Introduction
Gastric cancer (GC) is one of the most common
cancers worldwide, particularly in East Asia and East Europe.
Furthermore, the incidence and mortality rates of GC are high,
accounting for ~1,000,000 mortalities annually. Therefore, GC is a
significant problem in terms of global health (1). Despite advances in surgery and
chemotherapy for colon cancer, the outcomes of anticancer therapy
remain unsatisfactory, thus, further improvements are required. A
number of pharmacological experiments have demonstrated that
numerous naturally active components, isolated from plants and
herbs, exhibit antitumor effects and may be potent agents for
cancer treatment (2,3).
Podophyllotoxin is a lignan extracted from the
Podophyllum plant, with a molecular formula of
C22H22O8 and a molecular weight of
414 Da. This compound and its derivatives have great significance
as antineoplastic drugs and antiviral agents due to the biological
activities that they exhibit. Podophyllotoxin and its derivatives
are currently used in chemotherapy for various cancer types,
including cervical carcinoma, osteosarcoma, nasopharyngeal
carcinoma, colon cancer, breast cancer, prostate cancer, testicular
carcinoma, small cell lung cancer and lymphoma (4–8).
However, studies investigating the antitumor effect on GC are
limited (9) and the molecular
mechanism remains unclear. In the present study, the inhibition of
cell growth and apoptosis induced by podophyllotoxin was
investigated in the human GC SGC-7901 cell line, and the underlying
mechanism was studied through the mitochondrial pathway.
Materials and methods
Reagents
Podophyllotoxin, MTT, propidium iodide (PI), Hoechst
33258 and Rodamine 123 were all purchased from Sigma-Aldrich (St.
Louis, MO, USA). Hydroxycamptothecin (HCPT) was purchased from
Harbin Shengtai Pharmaceutical Co., Ltd. (Harbin, China); RPMI 1640
culture medium was purchased from Thermo Fisher Scientific Inc.
(Waltham, MA, USA); fetal bovine serum (FBS) was obtained from
Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.
(Hangzhou, China) and trypsin was purchased from Gibco-BRL
(Rockville, MD, USA). Additionally, mouse anti-human β-actin,
cytochrome c (cyt-c), caspase-9 and caspase-3 polyclonal
antibodies, alkaline phosphatase (AP)-conjugated goat anti mouse
polyclonal antibody, SDS-PAGE sample loading buffer, blocking
buffer, TBST, buffer5-bromo-4-chloro-3-indolyl-phosphate
(BCIP)/nitroblue tetrazolium (NBT) alkaline phosphatase color
development kit (Beyotime Institute of Biotechnology, Haimen,
China); detergent-compatible protein assay kit (Bio-Rad, Hercules,
CA, USA).
Apparatus
The CKX 41 inverted fluorescence microscope was
purchased from Olympus (Tokyo, Japan) and the mini electrophoresis
meter and microplate reader were purchased from Bio-Rad (Hercules,
CA, USA). The EPICS XL flow cytometer was purchased from Beckman
Coulter (Brea, CA, USA); the SP2 laser confocal scanning microscope
was purchased from Leica (Solms, Germany) and the CO-150
CO2 incubator was purchased from New Brunswick
Scientific (Edison, NJ, USA).
Cell culture
The human GC SGC-7901 cell line was provided by the
Center of Research and Development on Life Sciences and
Environmental Sciences of Harbin University of Commerce (Harbin,
China). SGC-7901 cells were grown in RPMI 1640 culture medium
containing 10% heat-inactivated FBS at 37°C in a humidified
atmosphere of 5% CO2.
Antitumor activity of podophyllotoxin on
SGC-7901 cells
Exponentially growing cells were washed, digested
with trypsin and suspended in RPMI 1640 medium until a
concentration of 1×105 cells/ml was achieved. In a
96-well plate, 100 μl cell suspension per well was cultured for 24
h. The cells were subsequently incubated with varying
concentrations of podophyllotoxin for a further 72 h; 12 wells were
used per concentration. Cells incubated with only 100 μl RPMI 1640
culture medium served as a control group. MTT was dissolved in
phosphate-buffered saline (PBS) to provide a solution with a final
concentration of 0.5 mg/ml. After 72 h, the cell suspension was
discarded and 200 μl MTT solution (0.5 mg/ml) was added to each
well and incubated at 37°C for 4 h. All media were removed and 150
μl dimethyl sulfoxide was added to each well in order to dissolve
the purple formazan crystals. The plate was agitated for 3 min and
the spectrophotometric absorbance at 570 nm was measured using a
microplate reader. The inhibitory rate and IC50 was
calculated.
