Introduction
Microglia are important cells that are involved in
initial responses to tissue damage in the central nervous system
(CNS). However, abnormally overactivated microglia promote the
expression of pro-inflammatory mediators, such as nitric oxide (NO)
and prostaglandin E2 (PGE2), as well as that
of pro-inflammatory cytokines, including interleukin-1β (IL-1β),
tumor necrosis factor-α (TNF-α) and monocyte chemoattractant
protein-1 and other factors that contribute to the development of
chronic inflammatory diseases (1–3).
In particular, microglia are activated by lipopolysaccharides
(LPS), β-amyloid, thrombin, or interferon-γ, and the secretion of
inflammatory molecules by abnormally activated microglia disturbs
the homeostasis of the immune system, thus inducing and promoting
degenerative CNS autoimmune diseases, such as multiple sclerosis,
Alzheimer’s disease and Parkinsonism (4–6).
Therefore, understanding congenital immune system disorders related
to the overactivation of microglia and controlling the inflammation
molecules secreted by abnormally activated microglia is an approach
to delaying chronic inflammatory diseases.
The transcription factor, nuclear factor-κB (NF-κB),
plays a central role in the regulation of several genes responsible
for the generation of pro-inflammatory mediators and cytokines. In
normal cells, NF-κB subunits are present in the cytosol bound to
the inhibitory protein IκB (IκB), which inactivates them (7,8).
However, in response to various stimuli, such as LPS, IκB is
rapidly degraded by the ubiquitin-proteasome pathway. The
degradation of IκB induces the translocation of NF-κB subunits into
the nucleus, and the NF-κB subunits bind to the promoter regions of
target genes, including inducible NO synthase (iNOS),
cyclooxygenase-2 (COX-2), TNF-α and IL-1β, and stimulate their
transcription. The activation of the phosphoinositide 3-kinase
(PI3K)/Akt signaling pathway plays an important role in regulating
LPS-induced pro-inflammatory responses by inducing NF-κB activation
through proteasome-dependent IκB degradation (9–14).
Therefore, inhibiting NF-κB activation through the PI3K/Akt pathway
results in anti-inflammatory effects.
Cnidium officinale (C. officinale)
Makino, which belongs to the Umbelliferae family, is a perennial
herb native to China. The dried rhizomes of C. officinale
have been used as one of the most commonly prescribed traditional
Oriental medicinal herbs in East Asian countries. In Korean
traditional medicine, they are widely used in the treatment of
menstrual disturbances and as a blood pressure depressant, as well
as for relieving pain from headaches and rheumatic arthralgia
(15–20). Although some pharmacological
beneficial effects of this herb and extracts of its rhizome have
recently been reported, including anticancer, anti-inflammatory and
antioxidant effects (21–24), its molecular mechanisms of action
have not yet been fully elucidated. Therefore, the present study
was conducted to evaluate the effects of an ethanol extract of
C. officinale rhizomes (EECO) on the production of
pro-inflammatory mediators and cytokines and the respective
regulatory genes with a focus on the underlying molecular
mechanisms in LPS-stimulated BV2 microglial cells.
Materials and methods
Reagents, chemicals and preparation of
EECO
LPS and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies
against COX-2, iNOS, TNF-α, IL-1β, NF-κB p65 and IκB-α were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Antibodies against phosphorylated PI3K (p-PI3K), PI3K,
phosphorylated Akt (p-Akt) and Akt were obtained from Cell
Signaling Technology (Beverly, MA, USA). Antibodies against
nucleolin and actin were obtained from Sigma-Aldrich.
Peroxidase-labeled goat anti-rabbit immunoglobulin was purchased
from Koma Biotechnology (Seoul, Korea). Other chemicals were
purchased from Sigma-Aldrich. To prepare EECO, the rhizomes of
C. officinale, which were obtained from Dongeui University
Oriental Hospital (Busan, Korea), were pulverized and extracted
twice with 10 volumes of 80% ethanol at 85–90°C in a reflux
condenser for 3 h, and then filtered with a 50 μm filter and
concentrated by vacuum evaporation at 60°C. The solid form of the
extract was dissolved in dimethyl sulfoxide.
Cell culture and viability assay
BV2 microglial cells were cultured at 37°C in 5%
CO2 in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 5% fetal bovine serum and antibiotics (WelGENE
Inc., Daegu, Korea). In all the experiments, the cells were
pre-treated with the indicated concentrations of EECO for 1 h prior
to the addition of LPS (500 ng/ml) in serum-free DMEM. Cell
viability was measured based on the formation of blue formazan that
was metabolized from colorless MTT by mitochondrial dehydrogenases,
which are active only in live cells. In brief, the BV2 cells were
seeded and treated with reagents for the indicated periods of time.
Following treatment, the medium was removed, and the cells were
incubated with 0.5 mg/ml of MTT solution for 2 h at 37°C and 5%
CO2, and then the supernatant was removed and the
formation of formazan was measured at 540 nm using a microplate
reader (Dynatech MR-7000; Dynatech Laboratories, Chantilly, VA,
USA).
Measurement of NO
The concentration of NO generated by BV2 cells
activated by LPS was detected using Griess reagent [1%
sulfanilamide in 5% phosphoric acid and 0.1%
N-(1-naphthyl)ethylenediamine dihydrochloride]. BV2 cells were
cultured for 24 h in a 6-well culture plate, pre-treated with
various concentrations of EECO for 1 h, and then treated again with
LPS (500 ng/ml). After 24 h of culture, the cell culture medium was
collected and the same quantity of Griess reagent was added to
induce a reaction at room temperature. The optical density of the
reaction solution was measured at 540 nm using a microplate reader
and the quantity of NO generated by the cells was calculated based
on the concentration of the sodium nitrite (NaNO2)
standard solution (standard curve).
Measurement of PGE2
To measure the quantity of PGE2 generated
by BV2 cells, medium from the cultures under the same conditions
was collected and the quantity of PGE2 generated was
measured using a PGE2 enzyme-linked immunosorbent assay
(ELISA) kit (Cayman Chemical Co., Ann Arbor, MI, USA). The
concentration (pg/ml) of PGE2 in the cell culture medium
was calculated based on the concentrations of the standard solution
as previously described (25).
Measurement of cytokines
The levels of IL-1β and TNF-α were measured using
ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the
manufacturer’s instructions. Briefly, BV2 cells were loaded in
24-well plates and pre-treated with the indicated EECO
concentrations for 1 h prior to stimulation with 500 ng/ml LPS for
24 h. A total of 100 μl of culture medium supernatant was collected
to determine IL-1β and TNF-α concentration by ELISA.
