1. Cardiovascular disease risk related to
low doses of ionizing radiation
Recognition of radiation-related
cardiovascular disease (CVD) risk
The recognition that exposure of the heart and the
vasculature to high doses of ionizing radiation can cause CVD began
in the late 1960s (1). This was
mainly related to the clinical observation of cardiovascular
complications in radiation-treated survivors of Hodgkin's lymphoma
and other childhood cancers. Later, larger-scale epidemiological
studies found a clear association between therapeutic doses of
thoracic irradiation and an increased risk of CVD in these
long-term cancer survivors, confirming the earlier observations
(2).
An excess risk of CVD was also observed after
post-operative radiotherapy for breast cancer. In these patients, a
part of the heart received accumulated doses of ≥40 Gy
(fractionated 20×2 Gy). After correction for fractionation effects
using the linear quadratic model and an α/β ratio of 1–3 Gy,
determined in experimental studies in the rat heart, Schultz-Hector
and Trott calculated that this corresponds to equivalent single
doses to the total heart of approximately 1–2 Gy (3). The Early Breast Cancer Trialists'
Collaborative Group performed a meta-analysis on mortality data of
>30.000 breast cancer patients 15 years after treatment. The
mortality of heart disease was increased by 27% in patients treated
with surgery and subsequent radiotherapy compared to patients
treated with surgery alone (4).
The evaluation of long-term mortality in breast cancer survivors
may however be influenced by the varying prognosis of the different
treatment regimens (surgery vs. radiotherapy). This can be
circumvented by comparing women irradiated for left-sided tumours
with women irradiated for right-sided tumours. Cardiac radiation
doses are larger in radiotherapy patients with left-sided tumours
than in radiotherapy patients with right-sided tumours (5). An analysis of 308,861 women with
breast cancer registered in the Surveillance, Epidemiology and
End-Results cancer registries database from the United States
revealed an increased heart disease mortality ratio for women
irradiated for left-sided breast cancer compared to those
irradiated for right-sided breast cancer (6). A study related to 72,134 women
diagnosed with breast cancer in Denmark and Sweden during the years
1976–2006 and a follow-up of 30 years revealed an increased risk of
ischemic heart disease (IHD), pericarditis and valvular disease in
irradiated women with left-sided tumours (mean cardiac dose 6.3 Gy)
compared to those with right-sided tumours (mean cardiac dose 2.7
Gy) (7).
In addition, patients with benign diseases, such as
peptic ulcers treated with radiotherapy form interesting study
cohorts. For instance, coronary heart disease-related mortality was
compared between peptic ulcer patients treated with radiotherapy
(n=1859) and those treated by other means (n=1860) (8). The calculated received
volume-weighted cardiac doses ranged from 1.6 to 3.9 Gy and the
portion of the heart directly in the radiation field received doses
of 7.6–18.4 Gy. A significantly increased risk of coronary heart
disease-related mortality was observed with the increasing dose.
Only recently, various epidemiological findings, in particular from
the Japanese atomic bomb survivors, have raised awareness of
possible CVD risk following exposure to low and moderate doses of
radiation (3). Below an overview
is given of the major epidemiological findings related to CVD risk
following low-dose exposure.
Low-dose exposed epidemiological
cohorts
Classification of CVD in
epidemiology
Reviewing the epidemiological literature related to
CVD and low-dose ionizing radiation is complicated by the different
classifications of CVD. Moreover, all types of CVD are often pooled
in one diagnosis in epidemiological studies. This hampers the
thorough understanding of radiation-related CVD risk, and
distinction should be made between the different clinical
manifestations. In addition, many epidemiological studies face the
problem of misclassification of the cause of death, except for
stroke, for which the diagnosis tends to be reasonably good
(9). In fact, stroke is not
considered to be a CVD, but a circulatory disease since it involves
the blood circulation in the brain and is unrelated to the heart.
It is defined by brain injury, which occurs when a blood vessel in
the brain ruptures, leading to haemorrhage, or when a blood vessel
is blocked, leading to ischemia following the loss of blood supply
in the brain area of concern.
Patients treated with radiation
therapy (RT)
External beam RT for breast cancer, Hodgkin's
lymphoma, or even peptic ulcer disease in the early days often
involves some incidental exposure of the heart. There are studies
pointing to late secondary cardiovascular effects due to this
scattered radiation exposure. Long-term follow-up was shown to be
essential, as the cardiovascular complications may manifest years
after the completion of RT.
Most peptic ulcers are caused by an infection with a
type of bacteria known as Helicobacter pylori and are
nowadays treated, at least partly, with antibiotics. However,
mid-last century peptic ulcer disease patients were irradiated.
Peptic ulcer disease patients treated with RT (n=1859) or by other
means (n=1860) at the University of Chicago Medical Center between
1936 and 1965, were followed through 1997 by Carr et al
(8). The irradiated patients
received volume-weighted cardiac doses ranging from 1.6 to 3.9 Gy
and the portion of the heart directly in the radiation field
received doses of 7.6–18.4 Gy. The observed numbers of
cause-specific deaths were compared with the expected numbers from
the general population rates. Greater than expected coronary heart
disease-related mortality was observed among the irradiated
patients. The excess coronary heart disease risk in patients who
received RT for peptic ulcer disease decades before indicates the
need for long-term follow-up of CVD after chest RT.
Over the last half century, RT has evolved to become
one of the cornerstones of treatment for various types of cancer.
It is estimated that >50% of patients with cancer are treated
with radiotherapy. Along with the development of novel
chemotherapeutic agents, RT has revolutionized the prognosis of
patients with various types of cancer. Cancers during childhood and
adolescence are now more and more successfully treated and these
patients go on to live an active and normal adult life, as evident
by an increasing number of cancer survivors. Late cardiovascular
effects are often observed in cancer survivors (10). Hodgkin's lymphoma was the earliest
paradigm for the study of radiation-induced vascular disease. A
first case report was published in 1924 about histological changes
in the human heart after irradiation for Hodgkin's lymphoma
(11). Amongst Hodgkin's lymphoma
patients who received radiation, CVD is of the most common causes
of death. Studies have shown that these patients have an increased
risk of coronary artery disease, valvular heart disease, congestive
heart failure, pericardial disease and sudden death. The risk is
particularly high in patients treated before the age of 40 years
(12–15).
In addition, RT for breast cancer often involves
some incidental exposure of the heart to ionizing radiation. Darby
et al (16) conducted a
population-based case-control study of major coronary events (i.e.,
myocardial infarction, coronary revascularization, or death from
IHD) in 2,168 women who underwent radiotherapy for breast cancer
between 1958 and 2001 in Sweden and Denmark. The overall average of
the mean doses to the whole heart was 4.9 Gy (range, 0.03–27.72).
The rates of major coronary events increased linearly with the mean
dose to the heart by 7.4%/gray [95% confidence interval (CI),
2.9–14.5; P<0.001], with no apparent threshold. The increase
began within the first 5 years after radiotherapy and continued
into the third decade after radiotherapy.
Due to improvements in radiation techniques (e.g.,
breathing-adapted RT, CyberKnife), the risk of cardiovascular
complications in relation to radiation are uncertain, but may be
expected to decline. However, patients with classical risk factors,
such as hypertension, smoking and hyperlipidaemia may be at an
increased risk of radiation-related cardiovascular complications,
and these risk factors should be treated aggressively (10). Younger patients should be
screened, as this patient population at risk usually has a
considerable life expectancy.
Survivors of the atomic bombings of
Hiroshima and Nagasaki
The most informative cohort is the Life Span Study
(LSS), consisting of 120,321 exposed and non-exposed individuals
selected from respondents to the national census of Japan in 1950
calling for survivors exposed to the bombings in Hiroshima and
Nagasaki, and from residential surveys in the cities after the
national census. Mortality in this population has been investigated
since the 1950s by collecting information through the national
population registry (koseki) and death certificates obtained
throughout Japan. Cancer incidence data was available from
population-based cancer registries since 1957 in Hiroshima and
since 1958 in Nagasaki (17).
Next to the availability of these data, the large size, the
presence of both genders and all ages, and well-characterized
individual dose estimates makes this cohort a valuable source for
risk estimation. Another cohort, the Adult Health Study (AHS), was
established in 1958 and consists of 19,961 subjects from the LSS
cohort. These survivors underwent biennial health examinations,
which provided additional clinical and sub-clinical information to
the death and cancer registries data. In this way, disease
morbidity for a variety of conditions can be investigated (18).
Preston et al evaluated non-cancer mortality
based on the LSS report 13 published by the Radiation Effects
Research Foundation (RERF), which covers the time period between
1950–1997 (19). In their study,
the weighted colon doses from the DS86 dosimetry system were used
for individual dose estimates. Only the period between 1968–1997
was included to account for the 'healthy survivor' selection
effect. Individuals had to be alive in 1950 to enter the LSS cohort
and have thus survived the difficult conditions after the bombing,
which means that the health experience of this cohort may not be
typical for a normal population. This is reflected as a decrease in
non-cancer mortality during 1950–1960 in the LSS members that
received doses below 2 Sv, as shown by Shimizu et al
(20). This 'healthy survivor'
selection effect had largely disappeared by the mid-1960s. To
exclude this confounding effect, Preston et al advised to
restrict the analyses to proximal survivors who were within 3 km of
the hypocenter of the bombing, and to a follow-up period starting
from 1968 (19). Based on the
linear no-threshold model (LNT) model, excess relative risk (ERR)
estimates were calculated to be 0.17 with 90% CI (0.08–0.26) for
heart disease and 0.12 (90% CI, 0.02–0.22) for stroke, for the
period between 1968–1997 (19).
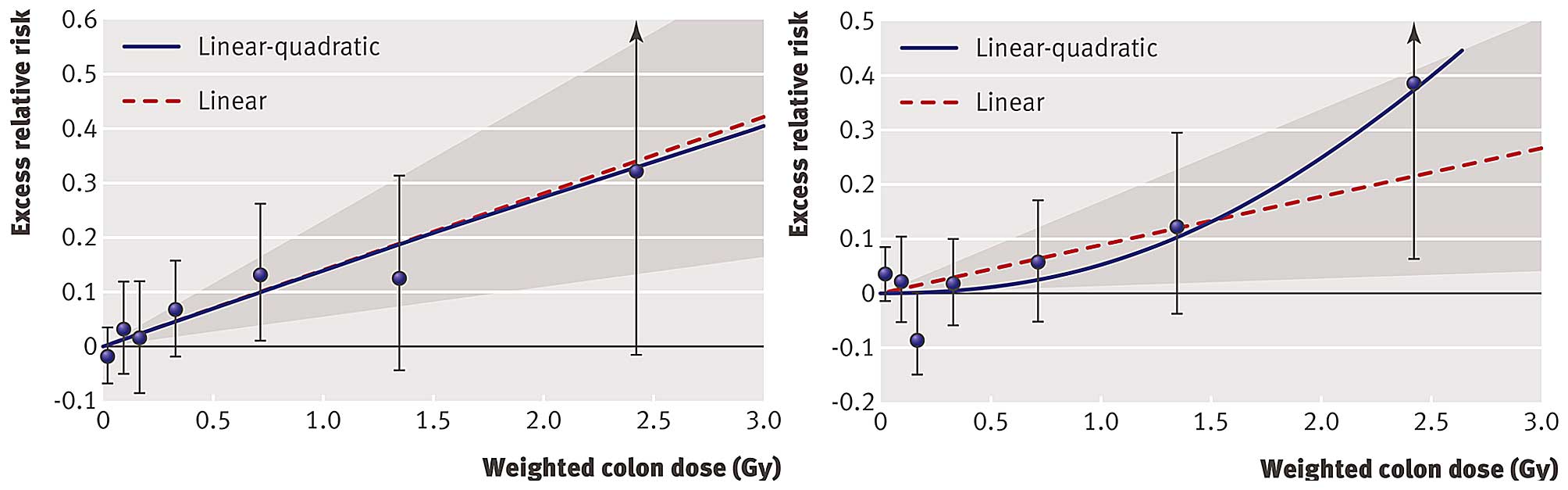
Shimizu et al evaluated ERR of mortality from
heart disease and stroke in the LSS cohort with a follow-up of 53
years (1950–2003) (21). For
individual dose estimates, weighted colon doses (Gy) from the DS02
dosimetry system were used. In addition, the authors obtained, by a
mail survey, information regarding sociodemographic (education,
occupation type), lifestyle (smoking, alcohol intake) and health
variables (obesity, diabetes mellitus) from 36,468 members of the
LSS cohort. This allowed them to evaluate the effect of these
confounding factors on ERR estimates. It should be noted that they
included the full follow-up period from 1950–2003 and all
survivors, thus not taking into account the 'healthy survivor'
selection effect. For the detrimental health outcomes of heart
diseases and stroke there is, however, no healthy survivor
selection effect. This has recently been confirmed by Schöllnberger
et al (23) following the
comments of Little et al (22). Shimizu et al (21) found an ERR of 0.14 (95% CI,
0.06–0.23) for heart disease and an ERR of 0.09 (95% CI, 0.01–0.17)
for stroke based on the LNT model. Whereas the LNT model fitted
best the data for heart disease, the quadratic model was best to
fit the data for stroke (Fig. 1).
The latter model implies relatively little risk at lower doses.
Indeed, the calculation of ERR for stroke over restricted dose
ranges revealed an ERR of 0.03 (95% CI, −0.10–0.16) for 0–1 Gy and
−0.07 (95% CI, −0.28–0.16) for 0–0.5 Gy. Furthermore, they showed
that the association of dose with CVD risk in the LSS cohort is
unlikely to be an artefact from confounding by sociodemographic,
lifestyle or disease risk factors.
The above-mentioned studies have used the LSS cohort
for CVD risk estimations. Takahashi et al examined the
association with dose and the incidence of stroke in the AHS cohort
(18). For their study,
information of health examinations from the follow-up from 1980
onwards has been used, resulting in 9,515 AHS participants. For
individual dose estimates, weighted colon doses (Gy) from the DS02
dosimetry system were used. In this study population, risk for
haemorrhagic stroke was observed to increase with dose. This was
across the full range of doses for men, while in women there seems
to be a threshold of approximately 1.3 Gy.
Occupational exposure
Studies on radiation workers are of interest since
they generally involve relatively low doses received over repeated
exposures, although in some cases, accumulated doses may be high.
Various studies have been performed, of which the most important
will be discussed. The largest studied cohort consists of 275,000
nuclear industry workers from 15 countries, referred to as the
15-country study (24). The
average cumulative dose received was 20.7 mSv. An overall
increasing trend, although not significant, for circulatory disease
mortality was observed. It was concluded that their findings are
compatible with both no increased risk and with an increased risk
comparable to that observed in A-bomb survivors. The Chernobyl
liquidator cohort consisted of 61,017 individuals with an average
cumulative dose of 0.109 Gy. An ERR/Gy of 0.41 (95% CI, 0.05–0.78)
was found for IHD morbidity and 0.45 (95% CI, 0.11–0.80) for the
morbidity of cerebrovascular diseases, though the outcomes were not
adjusted for recognized risk factors such as excessive weight,
hypercholesterolemia, smoking, alcohol consumption and others
(25). A study by Muirhead et
al revealed an increasing circulatory disease mortality risk
with dose, which was borderline significant, in the UK National
Registry of Radiation Workers in the industrial and medical field
(26). The average cumulative
dose received was 24.9 mSv. This finding should, however, be
interpreted with caution due to the lack of information on
confounding factors. Another large cohort consisted of 206,620
radiation workers in the industrial and medical field, registered
in the National Dose Registry of Canada (27). The average exposure of all workers
was 6.3 mSv, with large differences between males (10.6 mSv) and
females (1.7 mSv). A significant increasing trend of circulatory
disease mortality with dose was observed in males. Again, there is
a lack of information on confounding factors and there is also
incompleteness of dose records. Finally, a recent publication
studied a 34% increase in stroke incidence after a survey during
the years 1994–2008 of a cohort of 90,957 radiologic technologists
who worked with fluoroscopically guided interventional procedures
(28). In addition, mortality
from stroke was also modestly elevated, although not statistically
significant. No statistically significant excess risks of incidence
or mortality were observed from any other cardiovascular disorders
evaluated.
The Mayak cohort is of particular interest since it
includes information both on mortality and morbidity, and
information on confounding factors (29). In 1948, the first nuclear energy
enterprise in Russia, Mayak Plutonium Association, became
operational. Since 1948 the Mayak personnel undergo regular routine
medical examinations. In addition, every 3–5 years a more detailed
examination is carried out in a specialized hospital. This
examination system led to a unique archive of medical data, which
was used to create the 'Clinic' medical-dosimetric database. In
addition, from a dosimetric point of view, the database is sound.
