Introduction
For patients who have suffered spinal cord injury
(SCI), the disintegration and necrosis of a large number of
neuronal cells in tissues is one of the major causes of reduced
limb movement and sensory dysfunctions in the area below the injury
(1). Thus, promoting the
regeneration of nerve cells and recovering the structure and
function of the spinal cord are important and difficult issues with
which neuroscientists are concerned (2).
Bone marrow mesenchymal stem cells (BMSCs) are
non-hematopoietic stem cells in the bone marrow which are derived
from the mesoderm; they can self-proliferate and are capable of
multi-directional differentiation (3). A BMSC is a type of adult pluripotent
stem cell, isolated and cultured from the bone marrow, which can be
found in different species such as chickens, rats, rabbits, dogs
and other animals as well as humans (4). Its potential in multi-directional
differentiation has received much attention in studies on
Alzheimer's disease, strokes, myocardial infarction, peripheral
nerve injury, bone defects and nonunion (5). It has preciously been reported that
BMSCs have the ability to differentiate into neuroectodermal cells.
In a previous study, the BMSCs were injected into the hippocampus
of mice and the differentiation into astrocytes was observed; under
specific induction conditions, they can also be induced to
differentiate into neural cells which are capable of expressing
neuron-specific marker proteins (6).
Plumbagin is from the Plumbaginaceae family, genus
Plumbago, which is mainly distributed in the southwestern
provinces of China, and is usually used for treating sores,
swelling, clearing the meridians, and treating snakebites,
rheumatism, mastitis and chronic bronchitis (7). Previous pharmacological studies have
demonstrated that plumbagin is one of the most important active
antitumor components of Plumbago, which exerts
anti-bacterial, anti-inflammatory, anti-cancerous and other
beneficial effects (8,9). In the present study, we aimed to
explore the protective effect of a treatment which combined BMSCs
with plumbagin on SCI and also explore the potential mechanism of
the protective effects.
Materials and methods
Reagents
α-modified Eagle's medium (MEM) and Dulbecco's
modified Eagle's medium (DMEM) were provided by Thermo Scientific
(Waltham, MA, USA). Fetal bovine serum (FBS) was provided by Gibco
(Grand Island, NY, USA). Plumbagin was purchased from Sigma-Aldrich
(Hamburg, Germany). Myeloperoxidase (MPO), superoxide dismutase
(SOD), malondialdehyde (MDA), nuclear factor-κB (NF-κB) p65, tumor
necrosis factor-α (TNF-α) kits and hematoxylin were all purchased
from Invitrogen (Carlsbad, CA, USA).
Animals
Male Sprague-Dawley rats (SD rats) weighing 220±30 g
were used for the present study. The SD rats were maintained in
animal quarters with humidity ranging from 60–70%, a temperature of
23±1°C and a 12-h light/dark cycle. Food and water were made
available ad libitum. All experiments were carried out in
accordance with the criteria outlined in the Guide for the Care and
Use of Laboratory Animals prepared by the National Academy of
Sciences and were approved by the Ethics Committee of Zhengzhou
University (Zhengzhou, China).
Cell culture and identification of
BMSCs
The SD rats were sedated using chloral hydrate, and
their bilateral femurs and tibias were removed under sterile
conditions and separated. Subsequently, bone marrow tissues of the
bilateral femur and tibia were separated, and cell samples were
collected from these bone marrow tissues and seeded into a
25-cm2 plastic bottle to separate and obtain BMSCs using
the adherence method. BMSCs were cultivated in 5 ml α-MEM (Thermo
Scientific) and 10% FBS and cultivated in the plastic bottle for
incubation. After cultivation for 24–48 h, the non-adherent cells
were removed. When adherent cells reached 70–80% confluence,
adherent cells were then incubated with 0.25% trypsin to remove
them.
After paraformaldehyde (5%) was precooled, it was
used to fix BMSCs for 10–15 min. The fixed BMSCs were cultured with
hematoxylin (Invitrogen) for 3–10 min. Stained BMSCs were then
washed with tap water and distilled water for 5–10 min. Stained
BMSCs were dehydrated with 95% ethyl alcohol for 1–2 min, and
xylene was used to clear the stained cells for 5–10 min. BMSCs were
observed using a microscope (CFI60; Nikon, Tokyo, Japan).
Induction of SCI
A rat model of SCI was induced as described
previously (10). Briefly, SD
rats were intraperitoneally injected with 80 mg/kg ketamine and 10
mg/kg xylazine and then underwent a laminectomy during which the T8
and T9 vertebral peduncles were removed. After treatment was
completed, the SD rats were sacrificed using a lethal injection of
pentobarbital, and the spinal tissue was removed while on dry ice.
The tissue from the injury site of the spinal cord segments
(between T8 and T9) was microdissected and frozen on dry ice. The
spinal tissue samples were homogenized with Tris-HCl buffer (50 mM,
pH 7.4) on ice for future use.
