Introduction
Cellular migration and invasion play major roles in
several pathophysiological processes. Tumor cells are known for
their aggressive behavior during local invasion and distant
metastasis. Furthermore, the destruction of articular structures of
the joint, such as cartilage and bone, by synovial fibroblasts
(SFs) is a crucial event, particularly in rheumatoid arthritis (RA)
(1,2). The ‘tumor-like’ or ‘activated’ SFs
are localized in the hyperplastic synovium of patients with RA.
Supported by adhesion molecules, these rheumatoid arthritis
synovial fibroblasts (RASFs) attach to the cartilage, where matrix
degrading enzymes released by RASFs finally cause the destruction
of the joint.
Repellent factors of the Roundabout (Robo)- and
Slit-family are primarily known to be involved in regulating
cell-cell and cell-matrix interactions of migrating cells during
embryonic development (3) and by
mediating axon guidance through attraction or repulsion of growth
cones (4,5). The Robo/Slit system has also been
described to mediate cell adhesion of fibroblasts (6) and to induce tumor angiogenesis
(7). In previous studies we
analysed the role of these factors in melanoma invasion (8) as well as in processes of RASF
migration (9), and revealed that
Slit3 can potently inhibit cellular migration of both cell types.
Further studies, focusing, for example, on other types of cancer,
confirmed the strong inhibitory effects of Slit molecules on
cellular invasion (10–12).
There are 4 human Robo transmembrane receptors
(Robo1, Robo2, Robo3 and Robo4) that share fibronectin type III and
immunoglobulin-like domains (Ig), but vary in their cytoplasmatic
domains. The ligands for the Robo receptors are the secreted Slit
molecules (Slit1, Slit2 and Slit3) consisting of 4 leucine rich
repeat domains (D1–4), 7–9 epidermal growth factor (EGF)-like
domains, a laminin G domain and a C-terminal cysteine (Cys)-rich
domain (4). Slit binding to Robo
receptors is mediated by the second of the 4 highly conserved
leucine rich-repeat domains of the ligand Slit and the first
extracellular Ig domain of the Robo receptor (13–15). Slit molecules have a molecular
weight of approximately 170 kDa. Since shorter fragments might be
easier and more cost efficient to produce recombinantly, shorter
fragments are preferable for functional analysis and therapeutic
attempts.
In our study, we concentrated on generating smaller
Slit3 fragments, containing the second leucine rich-repeat domain
and testing their functionality. We determined whether the Slit3
fragments still show regulatory effects on migration in our 2 model
systems, RASF and melanoma cells, and whether these fragments could
be used as a pharmaceutical active ingredient to manufacture a
pharmaceutical product for the treatment of pathological processes
involving cellular invasion.
Materials and methods
Cell culture
Synovial tissue samples were obtained during
synovectomy and arthroplastic surgery from patients with RA
following their informed consent and the approval of the local
ethics committee. All RA patients fulfilled the American College of
Rheumatology 1987 criteria for the diagnosis of RA. In RA patients,
material was sampled from wrist or proximal interphalangeal joints
with the joints exhibiting florid synovitis and/or arthritic
destruction. Synovial tissue was minced mechanically, washed
extensively in sterile phosphate-buffered saline (PBS) and digested
with 150 mg/ml Dispase II (Roche Diagnostics GmbH, Mannheim,
Germany) for 1 h at 37°C under continuous agitation. The resulting
cell suspension was seeded into tissue culture dishes and cultured
in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL Life
Technologies, Basel, Switzerland) containing 10% fetal calf serum
and 100 U/ml penicillin per 100 μg/ml streptomycin in a humidified
atmosphere at 37°C followed by the addition of 5% CO2.
In the third passage, adhering fibroblasts were washed, trypsinized
and used for RNA isolation or in the assays.
The melanoma cell lines Mel Im and A375 were used as
previously described (16).
Briefly, cells were maintained in DMEM supplemented with penicillin
(400 U/ml), streptomycin (50 μg/ml), L-glutamine (300 μg/ml) and
10% fetal calf serum (FCS; Sigma, Deisenhofen, Germany) and split
at a 1:5 ratio every 3 days. Cells were detached for subcultivation
or assay with 0.05% trypsin, 0.02% EDTA in PBS.