Individual dosimetry for external gamma exposure was introduced at
the beginning of 1948 and for internal exposure during the 1960s
(29). Complete data are
available for 12,585 Mayak workers employed during the years
1948–1958 and followed-up until December 2000. The mean cumulated
external dose was 0.91±0.95 Gy (99% percentile 3.9 Gy) for men and
0.65±0.75 Gy (99% percentile 2.99 Gy) for women. In this cohort, a
significant increasing trend in IHD morbidity was observed with the
increasing total external dose [ERR/Gy=0.11 (95% CI, 0.049–0.168)].
The influence of confounding factors on this trend was minimal
(30). A follow-up study involved
the analysis of a cohort including 18,763 Mayak workers with an
additional follow-up of 5 years (31). Overall, risk estimates for IHD
were similar to the earlier study [ERR/Gy=0.10 (95% CI,
0.045–0.153)]. Remarkable though, a statistically significant
decrease in IHD incidence was found among workers exposed to
external doses of 0.2–0.5 Gy compared to workers exposed to
external doses below 0.2 Gy. This decreased risk is heavily
influenced by the observations in female workers. The authors
further noted that this finding should be interpreted with caution
since it has never been reported in other studies. The latter
analysis was further updated and extended by looking at the
lag-time to progression of IHD and by using the updated dosimetry
system MWDS-2008 (32). In that
study, it was observed that the main detrimental effects of
external radiation exposure occurred after >30 years. In
addition, a statistically significant risk was observed in men for
mortality caused by IHD [ERR/Gy=0.09 (95% CI, 0.02–0.16)] while the
risk was not significant for women. Recently, Azizova et al
published a study regarding incidence and mortality from IHD in an
extended cohort of 22,377 Mayak workers first employed during the
years 1948–1982 and followed-up to the end of 2008 (33). Risk analysis demonstrated a
significant increasing trend in IHD incidence, but not mortality,
with total dose from external gamma-rays after having adjusted for
non-radiation factors and dose from internal radiation. ERR/Gy for
IHD incidence in males was 6-fold higher than in females. In
addition, a significant increasing linear trend was observed in IHD
mortality, but not incidence, with total absorbed dose from
internal alpha radiation to the liver after having adjusted for
non-radiation factors and doses from external gamma-rays.
In the same Mayak population, the incidence of and
mortality from cerebrovascular disease was studied (34). The cohort consisted of 22,377
workers from the extended Mayak worker cohort that was followed-up
to the end of 2008. In this study, the workers were exposed to a
mean cumulated external dose of 0.54±0.76 Gy (95% percentile 2.21
Gy) for men and 0.44±0.65 Gy (95% percentile 1.87 Gy) for women.
After correction for confounding factors, a significant increasing
trend in cerebrovascular disease incidence was observed with
increasing total external dose [ERR/Gy=0.46 (95% CI, 0.37–0.57)].
In addition, the authors showed that the cerebrovascular disease
incidence was significantly higher in workers with a total external
dose >0.1 Gy when compared to those exposed to lower doses.
Restricting the analysis to a subcohort with negligible internal
exposure for incidence of cerebrovascular disease supports a dose
response sub-linear for low doses for incidence of cerebrovascular
disease (35). In that study, the
excess relative risk/dose was confirmed to be significantly higher
for the incidence of cerebrovascular disease in comparison to
cerebrovascular disease mortality and the incidence of stroke. The
authors hypothesized that this difference was based on the complex
nature of cerebrovascular diseases. The incidence was mainly
related to chronic forms of cerebrovascular disease, while the
mortality was mostly caused by the acute forms. Finally, having a
young age during exposure was observed to be an important,
aggravating modifier of radiation risk for incidence of
cerebrovascular disease and stroke.
It should be noted that apart from the classical
vascular-related confounding factors, occupational studies have to
deal with the 'healthy worker' selection effect, similar to the
'healthy survivor' selection effect in A-bomb survivors. The
'healthy worker' selection effect occurs when workers who are
healthier and have lower mortality and morbidity rates are
selectively retained in the workplace, as such accumulating higher
doses. One can adjust for this confounding factor by considering
the duration of employment as a confounding factor in the analysis,
as done in the 15-country study (24).
Meta-analysis of epidemiological
data
The Advisory Group on Ionizing Radiation (AGIR) from
the Health Protection Agency reviewed the available epidemiological
data for low- and moderate-dose exposure in 2010. Taking all the
studies together, they reported a small, but statistically
significant overall ERR/Gy of 0.09 (95% CI, 0.07–0.12). AGIR
noticed, however, that there was a lot of heterogeneity in risk
estimates of the different studies included in their meta-analysis
(9). Little et al recently
extended this meta-analysis (36). They estimated excess risks for
four subgroups of circulatory disease, classified according to the
'International Classification of Diseases, 10th revision': IHD,
non-IHD, cerebrovascular disease and all other circulatory
diseases. A significant effect of heterogeneity between the
different studies was found for cerebrovascular disease and other
circulatory diseases, but not for IHD and non-IHD. ERR were
calculated based on the LNT model, which implicitly assumes a
linear association of CVD risk at low doses and dose rates. They
noted that this assumption is reasonable since there is little
evidence for non-linearity in the Japanese atomic bomb survivors
and Mayak workers data. Furthermore, at least for IHD and non-IHD,
the ERR/Sv was consistent between the Japanese atomic bomb
survivors, Mayak workers and other occupational cohorts (36). Although it should be noted that
Schöllnberger et al and others advocate for the
consideration and testing of other dose-response models for
non-cancer effects (32,37). To conclude, the overall consensus
of the above-mentioned studies is that there is a significant
elevated CVD risk for doses >0.5 Gy (9,38).
Epidemiology alone is not the
answer
CVD is the leading cause of mortality and morbidity
and accounts for 30–50% of all deaths in most developed countries.
It is a multifactorial disease with many risk factors, such as
lifestyle and other personal factors (39). The most established risk factors
include the male gender, elevated low-density lipoprotein (LDL)
levels, smoking, hypertension, a family history of premature
coronary disease and diabetes mellitus (40). Epidemiological studies, as
presented above, have limited statistical power to detect a
possible excess risk of CVD following low-dose exposure (<0.5
Gy), due to the high background level of CVD in the population as a
whole and many potentially confounding risk factors (9). For example, it has been calculated
that, if the excess risk is in proportion to dose, a cohort of 5
million individuals would be needed to quantify the excess risk of
a 10 mSv dose (41). Other
factors that have an influence on epidemiological results are the
distribution of the dose range, the accuracy of dosimetry, the
duration of follow-up after exposure and correct assignment of
cause of mortality, as reviewed by Borghini et al (42).
Although epidemiological studies have led to a
better insight in radiation-related CVD risk, there are still many
uncertainties that need to be clarified. These include whether
there is a threshold dose; whether the latency of CVD development
is dependent on the dose; the identification of the sensitive
targets in the heart and vasculature; whether exposure has an
impact on CVD incidence or progression, or both; and the exact
impact of acute, fractionated or chronic exposure on risk
estimates. For an accurate dose risk assessment, these questions
need to be answered.
Classical epidemiological studies, as described
above, do not provide all the needed insight to answer these
questions. A more targeted approach, such as the integration of
epidemiology and biology is required. For example, the assessment
of subclinical endpoints and other cardiovascular biomarkers by
functional imaging in patients receiving radiotherapy may provide
insight into the development and progression of CVD following
radiation exposure (39,42). Single-photon emission computed
tomography (SPECT) or positron emission tomography (PET) imaging of
micro-vascular perfusion has already been applied in breast cancer
studies. The outcome differed between studies. For instance,
whereas in one study perfusion defects were observed within 6–12
months after radiotherapy (43),
no significant differences in perfusion defects were found in
another study (44). In addition,
the evaluation of cardiovascular biomarkers in patients receiving
radiotherapy may be useful. For instance, elevated levels of
N-terminal pro-B-type natriuretic peptide (NT-proBNP) in the blood
(45,46) have been shown to be predictive for
heart failure and/or CVD mortality across a broad range of
individuals (47). Higher values
of NT-proBNP were found in patients treated with radiotherapy for
left-sided breast cancer compared to patients treated with other
means (48).
Next to epidemiology, radiobiological research is
essential for understanding CVD risk specifically in the low-dose
region. Since epidemiological findings for low and moderate doses
are suggestive and not persuasive, their use in dose risk
assessment is limited. A thorough understanding of the biological
and cellular mechanisms gathered through experimental studies is
thus needed to complement the epidemiological findings. Once we
have a comprehensive understanding of the underlying biological
mechanisms, biologically-based dose-response models can be included
in the dose risk assessment. This may prove to be beneficial for an
accurate risk estimation in the low-dose region (49).
Societal concern
The possible excess risk of CVD following exposure
to low doses is of great societal concern. According to the ICRP, a
dose of 0.5 Sv may lead to approximately 1% of exposed individuals
developing cardiovascular or cerebrovascular disease >10 years
following exposure, in addition to the 30–50% suffering from
disease without being exposed to ionizing radiation (50). Although the assumed risk is rather
small, it may have serious implications for public health. Indeed,
seeing the high background rate of CVD, the absolute number of
excess cases would be substantial (42).
Various issues, such as occupational radiation
exposure, the future of nuclear power, manned space flights and the
threat of radiological terrorism, call for a thorough understanding
of low-dose health risks (41).
The main concern is, however, the increasing use of ionizing
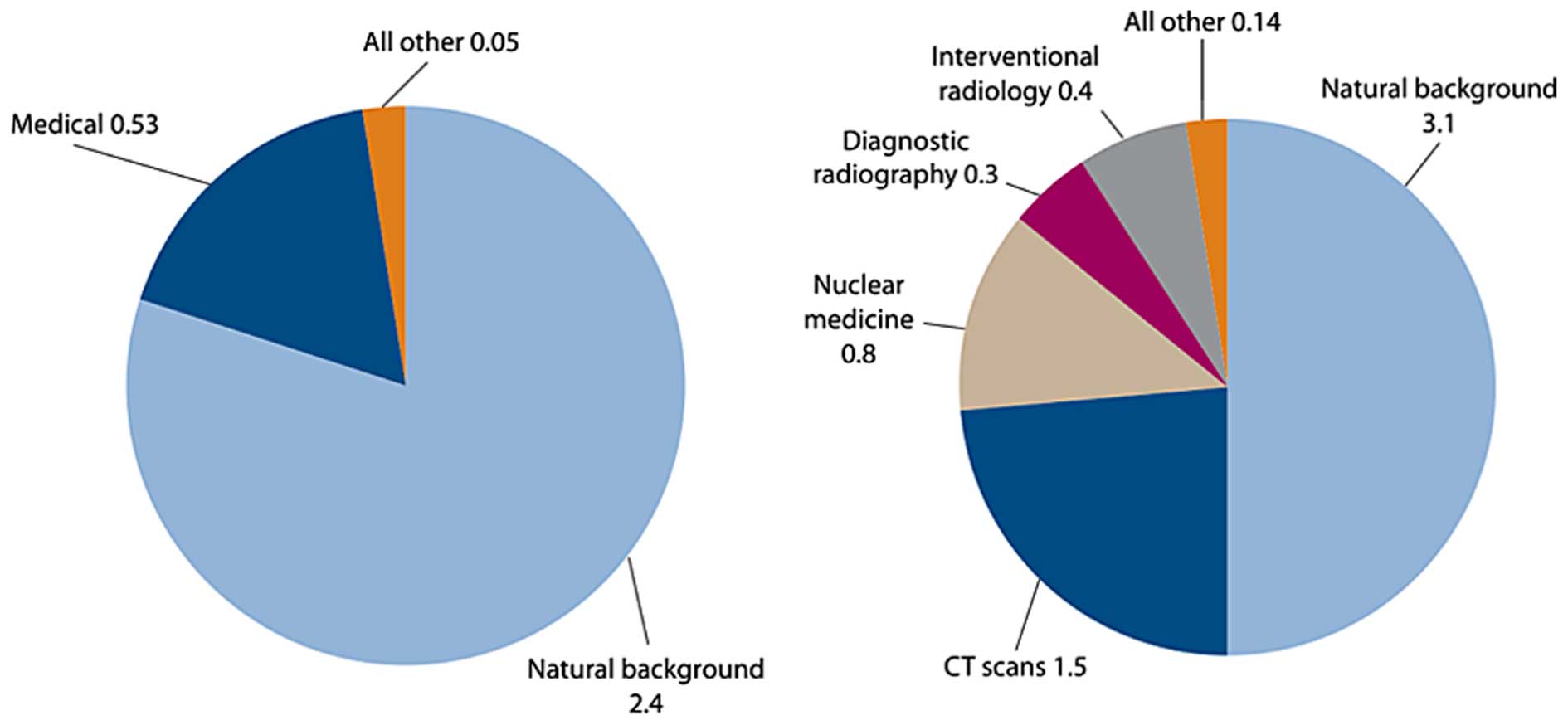
radiation for diagnostic medical purposes (Fig. 2) (51). For instance, since 1993, the
number of computed tomography (CT) scans has quadrupled in the US
and similar trends are observed in Europe (52).
In particular, the increased use of non-invasive
cardiovascular imaging techniques, such as cardiac CT scans and
myocardial perfusion imaging with radionuclides, are of importance
(53). Indeed, effective doses
range from 1 to 20 mSv depending on the procedure (Table I) (58). Although one cannot deny the huge
health benefits of these improved diagnostic procedures, concerns
are raised regarding the 'overuse' and potential associated health
risks (54). For example, it has
been observed that 14–22% of cardiac imaging tests are
inappropriate in the US (55,56).
 | Table IOverview of typical ionizing
radiation doses in cardiac imaging procedures. |
Table I
Overview of typical ionizing
radiation doses in cardiac imaging procedures.
| Examination | Representative
effective dose value (mSv) | Range of reported
effective dose values (mSv) | Administered
activity (MBq) |
|---|
| Chest X-ray
posteroanterior and lateral | 0.1 | 0.05–0.24 | N/A |
| Diagnostic invasive
coronary angiogram | 7 | 2–16 | N/A |
| Coronary CT
angiogram |
| 64-slice
multidetector, retrospective gating | 12 | 9–19 | N/A |
| 64-slice
multidetector, reduced tube voltage (100 kVp) | 6 | 3–8 | N/A |
| 64-slice
multidetector, prospective triggering | 3 | 2–4 | N/A |
| Dual-source high
pitch | <1 | <1 | N/A |
| 264 or 320
multidetector row CT | 4 | 2–8 | N/A |
| Nuclear medicine
studies |
| Myocardial
perfusion |
| Sestamibi (1-day)
stress/rest | 12 | N/A | 1480 |
| Tetrofosmin
(1-day) stress/rest | 10 | N/A | 1480 |
| Thallium
stress/redistribution | 29 | N/A | 130 |
| Rubidium-82
rest/stress | 10 | N/A | 2960 |
| Myocardial
viability | | | |
| PET F-18 FDG | 14 | N/A | 740 |
| Thallium
stress/reinjection | 41 | N/A | 185 |
Radiation protection of patients is not based on
dose limits, but on the principle of justification that states that
the benefits and risks from the use of ionizing radiation should be
carefully evaluated. However, this risk/benefit balance is highly
patient-dependent and the decision for the use of a specific
imaging test relies on the physician's judgment. Several guidelines
have been published by various societies, such as the European
Society of Cardiology and the American College of Cardiology
Foundation, to aid in this decision (53,57). These guidelines provide
information regarding the accuracy of the tests, the usefulness of
the information obtained from the test, and also regarding the
risks of the tests including those related to adverse radiation
health effects. In addition, the implementation of informed
consent, in which the possible risks are communicated to the
patient and in view of his or her consent, will stimulate
physicians to more carefully balance benefits and risks of a
specific imaging procedure (54,58). Furthermore, the development and
implementation of dose-lowering techniques may be beneficial not
only for the patient (59), but
also for the physician. In addition, the identification of
biomarkers of susceptibility allows screening for sensitive
patients, aiding in the evaluation of the risk/benefit balance
(60).
2. Clinical manifestation and pathology of
radiation-induced cardiovascular diseases
Overview
As mentioned above, patients treated with
radiotherapy who received doses >40 Gy to part of the heart may
develop cardiovascular complications later on in life (5). More recently, epidemiological
findings also point to an excess risk of CVD following exposure to
lower doses. CVD, also commonly referred to as heart disease,
comprises a broad range of different clinical manifestations. The
radiation-induced clinical manifestation of CVD is dependent on
various factors, such as dose, dose rate, the volume of the heart
exposed, age at exposure, latency of disease, length of follow-up
and other confounding factors (e.g., smoking and diet) (61). The major clinical manifestations
of radiation-related CVD (i.e., pericarditis, congestive heart
failure and coronary artery disease) are discussed below, together
with the animal models utilized to research these radiation-related
pathologies. Ionizing radiation may also cause valvular disease,
arrhythmias and conduction abnormalities, although a direct causal
relationship is not evidenced (5,62).
