Introduction
Ischemic stroke is recognized as one of the
catastrophic threats to the human health, and is associated with an
increasing morbidity worldwide. An estimated 700,000 cases of
ischemic stroke occur in the United States each year, costing
>$70 billion to society (1).
Cerebral ischemia can cause severe damage, such as neuronal
apoptosis or death, glial cell activation and proliferation,
inflammatory reaction and stress response (2,3).
Cerebral ischemia can also cause many remote organ dysfunctions
(4–12); a previous study indicated that
brain ischemia can cause lung injury (13).
Mesenchymal stem cells (MSCs) are a type of stem
cell derived from the mesoderm, and primarily exist in the
connective tissue of the body and organ interstitial space,
particularly the bone marrow. Bone marrow-derived mesenchymal stem
cells (BMSCs), are one of the main types of stem cells and have the
ability of self-proliferation (14), self-renewal and the potential to
differentiate into other cells in peripheral tissue (15). Recently, studies using BMSCs have
achieved positive results in the treatment of degenerative and
defective diseases, cardiovascular disease, liver transplantation,
pulmonary fibrosis and other diseases (14–17). Previous studies have reported that
transplanted BMSCs can home to damaged lung tissues and
differentiate into lung epithelial cells, as well as vascular
endothelial cells, and can then regulate the inflammatory response
in acute lung injury (ALI); thus, they are involved in repairing
injured lungs (18,19). Furthermore, studies have
demonstrated that BMSC transplantation has therapeutic effects of
ALI/acute respiratory distress syndrome (ARDS) by releasing soluble
factors, reducing pulmonary capillary endothelium permeability and
alveolar edema, alleviating lung inflammation, promoting cell
proliferation and inhibiting cell apoptosis (20,21). Although a large number of studies
have demonstrated that stem cell transplantation has some
protective effects on the lungs, the cell biology and molecular
biological mechanisms of action of BMSCs in vivo are not yet
entirely understood, and in particular, the mechanisms of BMSCs in
lung injury caused by cerebral ischemia.
Gene microarray (22), as a high throughput technology for
life sciences and microelectronics, has been widely investigated
and is widely used in various research areas of biology and
medicine in bioinformatics research in recent years. It provides
important theoretical and practical values in sequence analysis,
gene expression, genome research and the intensity of hybridization
signals of gene expression profiles. It is able to yield
large-scale, high-throughput information, integrating a range of
biological information (23).
Thus, in the present study, in order to elucidate the potential
molecular mechanisms responsible for the protective role of BMSC
transplantation into the lungs, gene microarray analysis was
used.
The present study was therefore undertaken to
investigate the protective effects of BMSC transplantation on rat
lung injury following permanent focal cerebral ischemia, and to
explore the related molecular mechanisms using Gene microarray and
Gene Ontology (http://www.geneontology.org/).
Materials and methods
Experimental animals and grouping
A total of 27 healthy adult female SD rats, 2 months
old, weighing 220±10 g were provided by the Experimental Animal
Center of Kunming Medical University, Kunming, China. Animal care
and all experimental protocols were approved by the Animal Care
Committee of Sichuan University, West China Hospital, Chengdu,
China and according to the guidelines of the Unites States National
Institutes of Health. All animals were raised in plastic cages
(n=2/cage) with soft bedding and free access to food and water in a
temperature (21–25°C) and humidity (45–50%)-controlled room. The
rats were randomly divided into the sham-operated group (Sham), the
brain ischemia (BI) group and the BMSC transplantation [BMSCs (t)]
group (n=8 rats in each group). The animals in the BI group were
subjected to permanent focal brain ischemia and treated with
culture medium. The animals in the BMSCs (t) group were subjected
to BI and injected with the BMSC suspension at 9 days following
injury. The animals in the sham-operated group were not subjected
to either BI or to transplant injections.
Animal model of permanent focal cerebral
ischemia
A model of permanent focal cerebral ischemia was
established by occlusion of the middle cerebral artery (MCAO) as
previously described (24).
Briefly, the SD rats were anesthetized deeply by an intraperitoneal
injection of 2% pentobarbital sodium (30 mg/kg) and immobilized in
the supine position for skin preparation. After the left external
carotid artery section was exposed, a small hole was created in the
free section and a thread (purchased from Beijing Cinontech Co.,
Ltd., Beijing, China) was inserted from the bifurcation of the
common carotid artery into the internal carotid artery, and then
into the beginning of the middle cerebral artery. Thread insertion
was approximately 18.5±0.5 mm deep. The sham-operated group
insertion was approximately 1 cm.
BMSC culture and purification
BMSCs were harvested from the femurs and tibias of 3
SD rats. Briefly, after the rats were euthanized with a mixture of
70% CO2 and 30% O2, the femurs and tibias
were dissected in a sterile environment and rinsed with D-Hanks
solution. The epiphyses of the femurs and tibias were removed, and
the marrow was then extruded using a syringe filled with DMEM/F12
containing 10% fetal bovine serum (FBS; Gibco, Gaithersburg, MD,
USA), and repeatedly beated into a single cell suspension with 5 ml
DMEM/F12 containing 10% FBS and penicillin/streptomycin. Following
centrifugation (800 × g for 5 min) and re-suspension, 5 ml of cell
suspension was collected, and the cells were plated in 25
cm2 culture flasks at a density of 3×105
cells/ml in an incubator (37°C, 95% humidity, 5% CO2).
After 24 h, the supernatant containing non-adherent cells was
removed and fresh medium was added. The medium was changed every
3–5 days, after the cells had grown to near confluence and the
density was approximately (4–5)×105 cells/cm2;
the cells were passaged 3 times and the suspended cells were
discarded. Subsequently, the pure adherent cells (BMSCs) were
cultivated for further analysis. The growth status of the cultured
BMSCs was observed under an inverted phase contrast microscope
(Leica Microsystems GmbH, Wetzlar, Germany).
Immunohistochemistry
In previous studies, it was found that the growth
status of BMSCs in passage 3 was better than that in passage 1 or 2
(25,26). Therefore, BMSCs at passage 3 were
used in this study. To identify the cultured BMSCs, the purified
BMSCs, derived from passage 3 before transplantation, were fixed
with 4% paraformaldehyde for 10 min, and phosphate buffered-saline
(PBS) (0.01 mol/l) was used to wash the cells 3 times (5 min/time).
The specific methods for identification are listed below, according
to immunohistochemical two-step staining: washing with 0.01 mol/l
PBS 3 times (5 min/time), incubating with 0.3% Triton X-100 for 30
min at 37°C, incubation with 5% goat serum for 30 min at 37°C to
block non-specific binding sites; incubation with primary antibody
to CD44 (1:100; ZA-0537; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) at 4°C for 24 h (the
negative control was only incubated with 2% sheep serum); washing
with 0.01 mol/l PBS 3 times (5 min/time); and incubation with Alexa
Fluor-labeled 594 goat anti-rabbit IgG (secondary antibody, 1:100;
ZF-0516; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.)
for 60 min at 37°C. The cells were then observed and iamged using a
fluorescent microscope (Leica Microsystems GmbH).
Cell transplantation
Cyclosporine A (10 mg/kg body weight) was
intraperitoneally injected 3 days prior to cell transplantation;
Hoechst 33342 (Beyotime Institute of Biotechnology, Jiangsu, China)
was used to stain the prepared cells prior to transplantation in
order to be able to trace the cells after transplantation.
Approximately 6 µl BMSCs (1×106/ml) at passage 3
were slowly transplanted to the tail vein at the 9th day
post-surgery. Cyclosporine A (10 mg/kg body weight) and penicillin
(20 U/one) were injected at 24 h post-cell transplantation until
day 3. Cyclosporine A was used before and after
transplantation.
Behavioral evaluation
In order confirm that the animal model was
successfully created and to detect behavioral changes post-surgery,
neurological deficit evaluation was performed before and 4 h
post-surgery, in accordance with the scoring method described in
the study by Menzies et al (27): 0 points, no neurological damage,
double forelimb symmetric stretching to the ground; 1 point,
contralateral forelimb sustained adduction; 2 points, contralateral
forelimb grip strength decreased; 3 points, a slight stimulation of
rat tails to the contralateral circling; 4 points, independent
continuous circular motion. The animals in the model group with a
score ≥1 were selected for analysis in further experiments and were
randomly equivalently divided into the BMSCs (t) group and the
culture medium injection (BI) group.
In order to better assess the effects of BMSC
transplantation on neurological function, a more detailed scoring
criteria that was double-blind was evaluated at 4:00 p.m. at 0, 3,
7 and 14 days post-transplantation. The improved neurological
evaluation score ranged between 0–18 points as follows: 0 points,
no neurological damage; 1–6 points, mild injury; 7–12 points,
moderate injury; 13–18 points, severe damage.
Tissue harvest
At 14 days post-transplantation, the experimental
and sham animals were sacrificed by cutting the abdominal aorta
following an intraperitoneal injection of 2% pentobarbital sodium.
The lung tissues were removed immediately after cutting the
abdominal aorta for further histological and molecular analyses.
The specific steps were as follows:
Hematoxylin and eosin (H&E)
staining
At 14 days post-transplantation, all the rats were
sacrificed as described above, and the harvested lung tissues were
fixed with 4% paraformaldehyde in 0.1 M ice-cold phosphate buffer,
pH 7.4, for at least 72 h at 4°C. The fixed lung tissues were then
embedded in paraffin and sectioned at a thickness of 5 µm,
transferred to glass slides, and stained with H&E (C0105;
Beyotime Institute of Biotechnology). Subsequently, the lung
morphological changes in each group were observed under a light
microscope (RX50 series biological microscope; Sunny Optical
Technology (Group), Co., Ltd.).