Isolation of total RNA and reverse
transcription-polymerase chain reaction (RT-PCR)
RT-PCR was conducted to examine the effects of EECO
on the expression of LPS-induced iNOS, COX-2, and inflammatory
cytokines at the transcription level. Total RNA was separated from
the BV2 cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer’s instructions and
reverse-transcribed using MMLV reverse transcriptase (Promega,
Madison, WI, USA) to produce cDNA. The cDNA was amplified by PCR
using specific primers: iNOS forward, 5′-CCT CCT CCA CCC TAC CAA
GT-3′ and reverse, 5′-CAC CCA AAG TGC TTC AGT CA-3′; COX-2 forward,
5′-AAG ACT TGC CAG GCT GAA CT-3′ and reverse, 5′-CTT CTG CAG TCC
AGG TTC AA-3′; IL-1β forward, 5′-ATG GCA ACT GTT CCT GAA CTC AAC
T-3′ and reverse, 5′-TTT CCT TTC TTA GAT ATG GAC AGG AC-3′; TNF-α
forward, 5′-GCG ACG TGG AAC TGG CAG AA-3′ and reverse, 5′-TCC ATG
CCG TTG GCC AGG AG-3′; and GAPDH forward, 5′-ACC ACA GTC CAT GCC
ATC AC-3′ and reverse, 5′-TCC ACC ACC CTG TTG CTG TA-3′. The
following PCR conditions were applied: iNOS, COX-2, IL-1β and
TNF-α: 25 cycles of denaturation at 94°C for 30 sec, annealing at
59°C for 30 sec, and extension at 72°C for 30 sec; GAPDH, 23 cycles
of denaturation at 94°C for 30 sec, annealing at 57°C for 30 sec
and extension at 72°C for 30 sec. The PCR products were
electrophoresed on 1.5% agarose gels and stained with ethidium
bromide. GAPDH was used as an internal control to evaluate relative
expression.
Protein extraction and western blot
analysis
The cells were washed three times with
phosphate-buffered saline (PBS) and lysed in lysis buffer [1%
Triton X-100, 1% deoxycholate, 0.1% sodium azide (NaN3)]
containing protease inhibitor cocktail tablets to isolate total
protein (Roche Diagnostics GmbH, Mannheim, Germany). In a parallel
experiment, cytoplasmic and nuclear extracts were prepared using
NE-PER nuclear and cytosolic extraction reagents (Pierce, Rockford,
IL, USA) according to the manufacturer’s instructions. The protein
concentrations were determined using a Bio-Rad protein assay kit
(Bio-Rad, Hercules, CA, USA). Equal amounts of protein were
separated on SDS-polyacrylamide gels and transferred onto
nitrocellulose membranes (Schleicher & Schuell, Inc., Keene,
NH, USA) by electroblotting. Proteins were detected using an
enhanced chemiluminescence detection system (Pierce).
Immunofluorescence
The prepared cells were washed twice with PBS and
fixed for 15 min at 4°C using 4% paraformaldehyde. The cells were
washed again with PBS, reactions were induced for 20 min at 4°C in
PBS that contained 0.3% Triton X-100, and then reactions were
induced for 1 h at room temperature in PBS containing 2% bovine
serum albumin (BSA) to suppress non-specific reactions. The
