Introduction
Atherosclerosis is a chronic inflammatory disease
characterized by inflammatory infiltrates and lipid accumulation
(1). It is known that monocytes
play an important role in the progression of the disease (2,3).
Monocytes first migrate into the arterial tissue in response to
locally produced chemokines and then differentiate into
macrophages. These macrophages act to augment the expression of
several pattern recognition receptors, leading to the accumulation
of cholesterol and lipids; thse cells then become foam cells
(4–6). Therefore, inflammatory infiltrates
and the accumulation of cholesterol and lipids in macrophages is a
key point in the initiation of atherosclerosis.
Over the past decade, the search for natural
compounds with the ability to prevent atherosclerosis has been a
main focus for many investigators. Several lines of evidence
suggest that resveratrol, a plant-derived polyphenol and
phytoalexin, exhibits cardioprotective and anti-inflammatory
properties (7–10). In the current study, we
investigated the potential atheroprotective, anti-inflammatory and
lipid-lowering effects of resveratrol. The oral administration of
resveratrol has been shown to affect lipid profiles and
inflammation markers (11).
However, the precise mechanisms involved remain unclear.
The balanced flow of lipids into and out of
macrophages is necessary to avoid lipid overload, and ultimately,
foam cell formation (12). 5′
AMP-activated protein kinase (AMPK) is an important
serine/threonine kinase well known for regulating cellular energy
levels by balancing nutrient availability and energy demand through
its control of several proteins involved in glucose and lipid
metabolism (13,14). Recent evidence has shown a
promising role for AMPK in the attenuation of atherosclerosis and
vascular dysfunction (14).
Resveratrol can activate the AMPK pathway in adipose tissue
(15). In addition, it has been
demonstrated that resveratrol exerts cardiometabolic effects by
increasing AMPK expression and the level of Silent information
regulator T1 (SIRT1) (16).
Conventional risk factors for atherosclerosis
trigger an inflammatory response in the artery wall, mediated by
complex molecular interactions in which chemokines play a critical
role (17). Monocyte chemotactic
protein-1 (MCP-1) is a potent chemoattractant for monocytes and
plays a pivotal role in early atherogenesis by promoting monocyte
infiltration to lesion-prone areas and penetrates between
endothelial cells into the inner arterial space (18,19). MCP-1 is synthesized by endothelial
cells and monocytes in response to diverse stimuli, including
interleukins and oxidized low-density lipoprotein (ox-LDL)
(18). Lipopolysaccharide (LPS)
is thought to be involved in cardiovascular disease, as it
contributes to the development of arterial plaques through
activated pro-inflammatory pathways by secreting cytokines,
including MCP-1 (20,21). The mechanisms which have been
suggested to be responsible for the anti-inflammatory effects of
resveratrol, include the inhibition of MCP-1 production (22).
In this study, we investigated the effects of
resveratrol on foam cell formation, as well as on the expression of
MCP-1 and AMPK in macrophages in order to elucidate the mechanisms
involved in its anti-atherosclerotic effects. In addition, the
expression of SIRT1 and nuclear peroxisome proliferator-activated
receptors (PPARs) was detected. The results of this study provide
important information regarding the initiation and prevention of
atherosclerosis.
Materials and methods
Materials
Resveratrol, Oil Red O and compound C were purchased
from Sigma-Aldrich, St. Louis, MO, USA; antibodies directed against
phospho-AMPKα (Thr172), AMPK, SIRT1 and β-actin were from Cell
Signaling Technology, Danvers, MA, USA; Texas-Red-conjugated goat
anti-mouse secondary antibody was obtained from Molecular Probes,
Eugene, OR, USA; LPS, phorbol 12-myristate 13-acetate (PMA) and
TRIzol reagent were from Sigma-Aldrich; the cDNA Synthesis kit was
obtained from Fermentas, St. Leon-Rot, Germany; the SYBR Premix Ex
Taq™ II kit was purchased from Takara Bio Inc., Otsu, Japan; the
Human MCP-1 ELISA kit was from R&D Systems, Minneapolis, MN,
USA; the BCA kit was from Pierce, Rockford, IL, USA; and Agilent
1100 series HPLC system was from Agilent Technologies, Palo Alto,
CA, USA.