Lung injury pathological score
In order to evaluate the lung injury following BI,
the pathological score was assessed by 3 researchers blinded to the
experiment. The extent of the pathological injury was determined
using the following 4 categories: alveolar septa, alveolar
hemorrhage and intra-alveolar fibrin, intra-alveolar infiltrations
per field. The final average total lung injury scores of the 4
categories were the mean values from the 3 researchers, as
previously described (28).
Total RNA extraction and microarray
analysis
Microarray analysis was performed by Shanghai
Kangcheng Biological Co (Shanghai, China). for a 'whole genome
expression profiling gene chip of rat' test. Briefly, total RNA was
extracted using TRIzol agent and DNA concentration and purity were
measured using a UV spectrophotometer and qualified by agarose gel
electrophoresis. The One-cycle cDNA synthesis kit (obtained from
Affymetrix, Santa Clara, CA, USA, P/N 900431; following the
operating instructions) was used to perform reverse transcription
to obtain cDNA, which was then purified. After using the Gene Chip
IVT labeling kit to synthesize biotin-labeled cDNA in vitro,
the cDNA were hybridized with Gene Chip following fragmentation.
This was followed by washing and staining, and finally the
fluorescence signal intensity of gene expression with a scanner was
obtained, and then corrected using the reference gene. Signal
processes were detected using the Gene Ontology database
(http://www.geneontology.ogy.org/); the
analysis includes biological process (BP) that are composed by
orderly composition of molecular function and a process of multiple
steps; cell components (CC), namely the position of the cell in
which the gene product is located in the cell or gene products
group (e.g., the rough endoplasmic reticulum, nucleus or ribosomes
and proteasomes); and molecular function (MF) that describes the
activity in molecular biology. Each section has 4 small parts: 1,
the analysis of gene number (count), namely the number of
differentially expressed genes measured in this function group; 2,
P-value trees, namely thecascade relationship of biological
pathway; 3, enrichment factor (fold enrichment), that is, the
proportion of the changes in genes than proportions in the GO
database in this function group (such as, the more proportion, the
more reliability, which was regarded as the more significant
changes in molecular function); 4, enrichment points, with
enrichment factor empathy. Pathway analysis was performed using the
KEGG database (http://www.genome.jp/kegg/pathway.html) or DAVID
Bioinformatics Resources (https://david.ncifcrf.gov/).
Data analysis
The Agilent Feature Extraction software (version
10.7.3.1) was used to analyze the acquired array images as
previously described (29).
Staistical analysis
In this study, SPSS software (version 17.0; SPSS
Inc., Chicago, IL, USA) was used to process the data, and the
experimental results are expressed as the means ± standard
deviation (SD); one-way analysis of variance (ANOVA) was used to
compare the differences between the sham-operated group and the
experimental groups; a value of P<0.05 was considered to
indicate statistically significant differences. For statistical
analysis and gene scanning, we used Microarray Suite version 5.0
software to analyze the strength and the ratio of hybridization
signals, and the chip data with the normalization process; SMA
software was used for the statistical analysis of the results,
according to the conventional standard q-value (%) 5% screening of
the differentially expressed genes.
Results
BMSC cell culture, purification and
identification
The BMSCs appeared to be in good condition, and some
adherent round cells could be observed at 24 h of culture (Fig. 1A); at 48–72 h of culture, the
cultured cells slowly grew into spindle- and polygonal-shaped cells
with an orbicular-ovate nucleus (Fig.
1B). The amount of BMSCs increased at 5 days of culture, and
the cells gradually developed into a spindle shape and were
distributed radially (Fig. 1C).
To identify the cultured cells, immunofluorescence staining of CD44
at passage 3 was performed and the results revealed that the 99.3%
of the cells were CD44-positive (Fig.
1E). These results confirmed that the cultured cells were BMSCs
at passage 3 (Fig. 1D).
Role of BMSC transplantation
The lung tissues harvested at 14 days
post-transplantation emitted a large blue Hoechst 33342
florescence, demonstrating the survival and migration of the
transplanted BMSCs in lung tissues (Fig. 2A). However, we did not find NeuN-
and GFAP-positive cells in the lung tissues (data not shown).
Neurological evaluation based on a 0–18 point scording system
revealed that all the animals before surgery had a score of
0.0000±0.00000 points, and there were no statistically significant
differences among the rats (P>0.05). Following surgery, at 3, 7
and 14 days post-transplantation, as compared with the
sham-operated group, the scores in the BI group were significantly
higher (P<0.05), while BMSC transplantation significantly
attenuated the neurological injury, and the neurological assessment
scores in this group were significantly lower than those in the BI
group (P<0.05; Fig. 2B).
In order to observe the effect of BMSC
transplantation on lung tissues following permanent focal cerebral
ischemia, we used the lung injury pathological scores to evaluate
lung injury. H&E staining of the lung tissues revealed evident
inflammatory cell infiltration and alveolar edema in the BI group,
compared with the sham-operated group, whereas the BMSC group
exhibited less inflammatory cell infiltration (Fig. 2C). In addition, the lung injury
scores indicated that the scores of the BI group were higher than
those of the sham-operated group (P<0.05), while the scores in
BMSCs (t) group were lower than that in the BI group (P<0.05)
(Fig. 2D).
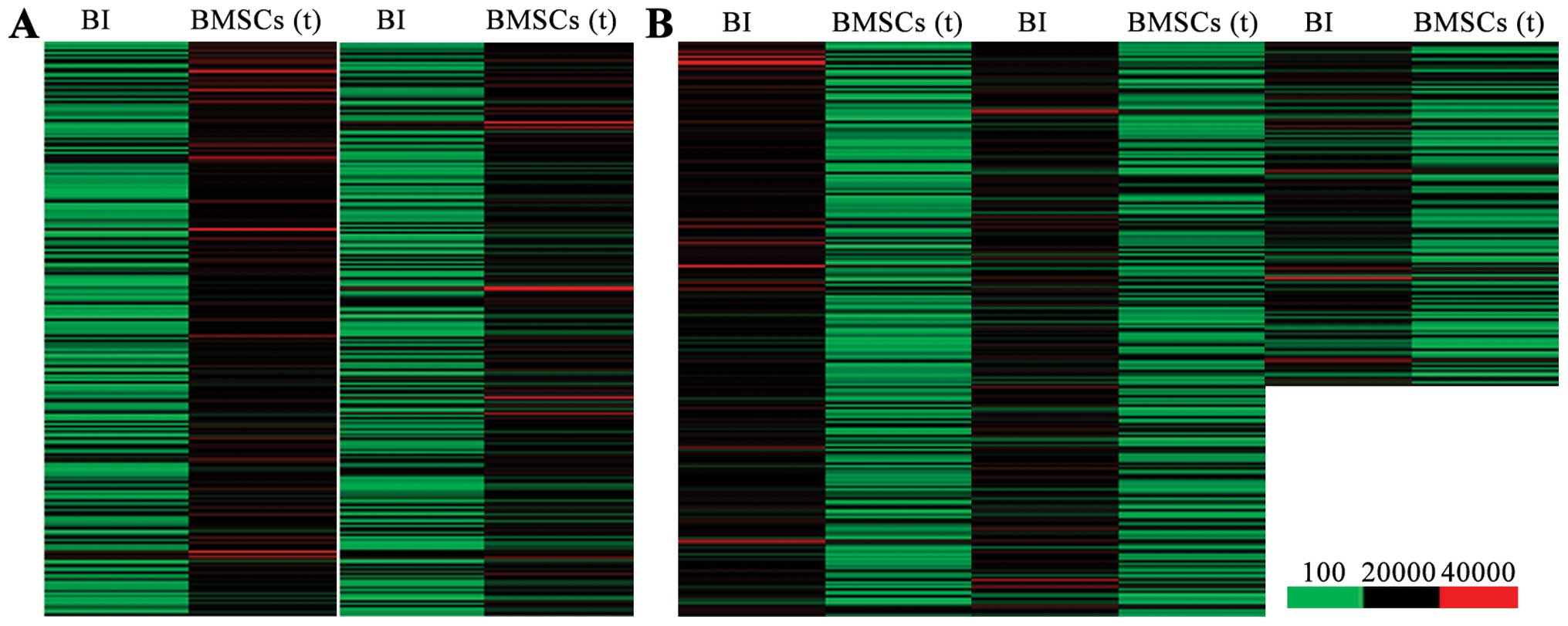
Differentially expressed genes between
the BMSC transplantation and BI groups
In order to elucidate the molecular mechanisms
responsiblef or the benefical effects of BMSC transplantation on
cerebral ischemia-induced lung injury, we used Agilent Feature
Extraction software to compare and analyze the differentially
expressed genes between the BI group and BMSC transplantation
group. Genes in signal intensity (normalized intensity) ratio >2
or 0.5 were defined as differentially expressed genes. We found
that, compared with the lung tissues from the rats in the BI group,
there were 1,836 upregulated genes (Fig. 3A) and 2,869 downregulated genes
(Fig. 3B) in the lung tissues
from the BMSC transplantation group.