anti-NF-κB p65 antibody was then diluted to 1:200 in a PBS solution
that contained 2% BSA to induce reactions for 2 h at room
temperature. The cells were washed three times in PBS and
fluorescein isothiocyanate (FITC)-conjugated IgG (Molecular Probes,
Eugene, OR, USA), which is a secondary antibody, was diluted to
1:100 to induce reactions for 1 h at room temperature. Samples of
the immunofluorescence-stained cells were observed under a confocal
laser scanning microscope (Olympus, Tokyo, Japan). A wavelength of
488 nm was used for FITC, and the images were reassembled into
final three-dimensional images according to the manufacturer’s
instructions (Olympus Fluoview 300, Olympus).
Statistical analysis
All results are expressed as the means ± standard
errors. Each experiment was repeated at least three times.
Statistical significances were identified between each treated
group by the paired Student’s t-test. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
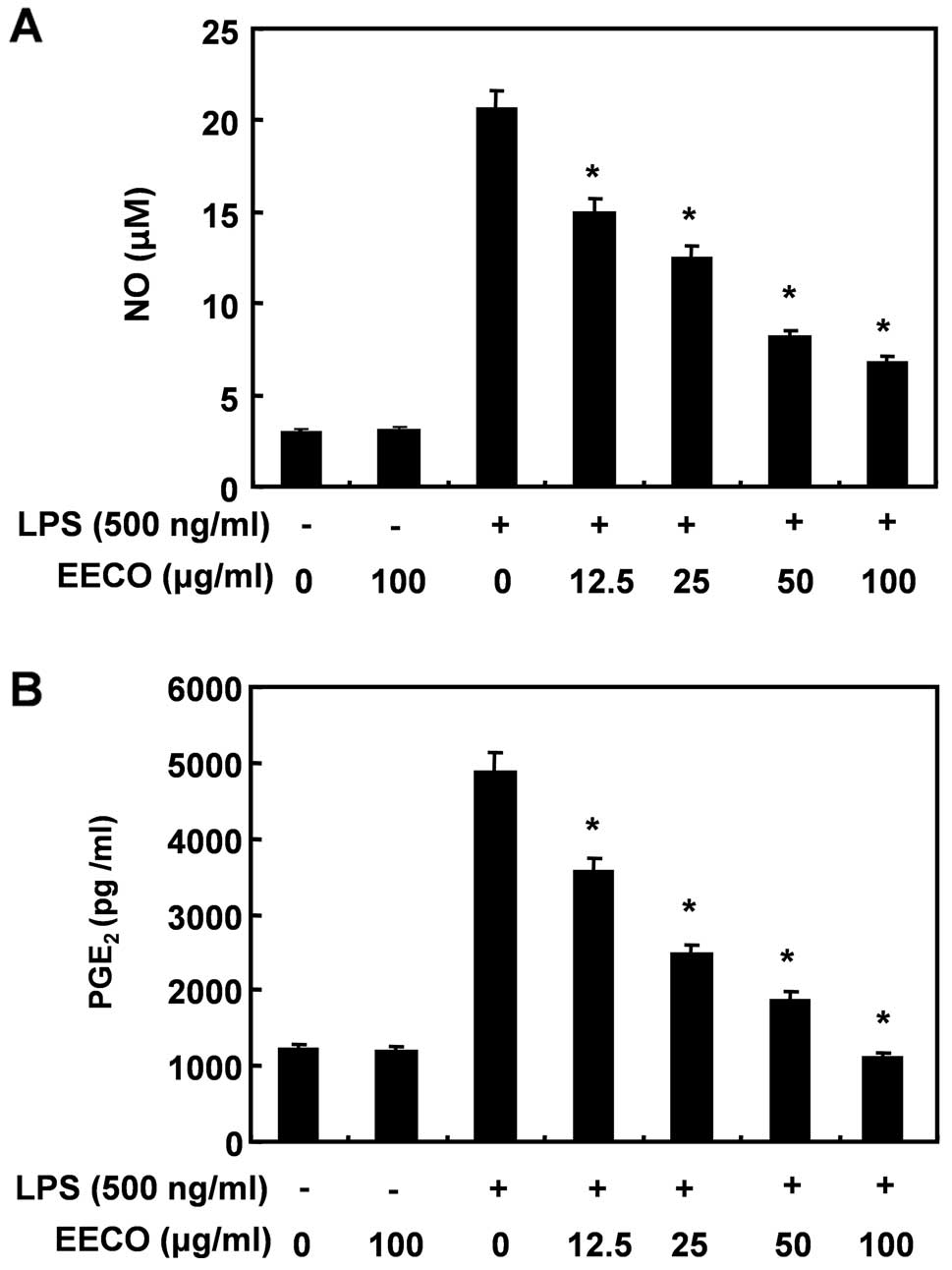
Inhibition of LPS-induced NO and
PGE2 production by EECO
To determine the inhibitory effects of EECO on
LPS-induced NO and PGE2 production, BV2 macroglial cells
were incubated with the indicated concentrations of EECO in the
presence or absence of LPS for 24 h, and the levels of NO and
PGE2 production were measured in the culture medium
using Griess reagent and ELISA, respectively. LPS triggered an
approximate 7-fold increase in NO production compared with that in
the untreated control group; however, pre-treatment with EECO
reduced LPS-induced NO production in a concentration-dependent
manner (Fig. 1A). The amount of
PGE2 present in the culture medium also increased after
24 h of exposure to LPS alone; however, a concentration-dependent
decrease was observed following pre-treatment with EECO (Fig. 1B).
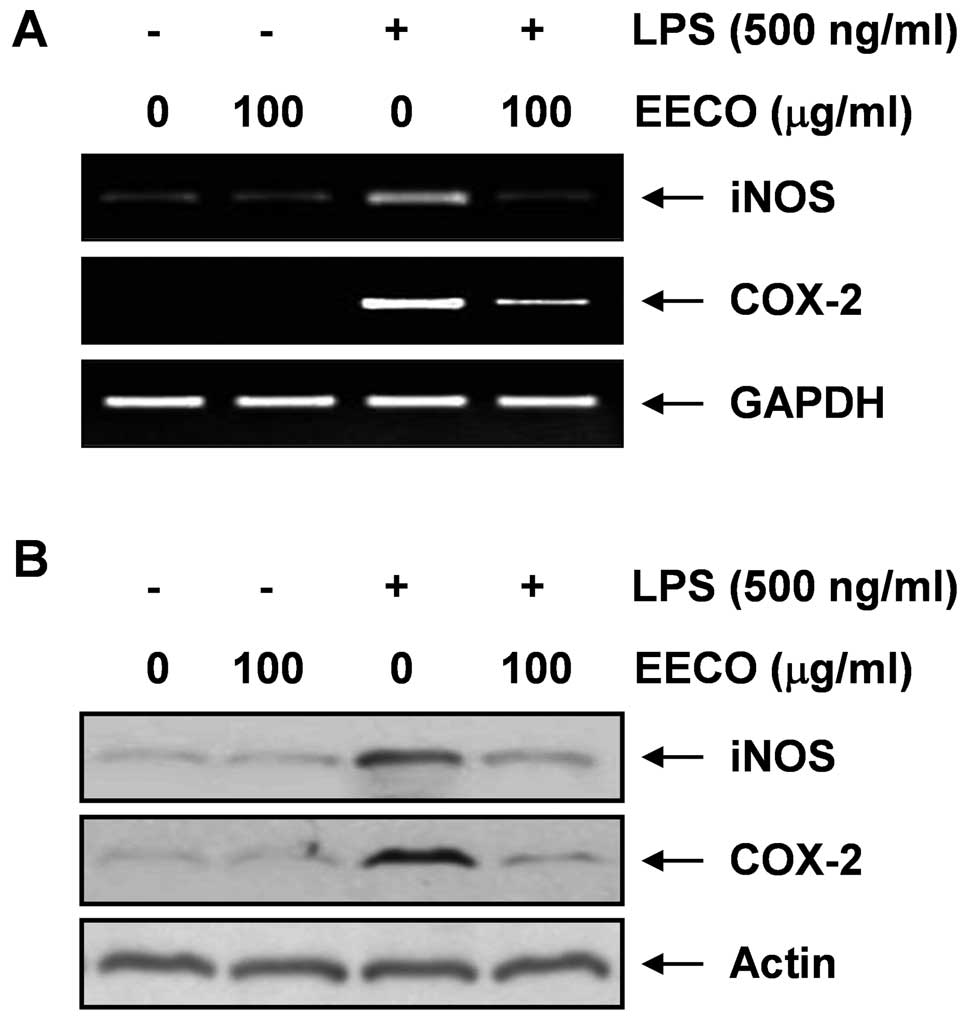
Inhibition of LPS-induced NO and
PGE2 expression by EECO
We then investigated whether the inhibitory effects
of EECO on NO and PGE2 production are associated with
decreased levels of iNOS and COX-2 expression, which are known to
induce NO and PGE2 production, using western blot
analysis and RT-PCR. The mRNA levels of iNOS and COX-2 were
markedly augmented in the presence of LPS alone; however, their
expression levels were markedly upregulated in the presence of EECO
(Fig. 2A). Western blot analyses
also revealed that treatment with LPS increased iNOS and COX-2
protein expression, whereas pre-treatment of the cells with EECO
attenuated the LPS-induced iNOS and COX-2 protein expression
(Fig. 3B). These results indicate
that EECO inhibits the LPS-induced release of NO and
PGE2 by suppressing iNOS and COX-2 expression at the
transcriptional level.
