Introduction
Esophageal cancer (EC) is a malignant cancer with
the 6th highest incidence rate and the 4th
highest mortality rate in China (1). The malignancy degree of EC is high,
and thus, even following comprehensive treatments, such as surgery,
radiotherapy and chemotherapy, the outcomes still remain poor for
patients with EC (2). Local
recurrence and distant metastasis are the main causes of death in
patients with EC, and effective treatments remain a challenge
(3). Therefore, investigating the
molecular targets and regulatory mechanisms responsible for the
proliferation, invasion and metastasis of EC would be of immense
practical significance.
Annexin A1 (ANXA1) is an important member of the
Annexin superfamily, it can be regulated by Ca2+, it can
bind with phospholipids, and participates a variety of
physiological-pathological reactions, thus affecting the
occurrence, proliferation and apoptosis, and the invasion and
metastasis of tumors (4,5); thus, it may be considered as a
possible candidate for targeted therapy. It has been indicated that
the expression of ANXA1 is associated with the prognosis of
esophageal adenocarcinoma (6);
however, ANXA1 may exhibit significant differences in expression in
different tumors, and opposing results have been obtained (7). Thus, the effects and mechanisms of
action of ANXA1 on tumor cells remain unclear. In addition,
microRNAs (miRNAs or miRs) can bind with messenger RNAs (mRNAs) of
target gene, thus negatively regulating gene expression and
affecting the occurrence and progression of a variety of tumors
(8,9), Luthra et al reported that
miR-196a negatively regulates the expression of the ANXA1 gene,
thus affecting the prognosis of esophageal adenocarcinoma (10). In China, the vast majority of EC
cases are esophageal squamous cell carcinoma (ESCC), which is
significantly different from Western countries, and the expression
of ANXA1 differs significantly between esophageal adenocarcinoma
and ESCC (11). Therefore, the
question of whether the expression of ANXA1 in ESCC affects the
proliferation, invasion and metastasis of ESCC cells, as well as
the prognosis of ESCC, and whether it is also negatively regulated
by miR-196a, is still worthy of investigation.
In this study, we constructed an ANXA1
overexpression plasmid, and then transfected this plasmid and
miR-196a mimics into ESCC Eca109 cells, in an aim to determine
whether the overexpression of ANXA1 and miR-196a affects cell
proliferation, migration and invasion, and to explore the molecular
mechanisms through which miR-196a regulates the expression of ANXA1
and affects the invasion and metastasis of ESCC cells. Our findings
may provide the basis for future research on ESCC and may aid in
the development of novel treatment strategies for ESCC.
Materials and methods
Cell and cell culture
The Eca109 cell line was purchased from the Shanghai
Institute of Biochemistry and Cell Biology, Chinese Academy of
Science (Shanghai, China), and placed in DMEM (Gibco-BRL, Carlsbad,
CA, USA) containing 10% fetal bovine serum (FBS), 2 mmol/l
L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin
(Amresco LLC, Solon, OH, USA) at 37°C and 5% of CO2. The
medium was changed once every 2 days, and cells in the logarithmic
growth phase were used for the experiments.
Construction of ANXA1 overexpression
plasmid
TRIzol reagent (Invitrogen Biotechnology Co., Ltd.,
Shanghai, China) was used to extract RNA from MDA-MB-231 human
breast cancer cells (purchased from the Shanghai Institute of
Biochemistry and Cell Biology). The AMV reverse transcription kit
(Promega, Madison, WI, USA) was then used for reverse transcription
to obtain the cDNA which was then used as a template, together with
ANXA1 primers (synthesized by Invitrogen Biotechnology Co., Ltd.)
for PCR amplification: the primer sequences were as follows: sense,
5′-ATGGCAATGGTATCAGAATTCCTC-3′ and antisense,
5′-TTAGTTTCCTCCACAAAGAGCCACC-3′. β-actin was tested as an internal
control, and the primers for β-actin were as follows: sense primer,
GCACCACACCTTCTACAATG and antisense primer, TGCTTGCTGATCCACATCTG
(synthesized by Invitrogen Biotechnology Co., Ltd.). PrimeSTAR HS
DNA polymerase was purchased from Takara Biotechnology Co., Ltd.