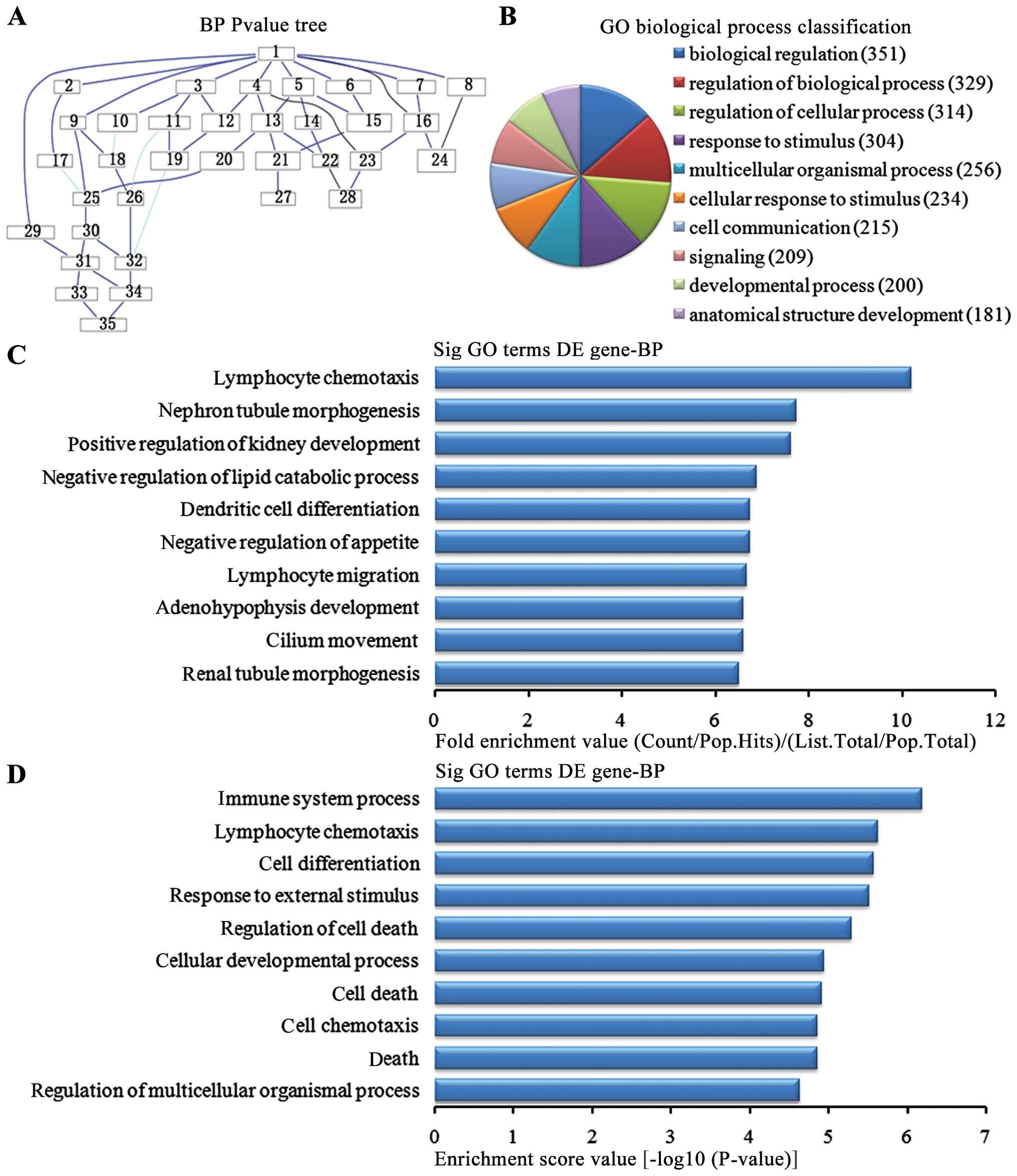
Biological process analysis of the
upregulated genes
In the 1,836 upregulated genes in the in the lung
tissues from the BMSC transplantation group, we could see the
cascade relationship of the biological pathways according to the
P-value tree (Fig. 4A). According
to the number of different genes measured, we selected the top 10
biological pathways for classification (Fig. 4B): 351 genes were involved in
biological regulation, 329 genes were involved in the biological
regulatory pathway, 314 genes were involved in the cell regulation
pathway, 304 genes were involved in the stress pathway, 256 genes
were involved in multi-cellular organisms pathways, 234 were
involved in the cellular stress pathway, 215 were involved in cell
communication pathways, 209 were involved in signaling pathways,
200 were involved in development pathways and 181 were involved in
the developmental pathways of anatomical structure. According to
the enrichment factor, we selected the top 10 biological pathways
(Fig. 4C): lymphocyte chemotaxis,
nephron tubule morphogenesis, positive regulation of kidney
development, negative regulation of lipid catabolic process,
negative regulation of appetite, dendritic cell differentiation,
migration and cilium movement of lymphocytes, adenohypophysis
development and renal tubule morphogenesis. According to the
enrichment points, we selected the top 10 biological pathways
(Fig. 4D): immune system process,
lymphocyte chemotaxis, cell differentiation pathway, the external
stress, cell death regulation pathways, cellular development
process, cell death, cell chemotaxis, regulation of death and
multicellular organisms pathways.
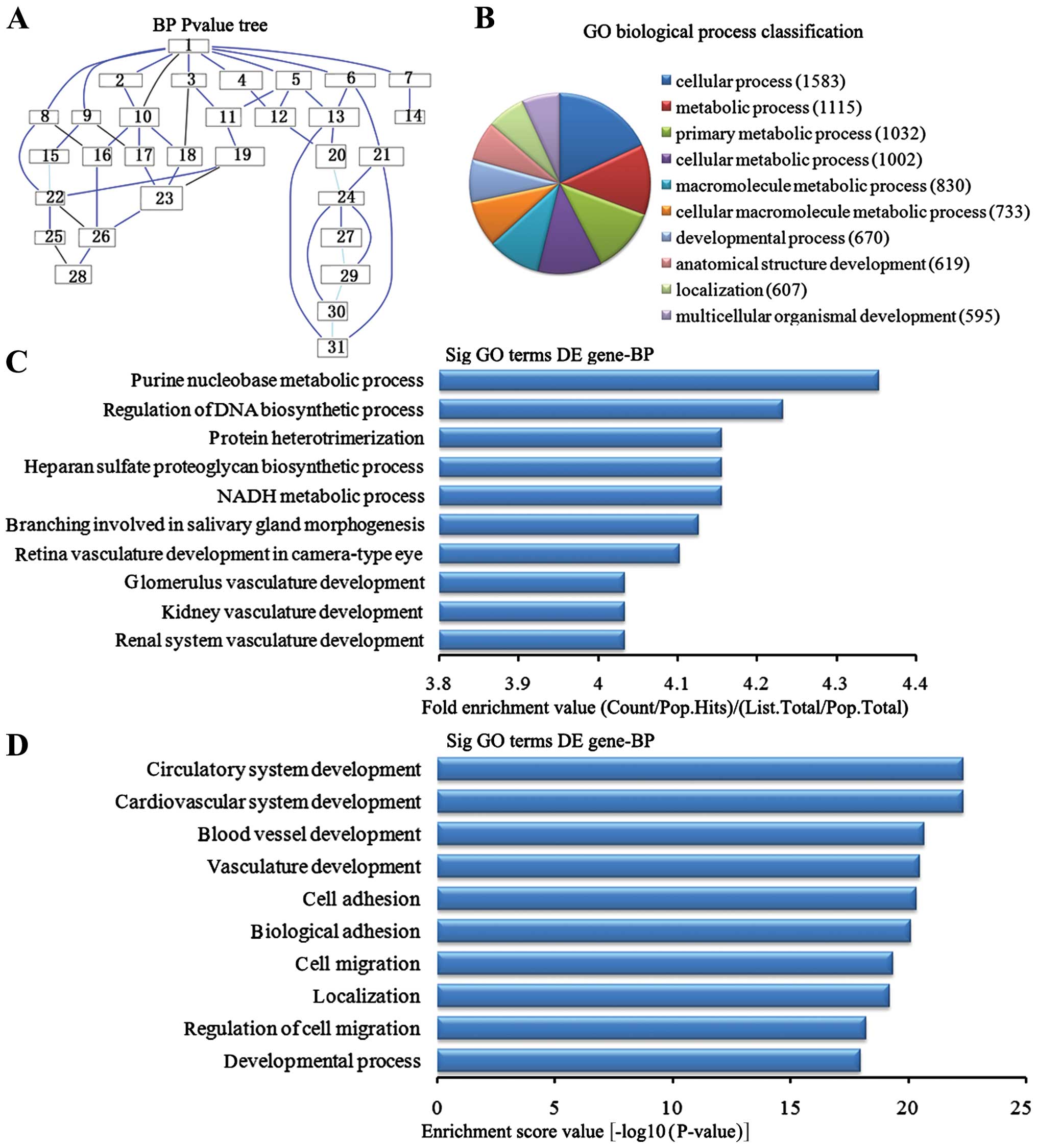
Biological process analysis of the
downregulated genes
In the 2,869 downregulated genes in the lung tissues
from the BMSC transplantation group, we could see the cascade
relationship of the biological process according to the P-value
tree (Fig. 5A). According to the
number of different genes measured, we selected the top 10
biological process for classification (Fig. 5B): 1,583 gene processes, 1,002
were involved in cell metabolic processes, 830 participated in
macromolecule metabolic processes, 733 were involved in cellular
macromolecule metabolic processes, 670 were involved in
developmental processes, 619 were involved in anatomical structure
development, 607 were involved in the localization of biological
processes and 595 participated in multicellular organism
development. According to the enrichment factor, we selected the
top 10 biological process (Fig.