Animal models
Next to epidemiology, animal models can be useful to
investigate radiation-induced cardiovascular disorders.
Nevertheless, it should be noted that translation to human
radiation-induced atherogenic risk should be done with care.
Indeed, the interaction of radiation exposure with other
atherogenic risk factors is difficult to study in these animal
models. For instance, gender-related differences in pro-atherogenic
risk are known to have an influence, as well as lifestyle habits,
such as smoking, diet and alcohol intake. Since genetic and
environmental factors play a significant role in cardiovascular
pathophysiology, it is difficult to match a particular disease with
a single experimental model (63). Therefore, experimenters should
select models that best reproduce the aspect of disease being
investigated. Additional considerations of cost, infrastructure and
the requirement for specialized personnel should also be taken into
account. Finally, also the length of the reproductive cycle, the
availability of genome-wide information and the ease of genome
manipulation can influence the selection of the model.
Due to the long experience of use, the available
infrastructure, the short reproductive cycle and large litters, the
mouse is often the animal model of choice. In addition, the mouse
has a well-known genome that is relatively easily to manipulate and
more recently, infrastructures for non-invasive imaging of small
animals have become available. However, normal rodents are
resistant to atherosclerosis since they have low plasma levels of
pro-atherosclerotic LDL. Therefore, atherosclerosis-prone animal
models have been developed. For example, apolipoprotein E
(ApoE)−/− and LDL receptor−/− mouse models
are commonly used (64,65). ApoE is an important glycoprotein
in the transport and metabolism of lipids and the lack of a
functional ApoE gene leads to an altered plasma lipid profile, and
the rapid development of atherosclerotic lesions (64,65). Mice that lack a functional LDL
receptor gene also have an altered plasma lipid profile, with
elevated LDL levels. LDL receptor-deficient mice will develop
atherosclerosis when fed a lipid-rich diet (64,65). These mouse model studies can
provide valuable information regarding the understanding of the
cellular and molecular pathways underlying radiation-induced
development and progression of atherosclerosis.
The high-cholesterol diet rabbit models have also
been used for experimental atherosclerosis (63). Cholesterol causes atherosclerotic
changes in the rabbit arterial intima, very similar to human
atherosclerosis. Atherosclerotic lesions also develop in
normolipidemic rabbits as a result of repeated, or continuous
intimal injury by catheters or balloons, or by nitrogen exposure
(66).
The pig is a very good model to study CVD as it has
a human-like cardiovascular anatomy (63). Pigs develop spontaneous
atherosclerotic lesions. Porcine models are probably the best way
to recreate human plaque instability and plaque rupture. However,
they require voluminous housing with high costs, they are difficult
to handle, and there are few genomic tools available for pigs.
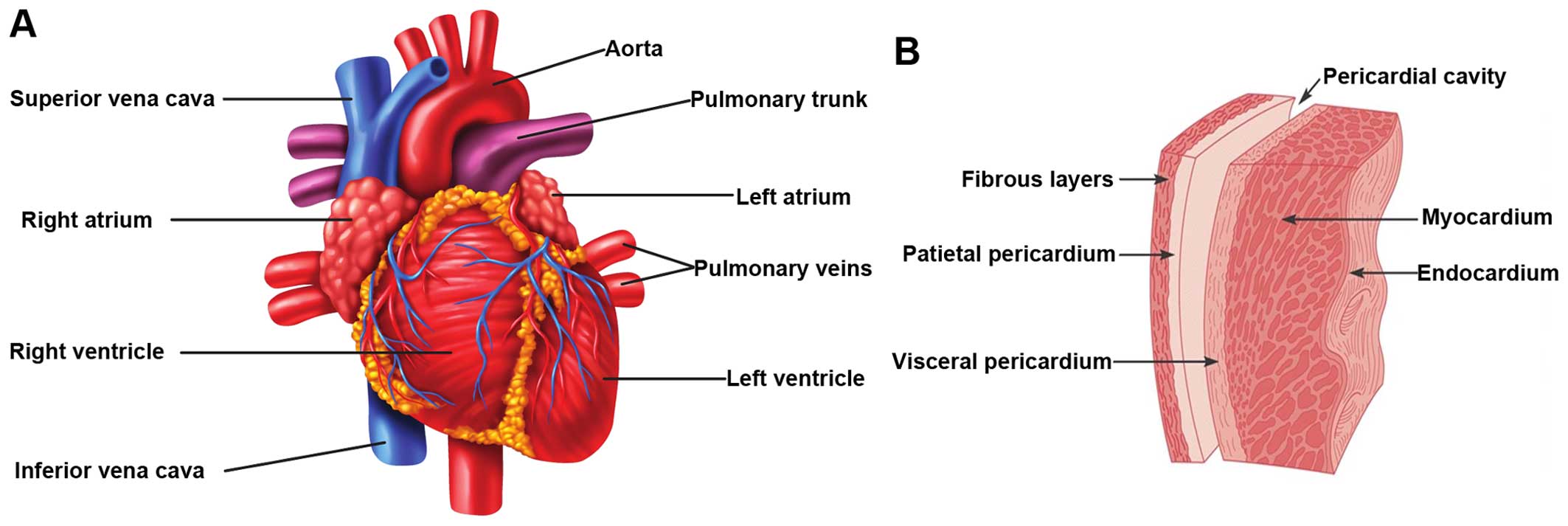
Pericarditis
The earliest sign of radiation-related heart disease
is acute pericarditis, which occurs already months after high-dose
irradiation of the heart (>40 Gy). Acute pericarditis is the
inflammation of the pericardium, the membrane that surrounds the
heart (Fig. 3), and is
characterized by the exudation of protein-rich fluid in the
pericardial sac. On the long-term, this can lead to chronic
constrictive pericarditis due to fibrin deposition, causing a
thickened, rigid pericardial sac (2,67).
The development of acute pericarditis has also been observed in
rabbits, rats and dogs after single doses to the heart of 16–20 Gy
(68–70). These experimental studies showed a
threshold dose of approximately 15 Gy with a steep dose-response
relationship (incidence of 100% at 20 Gy). Since 1970, advances in
radiotherapy treatments have led to a significant reduction in both
the dose and the volume of the heart exposed (3). Therefore, radiation-induced
pericarditis is uncommon these days.
Coronary artery disease
The obstruction of the blood flow in coronary
arteries, responsible for blood supply to the heart, is referred to
as coronary artery disease (9).
Mild obstruction due to narrowing of the coronary arteries leads to
angina (discomfort due to ischemia of the heart muscle), whereas
severe blockage leads to myocardial infarction (heart attack),
which on its turn leads to acute heart failure. Atherosclerosis is
the major underlying pathogenesis causing coronary artery disease.
It can be described as a chronic inflammatory disease of the
arterial wall in which the build-up of plaques in the intima
impairs normal vascular functioning (Fig. 4). These plaques are characterized
by the accumulation of lipids and fibrous elements (71). The development and progression of
atherosclerosis is a complex process with many players. The
presence of plaques leads to narrowing of the artery and, upon
rupture of a plaque, even to blockage of the artery (72). Nowadays, coronary artery disease
is considered the major cardiovascular complication in patients
that have received radiotherapy for thoracic malignancies (73).
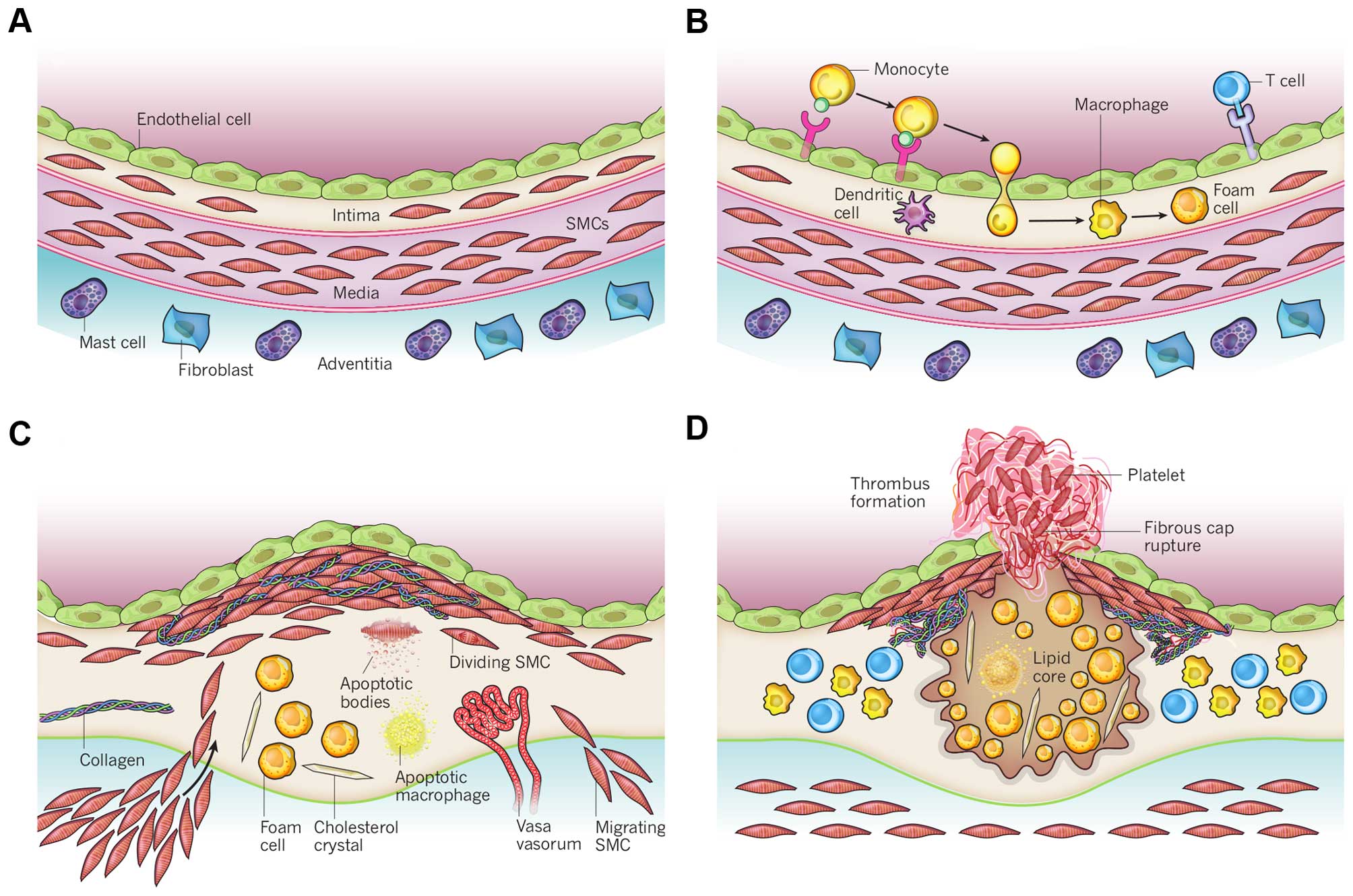 | Figure 4Schematic overview of the development
of an atherosclerotic lesion. In all steps, inflammation plays an
important role. (A) A healthy artery with a well-functioning intact
endothelium, a tunica intima, media and adventitia. VSMCs are
mainly found in the tunica media but also in the tunica intima. (B)
One of the initiating steps is the expression of adhesion molecules
on the endothelium and the subsequent attraction of inflammatory
blood cells (mainly monocytes). These monocytes will transmigrate
to the intima where they will maturate to macrophages which will
then transform to foam cells upon the uptake of ox-LDL. (C) Further
progression to an atherosclerotic plaque includes the
transmigration of VSMCs from the tunica media into the intima and
the proliferation of VSMCs in the intima. There is also an enhanced
production of extracellular matrix molecules, such as collagen,
elastin and proteoglycans. Macrophages, foam cells and VSMCs can
die, and released lipids will accumulate into the central region of
the plaque, also denoted the lipid or necrotic core. (D) When a
plaque ruptures it will induce thrombosis which is the major
complication. The blood component will come in contact with the
tissue factors present in the interior of the plaque triggering the
formation of a thrombus which will hamper or even obstruct blood
flow. The figure is based on a previous study (167). VSMCs, vascular smooth muscle
cells; ox-LDL, oxidized low-density lipoprotein. |
The effect of ionizing radiation on the development
and progression of atherosclerosis has been investigated in various
animal models, which has been reviewed (9). For example, Stewart et al
examined the development and progression of atherosclerotic lesions
in ApoE−/− mice after single-dose irradiation (14 Gy) of
the neck region (74). There was
no major increase in the total plaque burden in the exposed carotid
arteries, although the quality of the plaques was changed,
acquiring inflammatory characteristics. Indeed, the plaques showed
a macrophage-rich core, low collagen content and intraplaque
haemorrhage, which are known to render human atherosclerotic
plaques unstable and prone to rupture. They also observed the
presence of atypical swollen endothelial cells. It is hypothesized
that radiation-induced changes in endothelial function together
with radiation-induced endothelial cell death and the exposure of
thrombotic elements of the underlying subendothelium leads to
chronic inflammation and the development of a vulnerable plaque
(74). Gene expression profiling
of ApoE−/− mice exposed to an acute dose of 16 Gy also
revealed the upregulation of inflammation-related pathways
(75). Further research by the
same group revealed that a more clinically relevant fractioned
irradiation scheme (20×2 Gy in 4 weeks) also predisposed to the
formation of an inflammatory plaque (76). Remarkably, acute lower dose
irradiation (8 Gy) of ApoE−/− mice did not predispose to
an inflammatory plaque, but did accelerate the development of
atherosclerosis, as demonstrated by an increased number of plaques.
Overall, the authors concluded that exposure to high-dose ionizing
radiation accelerates the atherosclerotic process in the presence
of other risk factors (e.g., high-fat diet), and predisposes to the
development of a vulnerable inflammatory plaque prone to rupture
(9).
With low doses and dose rates of radiation the
response warrants further investigation. Mitchel et al
exposed ApoE−/− mice to low doses of radiation
(0.025–0.5 Gy) at either the high (150 mGy/min) or low (1 mGy/min)
dose rate (77). The mice were
exposed at an early stage of atherosclerotic disease (2 months old)
or at a late stage of atherosclerotic disease (8 months old). Doses
of 0.025–0.050 Gy, administered at both the low- and high-dose
rate, induced a protective effect by attenuating the formation of
new lesions and the increase in the size of existing lesions, in
mice exposed at an early stage. High-dose rate exposure however,
increased the progression of lesion severity. The effect for mice
exposed at a late stage of atherosclerotic disease with low-dose
rate was similar as that for mice exposed at an early stage. On the
other hand, high-dose rate exposures protected against the
progression of lesion severity, opposite to what was observed in
mice exposed at an early stage. Additional experiments with
ApoE−/− mice with reduced p53 functionality
(Trp53+/−) revealed an important role for p53 in
atherosclerosis progression (73). For example, protective effects of
low-dose radiation delivered at both low and high dose rate were
observed in Trp53 normal mice exposed at a late stage of
atherosclerotic disease. On the other hand, with the same
irradiation procedure, detrimental effects were observed in
Trp53+/− mice exposed at a late stage of atherosclerotic
disease. Overall, these findings raised the importance of dose-rate
effects and p53 functionality on the development of
atherosclerosis. Furthermore, their findings point out that a
linear extrapolation of the effects at high doses to low doses is
not appropriate.
In addition, the effects of chronic low-dose rate
vs. acute exposures were evaluated in female ApoE−/−
mice (60 days) that were chronically irradiated for 300 days with
gamma-rays at two different dose rates (1 and 20 mGy/day), with
total accumulated doses of 0.3 or 6 Gy (78). For comparison, age-matched
ApoE−/− females were acutely exposed to the same doses
and sacrificed 300 days post-irradiation. Mice acutely exposed to
0.3 or 6 Gy showed increased atherogenesis compared to the
age-matched controls, and this effect was persistent. When the same
doses were delivered at the low-dose rate over 300 days, again a
significant impact on global development of atherosclerosis was
observed, although at 0.3 Gy effects were limited to the descending
thoracic aorta. These data suggest that a moderate dose of 0.3 Gy
can have persistent detrimental effects on the cardiovascular
system, and that a high dose of 6 Gy poses high risks at both the
high- and low-dose rates. The results were clearly non-linear with
dose, suggesting that lower doses may be more damaging than
predicted by a linear dose response.