Treatment schedule
Firstly, 10 normal SD rats were defined as the
control group and injected with normal saline. Subsequently, 40
rats for the SCI model were randomly divided into 4 groups as
follows: SCI model group (SCI, n=10), BMSC-treated group (BMSCs,
n=10), plumbagin-treated group (plumbagin, n=10) and the BMSC and
plumbagin-treated group (BP, n=10). In the SCI model group, rats
were injected with saline and SCI was induced. In the BMSC-treated
group, rats with SCI were treated with BMSCs (3×105
cells) by intraspinal injection using a Hamilton syringe, as
previously described (11) for 5
consecutive days. In the plumbagin group, rats with SCI were
treated with 20 mg/kg plumbagin, as previously described, (12) for 5 consecutive days. In the group
treated with BMSCs and plumbagin, rats with SCI were treated with
BMSCs (3×105 cells) by intraspinal injection using a
Hamilton syringe and also 20 mg/kg plumbagin for 5 consecutive
days.
Locomotor recovery after SCI
Locomotor recovery was assessed as previously
described (13). Locomotor
behavior was evaluated beginning 1 day before injury and ending 5
days after. Scoring categories and attributes were identified,
operationally defined and ranked on the observed sequence of
locomotor recovery patterns using the Basso, Beattie and Bresnahan
(BBB) locomotor rating scale. In the BBB scale, 0 is defined as 'no
observable movement of the hindlimbs', 21 is defined as 'consistent
plantar stepping and coordinated gait, consistent movement of the
toes; paw position is predominantly parallel to the body during the
whole support stage; consistent trunk stability; consistent tail
elevation'. The BBB rating was carried out 1 day before SCI was
induced (0 day), 1 day after SCI was induced (1 day), and again 5
days later (5 day).
Measurement of spinal cord water content
after SCI
After treatment with BP for 5 consecutive days,
spinal cord water content was measured as previously described
(14). Briefly, thye SD rats were
sacrificed using a lethal injection of pentobarbital and the spinal
tissue was removed while on dry ice, and dried at 80°C for 24–48 h
before the dry weight was measured. Spinal cord water content was
calculated as follows: water content = [(wet weight ‒ dry
weight)/wet weight] ×100.
MPO activity
After treatment with BP for 5 consecutive days, and
after the rats were sacrificed and the spinal cord tissue removed,
the injured spinal cord segment was mechanically homogenized in
ice-cold tris-buffered saline containing 40 mM Tris-HCl (pH 7.5),
2% SDS, 2 mg/ml aprotinin, 2 mg/ml antipain, 2 mg/ml chymostatin, 2
mg/ml bestatin, 2 mg/ml pepstatin-A, 2 mg/ml leupeptin, 1 mM
phenylmethylsulfonyl fluoride, 1 mM dithiothreitol and 1 mM EDTA.
The homogenates were centrifuged at 12,000 × g for 10 min. Liquid
supernatant was defined as the quantity of enzyme degrading and was
expressed in U/g of wet tissue following the manufacturer's
protocol (Invitrogen).
Oxidative stress and inflammation
After treatment with BP for 5 consecutive days, and
after the rats were sacrificed and the spinal cord tissue removed,
the spinal tissue samples were centrifuged at 12,000 × g for 10
min. Liquid supernatant was used to assess SOD, MDA, NF-κB p65 and
TNF-α activity following the manufacturer's instructions (Beyotime,
Nanjing, China).
Western blot analysis
After treatment with BP for 5 consecutive days, and
after the rats were sacrificed and the spinal cord tissue removed,
the spinal tissue homogenates were centrifuged at 12,000 × g for 10
min. Liquid supernatant was subsequently used to measure the
protein concentration with the Coomassie (G250) binding method.
Fifty micrograms of sample proteins were loaded onto 12% sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and
then transferred to 0.22-mm nitrocellulose membranes (Bio-Rad,
Munich, Germany). The membranes were blocked with 5% non-fat milk
in Tris-buffered saline (pH 7.4) containing 0.1% Tween-20 (TBST)
for 2–3 h at room temperature. The membranes were subsequently
incubated with primary antibodies overnight at 4°C: antinuclear
factor erythroid 2-related factor 2 (Nrf2; sc-365949),
anti-phosphorylated (p-)Akt (sc-293125), anti-Akt (sc-135829),
p-p38 mitogen-activated protein kinase (MAPK; sc-7973),
anti-p-extracellular-signal-regulated kinase (ERK; sc-365234) and
anti-ERK (sc-514302) antibodies (all from Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). The membranes were washed with TBST and
incubated at 4°C with secondary antibody for 2–3 h, horseradish
peroxidase-linked anti-rabbit IgG (Santa Cruz Biotechnology,
Inc.).