5C): purine nucleotide metabolic process, regulation of DNA
biosynthesis process, NADH metabolic process, heparin sulfate
proteoglycan biosynthesis process, protein heterotrimerization,
branching involved in salivary gland morphogenesis, retinal
vasculature development camera-type eye, renal system vasculature
development, kidney vasculature development and glomerulus
vasculature development. According to the enrichment points, we
selected the top 10 biological process (Fig. 5D): cardiovascular system
development, circulatory system development, the blood vascular
development, vasculature development, cell adhesion, biological
adhesion, cell migration, localization, regulation of cell
migration and development process.
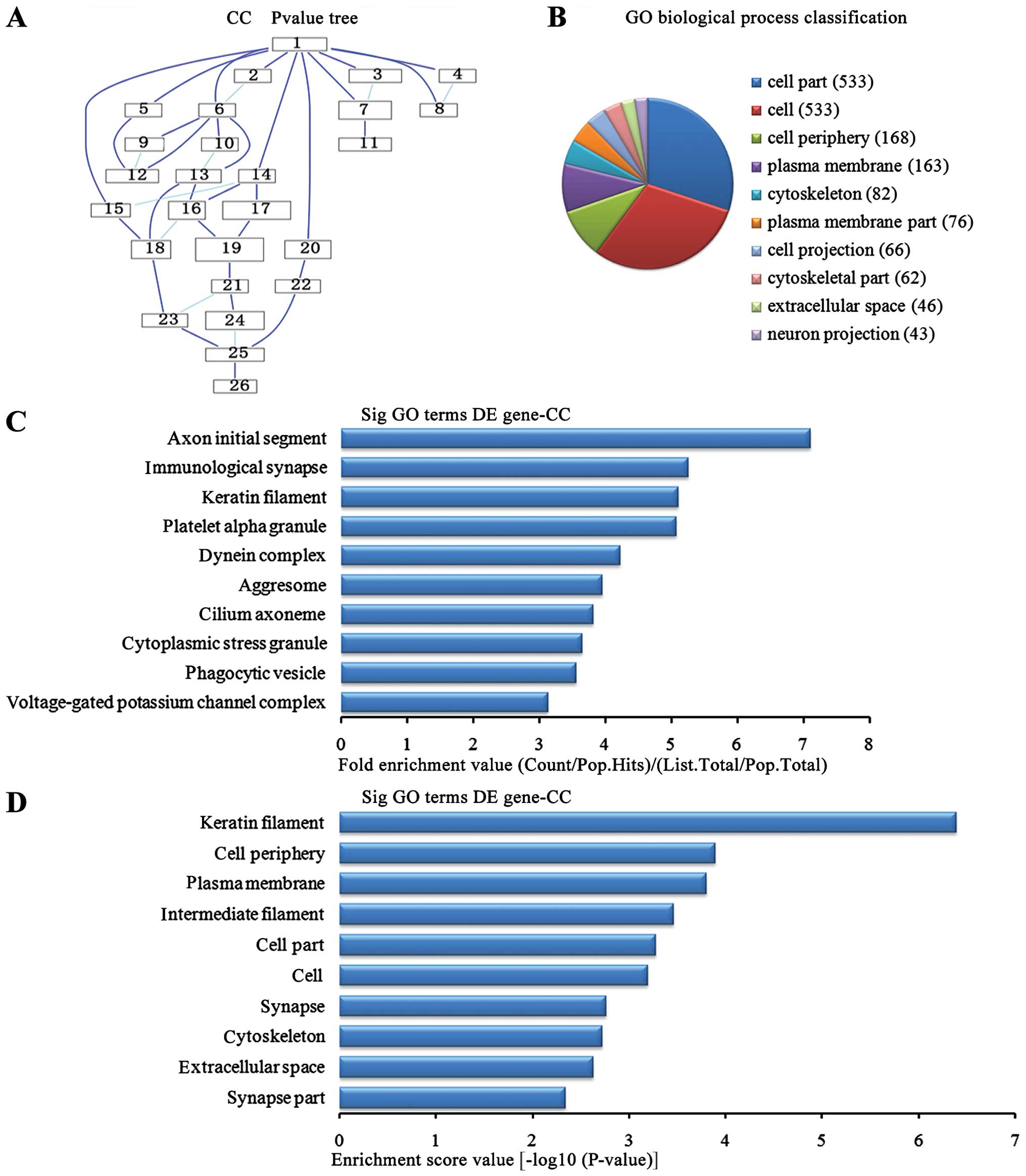
Cell components analysis of the
upregulated genes
In the 1,836 upregulated genes in the lung tissue
from the BMSC transplantation group, we could see the cascade
relationship of the cell components according to the P-value tree
(Fig. 6A), and according to the
number of different genes measured, the top 10 cell components were
selected for classification (Fig.
6B): 533 genes were involved in cell part, 533 were involved in
cell, 168 were involved in cell periphery, 163 were involved in
plasma membrane, 82 were involved in cytoskeleton, 76 were involved
in plasma membrane, 66 were involved in cell projection, 62 were
involved in the cytoskeleton, 46 were involved in extracellular
voids and 43 were involved in neuronal projection. According to the
enrichment factor, we selected the top 10 cell components (Fig. 6C): axon initial segment, the
immunological synapse, keratin fibers, platelet α granule potassium
channel complex, dynein complex, aggresome, cilium axoneme,
cytoplasmic stress granule, phagocytic vesicle, voltage-gated
potassium channel complex. According to the enrichment points, we
selected the top 10 cell components (Fig. 6D): keratin fibers, cell periphery,
plasma membrane, the intermediate filaments, cell part, cell,
synapse, extracellular space and synapse part.
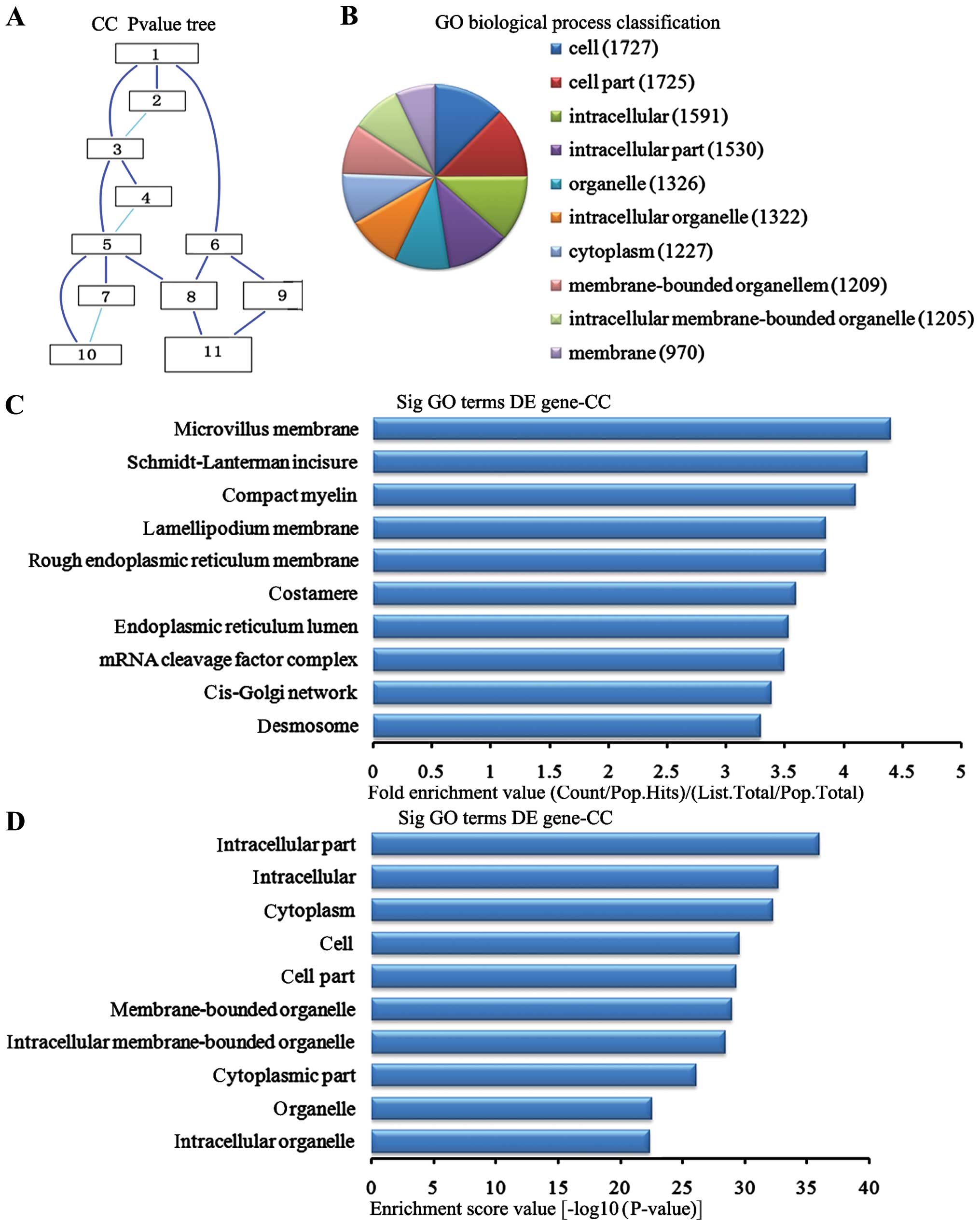
Cell components analysis of the
downregulated genes
In the 2,869 downregulated genes in the lung tissues
from the BMSC transplantation group, we can see the cascade
relationship of the cell components according to the P-value tree
(Fig. 7A). And according to the
number of different genes measured selected the top 10 cell
components for classification (Fig.
