Introduction
Invasive presurgical electroencephalogram (EEG)
recordings of seizures are required to define the optimal cortical
resection in patients with intractable epilepsy (1,2).
Alternative neurosurgical approaches that are minimally invasive
have been sought for a number of years. Recent advances in computed
tomography (CT) technology have enabled physicians to safely and
accurately manipulate a needle under CT fluoroscopy (3,4).
Radiofrequency thermocoagulation (RFTC), under the guidance of a
robot, is considered to be a safe and minimally invasive technique
that may be used to treat intractable epilepsy (5,6). The
methodology of deep electrode implantation was first developed by
Bancaud and Talairach (7);
however, the RF was not efficient in thermocoagulating the
epileptic foci (8,9). The deep electrodes are fundamental to
this method, as they are stereotactically implanted in the brain to
identify the exact locations of the epileptogenic zones (EZs) and
the pathways of seizure propagation (9). An advantage of this method is that it
is possible to define epileptogenic zones (EZs) without performing
a craniotomy. The deep electrodes in the mesial temporal lobe
record the discharges in the hippocampus and the deep electrodes in
the white matter of the frontal lobe record the discharges in the
gray matter of the frontal lobe.
The robot CAS-R-2 frameless stereotactic system is a
medical robot system recently developed by the Robotics Institute
of Beijing University of Aeronautics & Astronautics and the
Navy General Hospital (Beijing, China). The system provides
accurate RF treatment of the cranium and assists in deep electrode
implantation. In the present study, the efficacy and safety of this
robot-assisted system was evaluated by implanting deep electrodes
in the bilateral mesial temporal lobe through the frontal lobe. EZs
were located in patients with temporal epilepsy and deep EEG-guided
RFTC treatment was conducted, with control of the temperature and
time. CT scans were used to determine whether RFTC was performed
effectively and Engel classifications were assigned for
evaluation.
Patients and methods
Patients
The study was approved and registered by the Ethics
Committee of the Navy General Hospital of People’s Liberation Army
in February 2008. The Ethics Committee approved associated
treatment, data collection and patient follow-ups. All patients
provided written informed consent and all procedures were conducted
following the provisions of the Declaration of Helsinki.
A total of seven patients were selected successfully
following screening (female, 4; male, 3; age, 29.6±11.1 years; age
range, 16–45 years). The patients were selected according to the
following inclusion criteria: i) aged ≥15 years-old; ii)
experienced simple and/or complex partial seizures with or without
secondary generalization; iii) experienced ≥3 complex partial
seizures during the 3-month (12-week) baseline seizure diary
period, with ≥1 seizure occurring within the last 2 months. and iv)
electrographic evidence of seizures arising from one temporal lobe,
with radiographic evidence of mesial temporal sclerosis in the same
temporal lobe. Patients with normal magnetic resonance imaging
scans, bilateral hippocampal damage or cortical lesions were
excluded.
Since the data obtained from noninvasive presurgical
investigations were not congruent for sufficiently localizing the
EZs, intracerebral recordings of seizures, including video-deep EEG
and positron emission tomography/CT, were performed prior to
surgery. The necessary laboratory examinations were also performed,
including blood cell count, blood specimen collection,
alanineaminotransferase, aspartateaminotransferase, creatinine,
ureanitrogen, partial thromboplastin time, prothrombin time,
fibrinogen, ABO blood-group, blood grouping and crossmatching, HIV
antibodies, hepatitis C antibodies and hepatitis B surface
antigens.
Implantation procedure
Two recording deep electrodes (SD06R-SP10X-000,
Ad-Tech Medical Instrument Corp, Racine, WI, USA), were implanted
using the robot CAS-R-2 frameless stereotactic system. Following
the placement of four markers on the foreheads of the patients, MRI
scans at 3 mm intervals were collected. MRI scans of the head were
three-dimensionally reconstructed with a computer. Next, the
placement targets of the deep electrodes were marked in the
workstation. The deep electrodes were implanted through the
monitored designed path (transfrontal) and avoided the important
blood vessels and functional area. An assistant collaboratively
calibrated the mechanical arm to a zero position. Following the
registration of placement targets in the workstation, the
mechanical arm was calibrated, locked in position and the direction
and cranial point of craniotomy was determined.