Recombinant mouse Slit3 (R&D Systems,
Minneapolis, MN, USA) was used in all cell culture experiments at a
concentration of 0.1 μg/μl.
Cloning and expression of Slit3
fragments
Construct D1–4, including the Slit3 LRR domains 1–4,
was amplified using the following primers, 5′-GAC CAT ATG GCC CCT
GCC CCA CCA AGT GTA CC-3′ and 5′-GAC CCC GGG ATT GCA TTT GGC CAC
AAT G-3′; D2, 5′-GAC CAT ATG ATC TCC TGC CCT TCG CCC TGC-3′ and
5′-GAC CCC GGG GAA GCA CTC GCT GCT GAA CC-3′; D2 dNC, 5′-GAC CAT
ATG ATC GTC GAA ATA CGC CTA GAA C-3′ and 5′-GAC CCC GGG TGG GTT TTG
GGC TAA GTG GAG-3′, and D3, 5′-GAC CAT ATG GAC CTC GTG TGC CCC GAG
AAG-3′ and 5′-GAC CCC GGG GCT CAG CTG GCA GCT ACT CTC-3′. D2
dNCmut, in which the remaining Cys was exchanged by an alanine
(Ala) (Cys85Ala), was cloned by site-directed mutagenesis using the
QuikChange® Site-Directed Mutagenesis kit (Stratagene,
Amsterdam, The Netherlands) and the primers, 5′-CCA ACA AGA TCA ACG
CCC TGC GGG TTA ACA CGT TTC AGG-3′ and 5′-CCT GAA ACG TGT TAA CCC
GCA GGG CGT TGA TCT TGT TGG-3′. Fragments were cloned into the
NdeI and SmaI site of the expression vector
pIVEX2.3-MSC and the insert sequences were verified by sequencing.
C-terminal HIS tagged SLIT3 proteins were expressed in an RTS 500
system (Roche Molecular Biochemicals, Mannheim, Germany). In
vitro transcription and translation were performed according to
the manufacturer’s instructions as previously described (17). Protein expression was analyzed by
western blot analysis (SDS page) using different amounts of protein
generated with the RTS 500 expression system.
Protein analysis in vitro (western
blotting)
RTS-generated proteins were loaded and separated on
12% SDS-PAGE and subsequently blotted onto a PVDF membrane. After
blocking for 1 h with 3% BSA/PBS the membrane was incubated for 16
h with a primary antibody against 6xHis (1:2,000; Invitrogen Life
Technologies, Basel, Switzerland). Then the membrane was washed 3
times in PBS, incubated for 1 h with an alkaline phosphate-coupled
secondary sheep anti-mouse antibody (1:4,000; Chemicon, Hampshire,
UK) and then washed again. Finally immunoreactions were visualized
by NBT/BCIP (Sigma) staining.
Cloning and expression of Slit3 fragments
in E. coli
Codon-optimized cDNAs of D2, D2 dNCmut and D3 were
cloned into the NdeI and NotI site of the expression
vector pET22b(+) and the insert sequences were verified by
sequencing. C-terminal HIS tagged Slit3 constructs were expressed
in E. coli. For protein isolation E. coli cells were
treated with lysozyme followed by sonication. Proteins were
purified from the supernatant using immobilized metal affinity
chromatography (Clontech Laboratories, Mountain View, CA, USA)
according to the instructions of the manufacturer. Protein
expression was analyzed by standard western blot analysis using an
anti-His antibody (Qiagen, Hilden, Germany) and quantified by the
Bradford assay.
Migration assay
Migration assays were performed using Boyden
Chambers containing polycarbonate filters with 8 μm pore size
(Costar, Bodenheim, Germany), as previously described (16). Briefly, filters were coated with
gelatin. The lower compartment was filled with
fibroblast-conditioned medium, used as a chemo-attractant. Synovial
fibroblast or melanoma cells, respectively, were harvested with
trypsin incubation for 2 min, resuspended in DMEM without FCS at a
density of 3×104 cells/ml and injected in the upper
compartment of the chamber on the filter. Following incubation at
37°C for 4 h, all cells attached to the upper surface of the
membrane were removed. Cells adhering to the lower surface were
fixed, stained and counted. Recombinant proteins and peptides were
added to the upper chamber of the system. All experiments were
repeated at least 3 times.