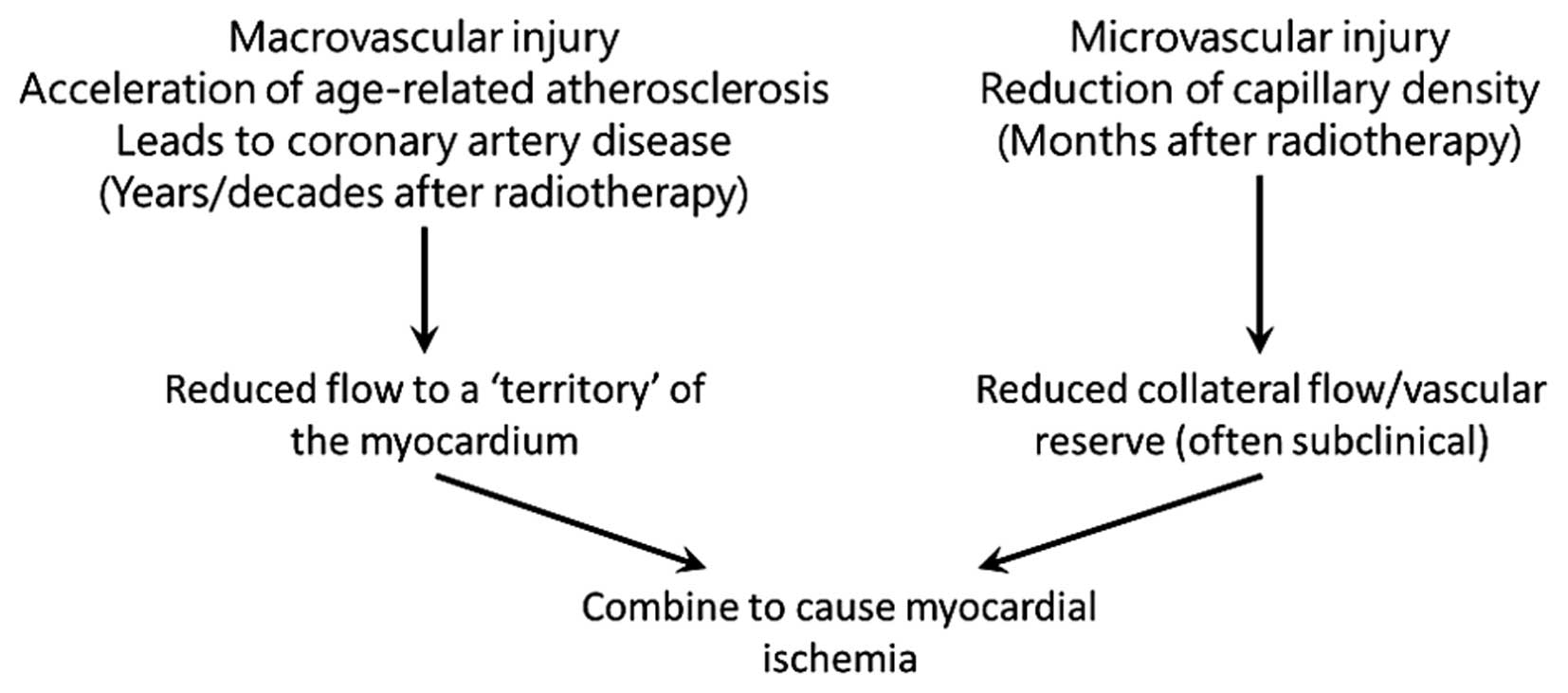
Darby et al formulated two hypotheses for
biological mechanisms that lead to increased morbidity and
mortality from coronary artery disease following radiation exposure
(5). The first hypothesis states
that radiation interacts with the pathogenesis of age-related
atherosclerosis, as such accelerating the development of
atherosclerosis. The second hypothesis is that radiation increases
the lethality of age-related myocardial infarction by decreasing
the heart tolerance to acute infarctions as a result of
microvascular damage in the myocardium. These hypotheses do not
stand alone, and both macro- and microvascular effects most likely
act together to produce clinical heart disease (Fig. 5).
Finally, Le Gallic et al investigated the
effects of a chronic internal exposure to 137Cs on
atherosclerosis in predisposed ApoE−/− mice (79). Mice were exposed daily to 0, 4, 20
or 100 kBq/l 137Cs in drinking water, corresponding to
range of concentrations found in contaminated territories, for 6 or
9 months. The results suggest that the low-dose chronic exposure of
137Cs in ApoE−/− mice enhances
atherosclerotic lesion stability by inhibiting pro-inflammatory
cytokine and matrix metalloproteinase (MMP) production, resulting
in collagen-rich plaques with greater smooth muscle cell and less
macrophage content.
Congestive heart failure
Congestive heart failure is described by a
compromised blood pumping function of the heart, due to a reduced
capacity of the heart muscles, causing under-perfusion of the body
tissues. The underlying pathologies are various and include IHD,
hypertension, valvular heart disease, cardiomyopathies and
congenital heart disease (80).
Rats that received single doses of at least 15 Gy to the heart have
been shown to develop congestive heart failure within their normal
lifespan (70). Further
radiobiological research has revealed an important role for
radiation-induced decrease in capillary density. Areas of decreased
capillary density in the heart are characterized by focal loss of
the endothelial cell marker, alkaline phosphatase (81). The progressive reduction of
capillary density leads to ischemic necrosis, fibrosis and the
death of cardiac myocytes (muscle cells) in these areas. This
myocardial degeneration is associated with the first symptomatic
signs of congestive heart failure, a slight decrease in left
ventricle ejection fraction (2).
This reduced cardiac function is maintained in a steady-state for a
certain period, due to in vivo compensatory mechanisms, and
fatal congestive heart failure is only observed on the long-term
(82). Indeed, in ex vivo
experiments, cardiac function has been shown to deteriorate more
rapidly (83).
Myocardial damage has also been observed with lower
doses. For instance, mild alterations in cardiac function in
ApoE−/− mice following exposure to 2 Gy have been
observed, which however did not deteriorate over time (84). Histological examination revealed
functional damage to the microvasculature as indicated by a focal
loss of alkaline phosphatase. More recently, Monceau et al
exposed the hearts of ApoE−/− and wild-type mice to
doses of 0.2 Gy (85). Mild but
significant alterations in cardiac function were observed in both
mouse strains following exposure to 0.2 Gy. The progression of
cardiac dysfunction remained, however, stable over the whole study
period (60 weeks), suggesting the occurrence of compensatory
mechanisms. Whereas in ApoE−/− mice cardiac damage was
the consequence of reactive fibrosis in response to inflammatory
signalling, this was the consequence of reparative fibrosis induced
by the loss of cardiac myocytes in wild-type mice. Overall,
ApoE−/− mice were more radiosensitive. This implies that
atherosclerosis predisposition enhances and accelerates the
structural deterioration of the heart following exposure to low
doses of ionizing radiation, and can thus be considered as a risk
factor.
3. Molecular and cellular mechanisms
underlying the observed radiation-induced cardiovascular
disorders
The mechanism through which radiation causes heart
disease is at present unknown, but it acts, at least in part, by
causing or promoting atherosclerosis. Atherosclerosis is a
multifactorial disease, resulting from interactions between genetic
and environmental factors which may be modified by radiation
exposure. For radiation doses >1–2 Gy, in vitro and in
vivo studies have shown that several mechanisms may play a
relevant role in radiation-induced cardiovascular effects (3,86–88). These effects include endothelial
dysfunction, inflammation, oxidative stress, alterations in
coagulation and platelet activity, DNA damage, senescence and cell
death. On the contrary, low-dose exposure produces both protective
and detrimental effects, suggesting that multiple mechanisms may
influence radiation-induced atherosclerosis (42).
Need for experimental studies,
particularly in the low-dose region
Due to reasons of statistical power, in exposure to
doses <0.5 Gy, an increased cardiovascular risk cannot be
evidenced by epidemiology alone, and a better understanding of the
underlying biological and molecular mechanisms is needed. If one
proves that there is an increased risk of CVD following low-dose
exposure, it may have a considerable impact on current low-dose
health risk estimates.
In contrast to cancer and hereditary effects,
knowledge of the underlying biological mechanisms for other
radiation-related non-cancer effects in the moderate and low-dose
range is limited and is assumed to be different from high-dose
exposure. Therefore, research to understand the mechanisms is
urgently necessary [Multidisciplinary European Low Dose Initiative,
Strategic Research Agenda 2015, http://www.melodi-online.eu/]. Further research is
essential to elucidate the low-dose effects on the cardiovascular
system, and the impact on CVD risk. This is, however, not
straightforward due to the subtlety of low-dose effects and the,
most likely, little impact on clinical outcome. Apart from the
integration of epidemiology and biology, as mentioned above, pure
radiobiological studies are needed. These include mechanistic
studies and in vitro studies focusing on the elucidation of
molecular signaling pathways and further in vivo studies. In
these studies, attention should be paid, not only to dose, but also
to dose-rate, fractionated exposures and radiation quality.
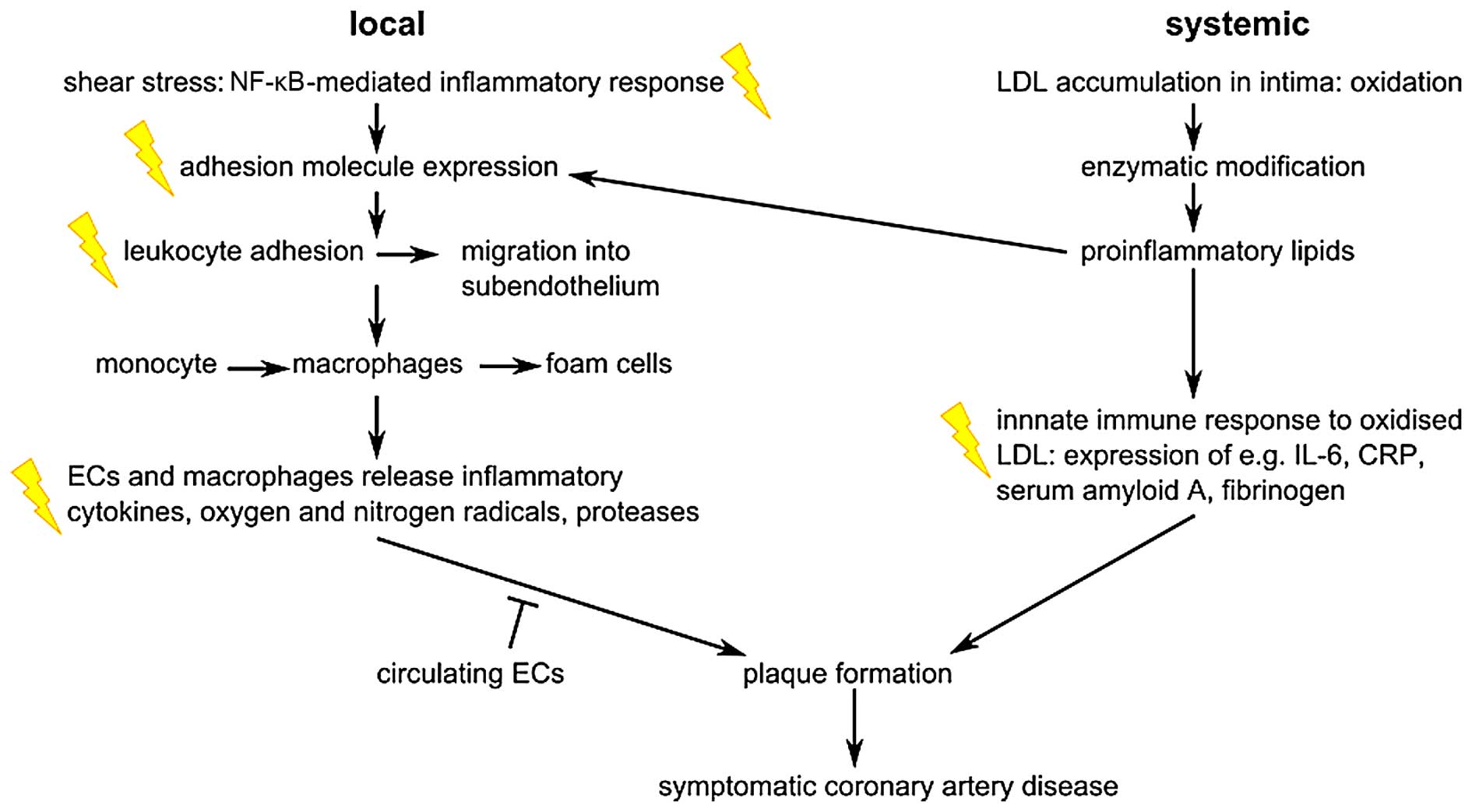
The role of inflammation
As indicated in Fig.
6, ionizing radiation acts on the atherosclerotic process by
enhancing pro-inflammatory signalling, as reviewed previously
(3,89). Atherosclerotic plaques are formed
by the migration of inflammatory cells from the bloodstream into
the intima where they transform to foam cells. The endothelial
expression of adhesion molecules plays an important role in this
process. Radiation has been shown to upregulate E-selectin,
intercellular adhesion molecule (ICAM)-1 and vascular cellular
adhesion molecule (VCAM)-1 following irradiation of endothelial
cells, in a time- and dose-dependent manner (3). For instance, the exposure of
endothelial cells to 5 Gy has been shown to induce an increase in
ICAM-1 and E-selectin expression 6 h after irradiation (90). Platelet endothelial cell adhesion
molecule (PECAM)-1, ICAM-1/2 and VCAM-1 were also observed to
increase in mouse heart cells 10 weeks after local thorax
irradiation with 8 Gy (91).
Interestingly, ICAM-1 and VCAM-1 remained upregulated 20 weeks
after irradiation. The transcription factor, nuclear factor-κB
(NF-κB), is involved in the radiation-induced upregulation of
adhesion molecules (92). Apart
from the induction of adhesion molecules, the levels of cytokines
such as interleukin (IL)-6 and IL-8, and other inflammatory
molecules, such as transforming growth factor-β (TGF-β), were shown
to increase after high and moderate irradiation (93,94). In addition, the Japanese atomic
bomb survivors' cohort showed signs of a general increased state of
inflammation, with increased levels of IL-6 and C-reactive protein
(CRP) (95). In addition to
pro-inflammatory responses, there is evidence of pro-thrombotic
changes after irradiation of the endothelium. For example, several
in vitro and in vivo studies have demonstrated
increased levels of von Willebrand factor (VWF) and decreased
levels of the anticoagulant, thrombomodulin (3,96).
Inflammation was also predicted from a proteomic
study as an immediate biological response in the cardiac tissue of
wild-type mice exposed to total body irradiation with 3 Gy gamma
radiation (97). Validated
proteomic data concerning cardiac microvascular endothelial cells
that were isolated from wild-type mice that received local X-ray
heart doses of 8 or 16 Gy and that were sacrificed after 16 weeks
also strongly suggested enhanced inflammation as the main causes of
radiation-induced long-term vascular dysfunction (98).
There is clinical evidence for anti-inflammatory
responses of low-dose radiation exposure in individuals who
experience inflammatory diseases. Indeed, for decades, low-dose
radiotherapy has been used for the treatment of benign inflammatory
diseases (99,100). However, due to the debate
regarding possible cancer and non-cancer risks of low-dose
radiation exposure, the use of low-dose radiotherapy has become
unfashionable nowadays (101).
In vitro experimental studies have confirmed the
anti-inflammatory effects of activated endothelial cells after
X-irradiation. Indeed, no induction of ICAM-1 and E-selectin was
observed up to 24 h after exposure to 0.3 and 1 Gy, and was even
decreased 4 h after irradiation (90). This results in a decreased
mononuclear cell adhesion onto the endothelium (90,102).
Endothelium as a critical target in
radiation-related CVD
Endothelium is the safeguard of normal
vascular functioning
The endothelium is a single layer of cells that
lines the interior of the vascular system and has thus a strategic
position between the blood and the surrounding tissues. Endothelial
cells are involved in a wide range of physiological processes, such
as the regulation of vascular tone, vascular permeability, blood
coagulation/fibrinolysis and inflammation, which are required to
maintain proper vascular functioning (Fig. 7) (103). Endothelial dysfunction has been
observed in patients with atherosclerosis and in patients that
exhibit CVD risk factors such as smoking, dyslipidaemia, obesity
and diabetes mellitus (104),
and is considered one of the first indicators of future
cardiovascular morbidity and mortality (105–108). It should be noted that the
endothelium is regarded as a critical target for radiation-induced
CVD.