Following the administration of 2 ml lidocaine (2%)
for anesthesia, patients were placed in a supine position for
surgery. The heads of the patients were immobilized with a molding
pillow. Percutaneous sphenotresia was performed following the
removal of the puncture needle to facilitate the implantation of
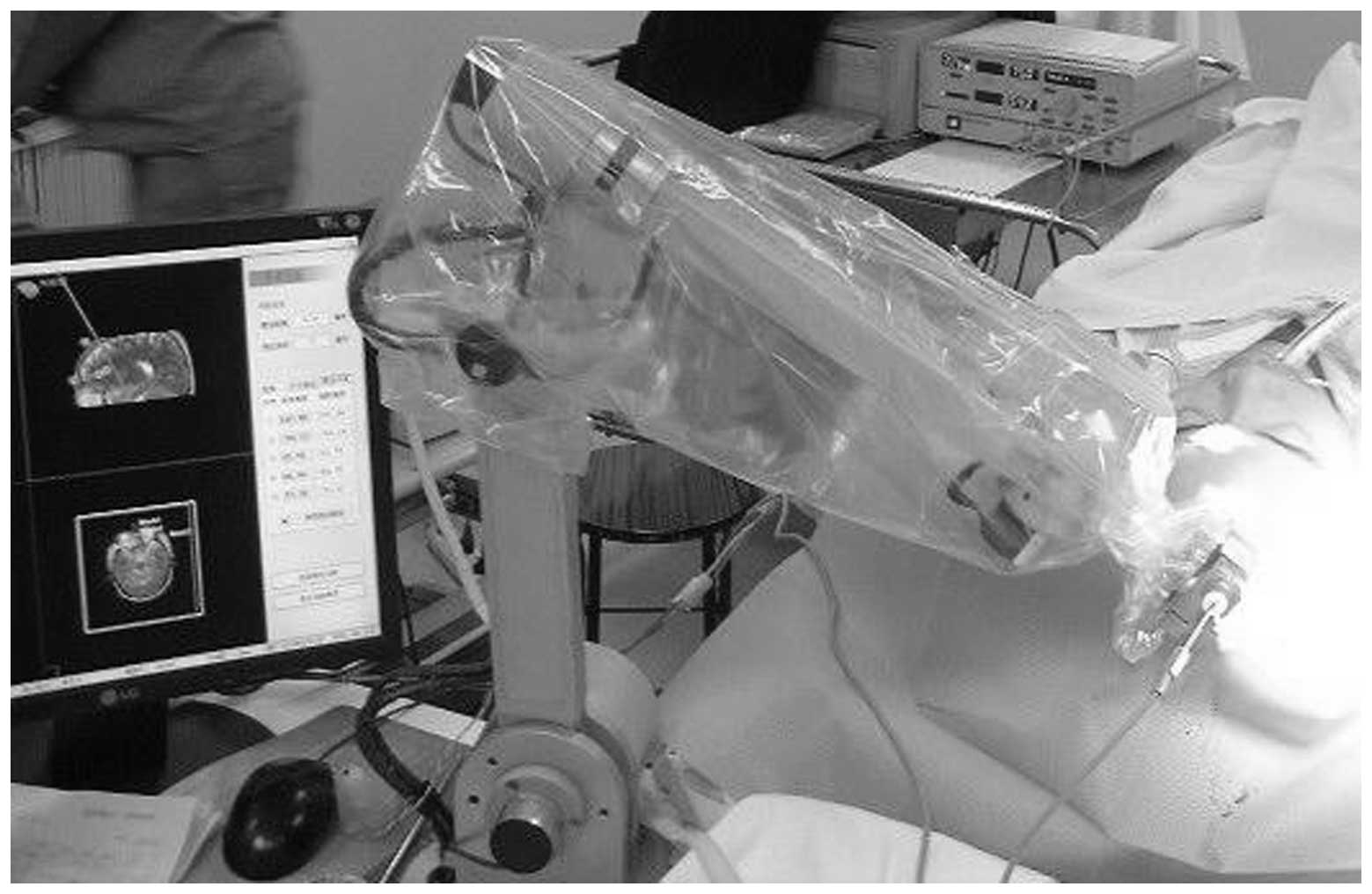
the deep electrodes (Fig. 1). Once
the electrodes had been confirmed to function normally, the wound
was sutured and covered by a sterile dressing. Postoperative
cranial CT scans were performed to confirm that the electrodes were
positioned accurately. Patients with no intracranial bleeding
complications were transferred to the EEG monitoring room.