(Dalian, China), and the PCR products were then purified to produce
the ANXA1 fragment using the QIAquick PCR Purification kit (Qiagen,
Hilden, Germany) following digestion using NdeI and
BamHI (both from MBI Fermentas, Burlington, ON, Canada). The
pCMV5-myc carrier (Takara Biotechnology Co., Ltd.) was connected
internally, via ligase, to generate the expression vector,
pCMV5-myc-ANXA1. This expression vector was transfected into DH5α
competent E. coli cells following amplification.
Subsequently, we used the plasmid DNA kit (purchased from Axygen
Biosciences, Union City, CA, USA) to obtain a sufficient amount of
expression plasmid, which was subjected to enzyme digestion for
identification and sequencing.
Transfection of ANXA1 expression plasmid
and miR-196a mimic
The Lipofectamine™ 2000 kit (purchased from
Invitrogen Biotechnology Co., Ltd.), was used for transfection.
Prior to transfection, the ANXA1 overexpression plasmid or miR-196a
mimic (designed and synthesized by Shanghai GenePharma Co., Ltd.,
Shanghai, China) were first mixed with liposomes, allowed to stand
at room temperature for 20 min so as to form a complex, and this
complex was then added to the culture wells, following the specific
steps included with the kit manual. A nonspecific miRNA mimic
(designated as Pre-NC), synthesized by Shanghai GenePharma Co.,
Ltd., was transfected as an appropriate negative control to
miR-196a mimic. The cells transfected with the ANXA1 overexpression
plasmid were designated as the ANXA1 group, and those transfected
with the miR-196a mimic was designated as the miRNA group; the
cells in the empty-vector group were only transfected with empty
vectors, and the cells in the control group were untransfected.
Western blot analysis
After the cells were collected, total proteins were
extracted using cell lysis, and the DC Protein Assay kit was then
used to determine the protein concentrations. A total of 50
µg/well protein was then used for gel electrophoresis on 10%
SDS-PAGE gels, and the proteins isolated after electrophoresis were
transferred onto polyvinylidene difluoride film (PVDF; Millipore,
Billerica, MA, USA). Blocking buffer was then added for 1 h at room
temperature, followed by the addition of the primary antibodies
(anti-ANXA1, Cat. no. SAB1405457, 1:1,000; anti-Snail, WH0006591M5,
1:2,000; anti-E-cadherin, WH0000999M1, 1:2,000; and the internal
control β-actin, A1978, 1:3,000) (all from Sigma-Aldrich, St.
Louis, MO, USA) for overnight culture at 4°C with mild shaking. The
membranes were then washed and 2% BSA-containing horseradish
peroxidase-labeled secondary antibody (goat anti-mouse, Cat. no.
SAB4600316, Sigma-Aldrich) was then added for 1 h at room
temperature. The membranes were then washed again, and then exposed
and developed. Quantity One image analysis software was then used
for analysis, and each experiment was repeated 3 times.
Methyl thiazolyl tetrazolium (MTT)
assay
The cells from each group were seeded into 96-well
plates, with 3 repeated wells for each group of cells. When the
cells were in the logarithmic growth phase, MTT solution (5 mg/ml,
Sigma-Aldrich) was added to each well, followed by culture for a
further 4 h before terminating the culture, and discarding the
culture medium. Subsequently, 150 µl of dimethyl sulphoxide
(DMSO) were added to each well, followed by oscillation in a
low-speed shaker for 10 min to fully dissolve the crystals. The
optical density (OD) of each well was then detected using an ELISA
detection instrument at 490 nm, and the OD value of each cell was
averaged by the 3 repeated wells. The proliferation rate of the
cells was then calculated using the following formula:
proliferation rate (%) = (mean OD value of the experiment
group/mean OD value of the control group) ×100.
Transwell cell migration/invasion
assay
For the migration assay, the cells were collected
and resuspended in serum-free DMEM, and then placed in Transwell
chambers (Corning Inc., Corning, NY, USA) in 24 well culture plates
for 2 h of culture. FBS-containing complete medium was added to the
lower chamber followed by culture for a further 8 h. The cells that
had penetrated the polycarbonate membrane of the bottom chamber
were then stained with crystal violet (Sigma-Aldrich), and counted
under a microscope (Olympus IX53; Olympus Corp., Tokyo, Japan). A
total of 10 high-power fields (HPFs) in each chamber were randomly
selected for counting, and the mean value was set as the number of
migrated cells. The procedures for invasion assay were basically
the same as those for the migration assay described above, with the
exception that a layer of Matrigel was added to the bottom chamber
before adding the cells to imitate the extracellular matrix under
physiological conditions. Similarly, each chamber was randomly
counted at 10 HPFs, and the mean value was set as the number of
invaded cells.