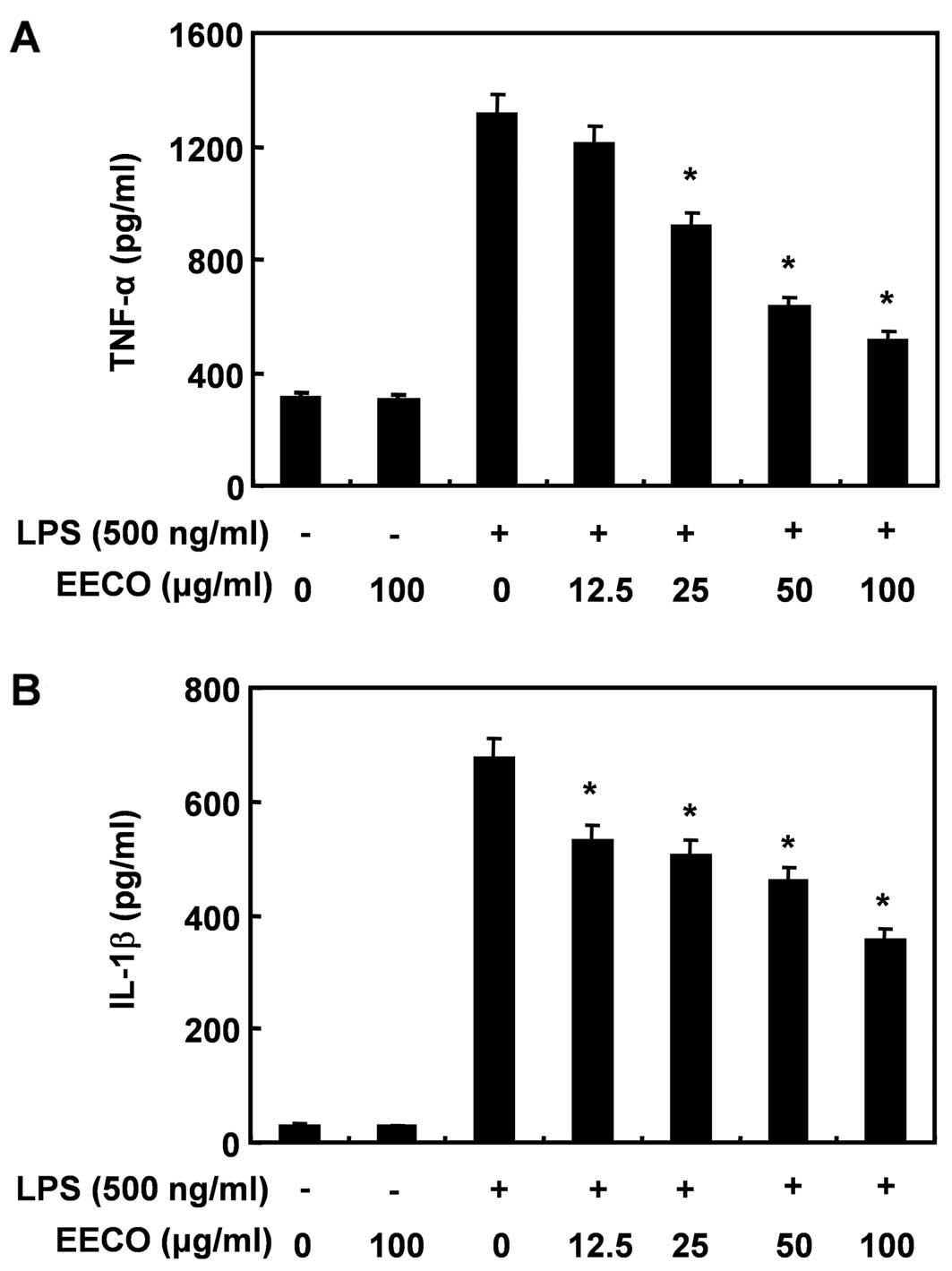
Inhibition of LPS-induced TNF-α and IL-1β
generation by EECO
We then determined the potential effects of EECO on
the production of pro-inflammatory cytokines, such as TNF-α and
IL-1β by ELISA. The levels of TNF-α significantly increased in the
culture medium of LPS-stimulated BV2 cells; however, the levels
decreased significantly in a dose-dependent manner following
pre-treatment with EECO (Fig.
3A). IL-1β production increased following stimulation with LPS
and EECO significantly decreased the levels of IL-1β in the
supernatant of LPS-stimulated BV2 cells (Fig. 3B).
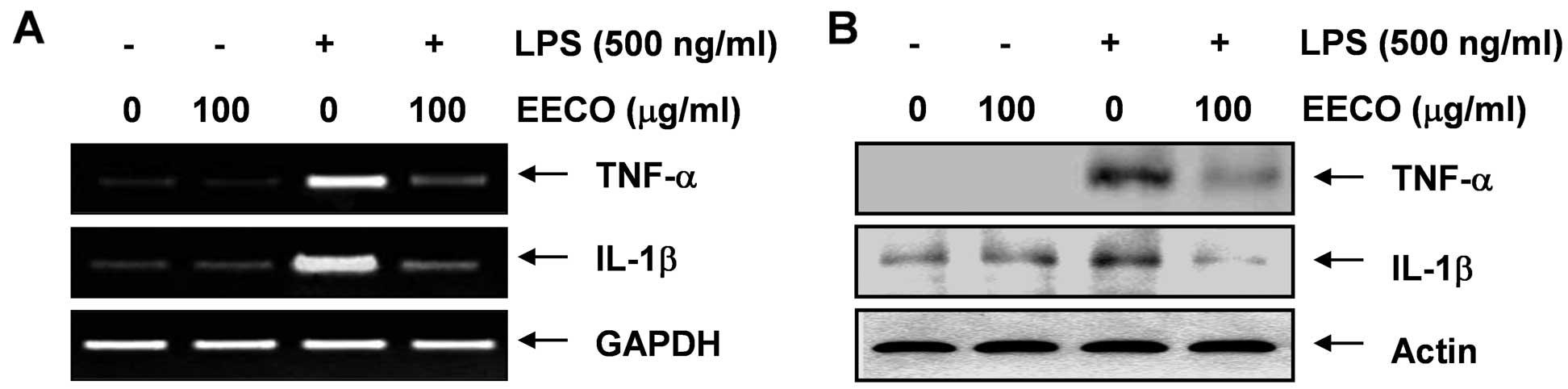
Inhibition of LPS-induced TNF-α and IL-1β
expression by EECO
RT-PCR and western blot analysis were performed in
parallel experiments to determine whether EECO inhibits TNF-α and
IL-1β expression. The increased expression of TNF-α and IL-1β
following treatment with LPS was markedly attenuated by
pre-treatment with EECO at both the transcriptional and
translational levels (Fig. 4).