Effect of podophyllotoxin on cell cycle
and apoptosis of SGC-7901 cells
Cells (5×104/ml; 1 ml) were planted in
24-well plates and cultured for 24 h with three wells per group.
Subsequently, the cells were treated with various drug
concentrations for 48 h. The cells were harvested via
trypsinization, washed twice with cold PBS and fixed in 70% cold
ethanol for 12 h at 4°C. The fixation fluid was then discarded and
the cells were washed with PBS twice. PI was added to the samples
for 30 min for staining and the samples were measured by flow
cytometry with an excitation wavelength of 488 nm.
Effect of podophyllotoxin on the
morphology of SGC-7901 cells
Cells (5×104/ml; 1 ml) were planted in
6-well plates, cultured for 24 h and treated with varying drug
concentrations for 48 h. Cells were harvested via trypsinization,
washed twice with cold PBS and fixed in 4% paraformaldehyde for 30
min at 4°C. The fixation fluid was then discarded and the cells
were washed with PBS twice. Hoechst 33258 was added for 20 min,
after which the dye was discarded and the cells were washed twice
with PBS. The cells were observed using an inverted fluorescence
microscope.
Effect of podophyllotoxin on the
mitochondrial membrane potential (MMP) in SGC-7901 cells
Cells (5×104/ml; 1 ml) were planted in
6-well plates, cultured for 24 h and treated with various drug
