Introduction
Skin tissue homeostasis is maintained by two kinds
of progenitor cells: epidermal stem cells (EpSCs) and their
progeny, known as the transit amplifying (TA) cells (1). EpSCs are responsible for replacing
the differentiated cells of the interfollicular epidermis, hair
follicles and sebaceous glands (2). While little is known about the
proliferation, differentiation and developmental processes of
EpSCs, these processes may be regulated by a program of
differential genes or different signaling pathways.
The Nanog gene is thought to be involved in the
differentiation and proliferation processes of different stem cells
(3–5). It was reported that the Nanog gene
plays a critical role in regulating the cell fate of the
pluripotent inner cell mass (ICM) during embryonic development,
maintaining the pluripotent epiblast and preventing differentiation
(6). The canonical Wnt/β-catenin
pathway is also involved in the control of gene expression, cell
behavior, cell adhesion, and cell polarity of different types of
cells (7). β-catenin plays a
central role in the Wnt signalling pathway, and components of the
β-catenin signaling pathway are often regulated by other signals.
Thus it was of great interest to study the relationship between the
Wnt/β-catenin pathway and other signaling pathways (8).
In this study, we aimed to evaluate the relationship
between β-catenin expression and Nanog expression. In brief, EpSCs
were isolated and identified by immunofluorescence. Cells were then
induced to be proliferated and differentiated. Western blotting and
real-time polymerase chain reaction (PCR) were respectively
performed during the proliferation and differentiation periods of
EpSCs. Our results showed that 10−7 M neuropeptide
substance P (10−7 M SP) could effectively induce
proliferation of EpSCs. Western blotting results showed that
expressions of β-catenin and Nanog both increased with culture time
during the proliferation period, and gained a robust increase 48 h
after the proliferation. The expression of β-catenin increased
steadily with culture time during the differentiation period, and
expression of the Nanog gene increased during the first 24 h of
cell culture but began to decrease after the initial periods. PCR
results were generally in accordance with the western blotting
results. Our results demonstrated the involvement of Nanog and
Wnt/β-catenin in the proliferation and differentiation process of
EpSCs and suggest the existence of a potential link between these
two signaling pathways.
Materials and methods
Cell culture
A skin tissue was obtained from the back of neonatal
SD mice by plastic surgical procedures, washed in
phosphate-buffered solution (PBS), and connective tissue and
subcutaneous fat were removed. The skin sample was sterilized with
70% ethanol, rinsed in PBS, and minced into 5-mm wide strips using
a sharp scalpel; the strips were treated with 0.25% Dispase (Roche
Co., Switzerland) solution at 4°C overnight. The epidermis was
mechanically separated from the dermis, and incubated in a solution
of 0.25% trypsin at 37°C for 30 min to dissociate the cells; enzyme
activity was then blocked with Dulbecco’s modified Eagle’s medium
(DMEM; Gibco Co., USA) containing 10% fetal bovine serum (FBS;
Gibco Co.) and the cells were suspended with a pipette. The cell
suspension was filtered through a stainless steel mesh attached to
a 60-mm cell culture plate to remove any remaining tissue pieces,
and the cells were transferred to a 15-ml centrifuge tube and
collected by centrifugation for 5 min at 1,000 rpm. To select stem
cells, 1×106 dissociated epidermal cells were plated
onto collagen type IV (Sigma Co., USA) (100 μg/ml)-coated dishes at
room temperature for 10 min. The unattached cells were removed, and
the rapidly adherent epidermal cells were cultured with
keratinocyte serum-free medium (K-SFM; Gibco Co.) supplemented with
epidermal growth factor (EGF) (K-SFM; Gibco Co.) and bovine
pituitary extract (BPE; Gibco Co.) and with 0.05 mM calcium
chloride (CaCl2, Sigma Co.) at 37°C, 5% CO2
in a humidified incubator for two days before replacing the medium.
The medium was changed every other day.