7B): 1,727 genes participated cell, 1,725 were involved in cell
part, 1,591 participated intracellular, 1,530 were involved in
intracellular part, 1,326 were participated in the organelle, 1,322
were involved in the intracellular organelle, 1,227 participated in
the cytoplasm, 1,209 were involved in membrane-bounded organelle,
1,205 participated in the intracellular membrane-bounded organelle
and 970 participated in the membrane. According to the enrichment
factor we selected the top 10 cell components (Fig. 7C): microchorillus membrane,
Schmidt-Lanterman incisures, compact myelin, lamellipodium
membrane, rough endoplasmic reticulum membrane, costamere,
endoplasmic reticulum lumen, RNA cleavage factor complex, cis-Golgi
network, and desmosomes. According to the enrichment points we
selected the top 10 cell components (Fig. 7D): intracellular part,
intracellular, cytoplasm, cell, cell part, membrane-bounded
organelles, intracellular membrane-bounded organelles cytoplasmic
part, organelle and intracellular organelles.
Molecular function analysis of the
upregulated genes
In the 1,836 upregulated genes in the lung tissue
from the BMSC transplantation group, we could see the cascade
relationship of the molecular functions according to the P-value
tree (Fig. 8A). According to the
number of different genes measured, we selected the top 10
molecular functions for classification (Fig. 8B): 409 genes were associated with
binding, 224 were associated with protein binding, 77 were
associated with purine ribonucleotide binding, 77 were associated
with ribonucleotide binding, 64 were associated with adenyl
ribonucleotide binding, 63 were associated with ATP binding, 56
were associated with transporter activity, 52 were associated with
transmembrane transporter activity, 50 were associated with
transfer activity. According to the enrichment factor, we selected
the top 10 molecular functions (Fig.
8C): DNA N-glycosylase activity, inorganic anion exchanger
activity, MAP tyrosine/serine/threonine kinase activity, RNA
polymerase II distal enhancer sequence-specific DNA binding, MAP
kinase activity, syntaxin-1 binding, neurotransmitter transporter
activity, translation elongation factor activity, neurotransmitter
transporter activity and enhancer sequence-specific DNA binding.
According to the enrichment points, we selected the top 10
molecular functions (Fig. 8D):
metal ion transmembrane transporter activity, inorganic cation
transmembrane transporter activity, cation transmembrane
transporter activity, ion transmembrane transport activity, protein
kinase activity, cation channel activity, DNA sequence-specific
bind, protein binding, G protein-coupled receptors binding and
chemokine activity.
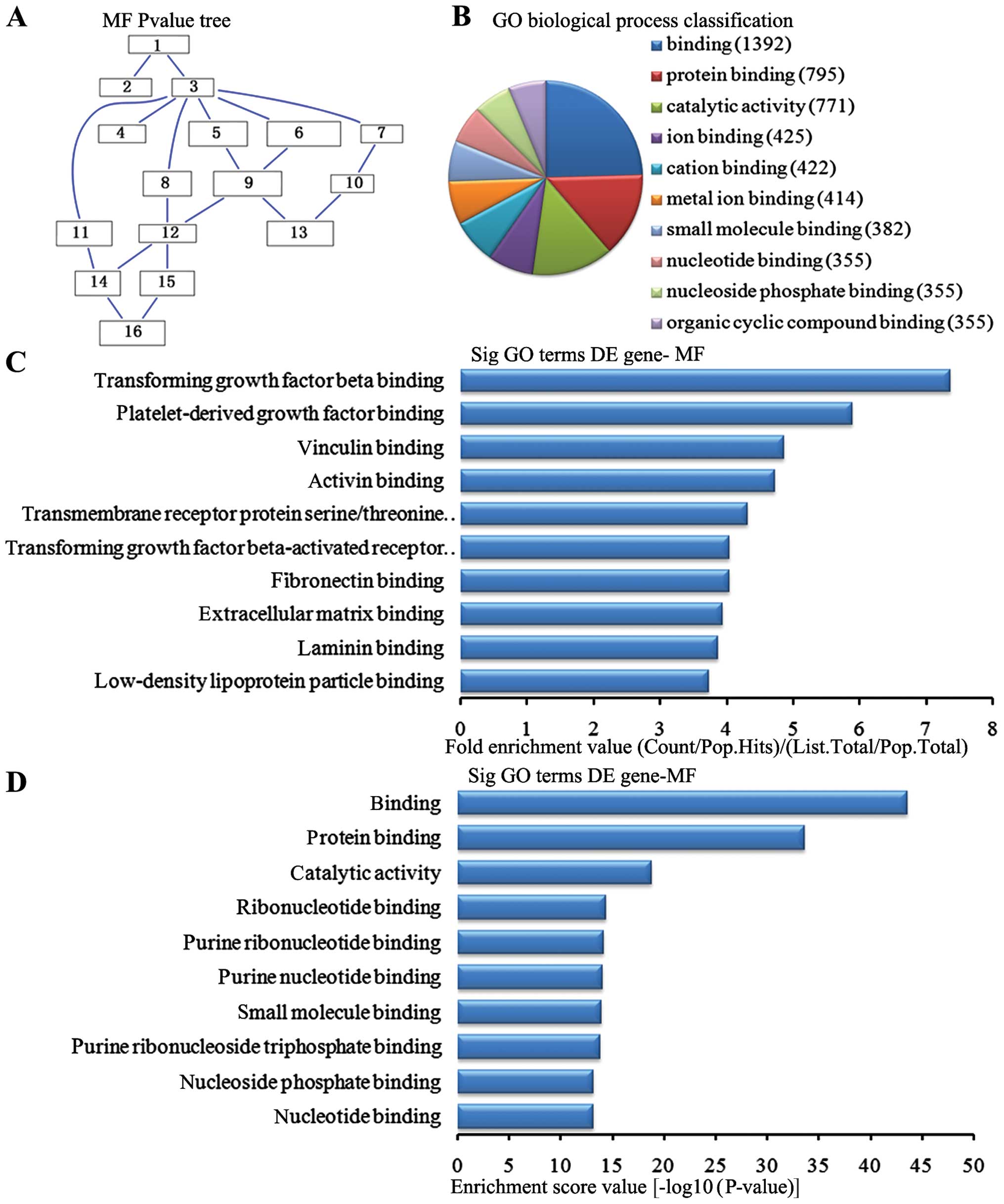
Molecular function analysis of the
downregulated genes
In the 2,869 downregulated genes in the lung tissue
from the BMSC transplantation group, we could see the cascade
relationship of the molecular functions according to the P-value
tree (Fig. 9A). According to the
number of different genes measured, we selected the top 10
molecular functions for classification (Fig. 9B): 1,392 genes were associated
with binding, 795 were associated with protein binding, 771 were
associated with catalytic activity, 425 were associated with ion
binding, 422 were associated with cation binding, 414 were
associated with metal ion binding, 382 were associated with small
molecule binding, 355 were associated with nucleoside phosphate
binding and 355 were associated with organic cyclic compound
binding. According to the enrichment factor, we selected the top 10
molecular functions (Fig. 9C):
transforming growth factor (TGF)-β binding, platelet-derived growth
factor binding, vinculin binding, activin binding, transmembrane
receptor protein serine/threonine kinase activity, TGF-β-activated
receptor activity, fibronectin binding, extracellular matrix
binding, laminin binding and low-density lipoprotein particle
binding. According to the enrichment points, we selected the top 10
molecular functions (Fig. 9D):
binding, protein binding, catalytic activity, ribonucleotide
binding, purine nucleotide binding, small molecule binding, purine
ribonucleoside triphosphate binding, nucleoside phosphate binding
and nucleoside binding.