Reverse transcriptrion-polymerase chain
reaction (RT-PCR)
Following transfection of the cells with miR-196a
mimic for 48 h, and digestion and centrifugation (1,500 × g, 20°C),
the cells were then collected. TRIzol reagent (Invitrogen
Biotechnology Co., Ltd.) was then added to extract the total RNA,
and ultraviolet spectrophotometry (Shimadzu UV-2550; Shimadzu
Scientific Instruments, Kyoto, Japan)was used to determine the
concentration and purity of the RNA. RNA samples with D260 nm/D280
nm ranging within 1.8 to 2.0 were then selected for reverse
transcription and PCR amplification according to the Takara One
Step RNA PCR kit (Takara Biotechnology Co., Ltd.) instructions; The
RT primer for miR-196a was 5′-GTCAGAAGGAATGATGCACAGCCAACAACA-3′,
and the PCR primers were 5′-CGTCAGAAGGAATGATGCACAG-3′ (forward) and
5′-ACCTGCGTAGGTAGTTTCATGT-3′ (reverse). U6 was used as an internal
reference and the U6 primer for RT was 5′-AACGCTTCACGAATTTGCGT-3′,
and the PCR primers were 5′-CTCGCTTCGGCAGCACA3′ (forward) and
5′-AACGCTTCACGAATTTGCGT-3′ (reverse). All those primers for
miR-196a and U6 were synthesized by Invitrogen Biotechnology Co.,
Ltd. The PCR reaction conditions were as follows: pre-denaturation
at 95°C, 15 min; denaturation at 95°C, 15 sec; annealing and
extension at 60°C, 1 min, 40 cycles. The 2−ΔΔCT value
(in which ΔCT = CTsample − CTinternal reference) was used to
represent the relative expression level of the target miRNA. The
experiment was repeated 3 times for the average.
Statistical analysis
The experimental data are expressed as the average
of triplicate experiments and are the means ± standard deviation.
Comparisons of the measurement data were made using the two-tailed
Student's t-test, and comparisons of the migration rates and
invasion rates of the cells in the different groups were made by
analysis of variance. A value of p<0.05 was considered to
indicate a statistically significant difference. The SPSS 19.0
software package (SPSS, Inc., Chicago, IL, USA) was used for
statistical analysis.
Results
Successful transfection of ANXA1 plasmid
into Eca109 cells
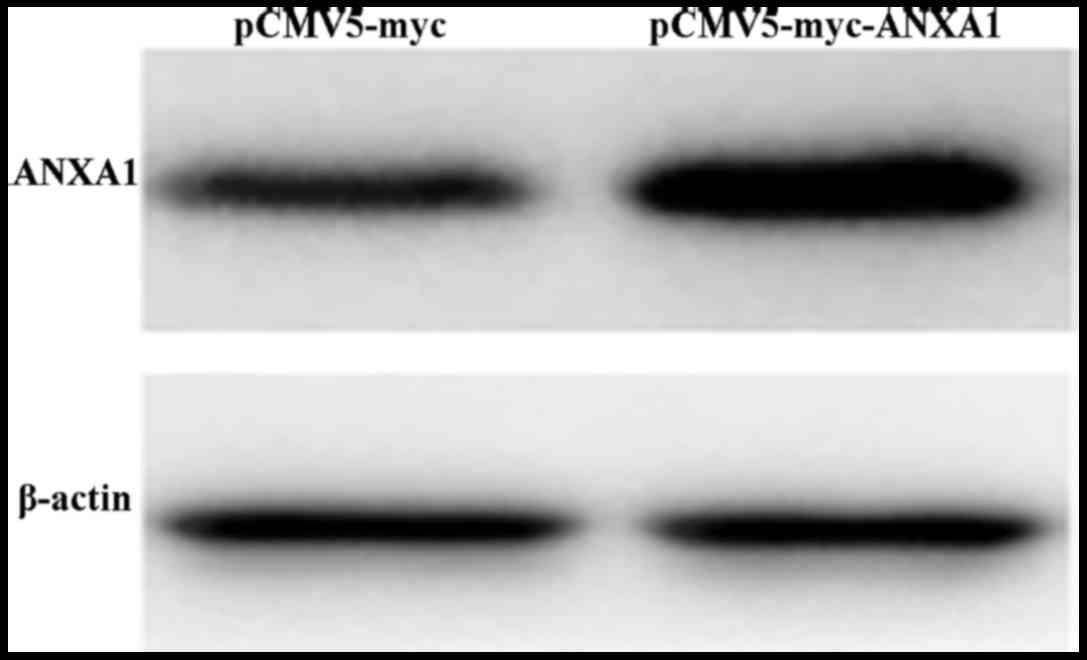
After the ANXA1 overexpression plasmid was
transfected into the Eca109 cells, western blot analysis was used
to measure the protein expression levels. The results revealed that
the cells in the ANXA1 group exhibited an obvious ANXA1 band
(Fig. 1), indicative of a
significantly higher protein expression compared with the cells in
the empty-vector group. This indicated that transfection with the
ANXA1 overexpression plasmid efficiently upregulated the expression
of ANXA1.