EpSCs proliferation
EpSCs were seeded in a 35-mm culture plate treated
with collagen type IV at a density of approximately
1×104 cells/plate for 24 h. Cells were then induced to
proliferate by adding 10−7 M SP (Sigma Co., USA) into
the K-SFM. After treatment with 10−7 M SP, the cells
were examined independently at three time points. The control cells
were cultured in the K-SFM without SP.
EpSCs differentiation
EpSCs were seeded in a 35-mm culture plate treated
with collagen type IV at a density of approximately
1×104 cells/plate for 24 h, and EpSCs were induced to
differentiate by culturing in Dulbecco’s modified Eagle’s medium
(DMEM; Gibco Co.) containing 10% fetal bovine serum (FBS; Gibco
Co.).
MTT assay
EpSCs were plated in 96-well plates treated with
collagen type IV at a density of 2×104 cells/well. Cells
were then divided into two groups, the first group was the control
group and the second group was exposed to 10−7 M SP to
induce cell proliferation. Forty-eight hours post-treatment, the
relative number of living cells was determined by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay. Cells were incubated in the medium containing 500 μg/ml MTT
(Sigma-Aldrich) for 4 h. The reaction was then terminated by
incubating the cells in dimethyl sulfoxide for 10 min. The specific
absorbance at 492 nm (A492) was determined. All the experiments
were performed at least three times independently.
Western blotting
Total cell lysates of EpSCs that underwent the
proliferation and differentiation processes were obtained at
different time points (0, 24, 48 and 72 h) by lysing the cells in
RIPA buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.1%
SDS, 0.5% sodium deoxycholate, 2 mM sodium fluoride, 1 mM EDTA, 1
mM EGTA and protease inhibitor cocktail. The protein concentration
was determined using the bicinchoninic acid protein assay (Pierce,
Rockford, IL). The proteins were separated by SDS-PAGE, transferred
to nitrocellulose, blocked, and incubated with the following
primary antibodies: mouse anti-β catenin (R&D Systems, USA)
diluted 1:500, mouse anti-Nanog (Beijing Biosynthesis Biotechnology
Co., Ltd.) diluted 1:500, and mouse anti-β-actin (Beijing
Biosynthesis Biotechnology Co., Ltd.) diluted 1:100 that was used
as a loading control. The membrane was washed and incubated with
the respective secondary antibodies conjugated with peroxidase.
Protein detection was performed with the chemiluminescence
detection system (Pierce).
PCR analysis
PCR analysis was performed 24, 48 and 72 h after
cell culture. Total-RNA was extracted using the TRIzol reagent
(Invitrogen, USA) according to the manufacturer’s protocol. RNA
concentration and purity were determined by A260 and A260/A280
ratios, respectively. The integrity of total-RNA was assessed on
standard 1% agarose/formaldehyde gels. The RNA samples were treated
with DNAse I to remove residual traces of DNA. cDNA was obtained
from 1 μg of total-RNA, using reverse transcriptase (Toyobo, Japan)
and random primers (Promega) in a final volume of 20 μl. cDNAs (1
μl for each sample) were amplified by PCR using the primer
sequences as follows: Nanog, 5′-GAGCGTGGATCTTTCTGG-3′ (sense) and
5′-TCTTGGTGAGGACCTTGTT-3′ (antisense); myc, 5′-CGCTACGTCCTTCTCCC-3′
(sense) and 5′-CCGCTC CACATACAGTCC-3′ (antisense); β-catenin,
5′-AGCAGTT CGTGGAGGGCGT-3′ (sense) and 5′-CATTCCTGGAGTGG
AGCAACTCT-3′ (antisense). Thermal cycle parameters were: 95°C for 2
min, 35 cycles of 95°C for 30 sec, 52–60°C (depending on the Tm of
each individual set of primers) for 1 min and 72°C for 30 sec.
β-actin, 5′-AGCCATGTACGTA GCCATCC-3′ (sense) and
5′-CTCTCAGCTGTGGTGGT GAA-3′ (antisense) was amplified as an
internal control. The RT-PCR products were separated by 2% agarose
gel electrophoresis, stained with ethidium bromide, and
photographed under UV illumination.