Preparation of ox-LDL
Human LDL was purified from the fresh plasma of
healthy donors by sequential centrifugation, according to the
method described in the study by Feng et al (23) with some modifications. In order to
produce ox-LDL, 200 μg/ml LDL were exposed to 20 μM
CuSO4 in phosphate-buffered saline (PBS) for oxidation
and the reaction was terminated with 40 μM butylhydroxytoluene in
ethanol. ox-LDL was then dialyzed against culture medium and
sterile-filtered.
Cell culture
The human monocytic cell line, THP-1 (ATCC,
Rockville, MD, USA), was grown in RPMI-1640 supplemented with 10%
(v/v) fetal bovine serum (FBS), 0.05 mM 2-mercaptoethanol, 10 mM
HEPES, 1 mM sodium pyruvate, 4.5 g/l glucose and 1.5 g/l
bicarbonate at 37°C in an atmosphere containing 5% CO2.
The THP-1 cells were stimulated by a 48-h exposure to 100 nM PMA to
induce their differentiation into adherent macrophages.
Differentiated THP-1 macrophages were extensively washed in PBS
before being used in experiments.
Resveratrol treatment
A stock concentration of 100 mM resveratrol in 50%
DMSO was produced fresh each time and diluted in culture medium to
the desired concentration. The controls received the same amount of
DMSO.
Foam cell formation assay
The experiments were performed in serum-free (SF)
experimental medium. The THP-1 cells were pre-treated with 2.5 μM
of resveratrol or the control (DMSO) for 1 h. Subsequently, both
the control and resveratrol-treated groups were treated with SF
medium alone or SF medium containing LPS (100 ng/ml) and ox-LDL (50
μg/ml) for 24 h. The formation of foam cells was determined by Oil
Red O staining. The cells were fixed with 4% formaldehyde for 15
min. Cell lipids were stained with Oil Red O (3 mg/ml in 60%
isopropanol) for 10 min, then observed under a microscope.
Quantitative reverse transcription PCR
(qRT-PCR)
Total cellular RNA was extracted from the
THP-1-derived macrophages using TRIzol reagent in accordance with
the manufacturer’s instructions, and dissolved in nuclease-free
water, prior to being reverse-transcribed to synthesize
first-strand cDNA with oligo(dT) primer using the cDNA Synthesis
kit. To correct for differences in cDNA loading among the samples,
the target PCRs were normalized to a reference PCR involving the
endogenous housekeeping gene, β-actin. Non-template controls were
included for each primer pair to check for any significant levels
of contaminants. A melting-curve analysis was performed to assess
the specificity of the amplified PCR products. qRT-PCR was
performed using the FastStart SYBR-Green reagent kit according to
the manufacturer’s instructions. The reaction conditions followed
the instructions provided by the manufacturer of the SYBR Premix Ex
Taq II kit using gene-specific primers for MCP-1, SIRT1, PPARs and
β-actin (Table I).
 | Table IPrimer sequences used in qRT-PCR. |
Table I
Primer sequences used in qRT-PCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| β-actin | F:
GATCATTGCTCCTCCTGAGC
R: ACTCCTGCTTGCTGATCCAC |
| SIRT1 | F:
GAGTGGCAAAGGAGCAGA
R: TCTGGCATGTCCCACTATC |
| PPARγ | F:
GCAGTGGGGATGTCTCATAATGC
R: CAGGGGGGTGATGTGTTTGAA |
| PPAR | β/δ F:
AATGCCTACCTGAAAAACTTCAAC
R: GTGCACGCTGATTCCTTGT |
| PPARα | F:
GAGAAAGCAAAACTGAAAGCAGAGA
R: GAAGGGCGGGTTATTGCTG |
| MCP-1 | F:
AGCCACCTTCATTCCCCAAG
R: CTCCTTGGCCACAATGGTCT |
Lipid analysis by high-performance liquid
chromatography (HPLC)
Cellular total cholesterol and triglyceride contents
were analyzed by lipid analysis by HPLC. Briefly, the cells were
washed 3 times in PBS and lysed by the addition of 0.9% NaOH
solution. Protein concentration was measured using the BCA kit.