Effect of the overexpression of ANXA1 on
the proliferation of Eca109 cells
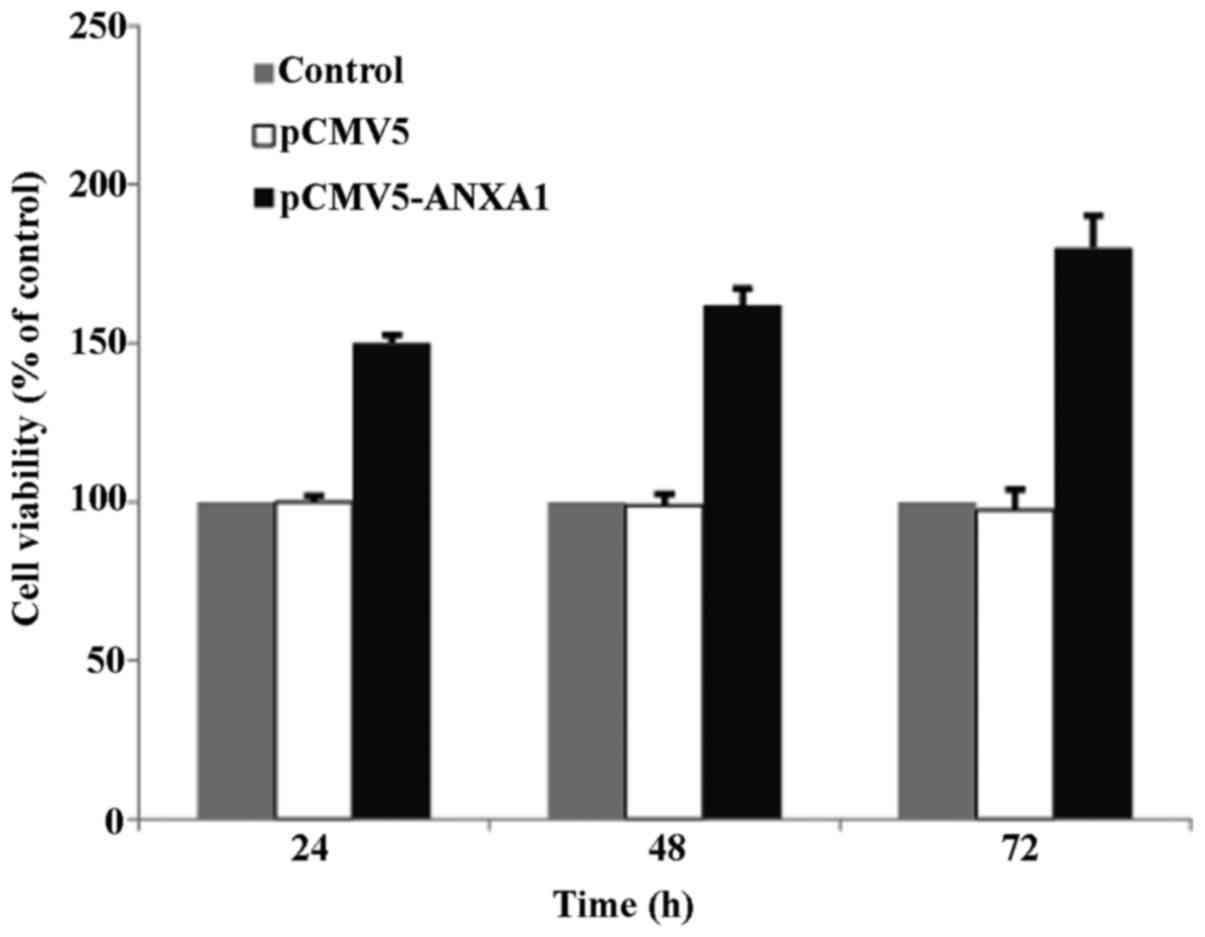
MTT assay at 24, 48 and 72 h post-transfection
revealed that the survival rate of the cells in the empty-vector
group was similar to that of the cells in the control group,
indicating that the transfection of the vector did not affect cell
proliferation. However, the survival rate of the cells in the ANXA1
group was significantly increased at 24–72 h post-transfection
(150–180%), suggesting that ANXA1 promotes cell proliferation; this
promoting effect on proliferation was more pronounced as time
progressed (Fig. 2).