Statistical analysis
The data were calculated using SPSS 17.0 software,
are expressed as the means ± SD, and were analyzed using one-way
analysis of variance (ANOVA) followed by Tukey's multiple
comparison test. A p-value <0.05 was considered to indicate a
statistically significant difference.
Results
BMSC culture and detection
As shown in Fig.
1, the cell morphology of BMSCs was observed: dark blue color,
spindle shape, and serial sub-cultivation, and homogeneity and
multiplicity were all observed. These features indicated that BMSCs
were successfully separated and cultivated.
Treatment with BP improves locomotor
recovery after SCI
In order to test the hypothesis that treatment with
BP improves locomotor recovery after SCI, BBB scores were
calculated. The results show that SCI caused a significant decrease
in BBB scores when compared to the control group, and these were
improved by BP treatment (Fig.
2). Five days after SCI induction, only the administration of
BMSCs augmented BBB scores in a non-statistically significant
manner. At day 5, the administration of plumbagin only
significantly increased the BBB scores when compared to the SCI
group. The administration of BMSCs and plumbagin (the BP group)
increased the BBB scores even more significantly (compared to the
SCI group) than the group treated only with plumbagin (Fig. 2).
Treatment with BP reduces spinal cord
water content after SCI
We then determined whether treatment with BP reduces
spinal cord water content after SCI. Spinal cord water content was
significantly elevated in the SCI group when compared to the
control group (Fig. 3). The
administration of BMSCs alone suppressed the spinal cord water
content in a non-statistically significant manner. Compared to the
rats in the SCI group, treatment with plumbagin significantly
lowered the spinal cord water content. However, in the rats in the
BP group, this decrease was even more significant (compared to the
SCI group) than that observed in the plumbagin-treated only group
after SCI induction (Fig. 3).
Treatment with BP affects MPO activity
after SCI
To clarify the effects of BP treatment on MPO
activity after SCI, MPO activity was measured. Compared with the
control group, MPO activity was increased after SCI (Fig. 4). BMSC treatment inhibited MPO
activity in a non-statistically significant manner. The increased
MPO activity was markedly decreased by treatment with plumbagin
when compared to the rats with SCI. In addition, MPO activity in
the BP-treated group was even lower than that in the
plumbagin-treated group (Fig.
4).
Treatment with BP affects oxidative
stress after SCI
In order to explore the effect of treatment with BP
on oxidative stress after SCI, SOD and MDA activity was analyzed.
SCI suppressed SOD activity and increased MDA activity compared
with the control group (Fig. 5).
Treatment with BMSCs slightly increased SOD activity and reduced
the promotion of MDA activity (neither was statistically
significant). The administration of plumbagin effectively reversed
the respective decrease in SOD activity and increase in MDA content
when compared to the SCI groups. Thus, treatment with BP
effectively enhanced the curative effects (Fig. 5).
Treatment with BP affects inflammation
after SCI
To further explore the effect of treatment with BP
on inflammation, NF-κB p65 and TNF-α activity was examined. We
demonstrated that the activity of NF-κB p65 and TNF-α in SCI rats
was markedly increased when compared to the control groups
(Fig. 6). The levels of
inflammatory factors in the BMSC-treated groups were very similar
to those of the SCI groups. However, the upregulation of NF-κB p65
and TNF-α was markedly reduced by administration of plumbagin when
compared to the SCI group. Moreover, compared to plumbagin
treatment, BP treatment decreased the activities of NF-κB p65 and
TNF-α even more considerably when compared to the SCI group
(Fig. 6).
Treatment with BP affects the Nrf2
pathway after SCI
To further investigate the possible mechanism of
treatment with BP, Nrf2 protein expression was appraised using
western blot analysis. We noted a significant upregulation of Nrf2
pathway protein expression (Fig.
7). There was no significant difference between the SCI group
and the BMSC-treated group. We also observed that Nrf2 protein
expression was significantly increased by treatment with plumbagin
only when compared to the SCI group. Moreover, treatment with BP
increased Nrf2 protein expression even more significantly when
compared to the SCI group (Fig.
7).
Treatment with BP affects the
phosphoinositide 3-kinase (PI3K)/Akt pathways after SCI
To further research the possible mechanism of
treatment with BP, p-Akt and Akt protein expression were evaluated
using western blot analysis. The results of the western blot
analysis showed that p-Akt/Akt expression in the SCI group was
significantly inhibited when compared to the control group
(Fig. 8). Administration of
plumbagin did not significantly affect p-Akt/Akt expression. In
addition, treatment with BMSCs significantly increased p-Akt/Akt
expression when compared to the SCI group. However, there was no
significant difference between the expression of p-Akt/Akt in the
BP-treated group and the BMSC-treated group (Fig. 8).