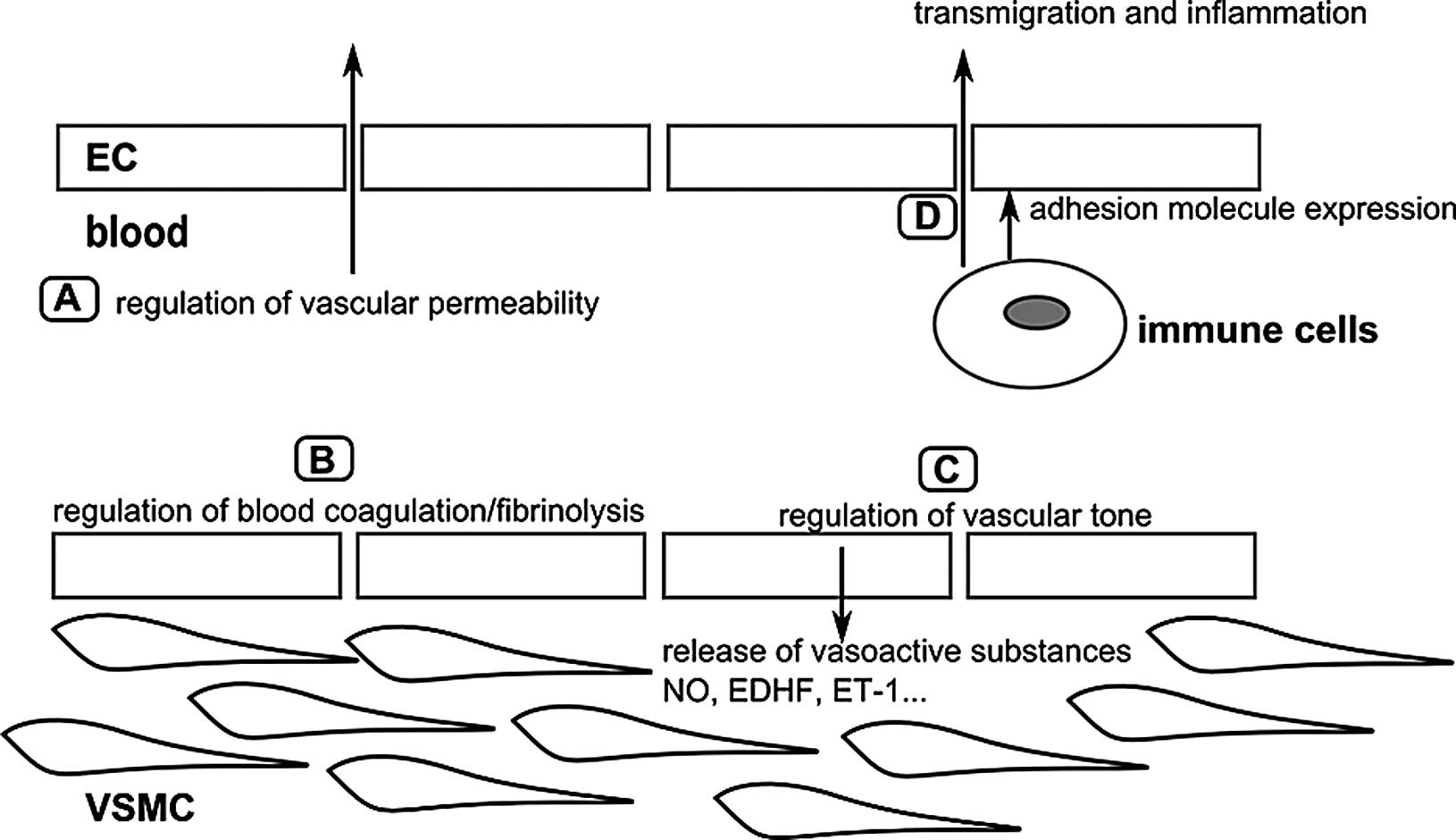 | Figure 7Overview of the major physiological
functions of the arterial endothelium. (A) The endothelium forms a
selective barrier regulating the solute flux and fluid permeability
between the blood and surrounding tissues (105). (B) The formation of a thrombus
or blood clot is referred to as coagulation and the breakdown of a
thrombus is referred to as fibrinolysis. Normal endothelium has
anti-thrombotic and pro-fibrinolysis properties, and actively
represses platelet adhesion and aggregation. Vessel damage or
exposure to pro-inflammatory molecules will shift the balance
towards more pro-thrombotic/anti-fibrinolysis actions (106,107). (C) To regulate vascular tone,
the endothelium releases various vasodilatory factors such as NO
and EDHF, or vasoconstrictive factors such as ET-1 which will
modify VSMC function (108). (D)
In the case of inflammation, endothelial permeability will be
increased. Endothelial cells will also recruit immune cells via the
expression of adhesion molecules, and mediate their transmigration
towards the inner vascular wall (107). The figure is based on a previous
study (103). ECs, endothelial
cells; VSMCs, vascular smooth muscle cells; NO, nitric oxide; EDHF,
endothelium-derived hyperpolarizing factor; ET-1, endothelin-1. |
The endothelium displays phenotypical and functional
heterogeneity depending on the vascular bed and tissue it is
situated in (109,110). The major function of arteries
and veins is the circulation of blood through the body.
Capillaries, on the other hand, are the major exchange vessels and
the capillary endothelium is thus very thin and usually fenestrated
to ensure the optimal diffusion of oxygen and nutrients between the
blood and underlying tissue (110). Arterial endothelial cells are
sensitive to disrupted flow at branching points and curvatures in
the arterial system, which are, consequently, 'hotspots' for
inflammation, coagulation and atherosclerosis (110). A healthy arterial endothelium
mediates vasodilatation and actively suppresses thrombosis,
vascular inflammation and inhibits vascular smooth muscle cell
proliferation and migration (111).
CVD risk factors associated with endothelial
dysfunction include hypertension, smoking, dyslipidaemia and aging.
A common underlying cellular mechanism leading to endothelial
dysfunction is oxidative stress (112). Oxidative stress is defined as an
imbalance between the generation of reactive oxygen species (ROS)
(Fig. 8) and the activity of
enzymatic and non-enzymatic antioxidant systems (113). At physiological levels, ROS are
important signalling molecules; however, at higher concentrations,
ROS cause cellular injury by inducing oxidative damage to DNA,
lipids and proteins, and may result in cell death (114). Apart from cellular damage,
oxidative stress leads to a decrease in nitric oxide
bioavailability, premature senescence and mitochondrial dysfunction
in endothelial cells.
In vitro endothelial models
The recognition of the endothelium as a central
regulator of the cardiovascular system significantly enhanced
endothelium-related research. One of the milestones was the first
successful isolation and subsequent cultivation and
characterization of endothelial cells in vitro, in the 1970s
(109). Jaffe et al
(115) and Gimbrone et al
(116) reported independently
the isolation of endothelial cells from human umbilical veins
[human umbilical vein endothelial cells (HUVECs)]. Since then, many
studies have relied on the use of HUVECs as they are relatively
easy to obtain. Although originating from large vessels, HUVECs are
unique since they exhibit endothelial properties that are
intermediate between those of large vessels (e.g., the aorta) and
those of the microvasculature (117). EA.hy926 cells, which are an
immortalized derivate of HUVECs, are commonly used as well
(118). However, it should be
taken into account when using these cells as models for studying
the endothelium radiation response, that there is a differential
response to ionizing radiation in primary and immortalized
endothelial cells. For example, EA.hy926 cells are more sensitive
to the induction of apoptosis and double-strand breaks (DSB) in
comparison to the same dose of ionizing radiation administered to
HUVECs (119).
It should be noted that in vitro endothelial
cell models are not limited to HUVECs and EA.hy926 cells.
Endothelial cells may be isolated from other sources in the human
body, such as the dermal microvasculature, coronary arteries and
the pulmonary vasculature. However, these are usually not easy to
obtain and at best only a small amount of material is available. In
addition, these primary cell cultures cannot be kept in long-term
cultures as they begin to lose their endothelial cell
characteristics. Therefore, immortalized derivatives of these
primary cells have been established; for example, EA.hy926 cells
are a derivate of HUVECs. Immortalized cells are characterized by
their ability to overcome senescence and can be kept in culture for
long periods of time (120).
These immortalized derivatives, however, also exhibit tumour cell
characteristics (121). For
instance, immortalized human coronary artery endothelial cells
[transfected with human telomerase reverse transcriptase (hTERT)]
showed 40% of aneuploidy at a low passage number and 100% of
aneuploidy at a high passage number (121).
Of course, in vitro endothelial models,
although useful, are not fully representative for the in
vivo situation. Yet, advances have been made, such as the
development of co-culture models where endothelial cells are
cultured with vascular smooth muscle cells to study the
atherosclerotic process (122,123). In addition, 3-D cultures, where
endothelial cells are grown in a matrix allowing the formation of
tubule-like structures are increasingly used to study angiogenesis
(124,125). A recent review described in
detail the use of these co-culture and 3-D culture models in
radiation research (126).
The effect of ionizing radiation on
the endothelium
Gaining insight into the endothelial response to
ionizing radiation exposure is not only of importance for
understanding the development of radiation-related CVD, but also
for optimizing cancer treatment. For instance, adverse reactions in
the surrounding healthy tissue of the tumour are related to the
radiation-response of the microvasculature in the tissue of
concern. In addition, tumour growth is highly dependent on an
abundant blood supply, which is maintained by a rich vascular
network (127). The tumour
vasculature thus became a potential target in radiotherapy
(128). Understanding the
effects of radiotherapy on the tumour endothelium can improve
treatment regimes. Since the tumour endothelium differs in many
aspects to the normal endothelium (129), this is not in the scope of this
review.
Below, an overview is given of classical cellular
radiation effects and how these may affect endothelial functioning.
Next, the impact of ionizing radiation on mitochondrial function
and on senescence is discussed. Finally, attention is paid to the
contribution of new technologies, such as high throughput
transcriptomic profiling, allowing a better understanding of the
underlying molecular signalling pathways:
i) DNA-targeted effects
Ionizing radiation is known to induce a wide range
of DNA lesions, such as base damage, DNA crosslinks, single-strand
breaks and DSB in a direct manner, but also indirectly through the
formation of ROS (130,131). Upon DNA damage, a DNA damage
response is initiated and the cells will activate cell cycle
checkpoints which can slow down or stop cell cycle progression
(132). This gives cells the
time to repair the damaged DNA or to prevent division when
chromosomes are damaged or incompletely replicated. If the cells
fail to repair the DNA, they can go into apoptosis (133).
In particular, DSB will lead to a high lethality of
the affected cells (134). At
the site of DSB damage, the histone H2AX is phosphorylated
(referred to as γ-H2AX) by kinases, such as ataxia telangiectasia
mutated (ATM) and ataxia telangiectasia and Rad3-related protein
(ATR), resulting in the formation of γ-H2AX foci (130). These foci will recruit DNA
repair proteins to the DSB sites. It has been shown that there is a
1:1 relationship between the amount of DSB and the γ-H2AX foci
formed (135). Visualization and
quantification of γ-H2AX foci has become a standard in assessing
radiation-induced DNA damage.
Irreparable DSB can cause cellular apoptosis, or
premature senescence (described below). Endothelial apoptosis has
implications on both the micro- and macrovascular. Namely,
endothelial cell death in the microvasculature leads to a decrease
in capillary density. In addition, endothelial apoptosis has been
related to the development of atherosclerosis (136,137) as it may compromise the
regulation of vascular tone, and increase the proliferation and
migration of vascular smooth muscle cells (VSMCs) (138). Furthermore, thrombosis, the
major complication of atherosclerosis, can be triggered by
endothelial cell death (137).
It should be noted that radiation-induced endothelial cell
apoptosis is not solely a consequence of DNA damage. Indeed,
ionizing radiation can act on the cellular membrane of endothelial
cells as well, generating ceramide which can induce apoptosis
(139).
Whereas high doses are known to induce the
apoptosis of endothelial cells (140), less is known about the effect of
low doses. There are indications that, regarding apoptosis,
endothelial cells display a non-linear dose relationship. Rödel
et al demonstrated a discontinuous induction of apoptosis
with a relative maximum at 0.3 and 3 Gy and a relative minimum at
0.5 Gy in endothelial cells stimulated with tumor necrosis factor
(TNF)-α (141). Another study
showed no increase in apoptotic endothelial cells following
exposure to 0.2 Gy, but only following exposure to 5 Gy (142). In a different study, a subtle
but a significant increase in DSBs was observed in HUVECs and
EA.hy926 cells 30 min after exposure to 0.05 Gy. In addition,
irradiation with 0.05 and 0.1 Gy induced relatively more DSB/Gy in
comparison to 0.5 and 2 Gy. Furthermore, a dose-dependent increase
in apoptotic cells was observed, down to 0.5 Gy in HUVECs and 0.1
Gy in EA.hy926 cells (119).
ii) Radiation-induced mitochondrial dysfunction
and metabolic changes
There is a great deal of interest in
radiation-induced mitochondrial dysfunction, seeing the
implications it has on CVD (143). Mitochondrial dysfunction is
closely related to oxidative stress, being both a target and a
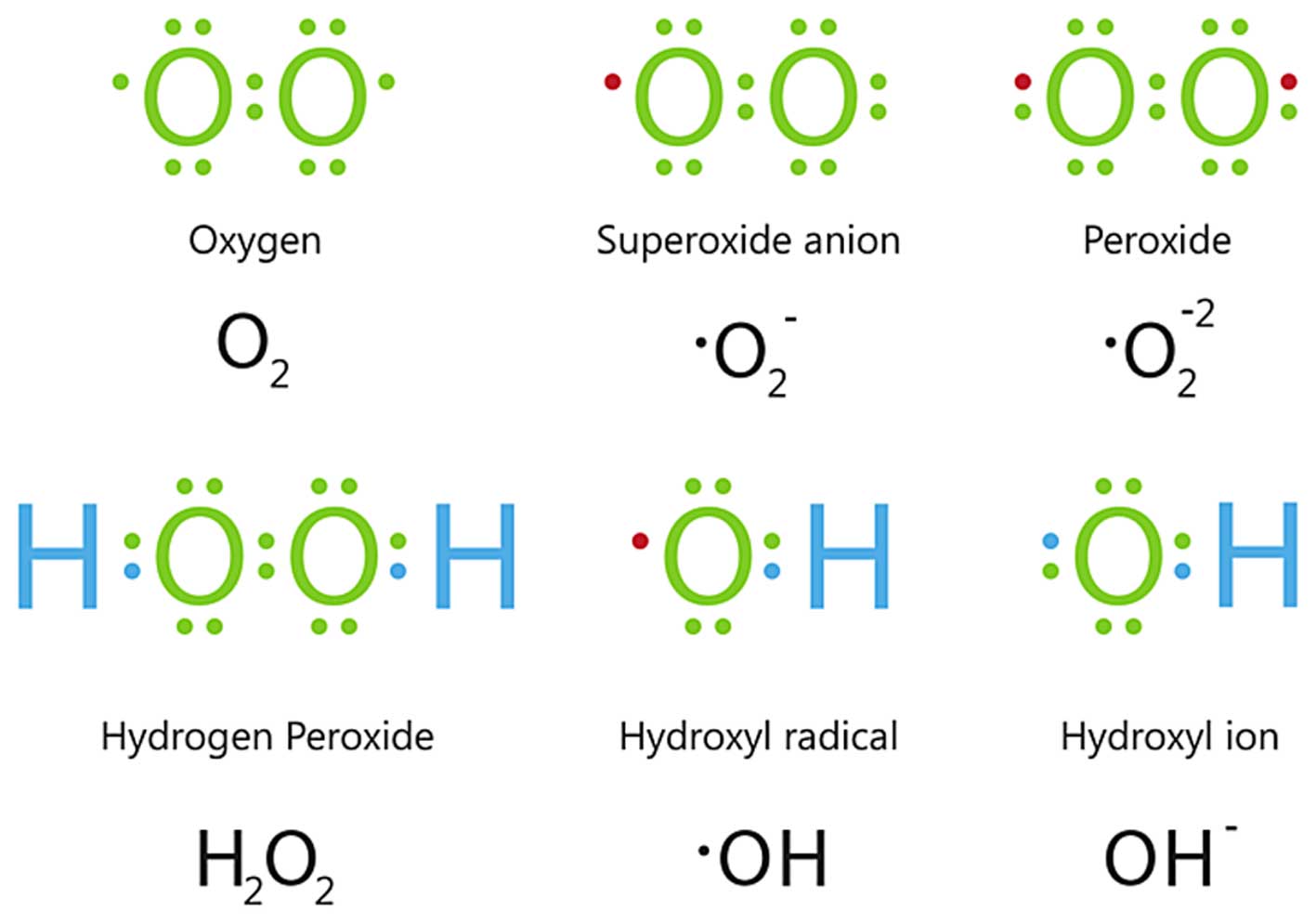
source of ROS. Initially, ionizing radiation causes the formation
of water radiolysis products, including hydroxyl radicals
(•OH), hydroperoxyl radicals
(HO2•) and hydrogen peroxide
(H2O2) (Fig.