Masterol was used as a standard curve first, and the extraction
procedure was then repeated. Samples were dissolved in 100 μl of
isopropanol-acetonitrile (v/v, 20:80), followed by incubation in an
ultrasonic water at room temperature for 5 min. Finally, the
samples were placed in the Agilent 1100 series HPLC system.
Western blot analysis
Protein concentrations were determined using bovine
serum albumin (BSA) as a standard protein with the BCA protein
assay. The same amounts of total proteins (15–20 μg/lane) were
loaded onto each lane and transferred to nitrocellulose membranes
at 80 V for 1 h. After blocking for 4 h in 5% skim milk, the
membranes were incubated overnight at 4°C with a 1:1,000 dilution
of primary antibody. Following incubation with the corresponding
secondary antibody, signals were detected using a chemiluminescent
detection system and quantified using Quantity One analysis
software.
ELISA
To evaluate the produced levels of MCP-1, the
THP-1-derived macrophages were pre-treated with resveratrol (10 μM)
for 1 h and then treated with LPS (10 μM) for 6 h. Supernatants
from the treated cells were collected and analyzed for MCP-1 using
a sandwich ELISA kit according to the manufacturer’s
instructions.
Data analysis
All results are expressed as the means ± standard
deviation (SD). All data were evaluated using SPSS 11.0 software. A
typical image from at least 3 similar experiments is presented.
Statistical analysis were carried out using t-tests. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Resveratrol inhibits foam cell
formation
We investigated foam cell formation in response to
exposure to LPS. As evidenced by Oil Red O staining, in the
presence of ox-LDL, the typical formation of foam cells was
observed (Fig. 1A-II vs. A-I).
Resveratrol effectively suppressed the foam cell formation induced
by LPS (Fig. 1A-III vs. A-II). We
investigated intracellular lipid accumulation by HPLC. LPS at 100
ng/ml without LDL loading increased lipid accumulation in the
macrophages (Fig. 1B, LPS vs. SF
medium, solid bars). In the presence of ox-LDL (50 μg/ml) loading,
LPS further increased lipid accumulation in the macrophages
(Fig. 1B, LPS ox-LDL vs. ox-LDL,
solid bars). These observations led us to hypothesize that
resveratrol may inhibit foam cell formation by regulating lipid
accumulation.
Resveratrol inhibits lipid accumulation
through SIRT1 and PPARs
SIRT1 reduces the accumulation of fatty acids by
suppressing the expression of PPAR-γ (24). PPAR-γ is one of the PPAR family
members, which comprises 3 isotypes: PPARα, PPARγ and PPARβ/δ
(25), PPARs play a central role
in the regulation of adipogenesis (26). Resveratrol is an activator of
SIRT1 (27). Thus, this prompted
us to investigate whether resveratrol regulates lipid accumulation
through SIRT1-PPARs.
To investigate the effects of resveratrol on SIRT1
and PPAR expression, the THP-1-derived macrophages were treated
with resveratrol (2.5 μM) for 6 h. As shown in Fig. 2A, resveratrol significantly
upregulated the mRNA expression of SIRT1 (P<0.05), and there
were analogous results obtained by western blot analysis (Fig. 2B).