Monitoring procedure
The duration of deep electrode EEG monitoring ranged
between 6 h and 7 days. At least one clinical seizure was recorded
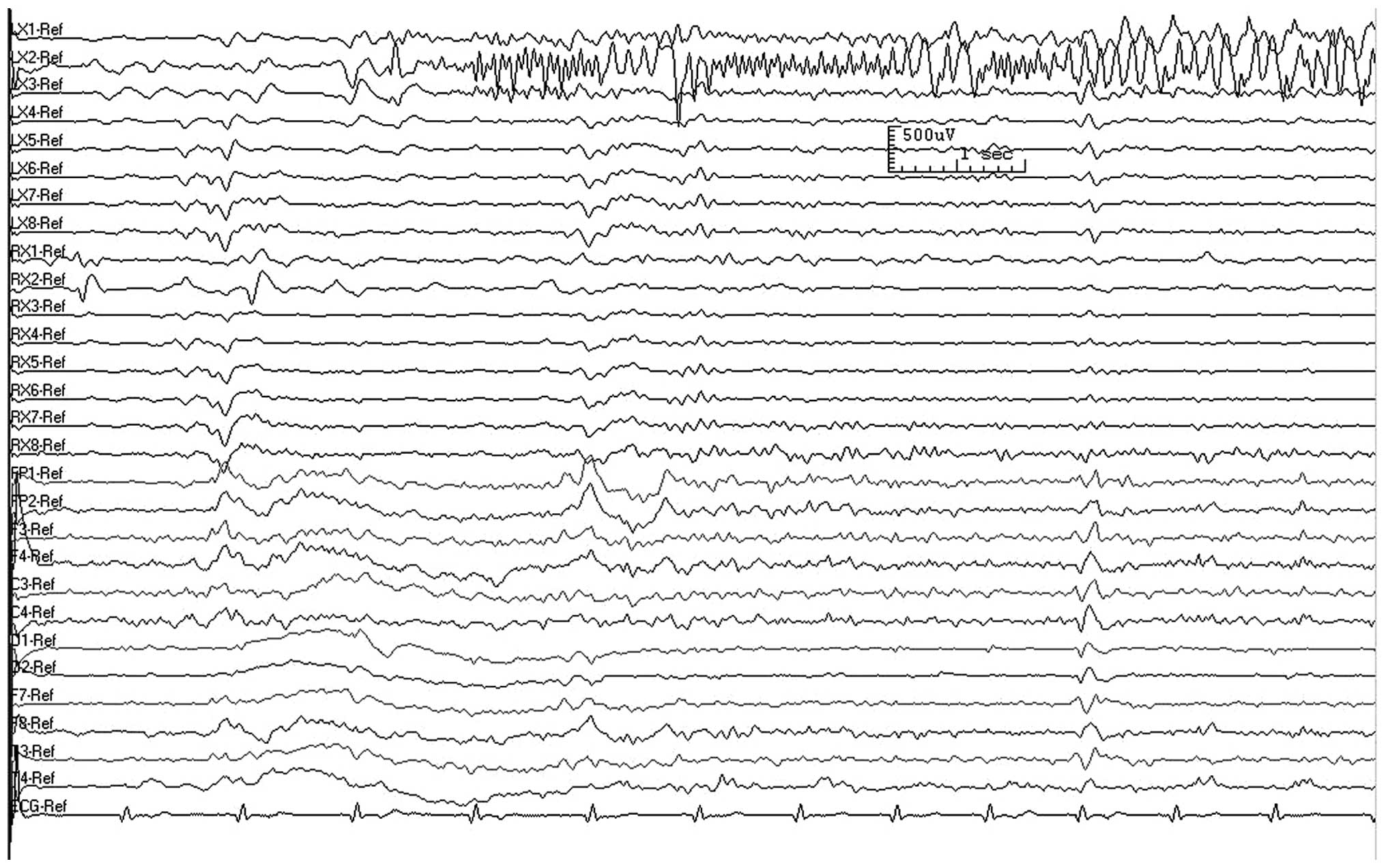
in each patient from which the EZs were determined. Fig. 2 shows a deep electrode EEG
recording of a seizure in the left medial temporal lobe. If the
patients had no natural seizures during the first 24 h of