Immunocytochemistry
The isolated cells were cultured in a 4-well plate
at a density of approximately 1×104 cells/well for 24 h.
The cells were then rinsed several times with PBS, fixed with 4%
paraformaldehyde solution for 15 min at room temperature, and
permeated with 0.2% Triton X-100-PBS solution for 5 min. The cells
were incubated with primary antibodies to anti-cytokeratin 15
antibody (CK15), anti-cytokeratin 18 antibody (CK18) (rabbit
polyclonal antibody, 1:100), anti-cytokeratin 19 antibody (CK19)
(rabbit polyclonal antibody, 1:100) and integrin β1 (rabbit
polyclonal antibody, 1:100) (obtained from Boster Biological
Technology, Ltd.) at 4°C overnight, and primary antibody binding
was detected via the corresponding goat anti-rabbit IgG: FITC and
goat anti-rabbit IgG Cy3 (Boster Biological Technology, Ltd.). The
cells were observed under a confocal microscope (Bio-Rad, Hercules,
CA, USA).
Statistical analysis
All data are presented as the mean values ± standard
deviation (SD). Statistical analyses were performed using SPSS11.0
statistics software. Differences between groups were compared using
Student’s t-test. Values of P<0.05 were considered statistically
significant.
Results
Cell morphology and
immunocytochemistry
Murine EpSCs were isolated using rapid substrate
attachment. Collagen type IV was used in this study to isolate
EpSCs because collagen type IV is the ligand of β1 integrin which
is a potent cell marker of EpSCs. The rapidly adherent epidermal
cells after 48 h of culture showed the stem cell characteristics of
immaturity, round shape, small size, few organelles (Fig. 1A). Cells formed different
morphologic colonies, such as bird nest-like (Fig. 1B) or slabstone-like (Fig. 1C). The expression of cytokeratin
15 (CK15), CK19, and integrin β1 was examined by
immunocytochemistry. Results showed that CK15 (Fig. 1D) and CK19 (Fig. 1E) were highly expressed in the
cytoplasm of the isolated cells but integrin β1 was expressed only
in the nucleus of the cells (Fig.
1F). These results showed that these isolated population
represented the EpSCs.
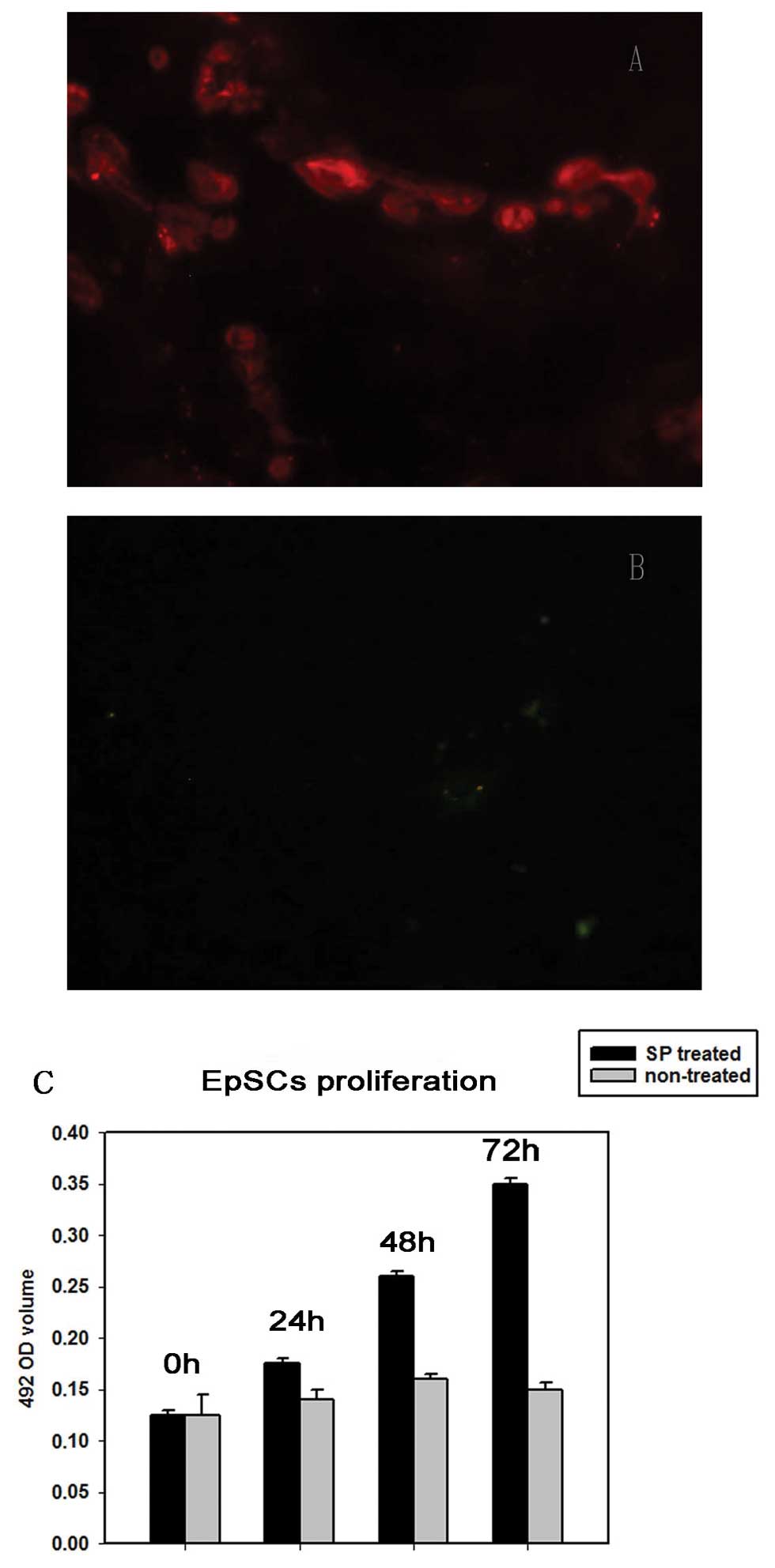
EpSCs proliferation
EpSCs were treated with 10−7 M SP to
induce cell proliferation. The effect of 10−7 M SP on