8). These are unstable and disappear within <10−3
sec, apart from H2O2 (144). However, following irradiation,
oxidative stress is observed after longer time periods due to an
increase in the endogenous cellular production of ROS (145). The mitochondria are believed to
be the major source of these radiation-induced secondary ROS,
although other sources may contribute as well. For instance, Leach
et al (147) demonstrated
that, between 1 and 10 Gy, the amount of ROS-producing cells
increased with the dose, which they suggested was dependent on the
radiation-induced propagation of mitochondrial permeability
transition via a Ca2+-dependent mechanism (146).
Since the mitochondria, in particular mitochondrial
DNA (mtDNA), are also a critical target of ROS, measurements of
mtDNA damage have been used to determine the deleterious effects of
ionizing radiation. The increased accumulation of common deletion
(CD) following exposure to ionizing radiation has been detected in
various studies (148–150). The measurement of CD by
quantitative (real-time) polymerase chain reaction (PCR) has been
proposed as a sensitive marker to detect low levels of oxidative
damage to the mtDNA (148). An
increased accumulation of the CD has been observed in several human
fibroblast cell lines following exposure to doses as low as 0.1 Gy
(150). Interestingly, increased
accumulation of the CD was also observed in bystander cells, i.e.,
cells that were cultured in conditioned medium derived from 0.1
Gy-irradiated cells.
However, the question of whether low doses of
ionizing radiation have an impact on mitochondrial function has not
been fully resolved yet. For instance, an in vivo study by
Barjaktarovic et al investigated the effects in vivo
of 0.2 and 2 Gy local heart irradiation on cardiac mitochondria
(151). In another study of
theirs, four weeks after exposure, cardiac mitochondria were
isolated from C57BL/6N mice and subjected to proteomic and
functional analysis. Whereas with 2 Gy both functional impairment
of mitochondria and alterations in the mitochondrial proteome were
observed, only a few alterations in the mitochondrial proteome and
no effect on mitochondrial function was observed with 0.2 Gy. After
40 weeks, the respiratory capacity of irradiated C57BL/6 cardiac
mitochondria was significantly reduced at 40 weeks (152). In parallel, protein
carbonylation was increased, suggesting enhanced oxidative stress.
In addition, considerable alterations were found in the levels of
proteins of the mitochondria-associated cytoskeleton, respiratory
chain, ion transport and lipid metabolism. High-dose radiation
induced similar, but less pronounced effects in the mitochondrial
proteome of ApoE−/− mice after 40 weeks upon local heart
X-irradiation. In ApoE−/−mice, no significant change was
observed in mitochondrial respiration or protein carbonylation. The
dose of 0.2 Gy had no significant effects on cardiac
mitochondria.
Alterations in cardiac proteins involved in lipid
metabolism and oxidative phosphorylation were shown in a proteomic
study of mice that received local heart irradiation with X-ray
doses of 8 and 16 Gy and were sacrificed at week 16 after exposure
(153). Ionizing radiation
markedly altered the phosphorylation and ubiquitination status of
peroxisome proliferator-activated receptor (PPAR) α, a
transcriptional regulator of lipid metabolism in heart tissue with
a possible role in the development of CVD. This was reflected as a
decreased expression of its target genes involved in energy
metabolism and mitochondrial respiratory chain confirming the
proteomics data. In addition, other proteomic studies of the same
group suggested the deregulation of mitochondrial proteins and
proteins involved in oxidative phosphorylation or
glycolysis/gluconeogenesis either in cells (2.5 Gy
gamma-irradiation of EA.hy926 cells, evaluation after 4 and 24 h)
or mouse hearts (total body irradiation with 3 Gy gamma-ray and
sacrificed after 5–24 h) (97).
Furthermore, post-translational acetylation alterations in primary
human cardiac microvascular endothelial cells 4 h after a gamma
radiation dose of 2 Gy indicated radiation-induced changes in,
amongst others, mitochondrial proteins (154). Finally, an impaired energy
metabolism and perturbation of the insulin/insulin-like growth
factor (IGF)-PI3K-Akt signalling pathway was identified by
validated proteomic data in cardiac microvascular endothelial cells
from wild-type mice that received a local X-ray dose of 8 or 16 Gy
and that were sacrificed after 16 weeks (98).
iii) Radiation-induced premature senescence
Ionizing radiation is a well-known stressor that
induces premature senescence in cells (158). The culprit is most likely severe
irreparable radiation-induced DSB (155), although radiation-induced
accelerated telomere attrition has also been suggested (156). In addition, oxidative stress is
a major player in radiation-induced senescence and is involved in
both radiation-induced DNA damage and accelerated telomere
attrition (156–158).
In several in vitro studies, it has been
demonstrated that ionizing radiation induces endothelial cell
senescence, mainly with exposure to higher doses of radiation
(159–162). Most studies confirm that
radiation-induced premature endothelial senescence is implemented
by the engagement of the classical DNA damage response pathways,
similar to replicative senescence (159,160,163). For instance, Kim et al
observed that exposure to 4 Gy led to a senescent phenotype in
endothelial cells. An increased formation of γ-H2AX foci and he
consequent activation of the DNA damage response was observed, as
indicated by the upregulation of p53 and p21 and the downregulation
of cyclins and Rb phosphorylation (162).
Interesting studies were carried out to examine the
effect of chronic low-dose rate irradiation (1.4, 2.4 and 4.1
mGy/h) (164,165). Endothelial cells were exposed
for 1, 3 and 6 weeks, to determine whether chronic low-dose rate
radiation changes the onset of replicative senescence, as measured
by SA-β-gal activity and the proliferation rate. Their findings are
indicative of a threshold dose rate for the induction of premature
senescence. Exposure to 1.4 mGy/h did not accelerate the onset of
senescence, whereas exposure to 2.4 and 4.1 mGy/h did. Remarkably,
a senescent profile was observed when the accumulated doses
received by the cells reached 4 Gy. Proteomic analysis revealed a
role for radiation-induced oxidative stress and DNA damage,
resulting in the induction of the p53/p21 pathway (164). A role of the PI3K/Akt/mTOR
pathway was also suggested (165).
In a related transcriptomic study, the gene
expression profile was studied in HUVECs that were exposed to
chronic low-dose rate ionizing radiation (1.4 and 4.1 mGy/h) for
either 1, 3 or 6 weeks. Using a dual approach, combining single
gene expression analysis and Gene Set Enrichment Analysis, an early
stress response with p53 signalling, cell cycle changes, DNA repair
and apoptosis were observed after 1 week of exposure to both dose
rates. This early response disappeared after 3 and 6 weeks of
chronic low-dose rate radiation exposure (4.1 mGy/h), and was
replaced by the development of an inflammation-related profile.
After a period of 6 weeks, the early stress response along with the
associated inflammation led to the induction of premature
senescence in the 4.1 mGy/h-exposed samples. This premature
senescence was stress-related and showed a possible role of IGF
binding protein 5 signalling, known to be involved in the
regulation of cellular senescence (166).
Validated proteomic data concerning cardiac
microvascular endothelial cells that were isolated form wild-type
mice that received local X-ray heart doses of 8 or 16 Gy and that
were sacrificed after 16 weeks also strongly suggested enhanced
inflammation as the main causes of radiation-induced long-term
vascular dysfunction (98).
4. Conclusion
We are all exposed to ionizing radiation, from
naturally occurring substances with unstable nuclei to X-rays for
medical diagnostics. A pressing question is whether or not exposure
to these very low doses can cause damage to our health. From
epidemiological studies, it is suggested that CVD may be a health
risk associated with radiation exposure. However, with exposure to
doses <0.5 Gy, an increased risk cannot be evidenced by
epidemiology alone, and a better understanding of the underlying
biological and molecular mechanisms is needed. In this way, the
current radiation protection system can be refined, making it
possible to more accurately assess the cardiovascular risk in the
low-dose region. The only way to unequivocally demonstrate the
cardiovascular effects of low-dose ionizing radiation is to use
dedicated or specialized in vitro and in vivo systems
representing the cardiovascular system. In these systems, the
endothelium is believed to be a critical target of ionizing
radiation exposure due to its crucial role in maintaining the
vascular homeostasis in the human body. Over the years, many
underlying causes of endothelial dysfunction following exposure to
ionizing radiation have been studied, such as nitric oxide
bioavailability, premature cell senescence and dysfunction of the
mitochondria. However, results in these fields are ambiguous and
more research is required to resolve their inconsistencies. These
efforts are not in vain, since not only can these findings be used
to ameliorate the current radiation protection system, but they can
also be used to devise cardiovascular risk-reducing strategies,
which can limit the number of patients suffering from CVD
worldwide.
Acknowledgments
This review is written in the context of a study
that was funded by the EU FP7 DoReMi (grant agreement 249689) on
'low dose research towards multidisciplinary integration', by the
EU FP7 Procardio project (grant agreement 295823), and by the
Federal Agency of Nuclear Control (FANC-AFCN, Belgium) (grant
agreement CO-90-13-3289-00). In addition, we would like to thank Dr
Markus Eidemüller, Dr Cristoforo Simonetto and Professor Helmut
Schöllnberger (all from Helmholtz Zentrum München) for their useful
comments. C. Rombouts was supported by a doctoral SCK•CEN/Ghent
University grant. B. Baselet is supported by a doctoral
SCK•CEN/Université catholique de Louvain grant.
References
|
1
|
Stewart JR and Fajardo LF:
Radiation-induced heart disease. Clinical and experimental aspects.
Radiol Clin North Am. 9:511–531. 1971.PubMed/NCBI
|
|
2
|
Stewart FA: Mechanisms and dose-response
relationships for radiation-induced cardiovascular disease. Ann
ICRP. 41:72–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schultz-Hector S and Trott KR:
Radiation-induced cardiovascular diseases: Is the epidemiologic
evidence compatible with the radiobiologic data? Int J Radiat Oncol
Biol Phys. 67:10–18. 2007. View Article : Google Scholar
|
|
4
|
Clarke M, Collins R, Darby S, Davies C,
Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al
Early Breast Cancer Trialists' Collaborative Group (EBCTCG):
Effects of radiotherapy and of differences in the extent of surgery
for early breast cancer on local recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 366:2087–2106. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Darby SC, Cutter DJ, Boerma M, Constine
LS, Fajardo LF, Kodama K, Mabuchi K, Marks LB, Mettler FA, Pierce
LJ, et al: Radiation-related heart disease: Current knowledge and
future prospects. Int J Radiat Oncol Biol Phys. 76:656–665. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Darby SC, McGale P, Taylor CW and Peto R:
Long-term mortality from heart disease and lung cancer after
radiotherapy for early breast cancer: Prospective cohort study of
about 300,000 women in US SEER cancer registries. Lancet Oncol.
6:557–565. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McGale P, Darby SC, Hall P, Adolfsson J,
Bengtsson NO, Bennet AM, Fornander T, Gigante B, Jensen MB, Peto R,
et al: Incidence of heart disease in 35,000 women treated with
radiotherapy for breast cancer in Denmark and Sweden. Radiother
Oncol. 100:167–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carr ZA, Land CE, Kleinerman RA, Weinstock
RW, Stovall M, Griem ML and Mabuchi K: Coronary heart disease after
radiotherapy for peptic ulcer disease. Int J Radiat Oncol Biol
Phys. 61:842–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Advisory Group on Ionising Radiation A:
Circulatory disease risk. Report of the independent Advisory Group
on Ionising Radiation. Health Protection Agency; London: 2010
|
|
10
|
Yusuf SW, Sami S and Daher IN:
Radiation-induced heart disease: A clinical update. Cardiol Res
Pract. 2011:3176592011.PubMed/NCBI
|
|
11
|
Schweizer E: Über spezifische
Röntgenschädigungen des Herzmuskels. Strahlentherapie. 18:812–828.
1924.In German.
|
|
12
|
Aleman BM, van den Belt-Dusebout AW,
Klokman WJ, Van't Veer MB, Bartelink H and van Leeuwen FE:
Long-term cause-specific mortality of patients treated for
Hodgkin's disease. J Clin Oncol. 21:3431–3439. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoppe RT: Hodgkin's disease: Complications
of therapy and excess mortality. Ann Oncol. 8(Suppl 1): 115–118.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ng AK, Bernardo MP, Weller E, Backstrand
KH, Silver B, Marcus KC, Tarbell NJ, Friedberg J, Canellos GP and
Mauch PM: Long-term survival and competing causes of death in
patients with early-stage Hodgkin's disease treated at age 50 or
younger. J Clin Oncol. 20:2101–2108. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Swerdlow AJ, Higgins CD, Smith P,
Cunningham D, Hancock BW, Horwich A, Hoskin PJ, Lister A, Radford
JA, Rohatiner AZ and Linch DC: Myocardial infarction mortality risk
after treatment for Hodgkin disease: A collaborative British cohort
study. J Natl Cancer Inst. 99:206–214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Darby SC, Ewertz M, McGale P, Bennet AM,
Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante
B, et al: Risk of ischemic heart disease in women after
radiotherapy for breast cancer. N Engl J Med. 368:987–998. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ozasa K, Shimizu Y, Sakata R, Sugiyama H,
Grant EJ, Soda M, Kasagi F and Suyama A: Risk of cancer and
non-cancer diseases in the atomic bomb survivors. Radiat Prot
Dosimetry. 146:272–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahashi I, Abbott RD, Ohshita T,
Takahashi T, Ozasa K, Akahoshi M, Fujiwara S, Kodama K and
Matsumoto M: A prospective follow-up study of the association of
radiation exposure with fatal and non-fatal stroke among atomic
bomb survivors in Hiroshima and Nagasaki 1980–2003. BMJ Open.
2:e0006542012. View Article : Google Scholar
|
|
19
|
Preston DL, Shimizu Y, Pierce DA, Suyama A
and Mabuchi K: Studies of mortality of atomic bomb survivors.
Report 13: Solid cancer and noncancer disease mortality: 1950–1997.
Radiat Res. 160:381–407. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimizu Y, Pierce DA, Preston DL and
Mabuchi K: Studies of the mortality of atomic bomb survivors.
Report 12, part II. Noncancer mortality: 1950–1990. Radiat Res.
152:374–389. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimizu Y, Kodama K, Nishi N, Kasagi F,
Suyama A, Soda M, Grant EJ, Sugiyama H, Sakata R, Moriwaki H, et
al: Radiation exposure and circulatory disease risk: Hiroshima and
Nagasaki atomic bomb survivor data, 1950–2003. BMJ. 340:b53492010.
View Article : Google Scholar
|
|
22
|
Little MP, Azizova TV, Bazyka D, Bouffler
SD, Cardis E, Chekin S, Chumak VV, Cucinotta FA, de Vathaire F,
Hall P, et al: Comment on 'dose-responses from multi-model
inference for the non-cancer disease mortality of atomic bomb
survivors' (Radiat. Environ. Biophys (2012) 51:165–178) by
Schöllnberger et al. Radiat Environ Biophys. 52:157–159. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schöllnberger H, Ozasa K, Neff F and
Kaiser JC: Cardiovascular disease mortality of A-bomb survivors and
the healthy survivor selection effect. Radiat Prot Dosimetry.