monitoring, 150 mg bemegride was injected intravenously at 25
mg/min. The injection was stopped immediately when the patient
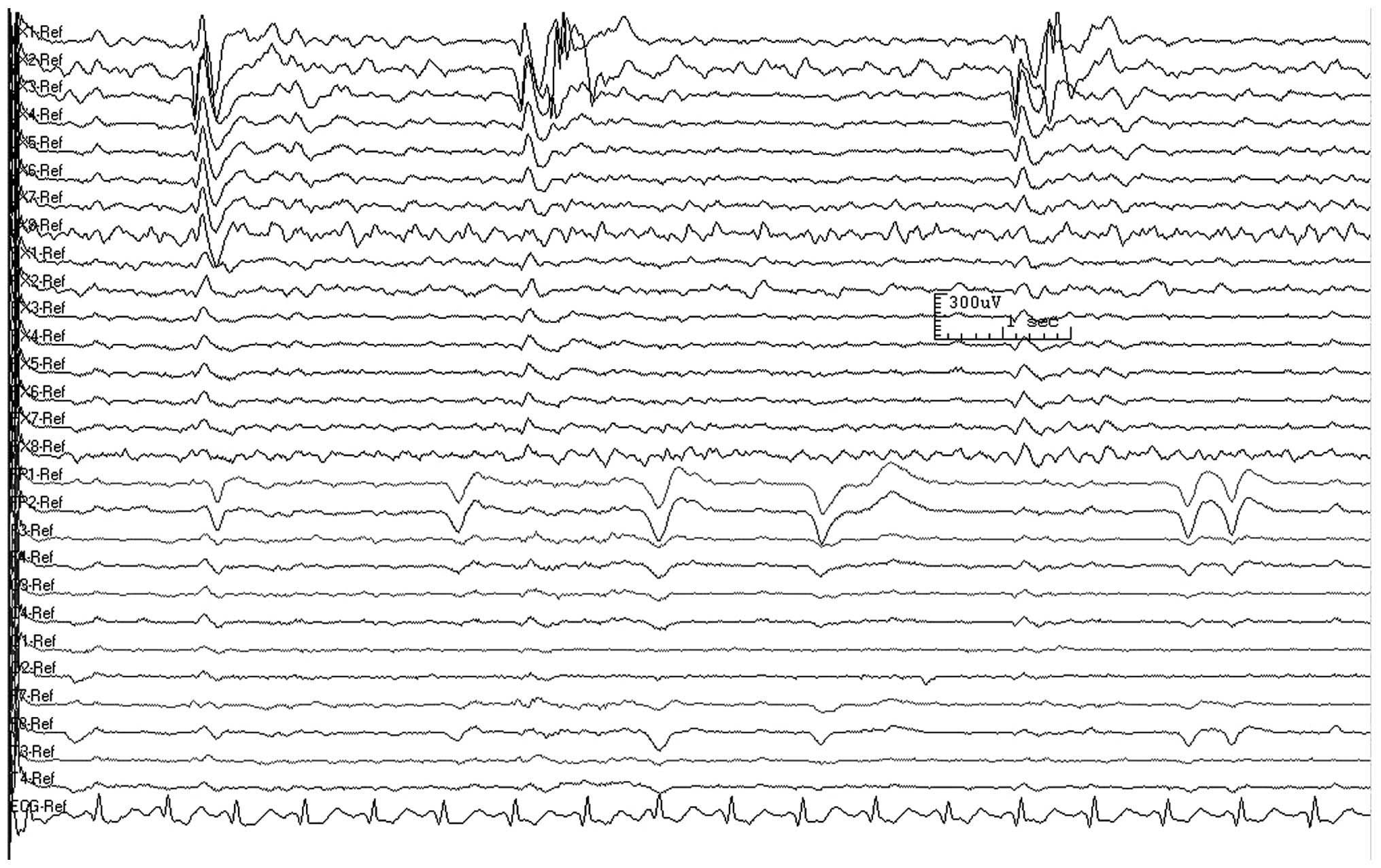
underwent seizure aura. Fig. 3
shows a deep electrode EEG recording following the injection of
bemegride. Phenobarbital (100–150 mg) was injected immediately into
patients experiencing a seizure to prevent status epilepticus.