concentrations for 24 h. Cells were harvested via trypsinization,
washed twice with cold PBS and loaded with Rhodamine 123 (5 μg/ml)
for 30 min at 37°C. The fluorescence intensity of the MMP was
observed using laser scanning confocal microscopy.
Effect of podophyllotoxin on the
expression of cyt-c, pro-caspase-9 and pro-caspase-3 in SGC-7901
cells
Cells (5×104/ml; 1 ml) were planted in
6-well-plates, cultured for 24 h and subsequently treated with
varying concentrations of podophyllotoxin for 48 h. Cytoplasm
extracts were prepared with 150 μl cell lysis buffer on ice for 30
min. Following centrifugation at 10,000 × g at 4°C for 10 min, the
supernatant was collected and the protein concentration was
quantified using the detergent-compatible protein assay kit.
Proteins were mixed with SDS-PAGE sample loading buffer (Beyotime
Institute of Biotechnology). In total, a 40-μg sample of protein
was separated in a 10% polyacrylamide gel and blotted on a
nitrocellulose membrane. The blots were blocked with blocking
buffer for 2 h at room temperature and incubated with
anti-cyt-c, anti-caspase-9 and anti-caspase-3 antibodies for
12 h at 4°C. Subsequently, the nitrocellulose membranes were washed
with TBST buffer three times and incubated with AP-conjugated goat
anti-mouse antibody in blocking buffer for 2 h at R.T. Then the
nitrocellulose membranes were washed with TBST buffer three times