EpSCs proliferation was measured by the MTT assay. SP of
10−7 M significantly promoted the proliferation of EpSCs
(Fig. 2C) and the EpSCs still
preserved stemness by expressing CK15 (Fig. 2A) and not CK18 (Fig. 2B).
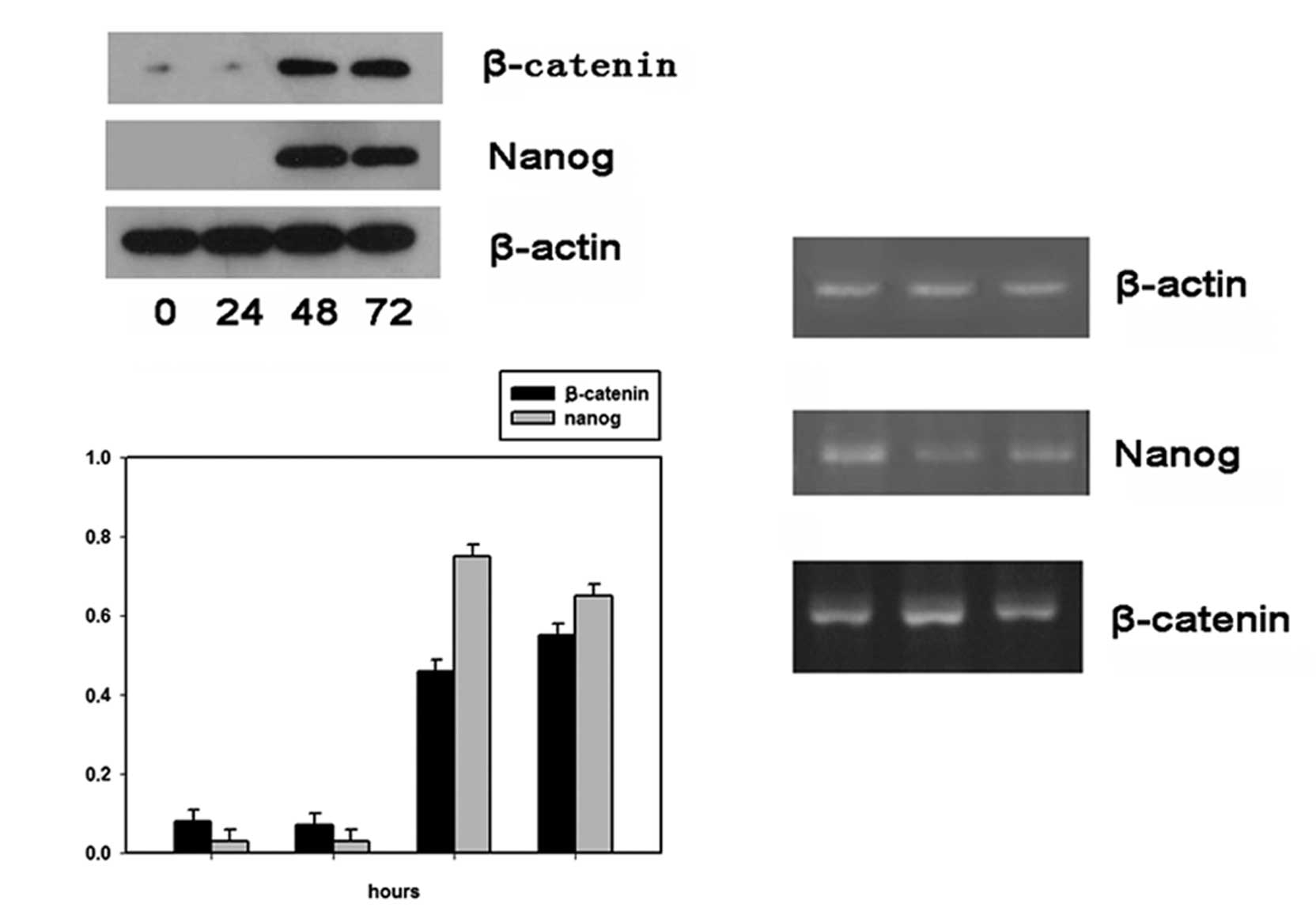
Nanog and β-catenin expression profiles
during the proliferation of EpSCs
At 0, 24, 48 and 72 h after cell culture, EpSCs of
the proliferation group induced by 10−7 M SP were
respectively analyzed for their Nanog and β-catenin expressions
both at the protein and mRNA levels. Results of western blot
analysis showed that protein levels of both Nanog and β-catenin
were low at the initial 24 h, but robustly increased 24 h after
cell culture (Fig. 3A). The PCR
results showed that Nanog mRNA expression levels decreased with
culture time while β-catenin mRNA expression levels remained high
throughout the whole proliferation process (Fig. 3B). Thus the western blotting and
PCR results suggested that both Nanog and β-catenin expression were
not in accordance at the protein and mRNA levels, possibly due to
asymmetric division of EpSCs.