These results indicate that EECO is effective in suppressing
pro-inflammatory cytokine production by altering the
transcriptional levels of TNF-α and IL-1β in LPS-activated
microglia.
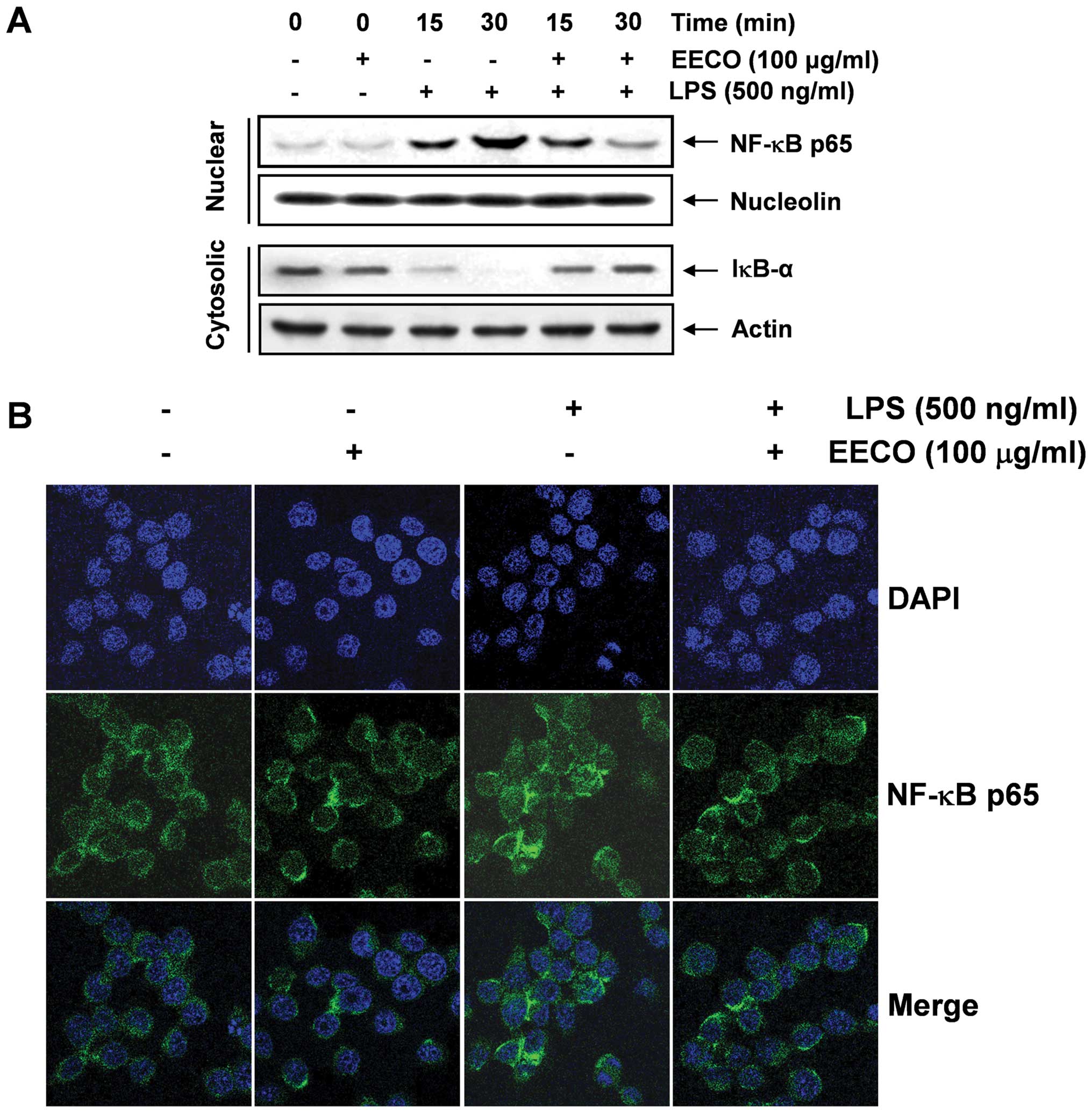
Inhibition of LPS-induced NF-κB
translocation by EECO
As NF-κB is a central transcription factor that
regulates the expression of a large number of inflammation-related
genes (7,26), the effects of EECO on the
LPS-stimulated nuclear translocation of NF-κB p65 subunits were
examined. The western blot analysis results in Fig. 5A indicate that the levels of NF-κB
p65 in the nucleus markedly increased within 15 min of exposure to
LPS; however, the LPS-induced p65 levels in the nuclear fraction
decreased following pre-treatment with EECO. In addition, IκB-α was
markedly degraded 15 min following exposure to LPS; however, the
LPS-induced IκB-α degradation was significantly reversed by EECO.
We also investigated whether EECO interferes with the translocation
of NF-κB in LPS-treated BV2 cells by immunofluorescence. The level
of NF-κB p65 in the nucleus decreased significantly by EECO,
indicating that EECO inhibits NF-κB activation in BV2 microglial
cells by suppressing IκB degradation and the nuclear translocation
of NF-κB (Fig. 4B).