and detected by BCIP/NBT alkaline phosphatase color development kit
(Beyotime Institute of Biotechnology). The bands were then
visualized and quantified using the Gel Doc XR imaging system
(Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data are expressed as the mean ± SD. Statistical
analyses were performed using analysis of variance to compare the
various groups. SPSS 15.0 software was used for statistical
analysis (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Antitumor activity of podophyllotoxin on
SGC-7901 cells
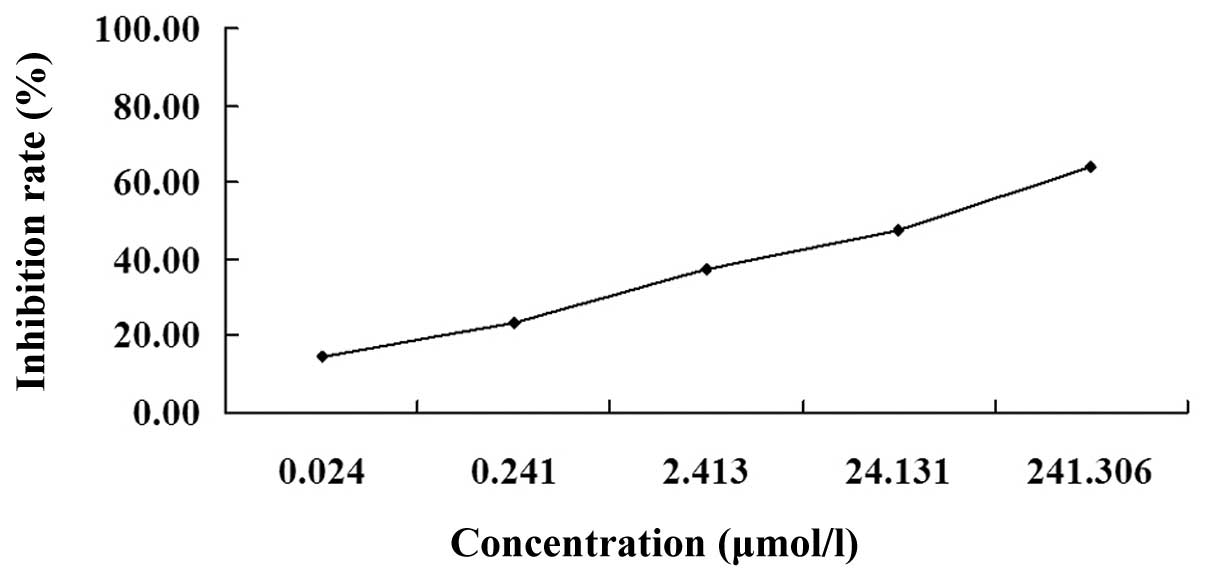
The MTT assay revealed that following treatment with
various concentrations of podophyllotoxin for 72 h, podophyllotoxin
exhibited an inhibitory effect on the proliferation of SGC-7901
cells in a concentration-dependent manner. The optical density
values of the treated groups exhibited statistically significant
differences when compared with the control group (P<0.01). The
IC50 was 26.30 μmol/l (Fig.
1).
Effect of podophyllotoxin on the cell
cycle and apoptosis of SGC-7901 cells
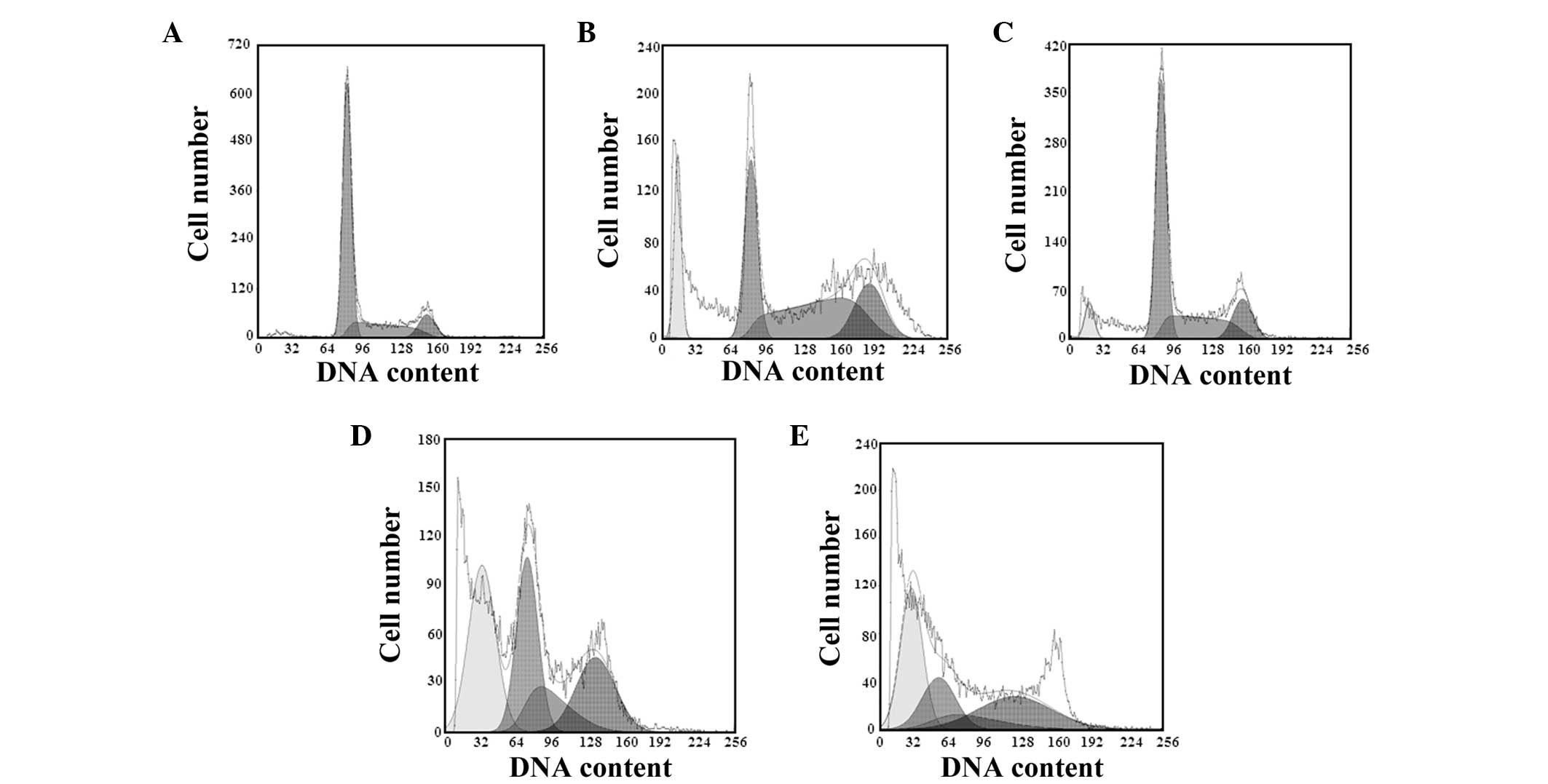
Following treatment with varying concentrations of
podophyllotoxin for 48 h, the cell cycle changed and the number of
cells in the G0/G1 and S phases decreased,
while the number of cells in the G2/M phase increased.
The apoptotic peaks appeared gradually in a concentration-dependent