Statistical analysis
Calculations were performed using the GraphPad Prism
software (GraphPad Software, Inc., San Diego, CA, USA). All results
are expressed as mean ± SD (range) or in %. Error bars represent
standard deviation. Comparison between groups was made using the
Student’s paired t-test (two-tailed).
Results
Recombinant expression of Slit3
fragments
Slit treatment revealed a strong negative effect on
the migration and cartilage destruction of active RASFs but not on
the SFs of healthy donors (9), we
therefore speculated about its therapeutic application. Full-length
Slit3 would require higher therapeutic doses and might be too
expensive for therapy due to their large size. Several publications
have previously shown that the second leucine-rich-repeat (LRR2,
D2) of Slit2 is necessary for the binding to the Robo receptor
(14). In addition, this region
is not glycosylated enabling recombinant expression in E.
coli. To determine whether D2 of Slit3 is sufficient to mediate
the inhibition on the migration and destruction by RASF cells and
to analyse whether the the N- and C-terminal region of the D2
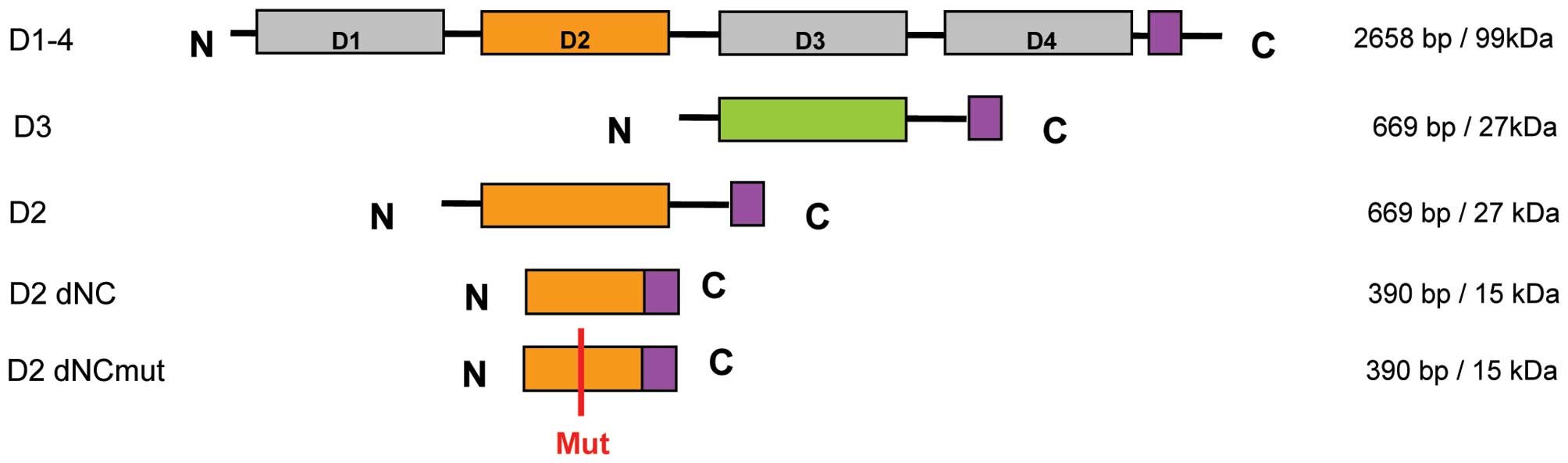
domain might be necessary for function and structure, fragments
were generated (Fig. 1). We
expressed all fragments recombinantly in a
transcription/translation system (RTS) and quantified protein
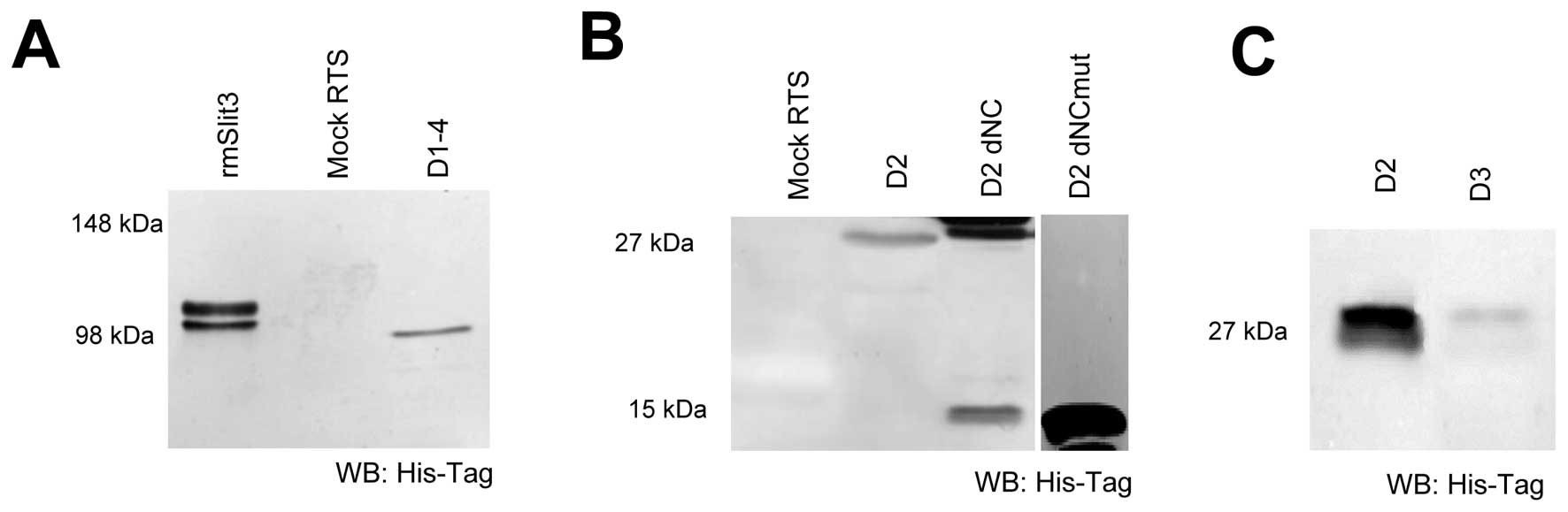
amount and protein quality by western blot analysis (Fig. 2). The D2 domain of Slit3 resulted
in a protein of 26 kDa (Fig. 2).
The shorter, N- and C-terminally truncated form of D2 (D2 dNC; 15
kDa) showed, in addition to the expected 15 kDa band, a 30 kDa
product as well, possibly occurring by dimerisation of 2 D2 dNC
molecules via a free Cys at position 393. To inhibit this
dimerization we mutated the free cysteine (D2 dNCmut) and revealed
expression of a 15 kDa construct not able to dimerize (Fig. 2B). This variant was used in
further studies instead of D2 dNC. As a control we further
expressed the Slit3 D3 domain as this domain is not expected to
bind to Robo receptors (Fig.
2C).