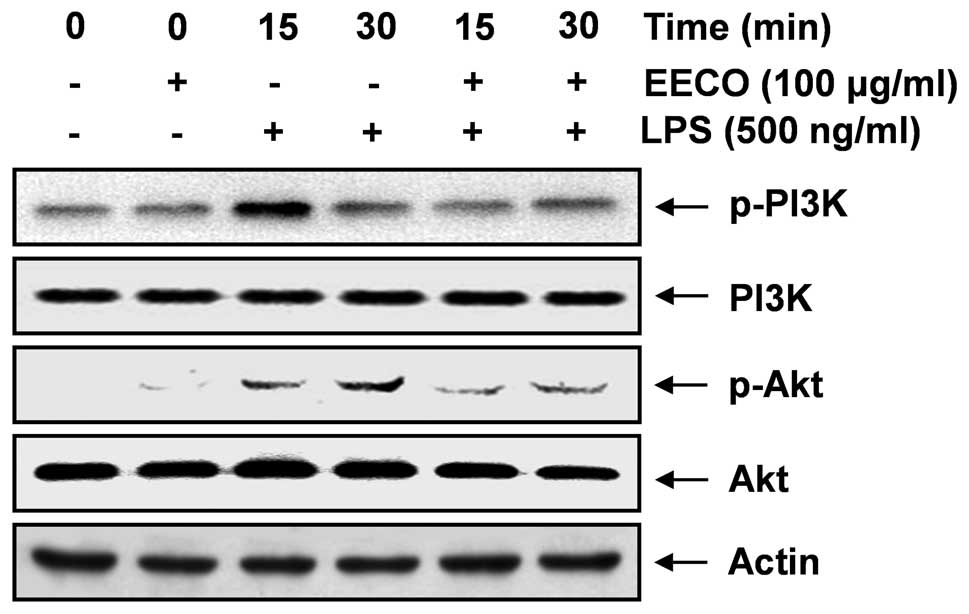
Inhibition of LPS-induced PI3K/Akt
activation by EECO
As the activation of the PI3K/Akt signaling pathway
leads to the production of inflammatory mediators and cytokines
through the activation of NF-κB (9–12),
we investigated the effects of EECO on the phosphorylation of PI3K
and Akt proteins in LPS-stimulated BV2 cells. Using western blot
analysis with anti-phospho-specific antibodies for PI3K and Akt, we
found that EECO suppressed the LPS-induced phosphorylation of PI3K
and Akt (Fig. 6), whereas the
levels of non-phosphorylated PI3K and Akt were unaffected by either
EECO or LPS treatment. These findings strongly suggest that the
anti-inflammatory effects of EECO in LPS-stimulated BV2 cells are
associated with the inactivation of the PI3K/Akt pathway.
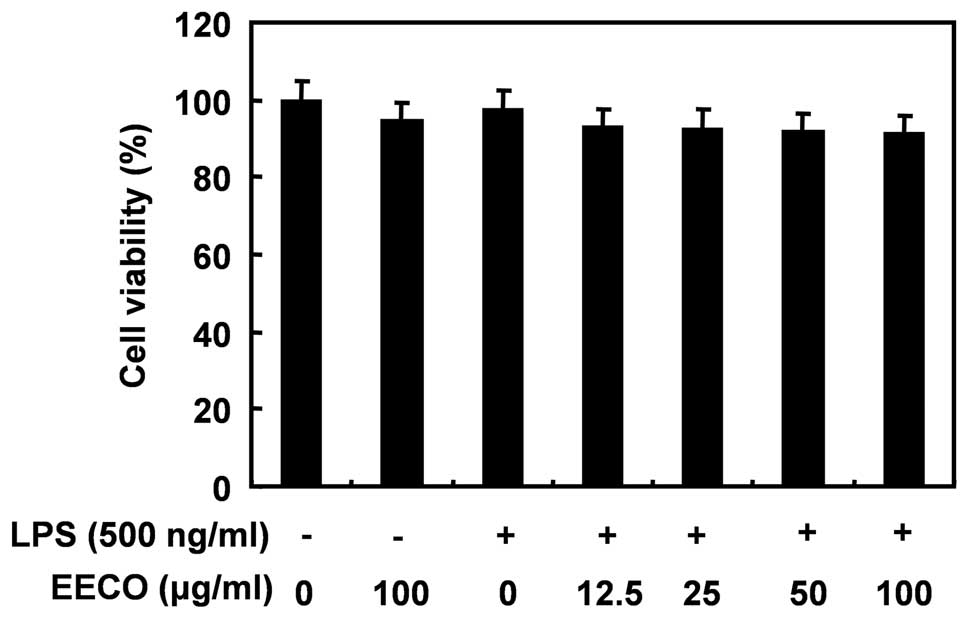
Effect of EECO on the viability of BV2
microglial cells
We evaluated the viability of BV2 cells incubated
with or without LPS in the absence or presence of EECO by MTT assay
to determine the cytotoxic effects (if any) of EECO on BV2
microglia. The concentrations (12.5 to 100 μM/ml) of EECO used to
inhibit LPS-induced inflammatory responses did not affect cell
viability, confirming that the anti-inflammatory effects of EECO in
LPS-stimulated BV2 cells are not due to the cytotoxicity of EECO
(Fig. 7).
Discussion
In the present study, we found that EECO impaired
LPS-induced gene expression and the secretion of pro-inflammatory
mediators (NO and PGE2), as well as that of the
inflammation-related genes, iNOS and COX-2, and cytokines (IL-1β
and TNF-α) in a BV2 microglia cell model. Further experiments
revealed that EECO attenuated the LPS-induced NF-κB activation by
suppressing the degradation of IκB-α, which was associated with the
inactivation of the PI3K/Akt signaling pathway.
Inflammation in the brain caused by activated
microglia plays an important role in the pathology of
neurodegenerative disorders (5,6).
iNOS and COX-2, which are responsible for synthesizing NO and
PGE2, respectively, are critical enzymes that mediate
inflammatory processes. The improper upregulation of iNOS and COX-2
has been associated with certain types of inflammatory disorders,
including neuronal degeneration (27,28). Pro-inflammatory cytokines also
play critical roles in the process of inflammation and the
increased production of these cytokines is associated with neuronal
dysfunction and neuronal loss (29,30). Therefore, the suppression of
neuroinflammation during microglial activation would theoretically
attenuate the progression of neurodegenerative disease. Thus, the
inhibition of pro-inflammatory mediators and cytokines by EECO in
LPS-stimulated BV2 microglia shown in the present study, may play a
beneficial role in the treatment of neurodegenerative diseases.
NF-κB is an important transcription factor that
regulates various cellular responses required for inducing the
expression of inflammation-related genes (7,26).
In an inactive state, NF-κB exists as a heterodimer of p65 and p50
subunits in the cytoplasm, but translocates to the nucleus once
activated through the phosphorylation and degradation of IκB, and
proceeds to transcribe the majority of pro-inflammatory genes, thus
contributing to the development of anaphylaxis, septic shock,
multiple organ failure and even cell death (31–33). In our experiments, the majority of
intracellular NF-κB p65 had translocated from the cytosol to the
nucleus following treatment with LPS, as demonstrated by strong
NF-κB p65 accumulation and staining in the nucleus. However, the
levels of NF-κB p65 in the nucleus decreased significantly
following pre-treatment with EECO. These results suggest that EECO
inhibits LPS-induced acute pro-inflammatory responses mediated by
the NF-κB signaling pathway.