manner, and the apoptosis rates were 11.06, 20.39 and 33.67% in the
13.15, 26.30 and 52.60 μmol/l podophyllotoxin groups, respectively,
as shown in Table I and Fig. 2.
 | Table IEffect of podophyllotoxin on the cell
cycle of SGC-7901 cells (3 wells). |
Table I
Effect of podophyllotoxin on the cell
cycle of SGC-7901 cells (3 wells).
| | Cell cycle (%) |
|---|
| |
|
|---|
| Group | Concentration
(μmol/l) |
G0/G1 | S | G2/M |
|---|
| Control | 0.00 | 65.166±0.125 | 26.754±0.114 | 14.080±1.074 |
| Podophyllotoxin | 26.30 | 43.049±0.102b | 23.526±0.081a | 22.425±0.202b |
| 52.60 | 34.737±0.082b | 18.004±0.142b | 27.259±0.736b |
| HCPT | 55.00 | 32.210±0.043b | 45.137±1.168b | 22.653±0.086b |
| 13.15 | 55.351±0.096a | 24.464±0.107 | 18.185±0.671a |
Effect of podophyllotoxin on the
morphology of SGC-7901 cells
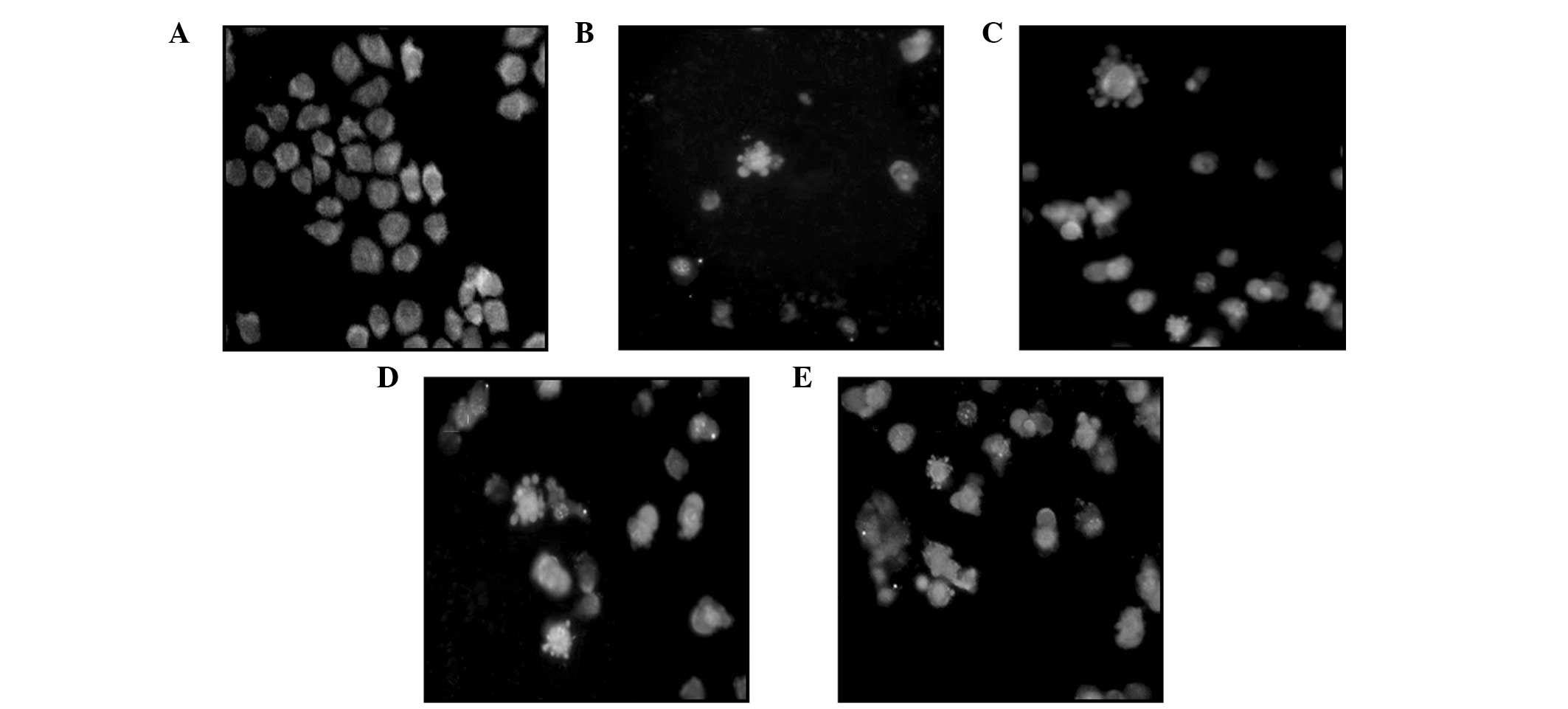
Under an inverted fluorescence microscope, the cells
in the control group were observed to grow normally and the
fluorescence in the cell nuclei was uniform. Following treatment
with various concentrations of podophyllotoxin for 48 h, a high
proportion of cells exhibited apoptosis-like changes, including
cell detachment, cytoplasmic condensation and the appearance of
apoptotic bodies, in a concentration-dependent manner (Fig. 3).
Effect of podophyllotoxin on the MMP in
SGC-7901 cells
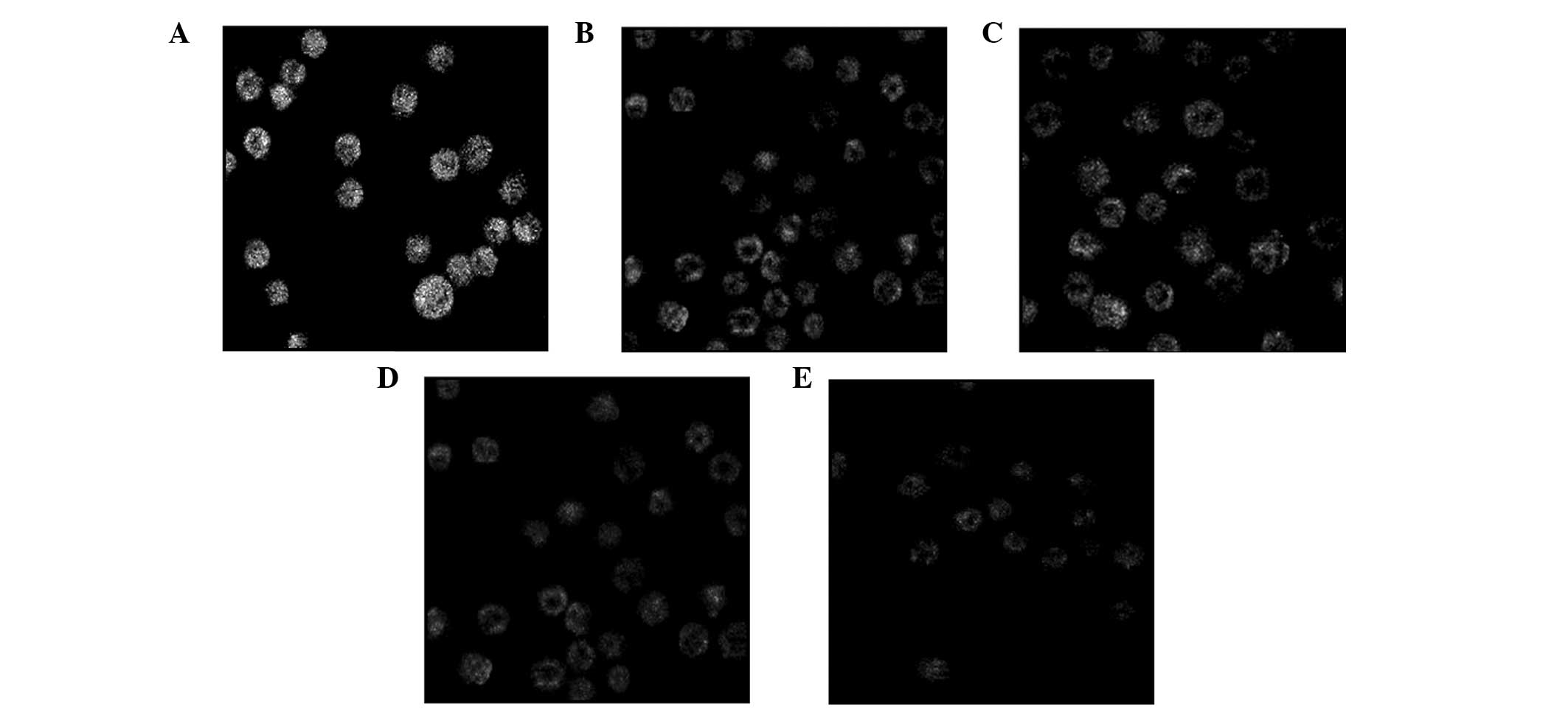