Effect of the overexpression of ANXA1 on
the migration and invasion of Eca109 cells
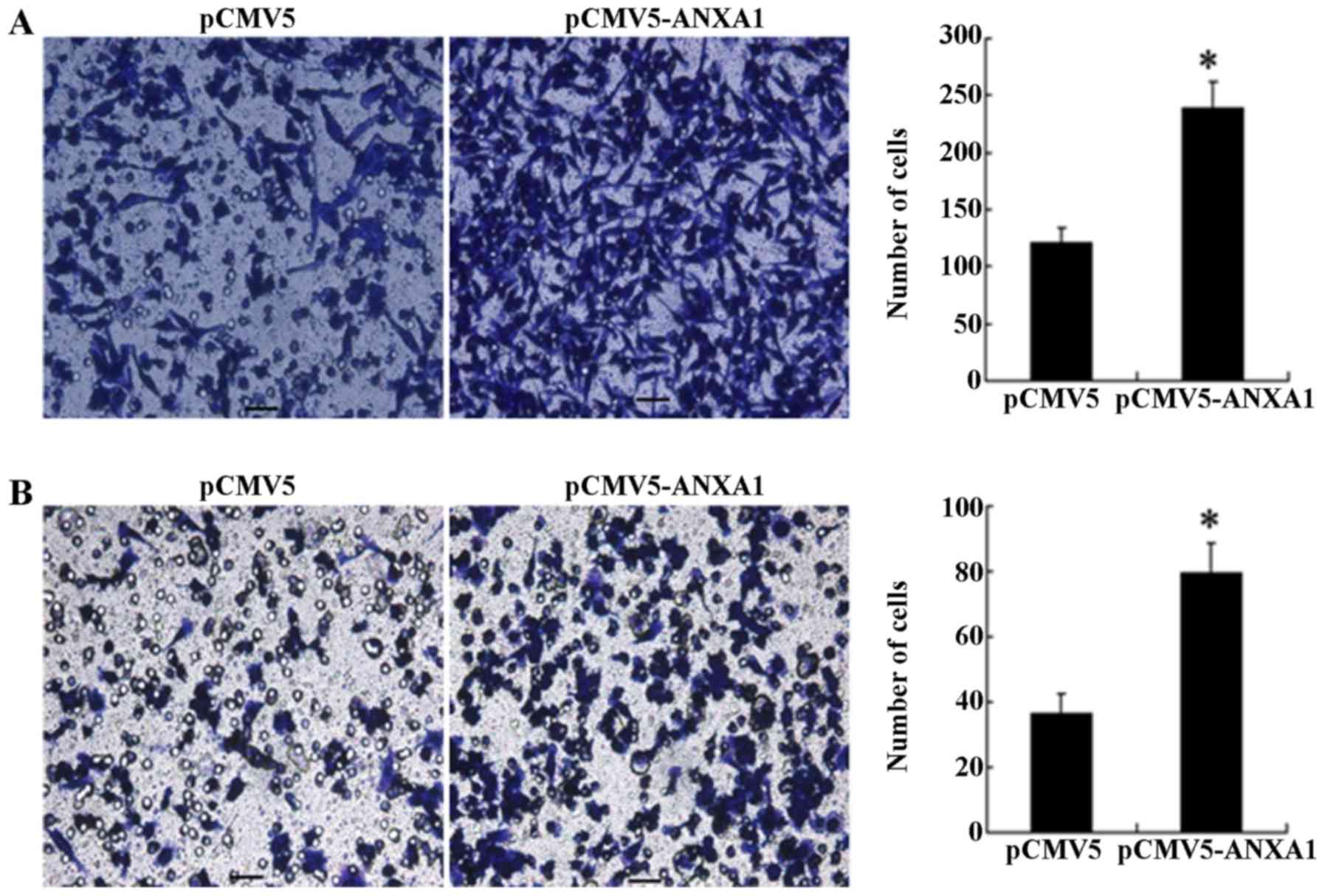
The results of Transwell migration assay revealed
that a significantly greater number of cells in the ANXA1 group had
migrated to the bottom surface of the chamber compared to the cells
in the empty-vector group (240 cells/HPF vs. 123 cells/HPF,
p<0.001; Fig. 3A). Similarly,
the results of invasion assay revealed that the number of cells in
the ANXA1 group that penetrated the basement membrane was
significantly increased than those in the empty-vector group (79
cells/HPF vs. 36 cells/HPF, p= 0.011; Fig. 3B), indicating that the
overexpression of ANXA1 enhanced the cell migration and invasion
ability.