Slit3 D2 and D2 dNC inhibit cellular
migration of melanoma cells and RASFs
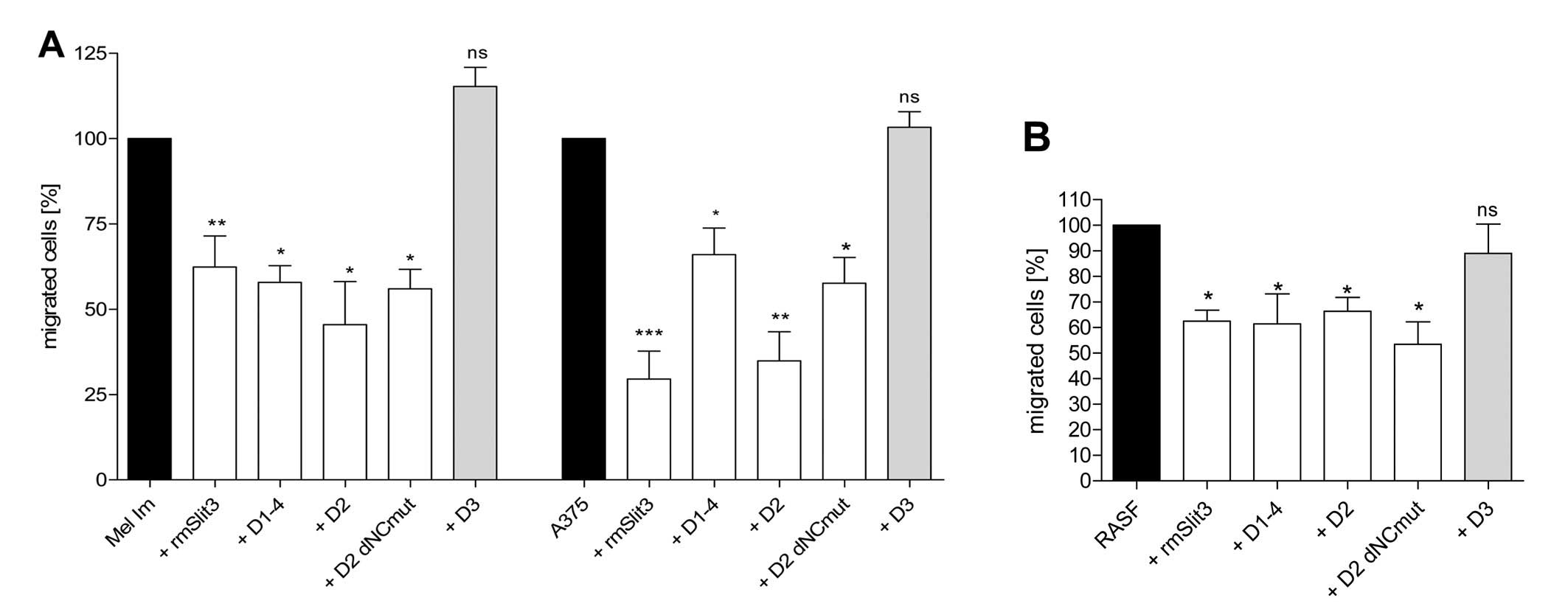
D1–4, D2, D3 and D2 dNCmut were tested in Boyden
Chamber Migration Assays on 2 melanoma cell lines (Fig. 3A) and RASFs (Fig. 3B) in comparison to recombinant
mouse (rm)Slit3. D1–4, D2 and D2 dNCmut were able to reduce
migration of the cells compared to control. Markedly, the D2 dNCmut
(Cys85Ala variant) protein which lacks the N- and C-terminal
stabilizing part of the SLIT3 protein sequence inhibits the
migration of SFs and melanoma cells indicating that only the
minimal binding domain of the protein comprising part of the
secondary structure is sufficient for the inhibitory effect. Both,
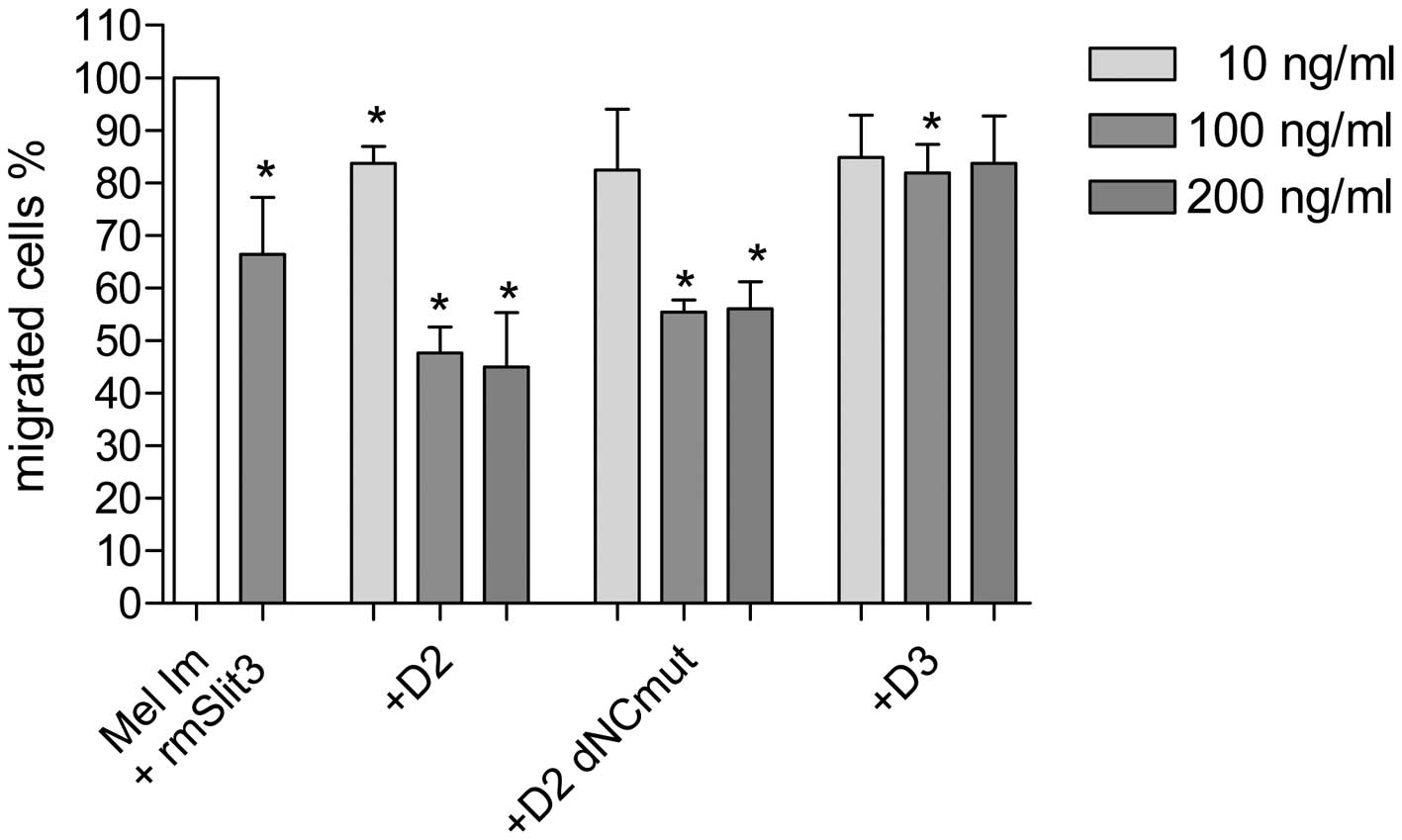
D2 and D2 dNCmut, revealed maximal activity at 100 ng/ml (Fig. 4).
Discussion
Slits are known to convey repulsive signals via Robo
receptors during development and possibly in several pathological
conditions. Previous studies demonstrated that Slit can inhibit
migration of neuronal cells, leucocytes and glioma cells (1,7,10).
We recently showed that Slit3 has a strong inhibitory impact on the
migration of aggressive SFs of patients suffering from RA and on
melanoma cells in vitro (8,9).
The modulation of Slit activity might therefore be a novel
therapeutic principle to prevent aggressive cells from destructing
healthy tissue in completely different groups of diseases such as
rheumatic diseases or cancer. The current RA therapy is mainly
based on anti-inflammatory agents such as glucocorticoids,
non-steroidal anti-rheumatic drugs (NSAIDs), disease-modifying
anti-rheumatic drugs (DMARDs) and in more recent approaches on
modulating the immune system (18). Malignant melanoma is usually
treated by surgical excision complemented by additional therapies
such as chemotherapy, radiation therapy or immunotherapy depending
on tumor stage (19,20). Chemotherapy, immunotherapy or
anti-inflammatory drugs in the treatment of RA or malignant
melanoma can frequently have adverse effects ranging from mild to
more serious conditions. The inhibition of migration of aggressive
synovial cells or melanoma cells using Slit3 or fragments of Slit3
might be a less damaging approach to treat RA or malignant
melanomas. Since cell proliferation of RASF cells was weakly
suppressed by Slit3 (9) mainly
migrating cells should theoretically be affected by therapeutic
intervention. Cell migration of constituting cells in target
tissues such as the dermal cells of the skin or different cell
types in joint tissue is not of major importance for tissue
homeostasis. Possible mechanisms for the induction of side-effects
may therefore be limited.