Following treatment with various concentrations of
podophyllotoxin for 24 h, the fluorescence intensity of Rhodamine
123 in cells decreased, indicating that the level of the MMP had
decreased. With increasing drug concentrations, the level of the
MMP decreased accordingly and statistically significant differences
were observed when compared with the control group (P<0.05), as
shown in Table II and Fig. 4.
 | Table IIEffect of podophyllotoxin on MMP in
SGC-7901 cells (20 wells). |
Table II
Effect of podophyllotoxin on MMP in
SGC-7901 cells (20 wells).
| Group | Concentration
(μmol/l) | Mean fluorescence
intensity |
|---|
| Control | 0.00 | 64.13±4.22 |
| Podophyllotoxin | 26.30 | 28.37±7.85b |
| 52.60 | 20.17±4.06b |
| HCPT | 55.00 | 27.55±9.16b |
| 13.15 | 44.80±1.97b |
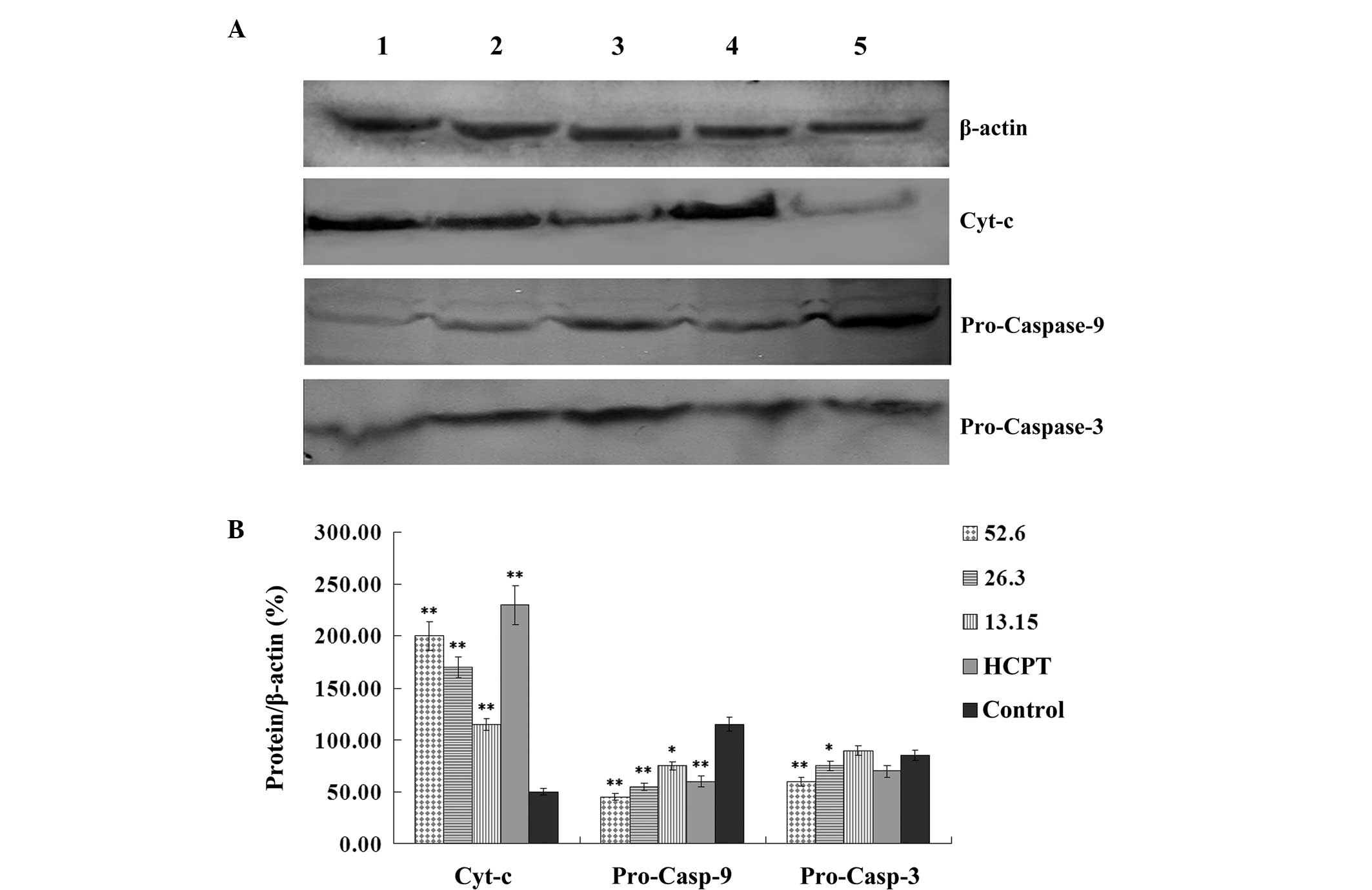
Effect of podophyllotoxin on the
expression levels of cyt-c, pro-caspase-9, pro-caspase-3 and
caspase-3 in SGC-7901 cells
Following treatment with various concentrations of
podophyllotoxin for 24 h, the expression levels of
apoptosis-related proteins in SGC-7901 cells changed. The
expression level of cyt-c increased, while the expression
levels of pro-caspase-9 and pro-caspase-3 decreased in a
concentration-dependent manner. Statistically significant
differences were observed when compared with the control group
(P<0.05), as shown in Fig.
5.
Discussion
Apoptosis is a rigorous, active and orderly process
of cell death that is regulated by numerous genes in order to
maintain the stability of the intracellular environment. The
mitochondrial pathway is one of three major apoptotic pathways.
This pathway involves the induction of apoptosis factors, the
life-or-death apoptosis switch, the start molecules of apoptosis
(cyt-c) and the effector molecules of apoptosis, including
caspase-9 and caspase-3.
A change in the permeability of the mitochondrial
membrane is the most significant event in apoptosis and this
permeability is controlled by the mitochondrial permeability
transition pore (MPTP), which is the key to the apoptotic
mitochondrial pathway (10). The
MMP is a result of the asymmetric distribution of protons and other
ions across the inner membrane of the mitochondrion, and is
necessary for sustaining mitochondrion function. The MMP is one of
the best indicators of mitochondrion permeability (11). In the present study, the
fluorescent probe Rhodamine 123 was used for staining. The
experimental results demonstrated that following the treatment of
SGC-7901 cells with podophyllotoxin for 24 h, the MPTP channels
opened, the MMP decreased and the permeability of the mitochondrial
membrane increased, resulting in irreversible cell apoptosis.
The release of cyt-c occurs in the early
stages of cell apoptosis (12).