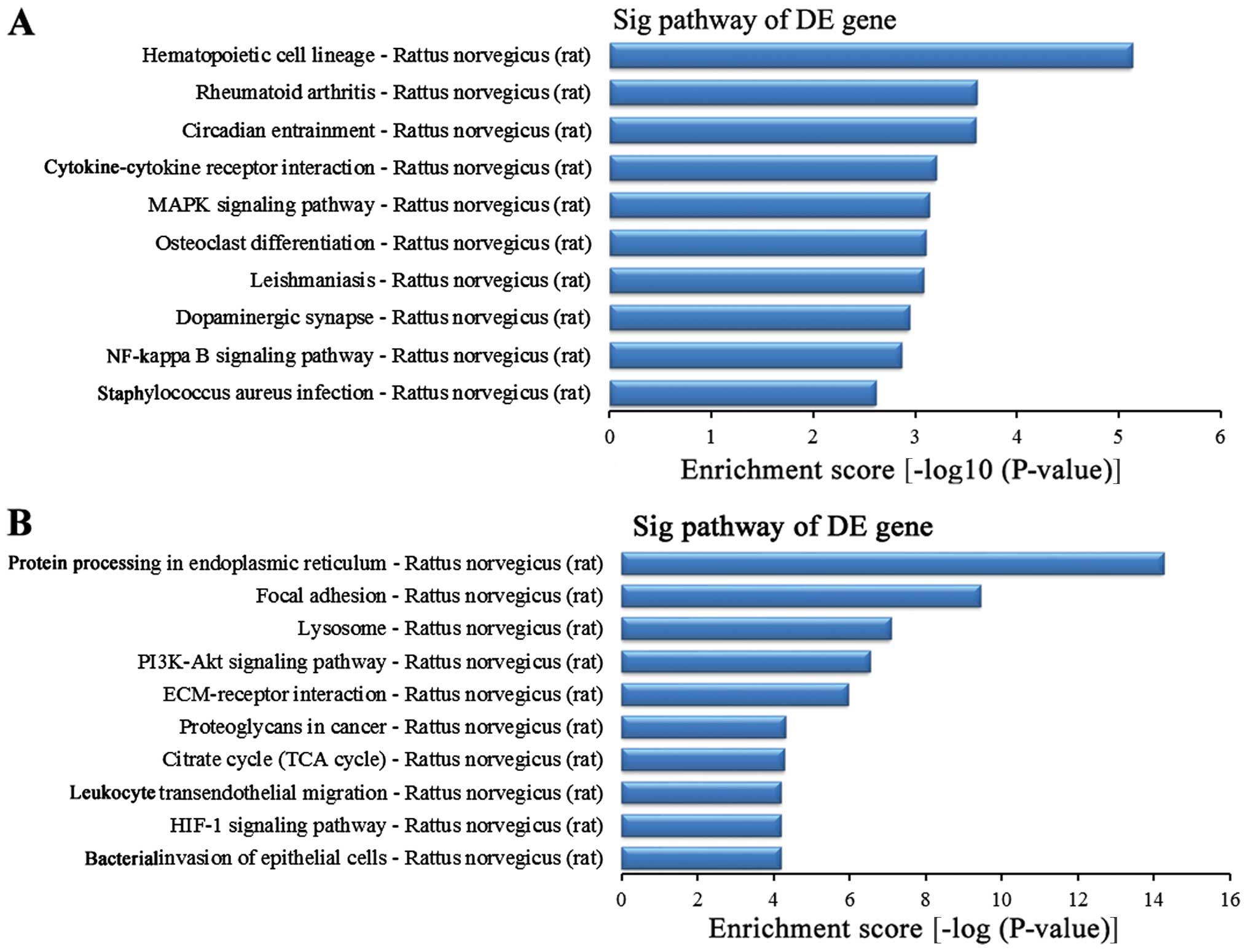
Related signaling pathway analysis of
differentially expressed genes
To further study the function of the differentially
expressed genes in the lung tissues between the BI group and the
BMSC (t) group, we used the KEGG database for pathway analysis. The
experimental results revealed that in this experiment, the striking
upregulated different genes in the BMSC (t) group were mainly
distributed in 40 signaling pathways when compared with the BI
group (data not shown), while the striking downregulated different
genes in the BMSC (t) group were mainly distributed in 59 signaling
pathways (data not shown). The upregulated differentially expressed
genes were sorted in descending order according to the enrichment
score. The top 10 signaling pathways (Fig. 10A) were as follows: the
hematopoietic cell lineage, rheumatoid arthritis, circadian
entrainment, cytokine-cytokine receptor interaction, MAPK signaling
pathway, osteoclast differentiation, leishmaniasis, dopaminergic
synapses, nuclear factor-κB (NF-κB) signaling pathway and
Staphylococcus infection. In addition, the top 10 signaling
pathways for the downregulated different genes (Fig. 10B) were as follows according to
the enrichment score: protein processing in the endoplasmic
reticulum, focal adhesion, lysosome, the phosphoinositide 3-kinase
(PI3K)-AKT signaling pathway, ECM-receptor interaction,
proteoglycans in cancer, citrate cycle (TCA cycle), leukocyte
transendothelial migration, hypoxia-inducible factor 1 (HIF-1)
signaling pathway and bacterial invasion of epithelial cells.
Discussion
The main finding of this study is that BMSC
transplantation via the rat tail vein to the lung tissues
attenuates lung injury induced by permanent focal cerebral
ischemia, associated with many differentially expressed genes. We
established a model of permanent focal cerebral ischemia-induced
lung injury model by MCAO, as indicated by the neurological
evaluation and lung injury scores. BMSC transplantation improved
neurological function and alleviated lung injury. In addition,
microarray analyses revealed a total of 1,836 upregulated genes
were and 2,869 downregulated genes. Biological process, cell
component, molecular function and the signaling pathway of the
differentially expressed genes were also analyzed and annotated.
Notably, downregulated TGF-β, PDGF and the PI3K-AKT pathway were
involved in the protective effects of BMSC transplantation.
Successful establishment of the model of
permanent focal cerebral ischemia-induced lung injury
In this study, we established a model of permanent
focal cerebral ischemia-induced lung injury model by MCAO.
Neurological evaluation and histological analysis of the lung
tissues were used to confirm the successful establishment of the
model. As previously reported, in addition to local damage to the
brain, permanent focal cerebral ischemia can lead to remote organ
dysfunction (30–32), particularly in the lungs,
resulting in ALI (33),
characterized by an excessive elevation of pro-inflammatory
cytokines and activated neutrophils (34–36). In the present study, BI caused
impaired neurological function, with higher behavioral scores.
Histological analysis by H&E staining indicated that BI also
contributed to lung injury, including the inflammatory reaction and
alveolar edema; in addition, the lung injury scores were increased
in the BI group. These findings confirmed that the model of
permanent focal cerebral ischemia-induced lung injury was
successfully established.
Protective effects of BMSC
transplantation on brain and lungs
In our experiment, it was found that 99.3% of the
cultured cells in passage 3 were CD44-positive by using CD44
immunohitochemical staining, indicating the high purity of our
cultured BMSCs. The cells reached the lung tissues through the
blood circulation at 14 days post-transplantation. Some scholars
(37–39) have used tail vein infusion of
different doses of BMSCs to study the effects on pulmonary fibrosis
in mice. It was shown that the injection of 1×106/ml
BMSCs via the tail vein reduced lung fibrosis, but
2×106/ml BMSCs aggravated this injury (37). In this study, the transplant dose
of 1×106/ml was used, which is an appropriate dose. As a
result, BMSC transplantation decreased the behavioral and lung
injury scores, and improved neurological and lung function,
indicating the protective role of BMSC transplantation
(1×106/ml). This improvement may have a certain
association with BMSCs migrating to the lung tissues and their
differentiation.
Gene microarray analysis
Furthermore, we used Go analysis and pathway
analysis to analyze the microarray results of the lung tissues.
Gene chip technology and data analysis has been successfully
applied in many fields, such as clinical diagnosis, medication
guide, drug screening (40), as
well as basic medicine (41,42) (expression profiling studies, gene
mutation studies and gene component type and sequencing). Gene chip
technology is able to analyze a high content of information, in a
high-throughput manner, and is rapid and accurate (42). In addition, microarray and
bioinformatics are complementary to Gene chip technology that were
developed to be able to rapidly obtain large amounts of genetic
information; they can provide the necessary database for
bioinformatics research, while data analysis of gene chips also
greatly depends on bioinformatics. Thus, the combination of both
provides a fast channel to study molecular biology (43,44).
Through gene microarray analysis, we found that,
compared with the BI group, 1,836 genes were upregulated and 2,869
were downregulated in the lung tissues from the BMSCs (t) group.
This indicated that the effect of BMSC transplantation therapy in
the lung tissue following cerebral ischemia was a multi-gene
regulated complex process. In addition, the functions of the
identified differentially expressed genes were involved in various
biological processes, cellular component and molecular function and
various related signal pathways.
Biological process analysis indicated that 1,836
upregulated genes in the BMSCs group were mainly involved in the
processes of biological regulation, stress, development,
immunological processes, lymphocyte chemotaxis, cell death
regulation and ciliary movement. The 2,869 downregulated genes were
mainly involved in metabolic processes, development process, DNA
biosynthesis adjustment process, the cardiovascular system, cell
adhesion, cell migration and positioning process. It has been shown
that under acute stress conditions, mesenchymal stem cells and
endothelial progenitor cells mobilize and provide a significant
response, while HPA axia response and its key effect
hormones-glucocorticoids exert an important regulatory effect
(45–47). Additionally, researchers have
found that the gene expression of early BMSCs played a supporting
role in nerve repair in the development of the nervous system
(48), and the endogenous ligand
of rat-derived BMSCs could be anti-apoptotic (49). Therefore, these studies combined
with ours, indicated that the effects of BMSC transplantation for
the treatment of cerebral ischemia and lung injury caused by
cerebral ischemia were mediated by the interacting effects of
multiple genes involved in the regulation of multiple systems.
In addition, molecular function analysis revealed
that in the 2,869 downregulated genes, TGF-β binding and PDGF
binding played a role in the repair of lung injury. It has been
shown that TGF-β regulates the transformation of human lung
fibroblasts into myofibroblasts through MAPK signal transduction
pathways, producing extracellular matrix proteins and resulting in
lung interstitial fibrosis (50).
Lu et al confirmed that Smad2 plays a role in pulmonary
vascular endothelial permeability induced by TGF-β1; thus,
increased TGF-β1 may promote lung injury (51). Moreover, it has been shown that
after experiencing hyoxia, the expression levels of PDGF-BB and
PDGFR-β were increased in the lung tissue, which suggested that
PDGF participates in hyperoxia-induced lung injury (52). In addition, studies have found
that PDGF stimulates mesenchymal cell hyperplasia and increases the
deposition of extracellular matrix, which promotes the formation of
pulmonary fibrosis (53,54). In the model of lung fibrosis
induced by radiation, radiation induces the high expression of
PDGF, resulting in the phosphorylation of PDGF receptors, which
activates downstream pathways; this process is inhibited by PDGF
receptor tyrosine kinase inhibitor, attenuating the process of
pulmonary fibrosis (55). In this
study, we found that BMSC transplantation significantly reduced the
TGF-β and PDGF levels, which may alleviate lung injury caused by
BI. Furthermore, related signaling pathway analysis indicated that
in the BMSCs (t) group, the PI3K-AKT signaling pathway was
downregulated, while the MAPK signaling pathway and NF-κB signaling
pathway were upregulated. PI3K/AKT signaling pathway activation
enhances the transcriptional activity of Smad3, the downstream
protein of TGF-β1, promoting the expression of type I collagen in
fibroblasts, and thereby contributing to lung fibrosis (56). It has been demonstrated that in
ALI, activated PAR2 in the alveolar epithelium increases the
expression of NF-κB through the PI3K/PKB signaling pathway, causing
the release of inflammatory factors, such as PGE2 and COX-2,
leading to alveolar epithelial injury; thus, the downregulation of
the PI3K/PKB signal pathway can reduce alveolar injury (57).