The PI3K/AKT signaling pathway also controls a
variety of cellular proliferation and survival processes. Upon
stimulation, PI3K phosphorylates specific phosphoinositide lipids,
which accumulate in the plasma membrane, creating docking sites for
Akt. Akt undergoes phosphorylation at the plasma membrane, leading
to its activation (34,35). Numerous studies have shown that
the PI3K/Akt signaling pathway plays an important role in
negatively regulating LPS-induced acute inflammatory responses in
microglia (13,14,36–38). Although the role of PI3K/Akt
signaling cascades in the regulation of NF-κB transactivation
remains controversial (8,39,40), certain studies have demonstrated
that LPS-induced NF-κB activation is directly regulated as a main
upstream molecule of NF-κB via the phosphorylation of Akt (41,42). Therefore, to further confirm the
inhibitory effects of NF-κB activation by EECO, we investigated the
effects of EECO on the levels of PI3K and Akt phosphorylation in
LPS-stimulated BV2 cells. As a result, we found that PI3K and Akt
phosphorylation was markedly suppressed by EECO. These results
suggest that EECO inhibits LPS-induced NF-κB activation by
inhibiting the activation of the PI3K/Akt pathway.
In conclusion, in this study, we demonstrate that
EECO inhibits pro-inflammatory mediator and cytokine production by
suppressing the activation of NF-κB in LPS-stimulated BV2
microglial cells. The regulation of NF-κB activity by EECO was also
associated with the inactivation of the PI3K/Akt signaling pathway
during the LPS-induced anti-inflammatory reaction. Therefore, the
present results provide a molecular basis for understanding the
inhibitory effects of C. officinale rhizomes on
endotoxin-mediated inflammation.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) grant funded by the Korean government (no. 2012046358) and
the R&D program of MKE/KEIT (10040391, Development of
Functional Food Materials and Device for Prevention of
Aging-associated Muscle Function Decrease).
References
|
1
|
Hailer NP: Immunosuppression after
traumatic or ischemic CNS damage: it is neuroprotective and
illuminates the role of microglial cells. Prog Neurobiol.
84:211–233. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kriz J: Inflammation in ischemic brain
injury: timing is important. Crit Rev Neurobiol. 18:145–157. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mirshafiey A and Jadidi-Niaragh F:
Prostaglandins in pathogenesis and treatment of multiple sclerosis.
Immunopharmacol Immunotoxicol. 32:543–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dheen ST, Kaur C and Ling EA: Microglial
activation and its implications in the brain diseases. Curr Med
Chem. 14:1189–1197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McGeer PL and McGeer EG: Inflammation,
autotoxicity and Alzheimer disease. Neurobiol Aging. 22:799–809.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu B, Gao HM and Hong JS: Parkinson’s
disease and exposure to infectious agents and pesticides and the
occurrence of brain injuries: role of neuroinflammation. Environ
Health Perspect. 111:1065–1073. 2003.
|
|
7
|
Atreya I, Atreya R and Neurath MF:
NF-kappaB in inflammatory bowel disease. J Intern Med. 263:591–596.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tas SW, Vervoordeldonk MJ and Tak PP: Gene
therapy targeting nuclear factor-kappaB: towards clinical
application in inflammatory diseases and cancer. Curr Gene Ther.
9:160–170. 2009. View Article : Google Scholar
|
|
9
|
Qi S, Xin Y, Guo Y, Diao Y, Kou X, Luo L
and Yin Z: Ampelopsin reduces endotoxic inflammation via repressing
ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways. Int
Immunopharmacol. 12:278–287. 2012.PubMed/NCBI
|
|
10
|
Kang CH, Jayasooriya RG, Dilshara MG, Choi
YH, Jeong YK, Kim ND and Kim GY: Caffeine suppresses
lipopolysaccharide-stimulated BV2 microglial cells by suppressing
Akt-mediated NF-κB activation and ERK phosphorylation. Food Chem
Toxicol. 50:4270–4276. 2012.PubMed/NCBI
|
|
11
|
Yasuda T: Hyaluronan inhibits Akt, leading
to nuclear factor-κB down-regulation in
lipopolysaccharide-stimulated U937 macrophages. J Pharmacol Sci.
115:509–515. 2011.PubMed/NCBI
|
|
12
|
Chiou WF, Don MJ, Liao JF and Wei BL:
Psoralidin inhibits LPS-induced iNOS expression via repressing
Syk-mediated activation of PI3K-IKK-IκB signaling pathways. Eur J
Pharmacol. 650:102–109. 2011.PubMed/NCBI
|
|
13
|
Lee HS, Kwon SH, Ham JE, Lee JY, Kim DH,
Shin KH and Choi SH: Zaprinast activates MAPKs, NFκB, and Akt and
induces the expressions of inflammatory genes in microglia. Int
Immunopharmacol. 13:232–241. 2012.PubMed/NCBI
|
|
14
|
Lee YH, Jeon SH, Kim SH, Kim C, Lee SJ,
Koh D, Lim Y, Ha K and Shin SY: A new synthetic chalcone
derivative, 2-hydroxy-3′,5,5′-trimethoxychalcone (DK-139),
suppresses the Toll-like receptor 4-mediated inflammatory response
through inhibition of the Akt/NF-κB pathway in BV2 microglial
cells. Exp Mol Med. 44:369–377. 2012.PubMed/NCBI
|
|
15
|
Bae KE, Choi YW, Kim ST and Kim YK:
Components of rhizome extract of Cnidium officinale Makino
and their in vitro biological effects. Molecules. 16:8833–8847.
2011.PubMed/NCBI
|
|
16
|
de Caires S and Steenkamp V: Use of
Yokukansan (TJ-54) in the treatment of neurological disorders: a
review. Phytother Res. 24:1265–1270. 2010.PubMed/NCBI
|
|
17
|
Bark KM, Heo EP, Han KD, Kim MB, Lee ST,
Gil EM and Kim TH: Evaluation of the phototoxic potential of plants
used in oriental medicine. J Ethnopharmacol. 127:11–18. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeong SI, Kwak DH, Lee S, Choo YK, Woo WH,
Keum KS, Choi BK and Jung KY: Inhibitory effects of Cnidium
officinale Makino and Tabanus fulvus Meigan on the high
glucose-induced proliferation of glomerular mesangial cells.