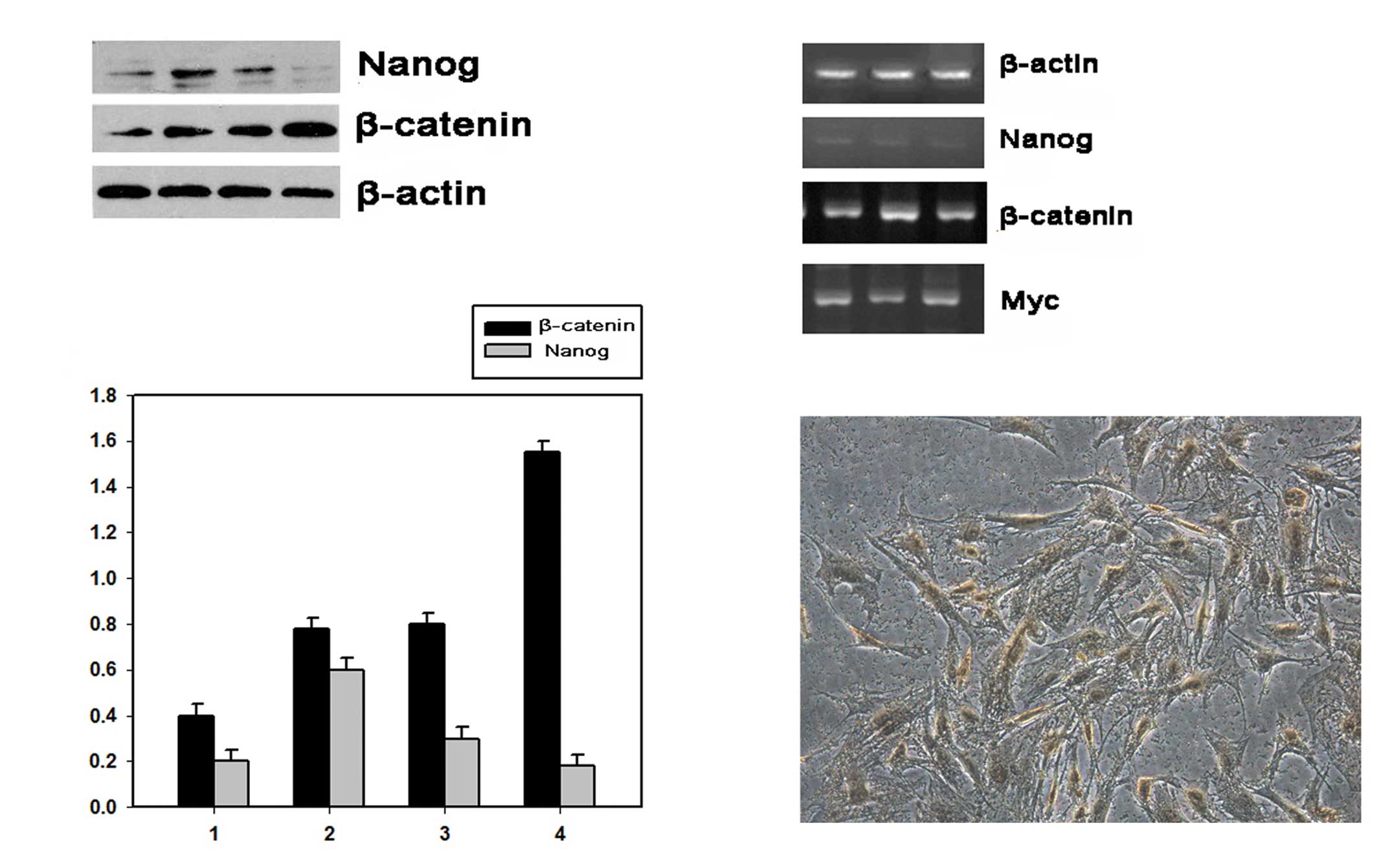
Nanog and β-catenin expression profiles
during the differentiation of EpSCs
EpSCs were isolated and cultured in serum-containing
culture medium DMEM containing 10% FBS to induce cell
differentiation. Differentiated EpSCs positively expressed CK18 as
demonstrated by immunocytochemistry (Fig. 4C). Western blotting results showed
that Nanog expression increased at the initial 48 h but with a
robust decrease 72 h after cell culture, while β-catenin expression
levels increased with culturing time for the whole process
(Fig. 4A). The PCR analysis was
performed 24, 48 and 72 h after cell culture. The Nanog mRNA
expression levels were low for the whole process, while β-catenin
and Myc mRNA expression levels showed a stable increase for the
whole cell culture period (Fig.
4B).
Discussion
The skin tissue is of great importance for human
beings. It protects internal organs from outer environmental
insults, functions in body temperature regulation and participates
in fluid balance (9). The
epidermis is the outer layer of the skin and consists of various
types of cells including stem cells such as EpSCs and TA cells
(10). EpSCs is characterized by
the unlimited self-renewing capacity and relatively low probability
of undergoing terminal differentiation. EpSCs are of great
importance in regulating human skin homeostasis. The balance
between EpSCs proliferation and EpSCs differentiation is precisely
controlled (11). However, the
molecular mechanisms underlying these processes remain unknown.