Treatment with BP affects the expression
of p-p38 MAPK after SCI
To further examine the possible mechanism of
treatment with BP on SCI, p-p38 MAPK protein expression was
evaluated using western blot analysis. We found that in the SCI
group p-p38 MAPK protein expression was significantly increased
when compared to the control group (Fig. 9). BMSCs inhibited the expression
in a non-statistically significant manner. Plumbagin significantly
inhibited the protein expression of p-p38 MAPK when compared to the
SCI group. Moreover, treatment with BP significantly inhibited the
protein expression of p-p38 MAPK compared with the SCI group
(Fig. 9).
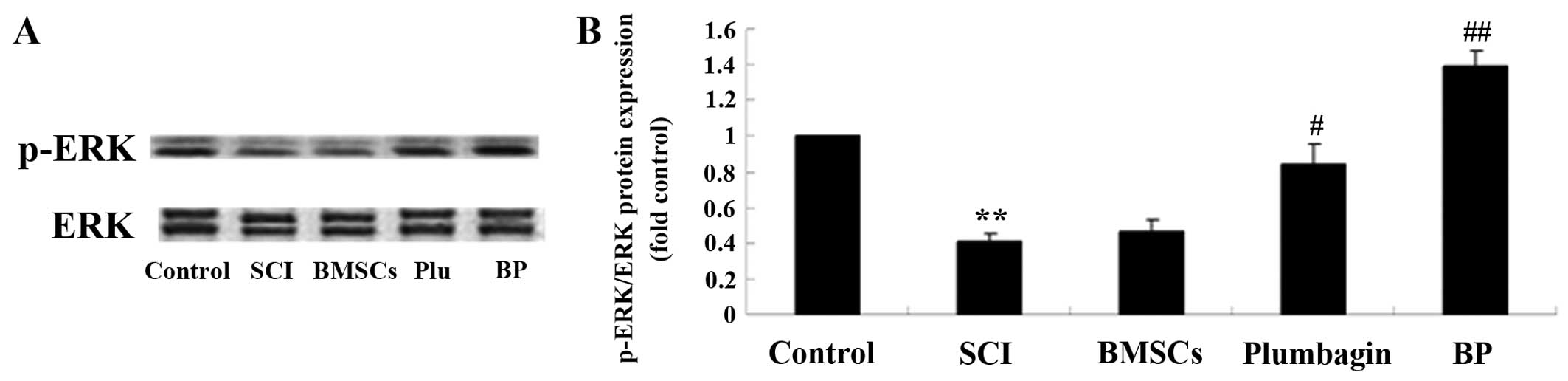
Treatment with BP affects the ERK
pathways after SCI
To further survey the possible effect of treatment
with BP on SCI, p-ERK and ERK protein expression were estimated
using western blot analysis. In the SCI group, p-ERK/ERK expression
was significantly inhibited when compared to the control group
(Fig. 10). We found no
significant inter-group difference between the SCI group and the
BMSC-treated group. The results revealed that plumbagin treatment
significantly increased the p-ERK/ERK rate when compared to the SCI
group. The p-ERK/ERK rate of expression in rats treated with BP was
considerably higher than in the SCI-treated group (Fig. 10).
Discussion
With the increasing economic development of society,
the incidence of SCI increases year by year, and it seriously
affects the quality of life of patients, and has serious adverse
effects on the patient's family and the community (15). The disintegration and necrosis of
a large number of neuronal cells in tissues of patients after SCI
is one of the major causes of the reduction in limb movement and
other sensory dysfunctions in the area below the injury (16). At present, there is no effective
method for paraplegics with SCI to gain restored function (17). Thus, promoting the regeneration of
nerve cells and recovering the structure and the function of spinal
cord are important and difficult issues which face neuroscientists
(18). In the present study,
treatment with BP was noted to improve locomotor recovery and
reduce spinal cord water content and MPO activity in rats with SCI.
Mansilla et al have previously reported that human
mesenchymal stem cells alleviated spinal cord injuries (19). Wang et al have demonstrated
that transplanted BMSCs reduce MPO activity in rats with
pancreatitis-associated lung injury (20). Zhang et al showed that
plumbagin protects against oxidative stress and inflammation in
rats with SCI (12). Thus, we
suggest that treatment with BP is a promising strategy for treating
SCI.
The SCI mechanism has not yet been fully elucidated,
but the present study indicates that oxidative stress plays an
important role in it. Currently, it is thought that many oxygen
free radicals (OFRs) are generated in SCI pathogenesis, which have
a trigger-like effect on damage to the body, and calcium overload
is a final common result of cellular damage (21). SOD is an enzyme that catalyzes the
disproportionation of superoxide anion, and is a major
antioxidative enzyme and free-radical scavenger in cells; it
protects cells against oxygen free radicals, and the level of SOD
indicates the level of protective effectiveness of cells from toxic
damage caused by free radicals (22). MDA is the end product of lipid
peroxidation, and MDA levels directly reflect the level of free
radicals, of which the content is an important indicator of tissue
damage (23). The determination
of SOD activity and MDA content indirectly reflect the ability of
the body's antioxidant system.
