters C, Schmitz S, Moellmann
J, Martincuks A, Heussen N, Lehrke M, Müller-Newen G, Marx G and
Schuerholz T: Soluble heparan sulfate in serum of septic shock
patients induces mitochondrial dysfunction in murine
cardiomyocytes. Shock. 44:569–577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang L, Zheng M, Zhou Y, Zhu J, Zhu M,
Zhao F and Cui S: Tanshinone IIA attenuates cardiac dysfunction in
endotoxin-induced septic mice via inhibition of NADPH oxidase
2-related signaling pathway. Int Immunopharmacol. 28:444–449. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng Y, Zou L, Chen C, Li D and Chao W:
Role of cardiac- and myeloid-MyD88 signaling in endotoxin shock: A
study with tissue-specific deletion models. Anesthesiology.
121:1258–1269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kimura A, Kitajima M, Nishida K, Serada S,
Fujimoto M, Naka T, Fujii-Kuriyama Y, Sakamato S, Ito T, Handa H,
Tanaka T, et al: NQO1 inhibits the TLR-dependent production of
selective cytokines by promoting IκB-ζ degradation. J Exp Med.
215:2197–2209. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu X, Wei C, Zhu X, Lu F, Sheng B and Zang
X: Effect of interleukin-31 on septic shock through regulating
inflammasomes and interleukin-1β. Exp Ther Med. 16:171–177.
2018.PubMed/NCBI
|
|
20
|
Li T, Sun XZ, Lai DH, Li X and He YZ:
Fever and systemic inflammatory response syndrome after retrograde
intrarenal surgery: Risk factors and predictive model. Kaohsiung J
Med Sci1. 34:400–408. 2018. View Article : Google Scholar
|
|
21
|
Kawamoto E, Masui-Ito A, Eguchi A, Soe ZY,
Prajuabjinda O, Darkwah S, Park EJ, Imai H and Shimaoka M: Integrin
and PD-1 ligand expression on circulating extracellular vesicles in
systemic inflammatory response syndrome and sepsis. Shock.
2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao W, Shen G, Ren H, Liang, Yu X, Zhang
Z, Huang J, Qiu T, Tang J, Shang Q, et al: Therapeutic potential of
microRNAs in osteoporosis function by regulating the biology of
cells related to bone homeostasis. J Cell Physiol. 2018.(Epub ahead
of print).
|
|
23
|
Kanthaje S, Makol A and Chakraborti A:
Sorafenib response in hepatocellular carcinoma: MicroRNAs as tuning
forks miRNAs as regulators of sorafenib response in HCC. Hepatol
Res. 38:5–14. 2018. View Article : Google Scholar
|
|
24
|
Thyagarajan A, Shaban A and Sahu RP: miRNA
directed cancer therapies: Implications in melanoma intervention. J
Pharmacol Exp Ther. 364:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ge C, Liu J and Dong S: MIRNA-214 protects
sepsis-induced myocardial injury. Shock. 50:112–118. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Diao H, Liu B, Shi Y, Song C, Guo Z, Liu
N, Song X, Lu Y, Lin X and Li Z: MicroRNA-210 alleviates oxidative
stress-associated cardiomyocyte apoptosis by regulating BNIP3.