Effect of the overexpression of
miRNA-196a on the expression of ANXA1
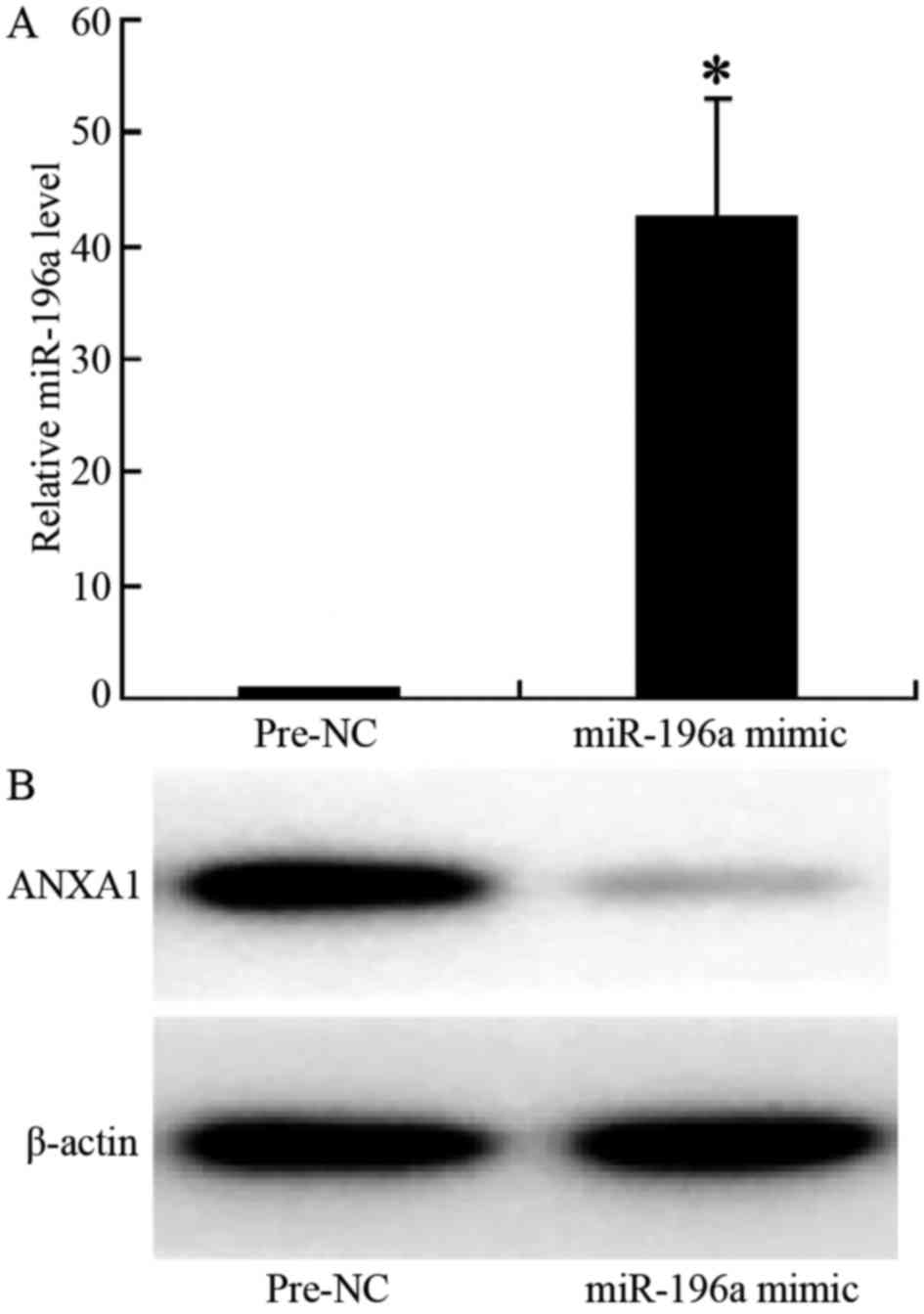
The results of RT-PCR indicated that following
transfection of the cells with miR-196a mimic, the expression of
miR-196a in the cells was significantly increased (relative
expression level, 1.1 vs. 43, p<0.001; Fig. 4A). The results of western blot
analysis revealed that ANXA1 protein expression was significantly
downregulated in the cells transfected with miR-196a mimic, with a
significantly lighter electrophoretic band (Fig. 4B), indicating that the
overexpression of miR-196a significantly decreased the expression
of ANXA1, thus confirming that miR-196a negatively regulates the
expression of ANXA1 in ESCC cells.