Inflammation plays an important role in the
pathogenesis of SCI, and the inflammatory response of local damaged
tissue increases the degree of secondary SCI (24). The secondary inflammatory
environment caused by local damage leads to cellular necrosis and
apoptosis at the SCI site, and irreversible pathological changes
occur, thereby increasing SCI (25). Previous research has reported that
neutrophils aggregate to the site of injury to release elastase and
other substances, increasing tissue edema, necrosis and promoting
neuronal and oligodendrocyte apoptosis, thus resulting in the
emergence of localized glial scars (26). The results from our study showed
that treatment with BP suppressed oxidative stress and inflammation
following SCI. Tu et al suggested that bone marrow-derived
mesenchymal stem cells attenuate oxidative stress and systemic
inflammation in rats with severe acute pancreatitis (27). Checker et al demonstrated
that plumbagin abrogates lipopolysaccharide-induced oxidative
stress, inflammatory oxidative stress, inflammation and endotoxic
shock (28). In the context of
these results, we found in the present study that the
anti-oxidative and anti-inflammatory effects of BP exert a certain
protective effect against damage caused by SCI.
In the body, the Nrf2/ARE signaling pathway acts as
an important endogenous anti-oxidative stress pathway, which
encodes and modulates the expression of various antioxidant genes,
enhances cellular resistance to oxidative stress, and thereby
reduces the damage to the body caused by oxidative stress (29). When the spinal cord suffers from
oxidative stress injury, the intracellular antioxidant defense
system is essential for reducing the neuronal damage caused, and
the Nrf2/ARE signaling pathway has been noted as the most important
endogenous anti-oxidative stress pathway. Thus far, it has been
noted that more than 200 endogenous genes can be encoded, and are
regulated by the Nrf2/ARE signaling pathway. As the major
regulatory protein of the Nrf2/ARE signaling pathway, antioxidant
enzymes do not only catalyze free radicals into non-toxic
substances, but also strengthen their water solubility to exclude
them, which is an important factor in maintaining the redox balance
of the body (30). In the present
study, we found that BP therapy increased the suppressed Nrf2
expression in rats with SCI. Son et al have demonstrated the
neuroprotective effects of plumbagin, and also demonstrated that it
protects against cerebral ischemia through activation of Nrf2/ARE
(31). Cho et al
demonstrated that mesenchymal stem cells promote the CCl4-inhibited
Nrf2 expression in rats with liver injury (32). In addition, we suggest that the
protective effect of BP on SCI damage is involved in the activation
of the Nrf2 signaling pathway.
Akt is an important factor in biological signaling
pathways, and constitutes an important part of the PI3K/Akt
signaling pathway, which inhibits apoptosis, promotes cell survival
and plays an important role in the angiogenesis process (33). The PI3K/Akt pathway is involved in
the growth and differentiation of neurons, inhibiting neuronal
apoptosis and promoting axonal growth and mediating synaptic
plasticity and many other cellular processes, and it plays an
important role in nerve physiological and pathological processes
(34). It has been previously
demonstrated that after optic nerve and hypoglossal nerve injury in
rodents, increased Akt expression causes anti-neuronal apoptosis.
After traumatic brain injury and ischemic brain injury in rats, Akt
activity in neurons was significantly increased, and neuronal
apoptosis was suppressed (35).
In the present study, we discovered that administration of BP
significantly increased the level of p-Akt/Akt compared to the SCI
group. Chen et al have reported that BMSCs upregulated p-ERK
and p-Akt through multiple pathways (36). Checker et al showed that
plumbagin inhibits inflammatory responses but does not affect the
phosphorylation of Akt (37).
Moreover, we suggest that the protective effect which BP exerts
against SCI damage involves the p-Akt signaling pathway.
MAPK is a type of serine/threonine protein kinase in
the cell, which exists in the majority of mammals in the cytoplasm
and nuclei, and can be stimulated for extracellular signaling to
the cells and their nuclei, and thus lead to cell biological
reactions, the regulation of cell proliferation, as well as
differentiation, development and apoptosis (38). It is known that p-p38 MAPK is
involved in nerve cell apoptosis and in the process of signal
transduction. It has also been noted that p-p38 MAPK is involved in
the acute inflammation caused by the SCI-induced apoptosis of
neurons and glial cells (39). In
the present study, treatment with BP significantly inhibited the
protein expression of p-p38 MAPK in rats with SCI. Wang et
al have suggested that the anti-inflammatory effect of
plumbagin mediates the inhibitory effect of MAPK signaling on
inflammation (40). Wang et
al have reported that BMSCs promote cell proliferation, but do
not influence p38 MAPK in rats (41). The reason for these various
conclusions is likely the dosage or disinfecting times of BMSCs,
and discussion in future studies is thus necessary.