Biosci Biotechnol Biochem. 81:1712–1720. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salati S, Salvestrini V, Carretta C,
Genovese E, Rontauroli S, Zini R, Rossi C, Ruberti S, Bianchi E,
Barbieri G, et al: Deregulated expression of miR-29a-3p, miR-494-3p
and miR-660-5p affects sensitivity to tyrosine kinase inhibitors in
CML leukemic stem cells. Oncotarget. 8:49451–49469. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Welten SM, Bastiaansen AJ, de Jong RC, de
Vries MR, Peters EA, Boonstra MC, Sheikh SP, La Monica N,
Kandimalla ER and Quax PH: Inhibition of 14q32 MicroRNAs miR-329,
miR-487b, miR-494, and miR-495 increases neovascularization and
blood flow recovery after ischemia. Circ Res. 115:696–708. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao R, Feng WT, Xu LJ, Zhong XM, Liu H,
Sun Y and Zhou LL: DUXAP10 regulates proliferation and apoptosis of
chronic myeloid leukemia via PTEN pathway. Eur Rev Med Pharmacol
Sci. 22:4934–4940. 2018.PubMed/NCBI
|
|
30
|
Sun G, Lu Y, Li Y, Mao J, Zhang J, Jin Y,
Li Y, Sun Y, Liu L and Li L: miR-19a protects cardiomyocytes from
hypoxia/reoxygenation-induced apoptosis via PTEN/PI3K/p-Akt
pathway. Biosci Rep. 37:2017. View Article : Google Scholar
|
|
31
|
Liu L, Yan X, Wu D, Yang Y, Li M, Su Y,
Yang W, Shan Z, Gao Y and Jin Z: High expression of Ras-related
protein 1A promotes an aggressive phenotype in colorectal cancer
via PTEN/FOXO3/CCND1 pathway. J Exp Clin Cancer Res. 37:1782018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Li L, Peng Y, Chen Y, Lv X, Li S,
Qin X, Yang H, Wu C and Liu Y: Surface chemistry induces
mitochondria-mediated apoptosis of breast cancer cells via
PTEN/PI3K/AKT signaling pathway. Biochim Biophys Acta.
1865:172–185. 2018. View Article : Google Scholar
|
|
33
|
Pero-Gascon R, Sanz-Nebot V, Berezovski MV
and Benavente F: Analysis of circulating microRNAs and their
post-transcriptional modifications in cancer serum by on-line
solid-phase extraction-capillary electrophoresis-mass spectrometry.
Anal Chem. 90:6618–6625. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gossack-Keenan KL and Kam AJ: Toxic shock
syndrome: Still a timely diagnosis. Pediatr Emerg Car. 2017.(Epub
ahead of print). View Article : Google Scholar
|
|
36
|
Zhang M, Zou L, Feng Y, Chen YJ, Zhou Q,
Ichinose F and Chao W: Toll-like receptor 4 is essential to
preserving cardiac function and survival in low-grade polymicrobial
sepsis. Anesthesiology. 121:1270–1280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Krome S: MikroRNA im septischen Schock
verändert. Anästhesiol Intensivmed Notfallmed Schmerzther. 51:654.
2016. View Article : Google Scholar
|
|
38
|
Geng L, Zhang T, Liu W and Chen Y:
miR-494-3p modulates the progression of in vitro and in vivo
Parkinson's disease models by targeting SIRT3. Neurosci Lett.
675:23–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, Ouyang M, Wang Q and Jian Z:
MicroRNA-142-3p inhibits hypoxia/reoxygenationinduced apoptosis and
fibrosis of cardiomyocytes by targeting high mobility group box 1.
Int J Mol Med. 38:1377–1386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Wen S, Yao X, Liu W, Shen J, Deng W,
Tang J, Li C and Liu K: MicroRNA-378 protects against intestinal
ischemia/reperfusion injury via a mechanism involving the
inhibition of intestinal mucosal cell apoptosis. Cell Death Dis.
8:e31272017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zheng HZ, Fu XK, Shang JL, Lu RX, Ou YF
and Chen CL: Ginsenoside Rg1 protects rat bone marrow mesenchymal
stem cells against ischemia induced apoptosis through miR-494-3p
and ROCK-1. Eur J Pharmacol. 822:154–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Su S, Luo, Liu X, Liu J, Peng F, Fang C
and Li B: miR-494 up-regulates the PI3K/Akt pathway via targetting
PTEN and attenuates hepatic ischemia/reperfusion injury in a rat
model. Biosci Rep. 37:2017. View Article : Google Scholar
|
|
43
|
Zhu H, Xie R, Liu X, Shou J, Gu W, Gu S
and Che X: MicroRNA-494 improves functional recovery and inhibits
apoptosis by modulating PTEN/AKT/mTOR pathway in rats after spinal
cord injury. Biomed Pharmacother. 92:879–887. 2017. View Article : Google Scholar : PubMed/NCBI
|