Robot-assisted RFTC procedure
General anesthesia was administered intravenously
prior to surgery. Ventilation was controlled with an anesthesia
machine and muscle relaxant was provided if necessary. Deep brain
resistance measurements and 0–6 V electrical stimulation (2 Hz)
were performed prior to RF. During this process, close attention
was paid to whether the patient experienced limb movement, and the
position of the nucleus of epileptic was confirmed. RF radiation
was targeted to the amygdala firstly and the actual EZs were
evaluated by intraoperative EEG. The selection of targets was
determined from video-deep EEG recordings regarding the
localization of the onset zone. Radiation was stopped once
interictal paroxysmal activities disappeared.
An RF generator system (Radionics Medical, HXKN
Ltd., China) was used to conduct RFTC in 2 or 3 mm intervals at
75°C for 120 sec. Once the epileptiform discharges disappeared from
the deep electrode EEG recordings, surgery was stopped. Following
compensation, if the epileptiform discharges remained, RFTC was
performed in the ipsilateral or contralateral amygdala hippocampus.
Deep EEG recordings were performed for ≥5 min prior to and
following the RFTC procedure. Surgery was terminated once the
frequency of the spike or sharp waves on the deep EEG recordings
was <75% of those recorded prior to robot-assisted RFTC.
Surgery was stopped immediately following a seizure
to prevent damage due to fracturing of the intracranial
needles.
Postoperative treatment and
follow-up
Antiepileptic drug treatment was not changed for 6
months following RFTC, but was modified at the 6 month follow-up if
required. The frequency of seizures and the possible adverse
effects of the RFTC procedure were recorded during the follow-up
period. During the follow-ups, patient complaints with regard to
memory, attention and language were recorded. Routine tests were
performed as part of the neurological evaluation and Engel
classifications (1993) (10) were
assigned for neurological function evaluation.
Results
Patients
The numbers of EZs treated, their locations and the
seizure frequencies prior to and following RFTC are listed in
Table I. The average disease
course was 14 years (range, 2–40 years). Five patients experienced
no pre-attack symptoms, one patient had fear as a seizure precursor
and one patient’s seizures were preceded by a feeling of discomfort
in the chest.
 | Table ITreatment outcomes of seven patients
who received robot-assisted RFTC. |
Table I
Treatment outcomes of seven patients
who received robot-assisted RFTC.
| Coagulations | RFTC targets | Follow-up time,
months | Seizure frequency
prior to RFTC, per month | Seizure frequency
after RFTC, per month |
|---|
| 4R/7L | Bilateral
hippocampus | 62 | 10 | 1 |
| 8L | Left amygdala and
hippocampus | 62 | 4 | 4.5 |
| 6L | Left hippocampus | 43 | 10 | 0 |
| 8L | Left amygdala and
hippocampus | 38 | 6 | 0 |
| 5R/5L | Bilateral
hippocampus | 38 | 10 | 5 |
| 6L | Left hippocampus | 35 | 2 | 0 |
| 6R/7L | Bilateral
hippocampus | 34 | 2.5 | 1 |
Patients received CT scans following deep electrode
implantation surgery. All the electrodes were implanted properly to
the designed locations. No permanent neurological or cognitive
impairments occurred following these procedures. However, one
patient had a mild subarachnoid hemorrhage (confirmed by CT scan),
but following the correct treatment did not suffer from
neurological function impairment. An average of 8.1±3.1 EZs (range,
6–13) were treated in one or more anatomical targets. The EZs were
located in the unilateral or bilateral hippocampus and/or amygdala.
The follow-up period ranged between 34 and 62 months, with a mean
follow-up period of 44.6±12.2 months.
Deep EEG results
All seven patients had interictal epileptiform
discharges in the bilateral mesial temporal lobe, as shown by the
deep EEG recordings. The clinical seizure onset zone of one patient
was the left frontal lobe, while for the other six patients it was
the unilateral or bilateral mesial temporal lobe. Due to the
shortened time between the brain electrical origin following
bemegride injection and the induction of an epileptic seizure,
positioning errors or difficulties may have occurred (data not
shown).