We also found the pathway which promotes lung tissue
survival: the hematopoietic cell lineage. Studies have indicated
that tetraspin CD37 can directly mediate the signal transduction of
survival and apoptosis (58),
promoting dendritic cell migration (59), and contributing to cellular
immunity and also promoting the long-term survival of plasma cells
(60). Additionally, in this
study, microarray analysis showed that BMSC transplantation can
downregulate protein processing in the endoplasmic reticulum
pathway, while ATF6 and Bcl-2-associated athanohene (BAG2) were
involved in this pathway. A previous study (61) demonstrated that ATF6 transcription
differed between an endoplasmic reticulum stress (ERS) state and a
no-ERS state. ATF6 upregulates the transcription and expression of
XBP1 in the no-ERS state, while ATF6 reduces the transcription and
expression of XBP1 in the ERS state. The BAG2 family can combine
with the anti-apoptotic protein, Bcl-2, thus it is also known as
the Bcl-2-related anti-apoptotic protein family. It is mainly
involved in the degration of intracellular proteins. Wang et
al (62) reported that in
thyroid cancer, the expression of BAG-2 promotes apoptosis in
vitro and its apoptotic activity can be induced by proteasome
inhibitor. Thus, the BMSC transplantation can improve behavioral
and lung function following permanent focal cerebral ischemia,
associated with the participation of multiple genes and signaling
pathways.
In conclusion, the findings of the present study
suggest that BMSC transplantation may repair lung injury following
permanent focal cerebral ischemia. The present study may provide a
new therapeutic strategy for BI-induced lung injury and may act as
a guide for fundamental research and clinical research in the
future.
Acknowledgments
We gratefully acknowledge the kind assistance and
suggestions provided by Dr Qing-Jie Xia from West China Hospital,
Sichuan University, China. This research was supported by a grant
from the China National Science Foundation (nos. 81471268;
81271358) together with the program Innovative Research Team In
Science and Technology in Yunnan Province.
References
|
1
|
Mozaffarian D, Benjamin EJ, Go AS, Arnett
DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ,
Howard VJ, et al American Heart Association Statistics Committee
and Stroke Statistics Subcommittee: Heart disease and stroke
statistics - 2015 update: A report from the American Heart
Association. Circulation. 131. pp. e29–e322. 2015, View Article : Google Scholar
|
|
2
|
Cojocaru IM, Cojocaru M, Tănăsescu R,
Iliescu I, Dumitrescu L and Silosi I: Expression of IL-6 activity
in patients with acute ischemic stroke. Rom J Intern Med.
47:393–396. 2009.PubMed/NCBI
|
|
3
|
Brüning CA, Prigol M, Luchese C, Jesse CR,
Duarte MM, Roman SS and Nogueira CW: Protective effect of diphenyl
diselenide on ischemia and reperfusion-induced cerebral injury:
Involvement of oxidative stress and pro-inflammatory cytokines.
Neurochem Res. 37:2249–2258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeppellini R, Salsa F, Gheno G and
Cucchini F: Cardiac injury in acute cerebral vasculopathy. Ann Ital
Med Int. 16:73–81. 2001.In Italian. PubMed/NCBI
|
|
5
|
Alaro D, Bashir A, Musoke R and Wanaiana
L: Prevalence and outcomes of acute kidney injury in term neonates
with perinatal asphyxia. Afr Health Sci. 14:682–688. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saeed F, Adil MM, Malik AA, Qureshi MH and
Nahab F: Worse in-hospital outcomes in patients with transient
ischemic attack in association with acute kidney injury: analysis
of nationwide in-patient sample. Am J Nephrol. 40:258–262. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sweetman DU, Riordan M and Molloy EJ:
Management of renal dysfunction following term perinatal
hypoxia-ischaemia. Acta Paediatr. 102:233–241. 2013. View Article : Google Scholar
|
|
8
|
Khatri M, Himmelfarb J, Adams D, Becker K,
Longstreth WT and Tirschwell DL: Acute kidney injury is associated
with increased hospital mortality after stroke. J Stroke
Cerebrovasc Dis. 23:25–30. 2014. View Article : Google Scholar
|
|
9
|
Zhao S, Rong R, Dan QQ and Zhang YH:
Expression of trkB gene in the pulmonary tissue of rats with lung
injury induced by cerebral ischemia. Sichuan Da Xue Xue Bao Yi Xue
Ban. 43:901–903. 2012.In Chinese.
|
|
10
|
Wu YH, Zhang X and Wang DH: Role of
asymmetric dimethylarginine in acute lung injury induced by
cerebral ischemia/reperfusion injury in rats. Nan Fang Yi Ke Da Xue
Xue Bao. 31:1289–1294. 2011.PubMed/NCBI
|
|
11
|
Liao F, Dan QQ, Du RF, Li JT and Zhang YH:
Expression of TNF-alpha in lung tissue of rats with lung injury
induced by brain ischemia. Sichuan Da Xue Xue Bao Yi Xue Ban.
43:914–917. 2012.In. Chinese.
|
|
12
|
Lv Q, Wang SL, Jiang L, He X, Hu YD, Wang
TH and Zhang YH: Identification of featured biomarkers between
human lung adenocarcinoma and inflammatory pseudotumor using gene
expression microarray. Idiscovery. 2:1–15. 2016.
|
|
13
|
Duan GX, Men XL and Peng J: The effects of
tetramethylpyrazine on lung injury induced by brain
ischemia/reperfusion. Zhongguo Ying Yong Sheng Li Xue Za Zhi.
22:361–362. 3782006.In Chinese.
|
|
14
|
Madala SK, Edukulla R, Schmidt S, Davidson
C, Ikegami M and Hardie WD: Bone marrow-derived stromal cells are
invasive and hyperproliferative and alter transforming growth
factor-α-induced pulmonary fibrosis. Am J Respir Cell Mol Biol.
50:777–786. 2014. View Article : Google Scholar :
|
|
15
|
Jansen BJ, Gilissen C, Roelofs H,
Schaap-Oziemlak A, Veltman JA, Raymakers RAP, Jansen JH, Kögler G,
Figdor CG, Torensma R, et al: Functional differences between
mesenchymal stem cell populations are reflected by their
transcriptome. Stem Cells Dev. 19:481–490. 2010. View Article : Google Scholar
|
|
16
|
Kitada M and Dezawa M: Induction system of
neural and muscle lineage cells from bone marrow stromal cells; a
new strategy for tissue reconstruction in degenerative diseases.
Histol Histopathol. 24:631–642. 2009.PubMed/NCBI
|
|
17
|
Vaquero J, Otero L, Bonilla C, Aguayo C,
Rico MA, Rodriguez A and Zurita M: Cell therapy with bone marrow
stromal cells after intracerebral hemorrhage: Impact of
platelet-rich plasma scaffolds. Cytotherapy. 15:33–43. 2013.
View Article : Google Scholar
|
|
18
|
Rippon HJ, Polak JM, Qin M and Bishop AE:
Derivation of distal lung epithelial progenitors from murine
embryonic stem cells using a novel three-step differentiation
protocol. Stem Cells. 24:1389–1398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Serikov VB, Popov B, Mikhailov VM, Gupta N
and Matthay MA: Evidence of temporary airway epithelial
repopulation and rare clonal formation by BM-derived cells
following naphthalene injury in mice. Anat Rec (Hoboken).
290:1033–1045. 2007. View
Article : Google Scholar
|
|
20
|
Pati S, Gerber MH, Menge TD, Wataha KA,
Zhao Y, Baumgartner JA, Zhao J, Letourneau PA, Huby MP, Baer LA, et
al: Bone marrow derived mesenchymal stem cells inhibit inflammation
and preserve vascular endothelial integrity in the lungs after
hemorrhagic shock. PLoS One. 6:e251712011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uccelli A, Moretta L and Pistoia V:
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
8:726–736. 2008. View
Article : Google Scholar
|
|
22
|
Shlyapnikov YM, Shlyapnikova EA and
Morozov VN: Carboxymethyl cellulose film as a substrate for
microarray fabrication. Anal Chem. 86:2082–2089. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Du JY, Yang H, Tian DR, Wang QM and He L:
Identification and functional analysis of differentially expressed
genes related to obesity using DNA microarray. Genet Mol Res.
13:64–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ansari S, Azari H, McConnell DJ, Afzal A
and Mocco J: Intraluminal middle cerebral artery occlusion (MCAO)
model for ischemic stroke with laser doppler flowmetry guidance in
mice. J Vis Exp. 51:28792011.
|
|
25
|
He QQ, He X, Wang YP, Zou Y, Xia QJ, Xiong
LL, Luo CZ, Hu XS, Liu J and Wang TH: Transplantation of bone
marrow-derived mesenchymal stem cells (BMSCs) improves brain
ischemia-induced pulmonary injury in rats associated to TNF-α
expression. Behav Brain Funct. 12:92016. View Article : Google Scholar
|
|
26
|
Zhang Z, Yan Y, Sun B, Liu J and Zhu YH:
Bone marrow stromal cells genetically modified by neurotrophin-4
gene improve the cognitive ability in Alzheimer's disease model
rats. Ibrain. 2:1–8. 2016.
|
|
27
|
Menzies SA, Hoff JT and Betz AL: Middle
cerebral artery occlusion in rats: a neurological and pathological
evaluation of a reproducible model. Neurosurgery. 31:100–106. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu MW, Su MX, Zhang W, Wang YQ, Chen M,
Wang L and Qian CY: Protective effect of Xuebijing injection on
paraquat-induced pulmonary injury via downregulating the expression
of p38 MAPK in rats. BMC Complement Altern Med. 14:4982014.