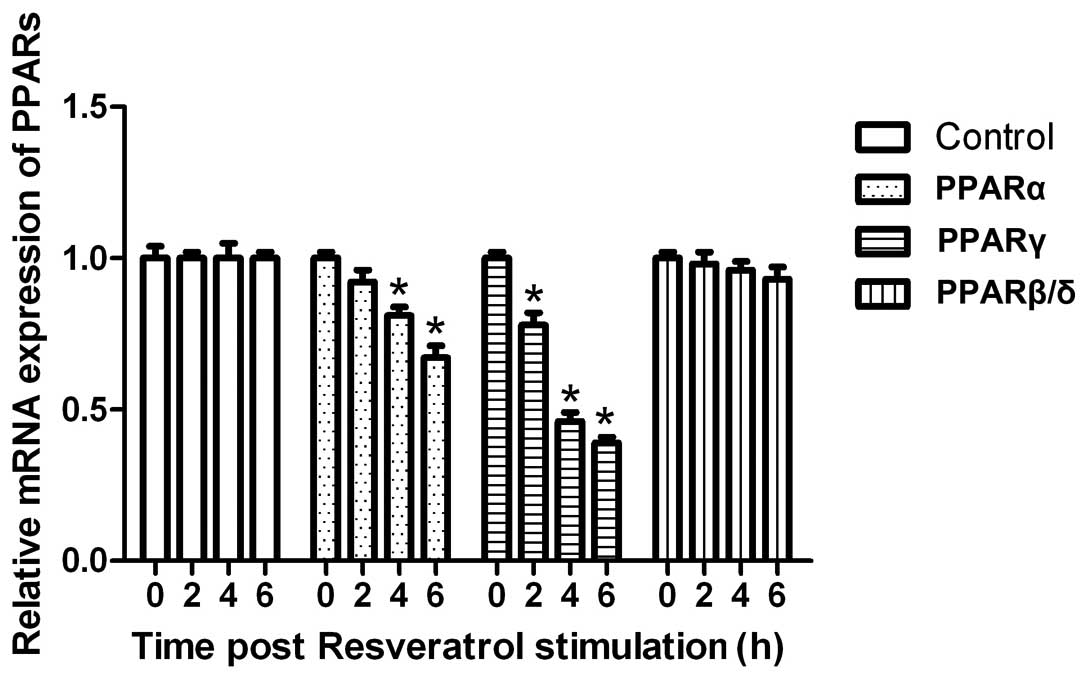
We then detected the expression of PPARs. As shown
in Fig. 3, resveratrol
significantly downregulated the mRNA expression of PPARγ
(P<0.05). As regards PPARα, resveratrol also significantly
downregulated the mRNA expression (P<0.05). By contrast,
resveratrol had no effect on the mRNA expression of PPARβ/δ
(P<0.05), suggesting that resveratrol regulates lipid
accumulation through SIRT1-PPARs.
Resveratrol regulates SIRT1-PPARs through
AMPK
As shown by our results, resveratrol regulates the
expression of SIRT1 and PPARs; however, the mechanisms involved are
not yet clear. AMPK, which acts upstream of SIRT1, controls several
proteins involved in glucose and lipid metabolism (13,14). The activation of AMPK activates
catabolic pathways, generating ATP, and ‘switches off’ a number of
processes that consume ATP, such as fatty acid, protein, or
cholesterol synthesis (28,29); AMPK also enhances SIRT1 activity
(30,31).
To investigate the effects of resveratrol on AMPK,
the THP-1-derived macrophages were treated with resveratrol (2.5
μM) for 6 h. The phosphorylated isoform is the active AMPK form;
thus, we determined the phosphorylated AMPK/total AMPK protein
ratio. Resveratrol significantly increased the phosphorylated
AMPK/total AMPK protein ratio; this increase demonstrated that AMPK
was significantly activated by resveratrol (Fig. 4), suggesting that resveratrol
regulates SIRT1-PPARs through AMPK.
Effects of AMPK inhibition on SIRT1 and
PPAR expression and lipid accumulation
To verify our hypothesis, we evaluated the effects
of AMPK inhibition (using compound C) on SIRT1 and PPAR expression
and lipid accumulation following incubation of THP-1-derived
macrophages in resveratrol (2.5 μM) for 6 h in the presence or
absence of 30 μM compound C for 4 h. Our results revealed that
pre-treatment with 30 μM compound C for 4 h inhibited the
resveratrol-induced increase in SIRT1 expression and blocked the
suppression of PPARγ/PPARα expression by resveratrol (Fig. 5A and B). We also examined lipid
accumulation; the suppression of lipid accumulation following
treatment with resveratrol was reversed when the cells were
pre-treated with 30 μM compound C (Fig. 5C).
Resveratrol blocks LPS-induced MCP-1
expression
Given the well-documented anti-inflammatory effects
of resveratrol, we focused on the effects of resveratrol on
LPS-induced MCP-1 expression in THP-1-derived macrophages.
Resveratrol downregulated the mRNA expression of MCP-1 in a
dose-dependent manner (Fig. 6A).