Engel classification outcome
Following the last consultation, four patients were
classified as Engel’s class I (three patients, Ia and one patient,
Id), two patients were classified as Engel class IVa and one
patient was classified as class IVc. Seizure frequency decreased by
≥50% in six patients and four patients were seizure-free.
Postoperative CT scans indicated that the robot-assisted RFTC
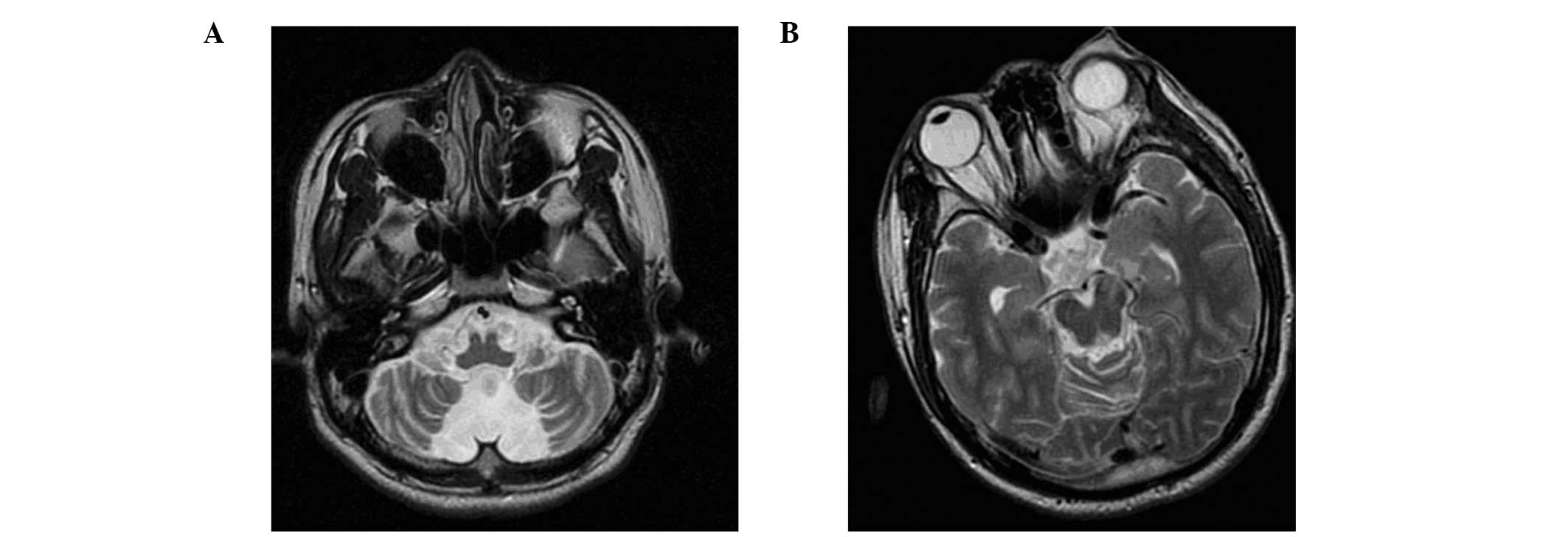
treatment was safe and effective (Table I). Fig. 4 compares the pre- and postoperative
CT scans of a patient who received robot-assisted RFTC
treatment.
Discussion
A deep electrode robot-assisted frameless system was
first reported by Eljamel (11).
Since then, a number of studies have been performed with the aim of
further improving this system (12–14).
The main characteristics of deep electrode implantation are firstly
that the electrodes are implanted by a stereotaxic method alone,
instead of using strip or grid electrodes. In addition,
epileptiform discharges are evaluated by space and time, and then
the cortical origin, form of communication and the focal cortical
areas involved are identified. In the present study, the deep
electrodes were implanted with the assistance of a robot. CT scans
showed that the implanted positions were consistent with the
preoperative planning. This preformation shortened the surgery
time, minimized pain and increased the precision. Furthermore, the
procedure was acceptable for the majority of patients in the Navy
General Hospital.
The CAS-R-2 robot-assisted frameless system was
designed by the Robotics Institute of Beijing University of
Aeronautics & Astronautics and the Navy General Hospital of
People’s Liberation Army. The system, without RFTC, has been
applied successfully in clinical practice for neural stem cell
therapy and, consistent with the results of the present study,
achieved satisfactory curative effects.