This study aimed to elucidate the molecular
mechanism that underlies EpSCs proliferation and EpSCs
differentiation from the Nanog and Wnt/β-catenin perspective and
tried to find out potential links between these two signaling
pathways.
A majority of the isolated EpSCs positively
expressed cytokeratin 15 (CK15), CK19 and integrin-β1 which
suggested that cells were perfectly isolated (12–14). Then EpSCs were induced to
proliferate by the addition of 10−7 M SP. MTT results
revealed that 10−7 M SP promoted EpSCs proliferation 24,
48 and 72 h after cell culture. Proliferated EpSCs were then
respectively tested for the CK15 and CK18 expressions by
immunofluorescence. Results demonstrated that EpSCs positively
expressed CK15 but negatively expressed CK18 which suggested that
10−7 M SP specifically promoted EpSCs proliferation but
not differentiation. EpSCs were then cultured in serum-containing
culture medium DMEM containing 10% FBS to induce cell
differentiation. Immunocytochemistry demonstrated that a majority
of EpSCs positively express CK18 which represented cell
differentiation (15). EpSCs that
underwent proliferation and differentiation were tested for Nanog
and β-catenin expression both at the protein and the mRNA
levels.
Nanog is a pluripotent state-specific transcription
factor which plays a critical role in regulating cell fate during
embryonic development, maintaining the pluripotent epiblast and
preventing differentiation. It is also proposed as a transcription
repressor that inhibits genes that are important for cell
differentiation (6).
Wnt signaling is another important signaling, Wnt
ligand binds to cell surface receptors, results in downstream
inactivation of glycogen synthase kinase (GSK) and translocation of
β-catenin to the nucleus (16).
Studies have demonstrated that Wnt/β-catenin signaling activation
results in generation of new hair follicles in adult epidermis
(17). The effect of the Wnt
signaling pathway on cell differentiation is still controversial,
possibly due to the various downstream Wnt signaling pathways
(18). Since β-catenin is the key
effector of the Wnt signaling pathway, we thus hypothesized that
there is a potential link between the Nanog pathway and the Wnt
pathway through β-catenin modulation.
Results of the western blotting demonstrated that
both Nanog and β-catenin were not expressed in the initial phase of
cell proliferation induced by 10−7 M SP but showed a
robust increase 48 and 72 h after cell proliferation. Results of
the PCR analysis demonstrated that the Nanog mRNA expression
decreased with the culture time while the β-catenin mRNA was high
for the whole cell proliferation period. Thus the EpSCs both
expressed Nanog protein and mRNA during the cell culture period,
which indicated the multipotency or pluripotency of EpSCs. Results
also showed that Nanog and β-catenin are positively involved in
regulating EpSCs proliferation.
Results of the western blotting demonstrated that
Nanog protein expression increased 24 and 48 h after cell
differentiation but with a slight decrease 72 h after cell
differentiation, while β-catenin protein expression increased
stably during the differentiation process. Results of the PCR
analysis during the differentiation period demonstrated that Nanog
mRNA expression decreased with culture time while the β-catenin
mRNA expression increased with culture time. It has been proposed
that Myc-induced differentiation acts as a fail-safe device to
prevent uncontrolled epidermal stem cell proliferation (19,20). Myc mRNA expression was also
increased during the cell differentiation process. From the results
we get to know that Nanog may be negatively involved in the
differentiation process of EpSCs, while β-catenin may be positively
involved in the differentiation process of EpSCs.
In summary, our study showed that both the Nanog
signaling pathway and the Wnt/β-catenin signaling pathway are
tightly involved in the proliferation and differentiation process
of EpSCs. These two signaling pathways may be linked to each other
through modulation of β-catenin. However, additional studied are
needed to elucidate the interaction mechanisms between these two
signaling pathways during the proliferation and differentiation
processes of EpSCs.