As shown in Fig. 6B, MCP-1 mRNA
expression increased following treatment with LPS in a
time-dependent manner. Pre-treatment with 10 μM resveratrol
markedly inhibited the mRNA expression of MCP-1 induced by LPS
(Fig. 6C). The induced expression
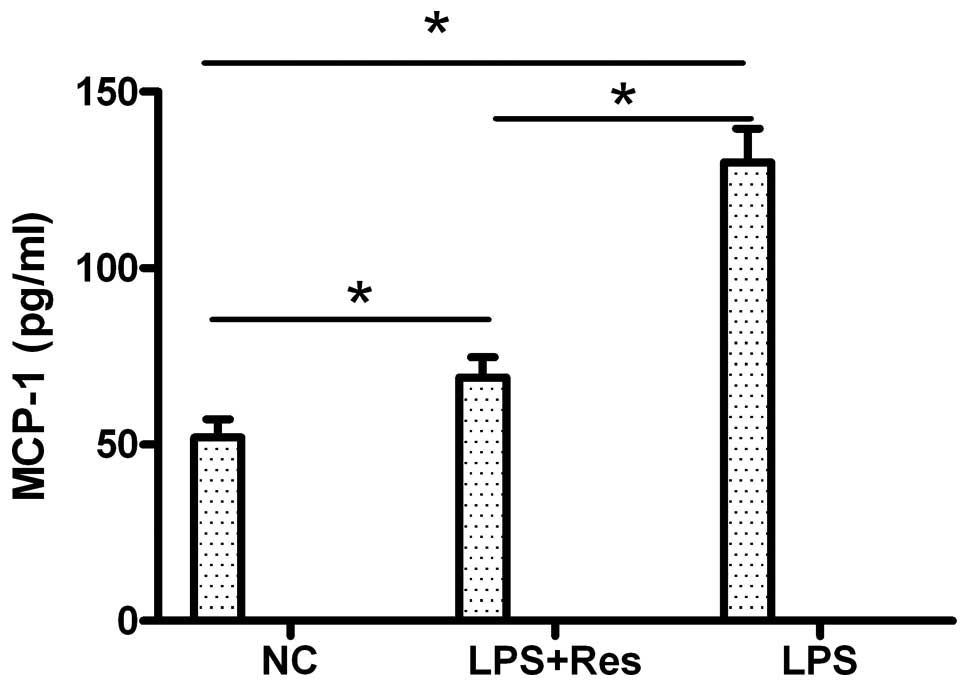
of MCP-1 was determined by ELISA (Fig. 7). Compared to the control group,
LPS induced a statistically significant upregulation of MCP-1
expression (P<0.05), and resveratrol downregulated the
LPS-induced MCP-1 expression. MCP-1 expression which was induced by
LPS was inhibited by resveratrol at both the transcriptional and
translational level. These results demonstrate that resveratrol
inhibits foam cell formation by regulating inflammatory cytokine
(MCP-1) production.
Discussion
Cardiovascular disease, which is currently the
leading cause of death and illness in developed countries, is a
preeminent health issue worldwide (32). Atherosclerosis, a progressive
inflammatory disease, produces arterial plaques characterized by
inflammatory infiltrates, lipid accumulation, cell death and
fibrosis (2,3,33).
Monocytes play an important role in the progression of the disease.
Monocytes first migrate, and then differentiate into macrophages.
Inflammatory infiltrates and the accumulation of cholesterol and
lipids in macrophages allows them to become foam cells; this is a
key point in the initiation of atherosclerosis.
Resveratrol is a polyphenol found in grapes, berries
and peanuts. It inhibits macrophage activation (34), one of the most important steps in
atherosclerosis, as well as lipid accumulation (34,35). In addition, resveratrol has been
suggested to exert anti-atherosclerotic effects (36). However, the precise mechanisms
responsible for the anti-atherosclerotic effects of resveratrol
remain unclear.
In this study, we found that THP-1-derived
macrophages treated with LPS and ox-LDL together resulted in the
typical formation of foam cells and resveratrol effectively
suppressed the foam cell formation induced by LPS. Based on our
findings, we suggest that resveratrol exerts anti-atherosclerotic
effects by suppressing foam cell formation. Experiments on the
effects of lipid deposition in THP-1-derived macrophages treated
with resveratrol, revealed that the cellular total cholesterol
content was suppressed by resveratrol, suggesting that resveratrol
protects against atherosclerosis by inhibiting lipid
accumulation.