View Article : Google Scholar
|
|
29
|
Luo H, Zhao X, Wan X, Huang S and Wu D:
Gene microarray analysis of the lncRNA expression profile in human
urothelial carcinoma of the bladder. Int J Clin Exp Med.
7:1244–1254. 2014.PubMed/NCBI
|
|
30
|
Sun L, Ai J, Wang N, Zhang R, Li J, Zhang
T, Wu W, Hang P, Lu Y and Yang B: Cerebral ischemia elicits
aberration in myocardium contractile function and intracellular
calcium handling. Cell Physiol Biochem. 26:421–430. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Min J, Farooq MU, Greenberg E, Aloka F,
Bhatt A, Kassab M, Morgan JP and Majid A: Cardiac dysfunction after
left permanent cerebral focal ischemia: The brain and heart
connection. Stroke. 40:2560–2563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Watanabe WK: Brain-heart signaling after
cerebral ischemia reperfusion injury. Int J Psychophysiol. 69:229.
2008. View Article : Google Scholar
|
|
33
|
Bratton SL and Davis RL: Acute lung injury
in isolated traumatic brain injury. Neurosurgery. 40:707–712. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sukhotnik I, Slijper N, Pollak Y,
Chemodanov E, Shaoul R, Coran AG and Mogilner JG: Parenteral
omega-3 fatty acids (Omegaven) modulate intestinal recovery after
intestinal ischemia-reperfusion in a rat model. J Pediatr Surg.
46:1353–1360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sayan H, Ozacmak VH, Sen F, Cabuk M, Atik
DY, Igdem AA and Ozacmak ID: Pharmacological preconditioning with
erythropoietin reduces ischemia-reperfusion injury in the small
intestine of rats. Life Sci. 84:364–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grommes J and Soehnlein O: Contribution of
neutrophils to acute lung injury. Mol Med. 17:293–307. 2011.
View Article : Google Scholar :
|
|
37
|
Rojas M, Xu J, Woods CR, Mora AL, Spears
W, Roman J and Brigham KL: Bone marrow-derived mesenchymal stem
cells in repair of the injured lung. Am J Respir Cell Mol Biol.
33:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kotton DN, Ma BY, Cardoso WV, Sanderson
EA, Summer RS, Williams MC and Fine A: Bone marrow-derived cells as
progenitors of lung alveolar epithelium. Development.
128:5181–5188. 2001.PubMed/NCBI
|
|
39
|
Ortiz LA, Gambelli F, McBride C, Gaupp D,
Baddoo M, Kaminski N and Phinney DG: Mesenchymal stem cell
engraftment in lung is enhanced in response to bleomycin exposure
and ameliorates its fibrotic effects. Proc Natl Acad Sci USA.
100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Srinivasan A, Leung KP, Lopez-Ribot JL and
Ramasubramanian AK: High-throughput nano-biofilm microarray for
antifungal drug discovery. MBio. 4:42013. View Article : Google Scholar
|
|
41
|
Jiang XS, Ni YQ, Liu TJ, Zhang M, Jiang R
and Xu GZ: Generation and characterization of immortalized rat
retinal microglial cell lines. J Neurosci Res. 92:424–431. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yoshida M, Watanabe Y, Yamanishi K,
Yamashita A, Yamamoto H, Okuzaki D, Shimada K, Nojima H, Yasunaga
T, Okamura H, et al: Analysis of genes causing hypertension and
stroke in spontaneously hypertensive rats: Gene expression profiles
in the brain. Int J Mol Med. 33:887–896. 2014.PubMed/NCBI
|
|
43
|
Fu LJ and Wang B: Investigation of the hub
genes and related mechanism in ovarian cancer via bioinformatics
analysis. J Ovarian Res. 6:922013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ng CF, Xu JY, Li MS and Tsui SK:
Identification of FHL2-regulated genes in liver by microarray and
bioinformatics analysis. J Cell Biochem. 115:744–753. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kinlein SA, Wilson CD and Karatsoreos IN:
Dysregulated hypothalamic-pituitary-adrenal axis function
contributes to altered endocrine and neurobehavioral responses to
acute stress. Front Psychiatry. 6:312015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu XY, Urbieta-Caceres V, Krier JD,
Textor SC, Lerman A and Lerman LO: Mesenchymal stem cells and
endothelial progenitor cells decrease renal injury in experimental
swine renal artery stenosis through different mechanisms. Stem
Cells. 31:117–125. 2013. View Article : Google Scholar
|
|
47
|
Brandl A, Meyer M, Bechmann V, Nerlich M
and Angele P: Oxidative stress induces senescence in human
mesenchymal stem cells. Exp Cell Res. 317:1541–1547. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nandoe Tewarie RD, Bossers K, Ritfeld GJ,
Blits B, Grotenhuis JA, Verhaagen J and Oudega M: Early passage
bone marrow stromal cells express genes involved in nervous system
development supporting their relevance for neural repair. Restor
Neurol Neurosci. 29:187–201. 2011.PubMed/NCBI
|
|
49
|
Zeng X, Yu SP, Taylor T, Ogle M and Wei L:
Protective effect of apelin on cultured rat bone marrow mesenchymal
stem cells against apoptosis. Stem Cell Res. 8:357–367. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Furukawa F, Matsuzaki K, Mori S, Tahashi
Y, Yoshida K, Sugano Y, Yamagata H, Matsushita M, Seki T, Inagaki
Y, et al: p38 MAPK mediates fibrogenic signal through Smad3
phosphorylation in rat myofibroblasts. Hepatology. 38:879–889.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lu Q, Harrington EO, Jackson H, Morin N,
Shannon C and Rounds S: Transforming growth factor-beta1-induced
endothelial barrier dysfunction involves Smad2-dependent p38
activation and subsequent RhoA activation. J Appl Physiol. 1985.
101:375–384. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Buch S, Han RN, Cabacungan J, Wang J, Yuan
S, Belcastro R, Deimling J, Jankov R, Luo X, Lye SJ, et al: Changes
in expression of platelet-derived growth factor and its receptors
in the lungs of newborn rats exposed to air or 60% O(2). Pediatr
Res. 48:423–433. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lin Q, Fang LP, Zhou WW and Liu XM:
Rosiglitazone inhibits migration, proliferation, and phenotypic
differentiation in cultured human lung fibroblasts. Exp Lung Res.
36:120–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kogan EA, Tyong FV and Demura SA: The
mechanism of lung tissue remodeling in the progression of
idiopathic pulmonary fibrosis. Arkh Patol. 72:30–36. 2010.In
Russian. PubMed/NCBI
|
|
55
|
Abdollahi A, Li M, Ping G, Plathow C,
Domhan S, Kiessling F, Lee LB, McMahon G, Gröne HJ, Lipson KE, et
al: Inhibition of platelet-derived growth factor signaling
attenuates pulmonary fibrosis. J Exp Med. 201:925–935. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Runyan CE, Schnaper HW and Poncelet AC:
The phosphatidylinositol 3-kinase/Akt pathway enhances
Smad3-stimulated mesangial cell collagen I expression in response
to transforming growth factor-beta1. J Biol Chem. 279:2632–2639.
2004. View Article : Google Scholar
|
|
57
|
Moriyuki K, Sekiguchi F, Matsubara K,
Nishikawa H and Kawabata A: Proteinase-activated
receptor-2-triggered prostaglandin E(2) release, but not
cyclooxygenase-2 upregulation, requires activation of the
phosphatidylinositol 3-kinase/Akt/nuclear factor-kappaB pathway in
human alveolar epithelial cells. J Pharmacol Sci. 111:269–275.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lapalombella R, Yeh YY, Wang L, Ramanunni
A, Rafiq S, Jha S, Staubli J, Lucas DM, Mani R, Herman SE, et al:
Tetraspanin CD37 directly mediates transduction of survival and
apoptotic signals. Cancer Cell. 21:694–708. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gartlan KH, Wee JL, Demaria MC, Nastovska
R, Chang TM, Jones EL, Apostolopoulos V, Pietersz GA, Hickey MJ,
van Spriel AB and Wright MD: Tetraspanin CD37 contributes to the
initiation of cellular immunity by promoting dendritic cell
migration. Eur J Immunol. 43:1208–1219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
van Spriel AB, de Keijzer S, van der
Schaaf A, Gartlan KH, Sofi M, Light A, Linssen PC, Boezeman JB,
Zuidscherwoude M, Reinieren-Beeren I, et al: The tetraspanin CD37
orchestrates the α(4)β(1) integrin-Akt signaling axis and supports
long-lived plasma cell survival. Sci Signal. 5:ra822012. View Article : Google Scholar
|
|
61
|
Guo FJ, Xiong Z, Lu X, Ye M, Han X and
Jiang R: ATF6 upregulates XBP1S and inhibits ER stress-mediated
apoptosis in osteoarthritis cartilage. Cell Signal. 26:332–342.
2014. View Article : Google Scholar
|
|
62
|
Wang HQ, Zhang HY, Hao FJ, Meng X, Guan Y
and Du ZX: Induction of BAG2 protein during proteasome
inhibitor-induced apoptosis in thyroid carcinoma cells. Br J
Pharmacol. 155:655–660. 2008. View Article : Google Scholar : PubMed/NCBI
|