References
|
1
|
FM WattKB JensenEpidermal stem cell
diversity and quiescenceEMBO Mol
Med1260267200910.1002/emmm.20090003320049729
|
|
2
|
L ChaiC CaoS BiSmall Rho GTPase Rac1
determines human epidermal stem cell fate in vitroInt J Mol
Med25723727201020372815
|
|
3
|
CR JeterB LiuX LiuNANOG promotes cancer
stem cell characteristics and prostate cancer resistance to
androgen
deprivationOncogene3038333845201110.1038/onc.2011.11421499299
|
|
4
|
A LatifiK AbubakerN CastrechiniCisplatin
treatment of primary and metastatic epithelial ovarian carcinomas
generates residual cells with mesenchymal stem cell-like profileJ
Cell Biochem11228502864201110.1002/jcb.23199
|
|
5
|
A ArzumanyanT FriedmanIO NgMM ClaytonZ
LianMA FeitelsonDoes the hepatitis B antigen HBx promote the
appearance of liver cancer stem cells?Cancer
Res7137013708201110.1158/0008-5472.CAN-10-395121464043
|
|
6
|
I ChambersD ColbyM RobertsonFunctional
expression cloning of Nanog, a pluripotency sustaining factor in
embryonic stem
cellsCell113643655200310.1016/S0092-8674(03)00392-112787505
|
|
7
|
RT MoonWnt/beta-catenin pathwaySci
STKE271cm1200515713948
|
|
8
|
FM WattCC LoV Silva-VargasEpidermal stem
cells: an updateCurr Opin Genet
Dev16518524200610.1016/j.gde.2006.08.00616919447
|
|
9
|
C RohS LyleCutaneous stem cells and wound
healingPediatr
Res59R100R103200610.1203/01.pdr.0000203572.51876.ba
|
|
10
|
M KamstrupA FaurschouR GniadeckiHC
WulfEpidermal stem cells - role in normal, wounded and pathological
psoriatic and cancer skinCurr Stem Cell Res
Ther3146150200810.2174/15748880878422308718473880
|
|
11
|
E ClaytonDP DoupeAM KleinDJ WintonBD
SimonsPH JonesA single type of progenitor cell maintains normal
epidermisNature446185189200710.1038/nature0557417330052
|
|
12
|
PH JonesS HarperFM WattStem cell
patterning and fate in human
epidermisCell808393199510.1016/0092-8674(95)90453-07813021
|
|
13
|
A LiPJ SimmonsP KaurIdentification and
isolation of candidate human keratinocyte stem cells based on cell
surface phenotypeProc Natl Acad Sci
USA9539023907199810.1073/pnas.95.7.39029520465
|
|
14
|
FM WattThe stem cell compartment in human
interfollicular epidermisJ Dermatol
Sci28173180200210.1016/S0923-1811(02)00003-811912004
|
|
15
|
M BrzoskaH GeigerS GauerP BaerEpithelial
differentiation of human adipose tissue-derived adult stem
cellsBiochem Biophys Res
Commun330142150200510.1016/j.bbrc.2005.02.14115781243
|
|
16
|
RT MoonB BowermanM BoutrosN PerrimonThe
promise and perils of Wnt signaling through
beta-cateninScience29616441646200210.1126/science.107154912040179
|
|
17
|
E FuchsT TumbarG GuaschSocializing with
the neighbors: stem cells and their
nicheCell116769778200410.1016/S0092-8674(04)00255-715035980
|
|
18
|
EJH vanN BarkerH CleversYou Wnt some, you
lose some: oncogenes in the Wnt signaling pathwayCurr Opin Genet
Dev132833200310.1016/S0959-437X(02)00012-612573432
|
|
19
|
KB JensenFM WattSingle-cell expression
profiling of human epidermal stem and transit-amplifying cells:
Lrig1 is a regulator of stem cell quiescenceProc Natl Acad Sci
USA1031195811963200610.1073/pnas.060188610316877544
|
|
20
|
FM WattM FryeSA BenitahMYC in mammalian
epidermis: how can an oncogene stimulate differentiationNat Rev
Cancer8234242200810.1038/nrc232818292777
|