AMPK regulates cellular energy levels by balancing
nutrient availability and energy demand through its control of
several proteins involved in glucose and lipid metabolism (13). SIRT1 is one of the 7 mammalian
homologs of the Sir2 family that catalyzes
NAD+-dependent protein deacetylation (37). Both AMPK and SIRT1 have emerged as
interesting targets as they are heavily involved in catabolic
metabolism, mitochondrial activation, angiogenesis and enhanced
cell survival (37–40). PPARs are central regulators of
adipogenesis (25). Previous
studies have shown that resveratrol negatively modulates PPARγ
protein levels in 3T3-L1 adipocytes (41) and it is known that resveratrol
affects both SIRT1 and AMPK (42). The data from these studies, as
well as ours, suggest that resveratrol regulates lipid metabolism
through the AMPK-SIRT1-PPAR signaling pathway.
In this study, we found that AMPK was significantly
activated by resveratrol and that resveratrol markedly upregulated
the expression of SIRT1 both at the mRNA and protein level. In
addition, resveratrol markedly downregulated the mRNA expression of
PPARγ and PPARα, but not that of PPARβ/δ. When the cells were
pre-treated with the AMPK inhibitor, compound C, the effects of
resveratrol on SIRT1, PPARγ and PPARα expression, as well as on
lipid accumulation were reversed, suggesting that resveratrol
suppresses lipid accumulation through the AMPK-SIRT1-PPARγ/PPARα
signaling pathway.
Inflammatory infiltrates in macrophages are a key to
foam cell formation. Thus, we hypothesized that resveratrol may
suppress foam cell formation by regulating inflammatory cytokines.
We selected MCP-1, a potent chemoattractant for monocytes. MCP-1
has been shown to be overexpressed in human and experimental
atheroma, and can recruit mononuclear phagocytes that
characteristically accumulate in the nascent atheroma (32,43). A recent study demonstrated that
LPS induces the expression of MCP-1 (44). Despite the evidence of a prominent
role of MCP-1 in the development of atherosclerosis, and although
resveratrol has been shown to inhibit the production of various
types of inflammatory cytokines (34,45), few studies have investigated the
direct effects of resveratrol on MCP-1. In this study, we found
that resveratrol downregulated the expression of MCP-1 in a
dose-dependent manner in THP-1-derived macrophages and that LPS
induced MCP-1 expression in THP-1-derived macrophages in a
time-dependent manner. Most importantly, we found that
pre-treatment of THP-1-derived macrophages with resveratrol
significantly blocked MCP-1 mRNA expression induced by LPS.
Therefore, we speculate that resveratrol plays a role in
anti-atherosclerosis by inhibiting the expression of MCP-1.
In conclusion, our results demonstrate that
resveratrol suppresses the foam cell formation induced by LPS.
Resveratrol inhibits foam cell formation by regulating the
expression of the inflammatory cytokine, MCP-1, and by activating
the AMPK-SIRT1-PPAR signaling pathway. These results suggest that
resveratrol may be a novel therapeutic agent for
atherosclerosis.
Acknowledgements
This study was supported by a grant from the Natural
Science Foundation of China (no. 81200633).
References
|
1
|
Andersson J, Libby P and Hansson GK:
Adaptive immunity and atherosclerosis. Clin Immunol. 134:33–46.
2010. View Article : Google Scholar
|
|
2
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weber C, Zernecke A and Libby P: The
multifaceted contributions of leukocyte subsets to atherosclerosis:
lessons from mouse models. Nat Rev Immunol. 8:802–815. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boring L, Gosling J, Cleary M and Charo
IF: Decreased lesion formation in CCR2−/− mice reveals a
role for chemokines in the initiation of atherosclerosis. Nature.
394:894–897. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gu L, Okada Y, Clinton SK, et al: Absence
of monocyte chemoattractant protein-1 reduces atherosclerosis in
low density lipoprotein receptor-deficient mice. Mol Cell.