166:320–323. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vrijheid M, Cardis E, Ashmore P, Auvinen
A, Bae JM, Engels H, Gilbert E, Gulis G, Habib R, Howe G, et al:
Mortality from diseases other than cancer following low doses of
ionizing radiation: Results from the 15-Country Study of nuclear
industry workers. Int J Epidemiol. 36:1126–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ivanov VK, Maksioutov MA, Chekin SY,
Petrov AV, Biryukov AP, Kruglova ZG, Matyash VA, Tsyb AF, Manton KG
and Kravchenko JS: The risk of radiation-induced cerebrovascular
disease in Chernobyl emergency workers. Health Phys. 90:199–207.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muirhead CR, O'Hagan JA, Haylock RG,
Phillipson MA, Willcock T, Berridge GL and Zhang W: Mortality and
cancer incidence following occupational radiation exposure: Third
analysis of the National Registry for Radiation Workers. Br J
Cancer. 100:206–212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ashmore JP, Krewski D, Zielinski JM, Jiang
H, Semenciw R and Band PR: First analysis of mortality and
occupational radiation exposure based on the National Dose Registry
of Canada. Am J Epidemiol. 148:564–574. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rajaraman P, Doody MM, Yu CL, Preston DL,
Miller JS, Sigurdson AJ, Freedman DM, Alexander BH, Little MP,
Miller DL and Linet MS: Incidence and mortality risks for
circulatory diseases in US radiologic technologists who worked with
fluoroscopically guided interventional procedures, 1994–2008. Occup
Environ Med. 73:21–27. 2016. View Article : Google Scholar
|
|
29
|
Azizova TV, Day RD, Wald N, Muirhead CR,
O'Hagan JA, Sumina MV, Belyaeva ZD, Druzhinina MB, Teplyakov II,
Semenikhina NG, et al: The 'clinic' medical-dosimetric database of
Mayak production association workers: Structure, characteristics
and prospects of utilization. Health Phys. 94:449–458. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Azizova TV, Muirhead CR, Druzhinina MB,
Grigoryeva ES, Vlasenko EV, Sumina MV, O'Hagan JA, Zhang W, Haylock
RG and Hunter N: Cardiovascular diseases in the cohort of workers
first employed at Mayak PA in 1948–1958. Radiat Res. 174:155–168.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Azizova TV, Muirhead CR, Moseeva MB,
Grigoryeva ES, Vlasenko EV, Hunter N, Haylock RG and O'Hagan JA:
Ischemic heart disease in nuclear workers first employed at the
Mayak PA in 1948–1972. Health Phys. 103:3–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simonetto C, Azizova TV, Grigoryeva ES,
Kaiser JC, Schöllnberger H and Eidemüller M: Ischemic heart disease
in workers at Mayak PA: Latency of incidence risk after radiation
exposure. PLoS One. 9:e963092014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Azizova TV, Grigoryeva ES, Haylock RG,
Pikulina MV and Moseeva MB: Ischaemic heart disease incidence and
mortality in an extended cohort of Mayak workers first employed in
1948–1982. Br J Radiol. 88:201501692015. View Article : Google Scholar
|
|
34
|
Azizova TV, Haylock RG, Moseeva MB,
Bannikova MV and Grigoryeva ES: Cerebrovascular diseases incidence
and mortality in an extended Mayak Worker Cohort 1948–1982. Radiat
Res. 182:529–544. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Simonetto C, Schöllnberger H, Azizova TV,
Grigoryeva ES, Pikulina MV and Eidemüller M: Cerebrovascular
Diseases in Workers at Mayak PA: The Difference in Radiation Risk
between Incidence and Mortality. PLoS One. 10:e01259042015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Little MP, Azizova TV, Bazyka D, Bouffler
SD, Cardis E, Chekin S, Chumak VV, Cucinotta FA, de Vathaire F,
Hall P, et al: Systematic review and meta-analysis of circulatory
disease from exposure to low-level ionizing radiation and estimates
of potential population mortality risks. Environ Health Perspect.
120:1503–1511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schöllnberger H, Kaiser JC, Jacob P and
Walsh L: Dose-responses from multi-model inference for the
non-cancer disease mortality of atomic bomb survivors. Radiat
Environ Biophys. 51:165–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Working Party on Research Implications on
Health and Safety SotAGoE: Emerging evidence for radiation induced
circulatory diseases. Radiation Protection No 158. EU Scientific
Seminar 2008; Luxembourg. November 25, 2008;
|
|
39
|
United Nations Scientific Committee on the
Effects of Atomic Radiation: Annex B: Epidemiological evaluation of
cardiovascular disease and other non-cancer diseases following
radiation exposure. (UNSCEAR 2006 Report Vol. 1). United Nations;
New York: 2006
|
|
40
|
Montgomery JE and Brown JR: Metabolic
biomarkers for predicting cardiovascular disease. Vasc Health Risk
Manag. 9:37–45. 2013.PubMed/NCBI
|
|
41
|
Brenner DJ, Doll R, Goodhead DT, Hall EJ,
Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS, et
al: Cancer risks attributable to low doses of ionizing radiation:
Assessing what we really know. Proc Natl Acad Sci USA.
100:13761–13766. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Borghini A, Gianicolo EA, Picano E and
Andreassi MG: Ionizing radiation and atherosclerosis: Current
knowledge and future challenges. Atherosclerosis. 230:40–47. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Seddon B, Cook A, Gothard L, Salmon E,
Latus K, Underwood SR and Yarnold J: Detection of defects in
myocardial perfusion imaging in patients with early breast cancer
treated with radiotherapy. Radiother Oncol. 64:53–63. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chung E, Corbett JR, Moran JM, Griffith
KA, Marsh RB, Feng M, Jagsi R, Kessler ML, Ficaro EC and Pierce LJ:
Is there a dose-response relationship for heart disease with
low-dose radiation therapy? Int J Radiat Oncol Biol Phys.
85:959–964. 2013. View Article : Google Scholar
|
|
45
|
Januzzi JL Jr: Natriuretic peptide
testing: A window into the diagnosis and prognosis of heart
failure. Cleve Clin J Med. 73:149–152. 155–147. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Palazzuoli A, Gallotta M, Quatrini I and
Nuti R: Natriuretic peptides (BNP and NT-proBNP): Measurement and
relevance in heart failure. Vasc Health Risk Manag. 6:411–418.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sabatine MS, Morrow DA, de Lemos JA,
Omland T, Sloan S, Jarolim P, Solomon SD, Pfeffer MA and Braunwald
E: Evaluation of multiple biomarkers of cardiovascular stress for
risk prediction and guiding medical therapy in patients with stable
coronary disease. Circulation. 125:233–240. 2012. View Article : Google Scholar :
|
|
48
|
D'Errico MP, Grimaldi L, Petruzzelli MF,
Gianicolo EA, Tramacere F, Monetti A, Placella R, Pili G, Andreassi
MG, Sicari R, et al: N-terminal pro-B-type natriuretic peptide
plasma levels as a potential biomarker for cardiac damage after
radiotherapy in patients with left-sided breast cancer. Int J
Radiat Oncol Biol Phys. 82:e239–e246. 2012. View Article : Google Scholar
|
|
49
|
Preston RJ1, Boice JD Jr, Brill AB,
Chakraborty R, Conolly R, Hoffman FO, Hornung RW, Kocher DC, Land
CE, Shore RE and Woloschak GE: Uncertainties in estimating health
risks associated with exposure to ionising radiation. J Radiol
Prot. 33:573–588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Authors on behalf of ICRP; Stewart FA,
Akleyev AV, Hauer-Jensen M, Hendry JH, Kleiman NJ, Macvittie TJ,
Aleman BM, Edgar AB, Mabuchi K, Muirhead CR, et al: ICRP
publication 118: ICRP statement on tissue reactions and early and
late effects of radiation in normal tissues and organ - threshold
doses for tissue reactions in a radiation protection context. Ann
ICRP. 41:1–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
United Nations Scientific Committee on the
Effects of Atomic Radiation: Annex A: Medical Radiation Exposures.
(UNSCEAR 2008 Report Vol. 1). United Nations; New York: 2006
|
|
52
|
Hall EJ and Brenner DJ: Cancer risks from
diagnostic radiology. Br J Radiol. 81:362–378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Einstein AJ and Knuuti J: Cardiac imaging:
Does radiation matter? Eur Heart J. 33:573–578. 2012. View Article : Google Scholar :
|
|
54
|
Shapiro BP, Mergo PJ, Snipelisky DF,
Kantor B and Gerber TC: Radiation dose in cardiac imaging: How
should it affect clinical decisions? AJR Am J Roentgenol.
200:508–514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Miller JA, Raichlin E, Williamson EE,
McCully RB, Pellikka PA, Hodge DO, Miller TD, Gibbons RJ and Araoz
PA: Evaluation of coronary CTA Appropriateness Criteria in an
academic medical center. J Am Coll Radiol. 7:125–131. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hendel RC, Cerqueira M, Douglas PS, Caruth
KC, Allen JM, Jensen NC, Pan W, Brindis R and Wolk M: A multicenter
assessment of the use of single-photon emission computed tomography
myocardial perfusion imaging with appropriateness criteria. J Am
Coll Cardiol. 55:156–162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Picano E and Vano E: The radiation issue
in cardiology: The time for action is now. Cardiovasc Ultrasound.
9:352011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Paterick TE, Jan MF, Paterick ZR, Tajik AJ
and Gerber TC: Cardiac imaging modalities with ionizing radiation:
The role of informed consent. JACC Cardiovasc Imaging. 5:634–640.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Halliburton SS and Schoenhagen P:
Cardiovascular imaging with computed tomography: Responsible steps
to balancing diagnostic yield and radiation exposure. JACC
Cardiovasc Imaging. 3:536–540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pernot E, Hall J, Baatout S, Benotmane MA,
Blanchardon E, Bouffler S, El Saghire H, Gomolka M, Guertler A,
Harms-Ringdahl M, et al: Ionizing radiation biomarkers for
potential use in epidemiological studies. Mutat Res. 751:258–286.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Adams MJ, Hardenbergh PH, Constine LS and
Lipshultz SE: Radiation-associated cardiovascular disease. Crit Rev
Oncol Hematol. 45:55–75. 2003. View Article : Google Scholar
|
|
62
|
Adams MJ, Lipshultz SE, Schwartz C,
Fajardo LF, Coen V and Constine LS: Radiation-associated
cardiovascular disease: Manifestations and management. Semin Radiat
Oncol. 13:346–356. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zaragoza C, Gomez-Guerrero C,
Martin-Ventura JL, Blanco-Colio L, Lavin B, Mallavia B, Tarin C,
Mas S, Ortiz A and Egido J: Animal models of cardiovascular
diseases. J Biomed Biotechnol. 2011:4978412011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zadelaar S, Kleemann R, Verschuren L, de
Vries-Van der Weij J, van der Hoorn J, Princen HM and Kooistra T:
Mouse models for atherosclerosis and pharmaceutical modifiers.
Arterioscler Thromb Vasc Biol. 27:1706–1721. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ohashi R, Mu H, Yao Q and Chen C: Cellular
and molecular mechanisms of atherosclerosis with mouse models.
Trends Cardiovasc Med. 14:187–190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yamashita A and Asada Y: A rabbit model of
thrombosis on atherosclerotic lesions. J Biomed Biotechnol.
2011:4249292011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Scherer E, Streffer C and Trott KR:
Radiopathology of Organs and Tissues. Springer Verlag; Berlin:
1991, View Article : Google Scholar
|
|
68
|
Fajardo LF and Stewart JR: Experimental
radiation-induced heart disease. I. Light microscopic studies. Am J
Pathol. 59:299–316. 1970.PubMed/NCBI
|
|
69
|
Gavin PR and Gillette EL: Radiation
response of the canine cardiovascular system. Radiat Res.
90:489–500. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lauk S, Kiszel Z, Buschmann J and Trott
KR: Radiation-induced heart disease in rats. Int J Radiat Oncol
Biol Phys. 11:801–808. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Virmani R, Kolodgie FD, Burke AP, Finn AV,
Gold HK, Tulenko TN, Wrenn SP and Narula J: Atherosclerotic plaque
progression and vulnerability to rupture: Angiogenesis as a source
of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol.
25:2054–2061. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mitchel RE, Hasu M, Bugden M, Wyatt H,
Hildebrandt G, Chen YX, Priest ND and Whitman SC: Low-dose
radiation exposure and protection against atherosclerosis in
ApoE(−/−) mice: The influence of P53 heterozygosity. Radiat Res.
179:190–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Stewart FA, Heeneman S, Te Poele J, Kruse
J, Russell NS, Gijbels M and Daemen M: Ionizing radiation
accelerates the development of atherosclerotic lesions in
ApoE−/− mice and predisposes to an inflammatory plaque
phenotype prone to hemorrhage. Am J Pathol. 168:649–658. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gabriels K, Hoving S, Seemann I, Visser
NL, Gijbels MJ, Pol JF, Daemen MJ, Stewart FA and Heeneman S: Local
heart irradiation of ApoE(−/−) mice induces microvascular and
endocardial damage and accelerates coronary atherosclerosis.
Radiother Oncol. 105:358–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hoving S, Heeneman S, Gijbels MJ, te Poele
JA, Russell NS, Daemen MJ and Stewart FA: Single-dose and
fractionated irradiation promote initiation and progression of
atherosclerosis and induce an inflammatory plaque phenotype in
ApoE(−/−) mice. Int J Radiat Oncol Biol Phys. 71:848–857. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mitchel RE, Hasu M, Bugden M, Wyatt H,
Little MP, Gola A, Hildebrandt G, Priest ND and Whitman SC:
Low-dose radiation exposure and atherosclerosis in
ApoE−/− mice. Radiat Res. 175:665–676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mancuso M, Pasquali E, Braga-Tanaka I III,
Tanaka S, Pannicelli A, Giardullo P, Pazzaglia S, Tapio S, Atkinson
MJ and Saran A: Acceleration of atherogenesis in ApoE−/−
mice exposed to acute or low-dose-rate ionizing radiation.
Oncotarget. 6:31263–31271. 2015.PubMed/NCBI
|
|
79
|
Le Gallic C, Phalente Y, Manens L,
Dublineau I, Benderitter M, Gueguen Y, Lehoux S and Ebrahimian TG:
Chronic internal exposure to low dose 137Cs induces
positive impact on the stability of atherosclerotic plaques by
reducing inflammation in ApoE−/− mice. PLoS One.
10:e01285392015. View Article : Google Scholar
|
|
80
|
Jeon YH, Kraus SG, Jowsey T and Glasgow
NJ: The experience of living with chronic heart failure: A
narrative review of qualitative studies. BMC Health Serv Res.
10:772010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lauk S: Endothelial alkaline phosphatase
activity loss as an early stage in the development of
radiation-induced heart disease in rats. Radiat Res. 110:118–128.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Schultz-Hector S: Radiation-induced heart
disease: Review of experimental data on dose response and
pathogenesis. Int J Radiat Biol. 61:149–160. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Franken NA, Camps JA, van Ravels FJ, van
der Laarse A, Pauwels EK and Wondergem J: Comparison of in vivo
cardiac function with ex vivo cardiac performance of the rat heart
after thoracic irradiation. Br J Radiol. 70:1004–1009. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Seemann I, Gabriels K, Visser NL, Hoving
S, te Poele JA, Pol JF, Gijbels MJ, Janssen BJ, van Leeuwen FW,
Daemen MJ, et al: Irradiation induced modest changes in murine
cardiac function despite progressive structural damage to the
myocardium and microvasculature. Radiother Oncol. 103:143–150.
2012. View Article : Google Scholar
|
|
85
|
Monceau V, Meziani L, Strup-Perrot C,
Morel E, Schmidt M, Haagen J, Escoubet B, Dörr W and Vozenin MC:
Enhanced sensitivity to low dose irradiation of ApoE−/−
mice mediated by early pro-inflammatory profile and delayed
activation of the TGFβ1 cascade involved in fibrogenesis. PLoS One.
8:e570522013. View Article : Google Scholar
|
|
86
|
Hendry JH, Akahoshi M, Wang LS, Lipshultz
SE, Stewart FA and Trott KR: Radiation-induced cardiovascular
injury. Radiat Environ Biophys. 47:189–193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Little MP, Tawn EJ, Tzoulaki I, Wakeford
R, Hildebrandt G, Paris F, Tapio S and Elliott P: A systematic
review of epidemiological associations between low and moderate
doses of ionizing radiation and late cardiovascular effects, and
their possible mechanisms. Radiat Res. 169:99–109. 2008. View Article : Google Scholar
|
|
88
|
Bhatti P, Sigurdson AJ and Mabuchi K: Can
low-dose radiation increase risk of cardiovascular disease? Lancet.
372:697–699. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hildebrandt G: Non-cancer diseases and
non-targeted effects. Mutat Res. 687:73–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hildebrandt G, Maggiorella L, Rödel F,
Rödel V, Willis D and Trott KR: Mononuclear cell adhesion and cell
adhesion molecule liberation after X-irradiation of activated
endothelial cells in vitro. Int J Radiat Biol. 78:315–325. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sievert W, Trott KR, Azimzadeh O, Tapio S,
Zitzelsberger H and Multhoff G: Late proliferating and inflammatory
effects on murine microvascular heart and lung endothelial cells
after irradiation. Radiother Oncol. 117:376–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hallahan DE, Virudachalam S and
Kuchibhotla J: Nuclear factor kappaB dominant negative genetic
constructs inhibit X-ray induction of cell adhesion molecules in
the vascular endothelium. Cancer Res. 58:5484–5488. 1998.PubMed/NCBI
|
|
93
|
Van Der Meeren A, Squiban C, Gourmelon P,
Lafont H and Gaugler MH: Differential regulation by IL-4 and IL-10
of radiation-induced IL-6 and IL-8 production and ICAM-1 expression
by human endothelial cells. Cytokine. 11:831–838. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Milliat F, François A, Isoir M, Deutsch E,
Tamarat R, Tarlet G, Atfi A, Validire P, Bourhis J, Sabourin JC and
Benderitter M: Influence of endothelial cells on vascular smooth
muscle cells phenotype after irradiation: Implication in
radiation-induced vascular damages. Am J Pathol. 169:1484–1495.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hayashi T, Morishita Y, Khattree R, Misumi
M, Sasaki K, Hayashi I, Yoshida K, Kajimura J, Kyoizumi S, Imai K,
et al: Evaluation of systemic markers of inflammation in
atomic-bomb survivors with special reference to radiation and age
effects. FASEB J. 26:4765–4773. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang J, Zheng H, Ou X, Fink LM and
Hauer-Jensen M: Deficiency of microvascular thrombomodulin and
upregulation of protease-activated receptor-1 in irradiated rat
intestine: Possible link between endothelial dysfunction and
chronic radiation fibrosis. Am J Pathol. 160:2063–2072. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Azimzadeh O, Scherthan H, Sarioglu H,
Barjaktarovic Z, Conrad M, Vogt A, Calzada-Wack J, Neff F, Aubele
M, Buske C, et al: Rapid proteomic remodeling of cardiac tissue
caused by total body ionizing radiation. Proteomics. 11:3299–3311.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Azimzadeh O, Sievert W, Sarioglu H,
Merl-Pham J, Yentrapalli R, Bakshi MV, Janik D, Ueffing M, Atkinson
MJ, Multhoff G and Tapio S: Integrative proteomics and targeted
transcriptomics analyses in cardiac endothelial cells unravel
mechanisms of long-term radiation-induced vascular dysfunction. J
Proteome Res. 14:1203–1219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Trott KR and Kamprad F: Radiobiological
mechanisms of anti-inflammatory radiotherapy. Radiother Oncol.