Cyt-c is released into the cytoplasm through mitochondrial
outer membrane permeabilization, which is regulated by MPTP or
members of the Bcl-2 family. The release of cyt-c results in
the activation of caspases. Caspases are a type of proenzyme that,
under normal conditions, contain no reactive site. In
caspase-dependent mitochondrial pathways, cyt-c is released
from the mitochondria and interacts with aminophospholipid
transferase and apoptotic peptidase activating factor 1,
subsequently converting these molecules to polymers and promoting
their interaction with caspase-9 to form apoptotic bodies (13). Cyt-c is capable of
activating caspase-9 by hydrolyzing its proenzyme, and the
activated caspase-9 further activates caspase-3, leading to cell
apoptosis (14). The results of
the present study demonstrate that podophyllotoxin facilitates the
release of cyt-c from the mitochondrion into the cytoplasm,
which decreases the expression levels of pro-caspase-9 and
pro-caspase-3, leading to the caspase cascade with the formation of
a caspase-dependent pathway. Therefore, apoptosis is induced in
SGC-7901 cells via the mitochondrial pathway.
In conclusion, podophyllotoxin induces apoptosis in
the human GC SGC-7901 cell line through the mitochondrial
pathway.
Acknowledgements
The study was supported by a grant from the Program
of Natural Science Foundation of Heilongjiang Province (no.
QC2011C100).
References
|
1
|
Hu Y, Fang JY and Xiao SD: Can the
incidence of gastric cancer be reduced in the new century? J Dig
Dis. 14:11–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ji YB, Ji CF and Zhang H: Laminarin
induces apoptosis of human colon cancer LOVO cells through a
mitochondrial pathway. Molecules. 17:9947–9960. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ji YB, Ji CF, Yue L and Xu H: Saponins
isolated from Asparagus induce apoptosis in human hepatoma cell
line HepG2 through a mitochondrial-mediated pathway. Curr Oncol.
19(Suppl 2): eS1–eS9. 2012.PubMed/NCBI
|
|
4
|
Wang B, Chen L, Zhen H, et al: Proteomic
changes induced by podophyllotoxin in human cervical carcinoma HeLa
cells. Am J Chin Med. 41:163–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang TM, Qi SN, Zhao N, et al: Induction
of apoptosis through caspase-independent or caspase-9-dependent
pathway in mouse and human osteosarcoma cells by a new nitroxyl
spin-labeled derivative of podophyllotoxin. Apoptosis. 18:727–738.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rojas-Sepúlveda AM, Mendieta-Serrana M,
Mojica MY, et al: Cytotoxic podophyllotoxin type-lignans from the
steam bark of Bursera fagaroides var. fagaroides. Molecules.
17:9506–9519. 2012.PubMed/NCBI
|
|
7
|
Xu H, Lv M and Tian X: A review on
hemisynthesis, biosynthesis, biological activities, mode of action,
and structure-activity relationship of podophyllotoxins: 2003–2007.
Curr Med Chem. 16:327–349. 2009.PubMed/NCBI
|
|
8
|
Hartmann JT and Lipp HP: Camptothecin and
podophyllotoxin derivatives: inhibitors of topoisomerase I and II -
mechanisms of action, pharmacokinetics and toxicity profile. Drug
Saf. 29:209–230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Zhou CS, Liu S, Chen H and Yang
C: Effect of podophyllotoxin on human gastric cancer cell line SGC
7901. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 33:718–722. 2008.(In
Chinese).
|
|
10
|
Martin LJ: The mitochondrial permeability
transition pore: a molecular target for amyotrophic lateral
sclerosis therapy. Biochim Biophys Acta. 1802:186–197. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lemeshko VV: Potential-dependent membrane
permeabilization and mitochondrial aggregation caused by anticancer
polyarginine-KLA peptides. Arch Biochem Biophys. 493:213–220. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scorrano L: Opening the doors to
cytochrome c: changes in mitochondrial shape and apoptosis. Int J
Biochem Cell Biol. 41:1875–1883. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
dos Santos AB, Dorta DJ, Pestana CR, et
al: Dehydromonocrotaline induces cyclosporine A-insensitive
mitochondrial permeability transition/cytochrome c release.
Toxicon. 54:16–22. 2009.PubMed/NCBI
|
|
14
|
Lee HJ, Lee HJ, Lee EO, et al:
Mitochondria-cytochrome c caspase-9 cascade mediates
isorhamnetin-induced apoptosis. Cancer Lett. 270:342–353. 2008.
View Article : Google Scholar : PubMed/NCBI
|