Previous research has demonstrated that ERK1/2
exists in the central nervous system, and it participates in a
variety of physiological and pathological processes (42). Research has shown that SCI,
excitotoxicity and transection injury leads to elevated levels of
p-ERK1/2, and in cases of contusion and excitotoxicity, ERK1/2
phosphorylation is increased significantly in the areas adjacent to
the damage (43). In the present
study, treatment with BP significantly increased p-ERK1/2 in SCI
rats. Zhang et al demonstrated that BMSCs promoted
osteoblast differentiation through upregulation of the ERK1/2
signaling pathway (44). Yang
et al suggested that plumbagin activates ERK1/2 in 3T3-L1
cells (45). Thus, we suggest
that the activation of ERK1/2 increases the protective effect which
BP exerts againsts SCI damage.
In conclusion, in the present study, we identified
that treatment with BP alleviates SCI through its effects on
oxidative stress, inflammation, anti-apoptotis, the activation of
Nrf2, suppression of PI3K/Akt and p-p38 MAPK, and the upregulation
of p-ERK1/2.
References
|
1
|
Jiang SH, Tu WZ, Zou EM, Hu J, Wang S, Li
JR, Wang WS, He R, Cheng RD and Liao WJ: Neuroprotective effects of
different modalities of acupuncture on traumatic spinal cord injury
in rats. Evid Based Complement Alternat Med. 2014:4315802014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nardone R, Höller Y, Brigo F, Orioli A,
Tezzon F, Schwenker K, Christova M, Golaszewski S and Trinka E:
Descending motor pathways and cortical physiology after spinal cord
injury assessed by transcranial magnetic stimulation: a systematic
review. Brain Res. 139–154. 2014.PubMed/NCBI
|
|
3
|
Chen X, He F, Zhong DY and Luo ZP:
Acoustic-frequency vibratory stimulation regulates the balance
between osteogenesis and adipogenesis of human bone marrow-derived
mesenchymal stem cells. BioMed Res Int. 2015:5407312015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin H, Luo X, Jin B, Shi H and Gong H: The
effect of EPO gene overexpression on proliferation and migration of
mouse bone marrow-derived mesenchymal stem cells. Cell Biochem
Biophys. Nov 14–2014.Epub ahead of print.
|
|
5
|
Munoz JL, Greco SJ, Patel SA, Sherman LS,
Bhatt S, Bhatt RS, Shrensel JA, Guan YZ, Xie G, Ye JH, et al:
Feline bone marrow-derived mesenchymal stromal cells (MSCs) show
similar phenotype and functions with regards to neuronal
differentiation as human MSCs. Differentiation. 84:214–222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song M, Jue SS, Cho YA and Kim EC:
Comparison of the effects of human dental pulp stem cells and human
bone marrow-derived mesenchymal stem cells on ischemic human
astrocytes in vitro. J Neurosci Res. 93:973–983. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian L, Yin D, Ren Y, Gong C, Chen A and
Guo FJ: Plumbagin induces apoptosis via the p53 pathway and
generation of reactive oxygen species in human osteosarcoma cells.
Mol Med Rep. 5:126–132. 2012.
|
|
8
|
Luo P, Wong YF, Ge L, Zhang ZF, Liu Y, Liu
L and Zhou H: Anti-inflammatory and analgesic effect of plumbagin
through inhibition of nuclear factor-κB activation. J Pharmacol Exp
Ther. 335:735–742. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Subramaniya BR, Srinivasan G, Sadullah SS,
Davis N, Subhadara LB, Halagowder D and Sivasitambaram ND:
Apoptosis inducing effect of plumbagin on colonic cancer cells
depends on expression of COX-2. PLoS One. 6:e186952011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Erşahın M, Toklu HZ, Erzık C, Akakin D,
Tetık S, Sener G and Yeğen BC: Ghrelin alleviates spinal cord
injury in rats via its anti-inflammatory effects. Turk Neurosurg.