Full-length Slit or Slit fragments containing
leucin-rich repeat domains D1–4 (7) may not be appropriate for therapeutic
use. Due to their large size they are difficult to express
recombinantly, and may be unstable and also difficult to apply
in vivo. Since several publications demonstrated the smaller
second domain of Slit2 D2 to be the domain necessary for Robo
binding (21,22), we analyzed the structure of this
domain closely and hypothesized that the N- and C-terminal region
may not be necessary for Robo binding. Several Slit3 fragments of
the D2 domain were designed and recombinantly expressed including
truncated and mutated fragments (Fig.
1). We analyzed the effects of these fragments on SFs of RA
patients and malignant melanoma cells in vitro in comparison
to rmSlit3.
Using Boyden Chamber migration assays, we showed
that the second domain of Slit3 (D2) is sufficient to exhibit
inhibitory activity towards RASF and melanoma cell migration as
effectively as recombinant Slit3 containing all 4 leucine rich
repeats (Fig. 3). Also, the
truncated form of D2 (D2 dNC) which lacks the N- and C-terminal
part of the domain as well as its mutated variant is adequate to
inhibit migration of RASFs and melanoma cells (Fig. 3). At doses of 100 ng/ml both
fragments D2 and D2 dNCmut were able to reduce migration of
melanoma cells to approximately 50% in the Boyden Chamber (Fig. 4). This means that Slit3 fragments
are sufficient to exert the inhibitory effect of Slit3 via the
Robo-receptor. Local application of Slit3 fragments into the joint
cavity of patients suffering from RA may therefore be a feasible
way to prevent joint destruction by aggressive SFs. Fortunately,
the D2 domain is not glycosylated enabling recombinant expression
in E. coli.
Taking into account the in vitro data
reported here, peptides derived from Slit3 may be a powerful
therapeutic tool to decelerate or even prevent tissue destruction
by aggressive cells in several pathological conditions.
Abbreviations:
|
SF
|
synovial fibroblast
|
|
RA
|
rheumatoid arthritis
|
Acknowledgements
This study was supported by the
Wilhelm Sander Foundation and the German Research Foundation (DFG),
P.B. and E.H. are employees of the Scil Technology GmbH. A.B. and
T.S. have a cooperation agreement with the Scil Technology
GmbH.
References
|
1.
|
CB GuanHT XuM JinXB YuanMM PooLong-range
Ca2+ signaling from growth cone to soma mediates
reversal of neuronal migration induced by
slit-2Cell1293853952007
|
|
2.
|
T YasudaCartilage destruction by matrix
degradation productsMod
Rheumatol16197205200610.3109/s10165-006-0490-616906368
|
|
3.
|
R AltenE Gromnica-IhleC PohlJ EmmerichJ
SteffgenR RoscherR SigmundB SchmolkeG SteinmannInhibition of
leukotriene B4-induced CD11B/CD18 (Mac-1) expression by BIIL 284, a
new long acting LTB4 receptor antagonist, in patients with
rheumatoid arthritisAnn Rheum
Dis63170176200410.1136/ard.2002.00449914722206
|
|
4.
|
BJ DicksonGF GilestroRegulation of
commissural axon pathfinding by slit and its Robo receptorsAnnu Rev
Cell Dev
Biol22651675200610.1146/annurev.cellbio.21.090704.15123417029581
|
|
5.
|
JH ChenL WenS DupuisJY WuY RaoThe
N-terminal leucine-rich regions in Slit are sufficient to repel
olfactory bulb axons and subventricular zone neuronsJ
Neurosci2115481556200111222645
|
|
6.