Phytomedicine. 12:648–655. 2005.
|
|
19
|
Kwon JH and Ahn YJ: Acaricidal activity of
butylidenephthalide identified in Cnidium officinale rhizome
against Dermatophagoides farinae and Dermatophagoides
pteronyssinus (Acari: Pyroglyphidae). J Agric Food Chem.
50:4479–4483. 2002.PubMed/NCBI
|
|
20
|
Tomoda M, Ohara N, Shimizu N and Gonda R:
Characterization of a novel heteroglucan from the rhizome of
Cnidium officinale exhibiting high reticuloendothelial
system-potentiating and anti-complementary activities. Biol Pharm
Bull. 17:973–976. 1994.PubMed/NCBI
|
|
21
|
Jeong JB, Ju SY, Park JH, Lee JR, Yun KW,
Kwon ST, Lim JH, Chung GY and Jeong HJ: Antioxidant activity in
essential oils of Cnidium officinale Makino and
Ligusticum chuanxiong Hort and their inhibitory effects on
DNA damage and apoptosis induced by ultraviolet B in mammalian
cell. Cancer Epidemiol. 33:41–46. 2009.PubMed/NCBI
|
|
22
|
Jeong JB, Park JH, Lee HK, Ju SY, Hong SC,
Lee JR, Chung GY, Lim JH and Jeong HJ: Protective effect of the
extracts from Cnidium officinale against oxidative damage
induced by hydrogen peroxide via antioxidant effect. Food Chem
Toxicol. 47:525–529. 2009.PubMed/NCBI
|
|
23
|
Kim SJ, Kwon do Y, Kim YS and Kim YC:
Peroxyl radical scavenging capacity of extracts and isolated
components from selected medicinal plants. Arch Pharm Res.
33:867–873. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramalingam M and Yong-Ki P: Free radical
scavenging activities of Cnidium officinale Makino and
Ligusticum chuanxiong Hort. methanolic extracts. Pharmacogn
Mag. 6:323–330. 2010.PubMed/NCBI
|
|
25
|
Bae DS, Kim YH, Pan CH, Nho CW, Samdan J,
Yansan J and Lee JK: Protopine reduces the inflammatory activity of
lipopolysaccharide-stimulated murine macrophages. BMB Rep.
5:108–113. 2012.PubMed/NCBI
|
|
26
|
Tas SW, Remans PH, Reedquist KA and Tak
PP: Signal transduction pathways and transcription factors as
therapeutic targets in inflammatory disease: towards innovative
antirheumatic therapy. Curr Pharm Des. 11:581–611. 2005. View Article : Google Scholar
|
|
27
|
Shie FS, Montine KS, Breyer RM and Montine
TJ: Microglial EP2 is critical to neurotoxicity from activated
cerebral innate immunity. Glia. 52:70–77. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aid S, Langenbach R and Bosetti F:
Neuroinflammatory response to lipopolysaccharide is exacerbated in
mice genetically deficient in cyclooxygenase-2. J
Neuroinflammation. 5:172008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leone S, Ottani A and Bertolini A: Dual
acting anti-inflammatory drugs. Curr Top Med Chem. 7:265–275. 2007.
View Article : Google Scholar
|
|
30
|
Mariani MM and Kielian T: Microglia in
infectious diseases of the central nervous system. J Neuroimmune
Pharmacol. 4:448–461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murphy K, Haudek SB, Thompson M and Giroir
BP: Molecular biology of septic shock. New Horiz. 6:181–193.
1998.
|
|
32
|
Pushparaj PN, Tay HK, H’ng SC, Pitman N,
Xu D, McKenzie A, Liew FY and Melendez AJ: The cytokine
interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci USA.
106:9773–9778. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Manukyan MC, Weil BR, Wang Y, Abarbanell
AM, Herrmann JL, Poynter JA and Meldrum DR: The phosphoinositide-3
kinase survival signaling mechanism in sepsis. Shock. 34:442–449.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ito K, Caramori G and Adcock IM:
Therapeutic potential of phosphatidylinositol 3-kinase inhibitors
in inflammatory respiratory disease. J Pharmacol Exp Ther. 321:1–8.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi YH and Park HY: Anti-inflammatory
effects of spermidine in lipopolysaccharide-stimulated BV2
microglial cells. J Biomed Sci. 19:312012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park HY, Han MH, Kim GY, Kim ND, Nam TJ
and Choi YH: Inhibitory effects of glycoprotein isolated from
Laminaria japonica on lipopolysaccharide-induced pro-inflammatory
mediators in BV2 microglial cells. J Food Sci. 76:T156–T162. 2011.
View Article : Google Scholar
|
|
38
|
Park HY, Kim GY and Choi YH: Naringenin
attenuates the release of pro-inflammatory mediators from
lipopolysaccharide-stimulated BV2 microglia by inactivating nuclear
factor-κB and inhibiting mitogen-activated protein kinases. Int J
Mol Med. 30:204–210. 2012.PubMed/NCBI
|
|
39
|
Takeshima E, Tomimori K, Kawakami H,
Ishikawa C, Sawada S, Tomita M, Senba M, Kinjo F, Mimuro H,
Sasakawa C, Fujita J and Mori N: NF-kappaB activation by
Helicobacter pylori requires Akt-mediated phosphorylation of
p65. BMC Microbiol. 9:362009.
|
|
40
|
Wei J and Feng J: Signaling pathways
associated with inflammatory bowel disease. Recent Pat Inflamm
Allergy Drug Discov. 4:105–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dan HC, Cooper MJ, Cogswell PC, Duncan JA,
Ting JP and Baldwin AS: Akt-dependent regulation of NF-kappaB is
controlled by mTOR and Raptor in association with IKK. Genes Dev.
22:1490–1500. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Minhajuddin M, Bijli KM, Fazal F, Sassano
A, Nakayama KI, Hay N, Platanias LC and Rahman A: Protein kinase
C-delta and phosphatidylinositol 3-kinase/Akt activate mammalian
target of rapamycin to modulate NF-kappaB activation and
intercellular adhesion molecule-1 (ICAM-1) expression in
endothelial cells. J Biol Chem. 284:4052–4061. 2009. View Article : Google Scholar
|