21:599–605. 2011.
|
|
11
|
Li F, Fei D, Sun L, Zhang S, Yuan Y, Zhang
L, Zhao K, Li R and Yu Y: Neuroprotective effect of bone marrow
stromal cell combination with atorvastatin in rat model of spinal
cord injury. Int J Clin Exp Med. 7:4967–4974. 2014.
|
|
12
|
Zhang W, Cheng L, Hou Y, Si M, Zhao YP and
Nie L: Plumbagin protects against spinal cord injury-induced
oxidative stress and inflammation in Wistar rats through Nrf-2
upregulation. Drug Res (Stuttg). 65:495–499. 2014. View Article : Google Scholar
|
|
13
|
Chio CC, Lin JW, Chang MW, Wang CC, Kuo
JR, Yang CZ and Chang CP: Therapeutic evaluation of etanercept in a
model of traumatic brain injury. J Neurochem. 115:921–929. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vink R, Young A, Bennett CJ, Hu X, Connor
CO, Cernak I and Nimmo AJ: Neuropeptide release influences brain
edema formation after diffuse traumatic brain injury. Acta
Neurochir Suppl. 86:257–260. 2003.
|
|
15
|
Wang T, Yuan W, Liu Y, Zhang Y, Wang Z,
Zhou X, Ning G, Zhang L, Yao L, Feng S and Kong X: The role of the
JAK-STAT pathway in neural stem cells, neural progenitor cells and
reactive astrocytes after spinal cord injury. Biomed Rep.
3:141–146. 2015.PubMed/NCBI
|
|
16
|
Chen MH, Ren QX, Yang WF, Chen XL, Lu C
and Sun J: Influences of HIF-lα on Bax/Bcl-2 and VEGF expressions
in rats with spinal cord injury. Int J Clin Exp Pathol.
6:2312–2322. 2013.
|
|
17
|
Bransford RJ, Chapman JR, Skelly AC and
Vanalstyne EM: What do we currently know about thoracic spinal cord
recovery and outcomes? A systematic review. J Neurosurg Spine.
17(Suppl 1): 52–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adams M and Cavanagh JF: International
Campaign for Cures of Spinal Cord Injury Paralysis (ICCP): another
step forward for spinal cord injury research. Spinal Cord.
42:273–280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mansilla E, Marin GH, Sturla F, Drago HE,
Gil MA, Salas E, Gardiner MC, Piccinelli G, Bossi S, Salas E, et
al: Human mesenchymal stem cells are tolerized by mice and improve
skin and spinal cord injuries. Transplant Proc. 37:292–294. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Tu XH, Zhao P, Song JX and Zou ZD:
Protective effect of transplanted bone marrow-derived mesenchymal
stem cells on pancreatitis-associated lung injury in rats. Mol Med
Rep. 6:287–292. 2012.PubMed/NCBI
|
|
21
|
Qayumi AK, Janusz MT, Jamieson WR and
Lyster DM: Pharmacologic interventions for prevention of spinal
cord injury caused by aortic crossclamping. J Thorac Cardiovasc
Surg. 104:256–261. 1992.PubMed/NCBI
|
|
22
|
Cuevas P, Reimers D, Carceller F and
Jimenez A: Ischemic reperfusion injury in rabbit spinal cord:
protective effect of superoxide dismutase on neurological recovery
and spinal infarction. Acta Anat (Basel). 137:303–310. 1990.
View Article : Google Scholar
|
|
23
|
Koc RK, Akdemir H, Karakücük EI, Oktem IS
and Menkü A: Effect of methylprednisolone, tirilazad mesylate and
vitamin E on lipid peroxidation after experimental spinal cord
injury. Spinal Cord. 37:29–32. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Chen C, Ma S, Wang Y, Zhang X and
Su X: Inhibition of monocyte chemoattractant peptide-1 decreases
secondary spinal cord injury. Mol Med Rep. 11:4262–4266.
2015.PubMed/NCBI
|
|
25
|
Yao AH, Jia LY, Zhang YK, Ma QR, Cheng P,
Liu L, Ju G and Kuang F: Early blockade of TLRs MyD88-dependent
pathway may reduce secondary spinal cord injury in the rats. Evid
Based Complement Alternat Med. 2012:5912982012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kadota R, Koda M, Kawabe J, Hashimoto M,
Nishio Y, Mannoji C, Miyashita T, Furuya T, Okawa A, Takahashi K
and Yamazaki M: Granulocyte colony-stimulating factor (G-CSF)
protects oligodendrocyte and promotes hindlimb functional recovery
after spinal cord injury in rats. PLoS One. 7:e503912012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tu XH, Song JX, Xue XJ, Guo XW, Ma YX,
Chen ZY, Zou ZD and Wang L: Role of bone marrow-derived mesenchymal
stem cells in a rat model of severe acute pancreatitis. World J
Gastroenterol. 18:2270–2279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Checker R, Patwardhan RS, Sharma D, Menon
J, Thoh M, Sandur SK, Sainis KB and Poduval TB: Plumbagin, a
vitamin K3 analogue, abrogates lipopolysaccharide-induced oxidative
stress, inflammation and endotoxic shock via NF-κB suppression.
Inflammation. 37:542–554. 2014. View Article : Google Scholar
|
|
29
|
Benedict AL, Mountney A, Hurtado A, Bryan
KE, Schnaar RL, Dinkova-Kostova AT and Talalay P: Neuroprotective
effects of sulforaphane after contusive spinal cord injury. J
Neurotrauma. 29:2576–2586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, de Rivero Vaccari JP, Wang H, Diaz
P, German R, Marcillo AE and Keane RW: Activation of the nuclear
factor E2-related factor 2/antioxidant response element pathway is
neuroprotective after spinal cord injury. J Neurotrauma.