2:275–281. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Edfeldt K, Swedenborg J, Hansson GK and
Yan ZQ: Expression of toll-like receptors in human atherosclerotic
lesions: a possible pathway for plaque activation. Circulation.
105:1158–1161. 2002.PubMed/NCBI
|
|
7
|
Fan EG, Zhang LJ, Jiang S and Bai YH:
Beneficial effects of resveratrol on atherosclerosis. J Med Food.
11:610–614. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palmieri D, Pane B, Barisione C, et al:
Resveratrol counteracts systemic and local inflammation involved in
early abdominal aortic aneurysm development. J Surg Res.
171:e237–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prasad K: Natural products in regression
and slowing of progression of atherosclerosis. Curr Pharm
Biotechnol. 11:794–800. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu X, Liu Q, Wang M, et al: Activation of
Sirt1 by resveratrol inhibits TNF-alpha induced inflammation in
fibroblasts. PLoS ONE. 6:e270812011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Militaru C, Donoiu I, Craciun A, Scorei
ID, Bulearca AM and Scorei RI: Oral resveratrol and calcium
fructoborate supplementation in subjects with stable angina
pectoris: effects on lipid profiles, inflammation markers, and
quality of life. Nutrition. 29:178–183. 2013. View Article : Google Scholar
|
|
12
|
Voloshyna I, Hai O, Littlefield MJ,
Carsons S and Reiss AB: Resveratrol mediates anti-atherogenic
effects on cholesterol flux in human macrophages and endothelium
via PPAR gamma and adenosine. Eur J Pharmacol. 698:299–309. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vingtdeux V, Chandakkar P, Zhao H, Davies
P and Marambaud P: Small-molecule activators of AMP-activated
protein kinase (AMPK), RSVA314 and RSVA405, inhibit adipogenesis.
Mol Med. 17:1022–1030. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fullerton MD, Steinberg GR and Schertzer
JD: Immunometabolism of AMPK in insulin resistance and
atherosclerosis. Mol Cell Endocrinol. 366:224–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siriwardhana N, Kalupahana NS, Cekanova M,
LeMieux M, Greer B and Moustaid-Moussa N: Modulation of adipose
tissue inflammation by bioactive food compounds. J Nutr Biochem.
24:613–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crandall JP and Barzilai N: Exploring the
promise of resveratrol: where do we go from here? Diabetes.
62:1022–1023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coll B, Alonso-Villaverde C and Joven J:
Monocyte chemoattractant protein-1 and atherosclerosis: is there
room for an additional biomarker? Clin Chim Acta. 383:21–29. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Charo IF and Taubman MB: Chemokines in the
pathogenesis of vascular disease. Circ Res. 95:858–866. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McLaren JE, Michael DR, Ashlin TG and
Ramji DP: Cytokines, macrophage lipid metabolism and foam cells:
implications for cardiovascular disease therapy. Prog Lipid Res.
50:331–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kallio KA, Buhlin K, Jauhiainen M, et al:
Lipopolysaccharide associates with pro-atherogenic lipoproteins in
periodontitis patients. Innate Immun. 14:247–253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vink A, Schoneveld AH, van der Meer JJ, et
al: In vivo evidence for a role of toll-like receptor 4 in the
development of intimal lesions. Circulation. 106:1985–1990. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park D-W, Kim J-S, Chin B-R and Baek S-H:
Resveratrol inhibits inflammation induced by heat-killed Listeria
monocytogenes. J Med Food. 15:788–794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng X, Zhang Y, Xu R, et al:
Lipopolysaccharide up-regulates the expression of Fcalpha/mu
receptor and promotes the binding of oxidized low-density
lipoprotein and its IgM antibody complex to activated human
macrophages. Atherosclerosis. 208:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Winnik S, Stein S and Matter CM: SIRT1 -
an anti-inflammatory pathway at the crossroads between metabolic
disease and atherosclerosis. Curr Vasc Pharmacol. 10:693–696. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Semple RK, Chatterjee VK and O’Rahilly S:
PPAR gamma and human metabolic disease. J Clin Invest. 116:581–589.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
dos Costa CS, Rohden F, Hammes TO, et al:
Resveratrol upregulated SIRT1, FOXO1, and adiponectin and
downregulated PPARgamma1–3 mRNA expression in human visceral
adipocytes. Obes Surg. 21:356–361. 2011.PubMed/NCBI
|
|
27
|
Nimmagadda VK, Bever CT, Vattikunta NR, et
al: Overexpression of SIRT1 protein in neurons protects against
experimental autoimmune encephalomyelitis through activation of
multiple SIRT1 targets. J Immunol. 190:4595–4607. 2013.