During the deep EEG recording process, the presence
of electrodecremental events, high frequency activity, irregular
sharp waves intermixed with slow activity, spike-wave activity and
rhythmic ictal transformation at the seizure onset determined the
onset zones of partial complex seizures (15). Park et al classified seizure
onset patterns as rhythmic activity, attenuation, repetitive spikes
or spike-wave complexes, and indicated that the common pattern of
seizure onset was rhythmic activity and repetitive spikes (16). The authors classified focal or
regional seizure onset based on the number of electrode contacts
that were involved in the ictal EEG and found that temporal
lobectomy lead to excellent outcomes for focal seizure onset.
Assisted by the robot system, the onset zones in the unilateral or
bilateral mesial temporal lobes were defined in all seven patients
in the current study. The discharges of the frontal lobe were
recorded by the electrodes in the white matter since the deep
electrodes were implanted in the planned targets properly. During
the complete robot-assisted RFTC process, complications were minor
and no long-term side-effects were observed. Guénot et al
(17) performed stereotactic EEGs
for 100 cases, and in each case, 5–15 (average, 11) deep electrodes
were implanted. Complications occurred in five cases with two
patients suffering from a skin infection, two patients with
electrode fracture and one patient who succumbed to an intracranial
hematoma. These results demonstrate the safety of stereotactic
EEG.
The application of stereo implantation in the
present study mainly focused on distinguishing the different lobe
of epilepsy in the frontal and temporal sides. The dorsolateral
prefrontal approach was adopted in the current study. The
appropriate angle of the deep electrode was adjusted and
epileptiform discharges in the white matter of the frontal lobe
were recorded by deep electrodes. Discharge in the white matter of
the frontal lobes has auxiliary effects on determining the origin
of epilepsy. If properly applied, deep electrode EEG has the
clinical diagnostic value of a cortex electrode (18).
The RFTC procedure on unilateral or bilateral mesial
temporal lobes was particularly favorable for patients whose
epileptic onset zone was the mesial temporal lobe. In the present
study, six of the seven patients benefited from RFTC with a
reduction of ≥50% in seizure frequency. The results obtained
compare favorably with those of other palliative therapeutic
procedures, including vagus nerve stimulation, multiple subpial
transaction, callosotomy or deep intracerebral stimulation
(19). Reported Engel I
classification rates were >70% (18,20),
but the effect varies in patients with different indications
(21,22). In the current study, robot-assisted
RFTC was performed in patients with intractable epilepsy.
Discharges existed in the frontal and temporal interictal
epileptiforms and deep electrode EEG recordings showed that the
medial temporal lobe was the seizure onset zone. Transfrontal RFTC
was performed in the deep temporal lobe structure using the
stereotactic system robot. The proportion of patients with an Engel
classification of I was 57% (4/7). However, further studies are
required to confirm these results.
In conclusion, the present study demonstrated that
robot-assisted RFTC is effective for patients with intractable
epilepsy; the key lies in the identification of the correct
epileptic locations. The robot-assisted stereotactic system is
capable of performing procedures with deep electrodes and RF and
demonstrates a good example of the transformation of engineering
technology to a medical application.
References
|
1
|
DuanYu N, GuoJun Z, Liang Q, LiXin C, Tao
Y and YongJie L: Surgery for perirolandic epilepsy: Epileptogenic
cortex resection guided by chronic intracranial
electroencephalography and electric cortical stimulation mapping.
Clin Neurol Neurosurg. 112:110–117. 2010. View Article : Google Scholar
|
|
2
|
Rosenow F and Lüders H: Presurgical
evaluation of epilepsy. Brain. 124:1683–1700. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koizuka S, Saito S, Tobe M, Sekimoto K,
Obata H and Koyama Y: Technical communication: percutaneous
radiofrequency mandibular nerve rhizotomy guided by high-speed
real-time computed tomography fluoroscopy. Anesth Analg.