|
S KrasnokutskyJ SamuelsSB
AbramsonOsteoarthritis in 2007Bull NYU Hosp Jt Dis652222282007
|
|
7.
|
JY WuL FengHT ParkN HavliogluL WenH TangKB
BaconZ JiangX ZhangY RaoThe neuronal repellent Slit inhibits
leukocyte chemotaxis induced by chemotactic
factorsNature410948952200110.1038/3507361611309622
|
|
8.
|
AE DenkS BraigT SchubertAK BosserhoffSlit3
inhibits activator protein 1-mediated migration of malignant
melanoma cellsInt J Mol Med28721726201121743955
|
|
9.
|
AE DenkS KaufmannK StarkJ SchedelT LowinT
SchubertAK BosserhoffSlit3 inhibits Robo3-induced invasion of
synovial fibroblasts in rheumatoid arthritisArthritis Res
Ther12R45201010.1186/ar295520298552
|
|
10.
|
S MertschN SchmitzA JeibmannJG GengW
PaulusV SennerSlit2 involvement in glioma cell migration is
mediated by Robo1 receptorJ
Neurooncol8717200810.1007/s11060-007-9484-217968499
|
|
11.
|
MC StellaL TrusolinoPM ComoglioThe
Slit/Robo system suppresses hepatocyte growth factor-dependent
invasion and morphogenesisMol Biol
Cell20642657200910.1091/mbc.E08-03-032119005219
|
|
12.
|
HK KimH ZhangH LiTT WuS SwisherD HeL WuJ
XuCA ElmetsM AtharSlit2 inhibits growth and metastasis of
fibrosarcoma and squamous cell
carcinomaNeoplasia1014111420200819048120
|
|
13.
|
E HohenesterS HussainJA HowittInteraction
of the guidance molecule Slit with cellular receptorsBiochem Soc
Trans34418421200610.1042/BST034041816709176
|
|
14.
|
C MorlotNM ThielensRB RavelliW HemrikaRA
RomijnP GrosS CusackAA McCarthyStructural insights into the
Slit-Robo complexProc Natl Acad Sci
USA1041492314928200710.1073/pnas.070531010417848514
|
|
15.
|
U Muller-LadnerC OspeltS GayO DistlerT
PapCells of the synovium in rheumatoid arthritisSynovial
fibroblasts Arthritis Res Ther9223200710.1186/ar2337
|
|
16.
|
AE DenkM BettstetterPJ WildK HoekF
BatailleW DietmaierAK BosserhoffLoss of maspin expression
contributes to a more invasive potential in malignant
melanomaPigment Cell
Res20112119200710.1111/j.1600-0749.2007.00363.x17371437
|
|
17.
|
R StollS LodermeyerAK BosserhoffDetailed
analysis of MIA protein by mutagenesisBiol
Chem38716011606200610.1515/BC.2006.19917132106
|
|
18.
|
V MajithiaSA GeraciRheumatoid arthritis:
diagnosis and managementAm J
Med120936939200710.1016/j.amjmed.2007.04.005
|
|
19.
|
R MouawadM SebertJ MichelsJ BlochJP SpanoD
KhayatTreatment for metastatic malignant melanoma: old drugs and
new strategiesCrit Rev Oncol
Hematol742739201010.1016/j.critrevonc.2009.08.00519781957
|
|
20.
|
E AtallahL FlahertyTreatment of metastatic
malignant melanomaCurr Treat Options
Oncol6185193200510.1007/s11864-005-0002-5
|
|
21.
|
S RajagopalanE NicolasV VivancosJ BergerBJ
DicksonCrossing the midline: roles and regulation of Robo
receptorsNeuron28767777200010.1016/S0896-6273(00)00152-511163265
|
|
22.
|
KT Nguyen Ba-CharvetK BroseV MarillatT
KiddCS GoodmanM Tessier-LavigneC SoteloA ChedotalSlit2-Mediated
chemorepulsion and collapse of developing forebrain
axonsNeuron22463473199910197527
|