|
|
28
|
Cantó C, Jiang LQ, Deshmukh AS, et al:
Interdependence of AMPK and SIRT1 for metabolic adaptation to
fasting and exercise in skeletal muscle. Cell Metab. 11:213–219.
2010.PubMed/NCBI
|
|
29
|
Hardie DG: AMP-activated/SNF1 protein
kinases: conserved guardians of cellular energy. Nat Rev Mol Cell
Biol. 8:774–785. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Canto C, Gerhart-Hines Z, Feige JN, et al:
AMPK regulates energy expenditure by modulating NAD+
metabolism and SIRT1 activity. Nature. 458:1056–1060. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Costford SR, Bajpeyi S, Pasarica M, et al:
Skeletal muscle NAMPT is induced by exercise in humans. Am J
Physiol Endocrinol Metab. 298:E117–E126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar
|
|
33
|
Hansson GK and Libby P: The immune
response in atherosclerosis: a double-edged sword. Nat Rev Immunol.
6:508–519. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park D-W, Baek K, Kim J-R, et al:
Resveratrol inhibits foam cell formation via NADPH oxidase
1-mediated reactive oxygen species and monocyte chemotactic
protein-1. Exp Mol Med. 41:171–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li H, Song Y, Li F, et al: Identification
of lipid droplet-associated proteins in the formation of
macrophage-derived foam cells using microarrays. Int J Mol Med.
26:231–239. 2010.PubMed/NCBI
|
|
36
|
Hou X, Xu S, Maitland-Toolan KA, et al:
SIRT1 regulates hepatocyte lipid metabolism through activating
AMP-activated protein kinase. J Biol Chem. 283:20015–20026. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lagouge M, Argmann C, Gerhart-Hines Z, et
al: Resveratrol improves mitochondrial function and protects
against metabolic disease by activating SIRT1 and PGC-1alpha. Cell.
127:1109–1122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rodgers JT, Lerin C, Haas W, Gygi SP,
Spiegelman BM and Puigserver P: Nutrient control of glucose
homeostasis through a complex of PGC-1alpha and SIRT1. Nature.
434:113–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reverchon M, Cornuau M, Cloix L, et al:
Visfatin is expressed in human granulosa cells: regulation by
metformin through AMPK/SIRT1 pathways and its role in
steroidogenesis. Mol Hum Reprod. 19:313–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Morita Y, Wada-Hiraike O, Yano T, et al:
Resveratrol promotes expression of SIRT1 and StAR in rat ovarian
granulosa cells: an implicative role of SIRT1 in the ovary. Reprod
Biol Endocrinol. 10:142012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Picard F, Kurtev M, Chung N, et al: Sirt1
promotes fat mobilization in white adipocytes by repressing
PPAR-gamma. Nature. 429:771–776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baur JA, Pearson KJ, Price NL, et al:
Resveratrol improves health and survival of mice on a high-calorie
diet. Nature. 444:337–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
von Grote EC, Venkatakrishnan V, Duo J and
Stenken JA: Long-term subcutaneous microdialysis sampling and
qRT-PCR of MCP-1, IL-6 and IL-10 in freely-moving rats. Mol
Biosyst. 7:150–161. 2011.PubMed/NCBI
|
|
44
|
Wang J, Si Y, Wu C, et al:
Lipopolysaccharide promotes lipid accumulation in human adventitial
fibroblasts via TLR4-NF-kappaB pathway. Lipids Health Dis.
11:1392012. View Article : Google Scholar
|
|
45
|
Culpitt SV, Rogers DF, Fenwick PS, et al:
Inhibition by red wine extract, resveratrol, of cytokine release by
alveolar macrophages in COPD. Thorax. 58:942–946. 2003. View Article : Google Scholar : PubMed/NCBI
|