111:763–767. 2010. View Article : Google Scholar
|
|
4
|
Nakajima K, Koizuka S, Yanagisawa A and
Saito S: Radiofrequency thermocoagulation of the thoracic nerve
root guided by high-speed real-time computed tomography
fluoroscopy. Anaesthesia. 67:675–676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malikova H, Liscak R, Vojtech Z, et al:
Stereotactic radiofrequency amygdalohippocampectomy: does reduction
of entorhinal and perirhinal cortices influence good clinical
seizure outcome? Epilepsia. 52:932–940. 2011. View Article : Google Scholar
|
|
6
|
Allen SJ and Sidebotham DA: Seizures and
shock after radiofrequency ablation for atrial fibrillation. J
Cardiothorac Vasc Anesth. 24:716–718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bancaud J and Talairach J: Methodology of
stereo EEG exploration and surgical intervention in epilepsy. Rev
Otoneuroophtalmol. 45:315–328. 1973.(In French).
|
|
8
|
Guénot M, Isnard J, Ryvlin P, Fischer C,
Mauguière F and Sindou M: SEEG-guided RF thermocoagulation of
epileptic foci: feasibility, safety, and preliminary results.
Epilepsia. 45:1368–1374. 2004.PubMed/NCBI
|
|
9
|
Catenoix H, Mauguière F, Guénot M, et al:
SEEG-guided thermocoagulations: a palliative treatment of
nonoperable partial epilepsies. Neurology. 71:1719–1726. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Engel J Jr: Clinical neurophysiology,
neuroimaging, and the surgical treatment of epilepsy. Curr Opin
Neurol Neurosurg. 6:240–249. 1993.PubMed/NCBI
|
|
11
|
Eljamel MS: Robotic application in
epilepsy surgery. Int J Med Robot. 2:233–237. 2006. View Article : Google Scholar
|
|
12
|
Spire WJ, Jobst BC, Thadani VM, Williamson
PD, Darcey TM and Roberts DW: Robotic image-guided depth electrode
implantation in the evaluation of medically intractable epilepsy.
Neurosurg Focus. 25:E192008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Centeno RS, Yacubian EM, Caboclo LO,
Júnior HC and Cavalheiro S: Intracranial depth electrodes
implantation in the era of image-guided surgery. Arq
Neuropsiquiatr. 69:693–698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bell B, Gerber N, Williamson T, et al: In
vitro accuracy evaluation of image-guided robot system for direct
cochlear access. Otol Neurotol. 34:1284–1290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alarcon G, Binnie CD, Elwes RD and Polkey
CE: Power spectrum and intracranial EEG patterns at seizure onset
in partial epilepsy. Electroencephalogr Clin Neurophysiol.
94:326–337. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park YD, Murro AM, King DW, Gallagher BB,
Smith JR and Yaghmai F: The significance of ictal depth EEG
patterns in patients with temporal lobe epilepsy.
Electroencephalogr Clin Neurophysiol. 99:412–415. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guénot M, Isnard J, Ryvlin P, et al:
Neurophysiological monitoring for epilepsy surgery: the Talairach
SEEG method. Stereoelectroencephalography Indications, results,
complications and therapeutic applications in a series of 100
consecutive cases. Stereotact Funct Neurosurg. 77:29–32. 2001.
|
|
18
|
Guenot M and Isnard J: Multiple
SEEG-guided RF-thermolesions of epileptogenic foci. Neurochirurgie.
54:441–447. 2008.(In French).
|
|
19
|
Elliott RE, Morsi A, Geller EB, Carlson
CC, Devinsky O and Doyle WK: Impact of failed intracranial epilepsy
surgery on the effectiveness of subsequent vagus nerve stimulation.
Neurosurgery. 69:1210–1217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Malikova H, Vojtech Z, Liscak R, et al:
Stereotactic radiofrequency amygdalohippocampectomy for the
treatment of mesial temporal lobe epilepsy: correlation of MRI with
clinical seizure outcome. Epilepsy Res. 83:235–242. 2009.
View Article : Google Scholar
|
|
21
|
Kameyama S, Murakami H, Masuda H and
Sugiyama I: Minimally invasive magnetic resonance imaging-guided
stereotactic radiofrequency thermocoagulation for epileptogenic
hypothalamic hamartomas. Neurosurgery. 65:438–449. 2009. View Article : Google Scholar
|
|
22
|
Schmitt FC, Voges J, Buentjen L, et al:
Radiofrequency lesioning for epileptogenic periventricular nodular
heterotopia: a rational approach. Epilepsia. 52:e101–e105. 2011.
View Article : Google Scholar : PubMed/NCBI
|