29:936–945. 2012. View Article : Google Scholar :
|
|
31
|
Son TG, Camandola S, Arumugam TV, Cutler
RG, Telljohann RS, Mughal MR, Moore TA, Luo W, Yu QS, Johnson DA,
et al: Plumbagin, a novel Nrf2/ARE activator, protects against
cerebral ischemia. J Neurochem. 112:1316–1326. 2010. View Article : Google Scholar :
|
|
32
|
Cho KA, Woo SY, Seoh JY, Han HS and Ryu
KH: Mesenchymal stem cells restore CCl4-induced liver injury by an
antioxidative process. Cell Biol Int. 36:1267–1274. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu W, Zhang L, Shi J, Liu Y, Zhou L, Hou
K, Qu X and Teng Y: Clinical significance of pAkt and pErk1/2
expression in early-stage breast cancer patients treated with
anthracycline-based adjuvant chemotherapy. Oncol Lett. 9:1707–1714.
2015.PubMed/NCBI
|
|
34
|
Ha KS, Kim KM, Kwon YG, Bai SK, Nam WD,
Yoo YM, Kim PK, Chung HT, Billiar TR and Kim YM: Nitric oxide
prevents 6-hydroxydopamine-induced apoptosis in PC12 cells through
cGMP-dependent PI3 kinase/Akt activation. FASEB J. 17:1036–1047.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chuenkova MV and Pereiraperrin M:
Neurodegeneration and neuroregeneration in Chagas disease. Adv
Parasitol. 76:195–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen TL, Zhu GL, Wang JA, Wang Y, He XL
and Jiang J: Apoptosis of bone marrow mesenchymal stem cells caused
by hypoxia/reoxygenation via multiple pathways. Int J Clin Exp Med.
7:4686–4697. 2014.
|
|
37
|
Checker R, Sharma D, Sandur SK,
Subrahmanyam G, Krishnan S, Poduval TB and Sainis KB: Plumbagin
inhibits proliferative and inflammatory responses of T cells
independent of ROS generation but by modulating intracellular
thiols. J Cell Biochem. 110:1082–1093. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shin BA, Yoo HG, Kim HS, Kim MH, Hwang YS,
Chay KO, Lee KY, Ahn BW and Jung YD: p38 MAPK pathway is involved
in the urokinase plasminogen activator expression in human gastric
SNU-638 cells. Oncol Rep. 10:1467–1471. 2003.PubMed/NCBI
|
|
39
|
Galan-Arriero I, Avila-Martin G,
Ferrer-Donato A, Gomez-Soriano J, Bravo-Esteban E and Taylor J:
Oral administration of the p38α MAPK inhibitor, UR13870, inhibits
affective pain behavior after spinal cord injury. Pain.
155:2188–2198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang T, Wu F, Jin Z, Zhai Z, Wang Y, Tu B,
Yan W and Tang T: Plumbagin inhibits LPS-induced inflammation
through the inactivation of the nuclear factor-kappa B and mitogen
activated protein kinase signaling pathways in RAW 264.7 cells.
Food Chem Toxicol. 64:177–183. 2014. View Article : Google Scholar
|
|
41
|
Wang Y, Wang J, Bai D, Song J, Ye R, Zhao
Z, Lei L, Hao J, Jiang C, Fang S, et al: Cell proliferation is
promoted by compressive stress during early stage of chondrogenic
differentiation of rat BMSCs. J Cell Physiol. 228:1935–1942. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pernet V, Hauswirth WW and Di Polo A:
Extracellular signal-regulated kinase 1/2 mediates survival, but
not axon regeneration, of adult injured central nervous system
neurons in vivo. J Neurochem. 93:72–83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shi B, Ding J, Liu Y, Zhuang X, Zhuang X,
Chen X and Fu C: ERK1/2 pathway-mediated differentiation of
IGF-1-transfected spinal cord-derived neural stem cells into
oligodendrocytes. PLoS One. 9:e1060382014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang P, Wu Y, Jiang Z, Jiang L and Fang
B: Osteogenic response of mesenchymal stem cells to continuous
mechanical strain is dependent on ERK1/2-Runx2 signaling. Int J Mol
Med. 29:1083–1089. 2012.PubMed/NCBI
|
|
45
|
Yang SJ, Chang SC, Wen HC, Chen CY, Liao
JF and Chang CH: Plumbagin activates ERK1/2 and Akt via superoxide,
Src and PI3-kinase in 3T3-L1 cells. Eur J Pharmacol. 638:21–28.
2010. View Article : Google Scholar : PubMed/NCBI
|