51:197–203. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Seegenschmiedt MH, Katalinic A, Makoski
HB, Haase W, Gademann G and Hassenstein E: Radiotherapy of benign
diseases: A pattern of care study in Germany. Strahlenther Onkol.
175:541–547. 1999.In German. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Rödel F, Keilholz L, Herrmann M, Sauer R
and Hildebrandt G: Radiobiological mechanisms in inflammatory
diseases of low-dose radiation therapy. Int J Radiat Biol.
83:357–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kern PM, Keilholz L, Forster C, Hallmann
R, Herrmann M and Seegenschmiedt MH: Low-dose radiotherapy
selectively reduces adhesion of peripheral blood mononuclear cells
to endothelium in vitro. Radiother Oncol. 54:273–282. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hirase T and Node K: Endothelial
dysfunction as a cellular mechanism for vascular failure. Am J
Physiol Heart Circ Physiol. 302:H499–H505. 2012. View Article : Google Scholar
|
|
104
|
Flammer AJ and Lüscher TF: Three decades
of endothelium research: From the detection of nitric oxide to the
everyday implementation of endothelial function measurements in
cardiovascular diseases. Swiss Med Wkly. 140:w131222010.PubMed/NCBI
|
|
105
|
Triggle CR, Samuel SM, Ravishankar S,
Marei I, Arunachalam G and Ding H: The endothelium: Influencing
vascular smooth muscle in many ways. Can J Physiol Pharmacol.
90:713–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
van Hinsbergh VW: Endothelium - role in
regulation of coagulation and inflammation. Semin Immunopathol.
34:93–106. 2012. View Article : Google Scholar
|
|
107
|
Michiels C: Endothelial cell functions. J
Cell Physiol. 196:430–443. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sandoo A, van Zanten JJ, Metsios GS,
Carroll D and Kitas GD: The endothelium and its role in regulating
vascular tone. Open Cardiovasc Med J. 4:302–312. 2010. View Article : Google Scholar
|
|
109
|
Aird WC: Phenotypic heterogeneity of the
endothelium: I. Structure, function, and mechanisms. Circ Res.
100:158–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Aird WC: Phenotypic heterogeneity of the
endothelium: II. Representative vascular beds. Circ Res.
100:174–190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Landmesser U, Hornig B and Drexler H:
Endothelial function: A critical determinant in atherosclerosis?
Circulation. 109(Suppl 1): II27–II33. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Mudau M, Genis A, Lochner A and Strijdom
H: Endothelial dysfunction: The early predictor of atherosclerosis.
Cardiovasc J Afr. 23:222–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Shah AM and Channon KM: Free radicals and
redox signalling in cardiovascular disease. Heart. 90:486–487.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lum H and Roebuck KA: Oxidant stress and
endothelial cell dysfunction. Am J Physiol Cell Physiol.
280:C719–C741. 2001.PubMed/NCBI
|
|
115
|
Jaffe EA, Nachman RL, Becker CG and Minick
CR: Culture of human endothelial cells derived from umbilical
veins. Identification by morphologic and immunologic criteria. J
Clin Invest. 52:2745–2756. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Gimbrone MA Jr, Cotran RS and Folkman J:
Human vascular endothelial cells in culture. Growth and DNA
synthesis. J Cell Biol. 60:673–684. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Bicknell R: Endothelial cell culture.
Cambridge University Press; 1996, View Article : Google Scholar
|
|
118
|
Edgell CJ, McDonald CC and Graham JB:
Permanent cell line expressing human factor VIII-related antigen
established by hybridization. Proc Natl Acad Sci USA. 80:3734–3737.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Rombouts C, Aerts A, Beck M, De Vos WH,
Van Oostveldt P, Benotmane MA and Baatout S: Differential response
to acute low dose radiation in primary and immortalized endothelial
cells. Int J Radiat Biol. 89:841–850. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu
JY, Weinberg RA, Louis DN, Li FP and Rheinwald JG: Human
keratinocytes that express hTERT and also bypass a
p16(INK4a)-enforced mechanism that limits life span become immortal
yet retain normal growth and differentiation characteristics. Mol
Cell Biol. 20:1436–1447. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Bouïs D, Hospers GA, Meijer C, Molema G
and Mulder NH: Endothelium in vitro: A review of human vascular
endothelial cell lines for blood vessel-related research.
Angiogenesis. 4:91–102. 2001. View Article : Google Scholar
|
|
122
|
Wallace CS and Truskey GA: Direct-contact
co-culture between smooth muscle and endothelial cells inhibits
TNF-alpha-mediated endothelial cell activation. Am J Physiol Heart
Circ Physiol. 299:H338–H346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Rainger GE and Nash GB: Cellular pathology
of atherosclerosis: Smooth muscle cells prime cocultured
endothelial cells for enhanced leukocyte adhesion. Circ Res.
88:615–622. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Dietrich F and Lelkes PI: Fine-tuning of a
three-dimensional microcarrier-based angiogenesis assay for the
analysis of endothelial-mesenchymal cell co-cultures in fibrin and
collagen gels. Angiogenesis. 9:111–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Vernon RB and Sage EH: A novel,
quantitative model for study of endothelial cell migration and
sprout formation within three-dimensional collagen matrices.
Microvasc Res. 57:118–133. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Acheva A, Aerts A, Rombouts C, Baatout S,
Salomaa S, Manda K, Hildebrandt G and Kämäräinen M: Human 3-D
tissue models in radiation biology: Current status and future
perspectives. Int J Radiat Res. 12:81–98. 2014.
|
|
127
|
Goel S, Duda DG, Xu L, Munn LL, Boucher Y,
Fukumura D and Jain RK: Normalization of the vasculature for
treatment of cancer and other diseases. Physiol Rev. 91:1071–1121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhang HP, Takayama K, Su B, Jiao XD, Li R
and Wang JJ: Effect of sunitinib combined with ionizing radiation
on endothelial cells. J Radiat Res (Tokyo). 52:1–8. 2011.
View Article : Google Scholar
|
|
129
|
Dudley AC: Tumor endothelial cells. Cold
Spring Harb Perspect Med. 2:a0065362012. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Jeggo P and Löbrich M: Radiation-induced
DNA damage responses. Radiat Prot Dosimetry. 122:124–127. 2006.
View Article : Google Scholar
|
|
131
|
Bolus NE: Basic review of radiation
biology and terminology. J Nucl Med Technol. 29:67–77.
2001.PubMed/NCBI
|
|
132
|
Norbury CJ and Hickson ID: Cellular
responses to DNA damage. Annu Rev Pharmacol Toxicol. 41:367–401.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Clarke PR and Allan LA: Cell-cycle control
in the face of damage - a matter of life or death. Trends Cell
Biol. 19:89–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Dikomey E, Dahm-Daphi J, Brammer I,
Martensen R and Kaina B: Correlation between cellular
radiosensitivity and non-repaired double-strand breaks studied in
nine mammalian cell lines. Int J Radiat Biol. 73:269–278. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Kuo LJ and Yang LX: Gamma-H2AX - a novel
biomarker for DNA double-strand breaks. In Vivo. 22:305–309.
2008.PubMed/NCBI
|
|
136
|
Stoneman VE and Bennett MR: Role of
apoptosis in atherosclerosis and its therapeutic implications. Clin
Sci (Lond). 107:343–354. 2004. View Article : Google Scholar
|
|
137
|
Mercer J, Mahmoudi M and Bennett M: DNA
damage, p53, apoptosis and vascular disease. Mutat Res. 621:75–86.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Choy JC, Granville DJ, Hunt DW and McManus
BM: Endothelial cell apoptosis: Biochemical characteristics and
potential implications for atherosclerosis. J Mol Cell Cardiol.
33:1673–1690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Haimovitz-Friedman A, Kan CC, Ehleiter D,
Persaud RS, McLoughlin M, Fuks Z and Kolesnick RN: Ionizing
radiation acts on cellular membranes to generate ceramide and
initiate apoptosis. J Exp Med. 180:525–535. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Langley RE, Bump EA, Quartuccio SG,
Medeiros D and Braunhut SJ: Radiation-induced apoptosis in
microvascular endothelial cells. Br J Cancer. 75:666–672. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Rödel F, Frey B, Capalbo G, Gaipl U,
Keilholz L, Voll R, Hildebrandt G and Rödel C: Discontinuous
induction of X-linked inhibitor of apoptosis in EA.hy.926
endothelial cells is linked to NF-κB activation and mediates the
anti-inflammatory properties of low-dose ionising-radiation.
Radiother Oncol. 97:346–351. 2010. View Article : Google Scholar
|
|
142
|
Pluder F, Barjaktarovic Z, Azimzadeh O,
Mörtl S, Krämer A, Steininger S, Sarioglu H, Leszczynski D, Nylund
R, Hakanen A, et al: Low-dose irradiation causes rapid alterations
to the proteome of the human endothelial cell line EA.hy926. Radiat
Environ Biophys. 50:155–166. 2011. View Article : Google Scholar
|
|
143
|
Yu E, Mercer J and Bennett M: Mitochondria
in vascular disease. Cardiovasc Res. 95:173–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Riley PA: Free radicals in biology:
Oxidative stress and the effects of ionizing radiation. Int J
Radiat Biol. 65:27–33. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Yamamori T, Yasui H, Yamazumi M, Wada Y,
Nakamura Y, Nakamura H and Inanami O: Ionizing radiation induces
mitochondrial reactive oxygen species production accompanied by
upregulation of mitochondrial electron transport chain function and
mitochondrial content under control of the cell cycle checkpoint.
Free Radic Biol Med. 53:260–270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Bernardi P: The mitochondrial permeability
transition pore: A mystery solved? Front Physiol. 4:952013.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Leach JK, Van Tuyle G, Lin PS,
Schmidt-Ullrich R and Mikkelsen RB: Ionizing radiation-induced,
mitochondria-dependent generation of reactive oxygen/nitrogen.
Cancer Res. 61:3894–3901. 2001.PubMed/NCBI
|
|
148
|
Prithivirajsingh S, Story MD, Bergh SA,
Geara FB, Ang KK, Ismail SM, Stevens CW, Buchholz TA and Brock WA:
Accumulation of the common mitochondrial DNA deletion induced by
ionizing radiation. FEBS Lett. 571:227–232. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wang L, Kuwahara Y, Li L, Baba T, Shin RW,
Ohkubo Y, Ono K and Fukumoto M: Analysis of Common Deletion (CD)
and a novel deletion of mitochondrial DNA induced by ionizing
radiation. Int J Radiat Biol. 83:433–442. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Schilling-Tóth B, Sándor N, Kis E, Kadhim
M, Sáfrány G and Hegyesi H: Analysis of the common deletions in the
mitochondrial DNA is a sensitive biomarker detecting direct and
non-targeted cellular effects of low dose ionizing radiation. Mutat
Res. 716:33–39. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Barjaktarovic Z, Schmaltz D, Shyla A,
Azimzadeh O, Schulz S, Haagen J, Dörr W, Sarioglu H, Schäfer A,
Atkinson MJ, et al: Radiation-induced signaling results in
mitochondrial impairment in mouse heart at 4 weeks after exposure
to X-rays. PLoS One. 6:e278112011. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Barjaktarovic Z, Shyla A, Azimzadeh O,
Schulz S, Haagen J, Dörr W, Sarioglu H, Atkinson MJ, Zischka H and
Tapio S: Ionising radiation induces persistent alterations in the
cardiac mitochondrial function of C57BL/6 mice 40 weeks after local
heart exposure. Radiother Oncol. 106:404–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Azimzadeh O, Sievert W, Sarioglu H,
Yentrapalli R, Barjaktarovic Z, Sriharshan A, Ueffing M, Janik D,
Aichler M, Atkinson MJ, et al: PPAR alpha: A novel radiation target
in locally exposed Mus musculus heart revealed by quantitative
proteomics. J Proteome Res. 12:2700–2714. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Barjaktarovic Z, Kempf SJ, Sriharshan A,
Merl-Pham J, Atkinson MJ and Tapio S: Ionizing radiation induces
immediate protein acetylation changes in human cardiac
microvascular endothelial cells. J Radiat Res (Tokyo). 56:623–632.
2015. View Article : Google Scholar
|
|
155
|
Vávrová J and Rezáčová M: The importance
of senescence in ionizing radiation-induced tumour suppression.
Folia Biol (Praha). 57:41–46. 2011.
|
|
156
|
Sabatino L, Picano E and Andreassi MG:
Telomere shortening and ionizing radiation: A possible role in
vascular dysfunction? Int J Radiat Biol. 88:830–839. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Kurz DJ, Decary S, Hong Y, Trivier E,
Akhmedov A and Erusalimsky JD: Chronic oxidative stress compromises
telomere integrity and accelerates the onset of senescence in human
endothelial cells. J Cell Sci. 117:2417–2426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Campisi J and d'Adda di Fagagna F:
Cellular senescence: When bad things happen to good cells. Nat Rev
Mol Cell Biol. 8:729–740. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Oh CW, Bump EA, Kim JS, Janigro D and
Mayberg MR: Induction of a senescence-like phenotype in bovine
aortic endothelial cells by ionizing radiation. Radiat Res.
156:232–240. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Panganiban RA, Mungunsukh O and Day RM:
X-irradiation induces ER stress, apoptosis, and senescence in
pulmonary artery endothelial cells. Int J Radiat Biol. 89:656–667.
2013. View Article : Google Scholar
|
|
161
|
Igarashi K, Sakimoto I, Kataoka K, Ohta K
and Miura M: Radiation-induced senescence-like phenotype in
proliferating and plateau-phase vascular endothelial cells. Exp
Cell Res. 313:3326–3336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Kim KS, Kim JE, Choi KJ, Bae S and Kim DH:
Characterization of DNA damage-induced cellular senescence by
ionizing radiation in endothelial cells. Int J Radiat Biol.
90:71–80. 2014. View Article : Google Scholar
|
|
163
|
Suzuki K, Mori I, Nakayama Y, Miyakoda M,
Kodama S and Watanabe M: Radiation-induced senescence-like growth
arrest requires TP53 function but not telomere shortening. Radiat
Res. 155:248–253. 2001. View Article : Google Scholar
|
|
164
|
Yentrapalli R, Azimzadeh O, Barjaktarovic
Z, Sarioglu H, Wojcik A, Harms-Ringdahl M, Atkinson MJ, Haghdoost S
and Tapio S: Quantitative proteomic analysis reveals induction of
premature senescence in human umbilical vein endothelial cells
exposed to chronic low-dose rate gamma radiation. Proteomics.
13:1096–1107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Yentrapalli R, Azimzadeh O, Sriharshan A,
Malinowsky K, Merl J, Wojcik A, Harms-Ringdahl M, Atkinson MJ,
Becker KF, Haghdoost S and Tapio S: The PI3K/Akt/mTOR pathway is
implicated in the premature senescence of primary human endothelial
cells exposed to chronic radiation. PLoS One. 8:e700242013.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Rombouts C, Aerts A, Quintens R, Baselet
B, El-Saghire H, Harms-Ringdahl M, Haghdoost S, Janssen A, Michaux
A, Yentrapalli R, et al: Transcriptomic profiling suggests a role
for IGFBP5 in premature senescence of endothelial cells after
chronic low dose rate irradiation. Int J Radiat Biol. 90:560–574.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Libby P, Ridker PM and Hansson GK:
Progress and challenges in translating the biology of
atherosclerosis. Nature. 473:317–325. 2011. View Article : Google Scholar : PubMed/NCBI
|