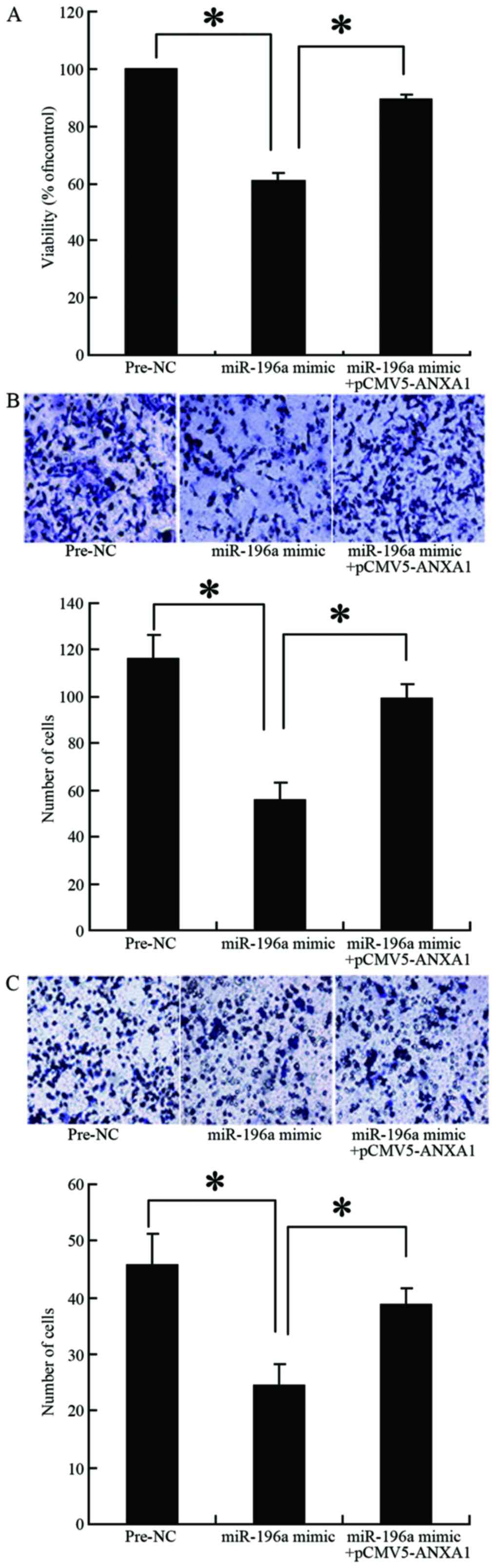
Effect of miR-196a overexpression on the
proliferation, migration and invasion of Eca109 cells
The results of MTT assay revealed that the survival
rate of the cells transfected with the miR-196a mimic was
significantly lower than that of the cells in the control group
(Pre-NC group; 57 vs. 100%, p=0.027) (Fig. 5A). However, co-transfection of the
cells with the miR-196a mimic and the ANXA1 overexpression plasmid
reversed the inhibitory effects of miR-196a on cell proliferation
(90 vs. 57%, p=0.034) (Fig. 5A).
The results of Transwell chamber assay revealed that cell migration
and invasion were significantly decreased when miR-196a was
overexpressed in the cells (56 vs. 116, p=0.009; 24 vs. 46,
p=0.021, respectively) (Fig. 5B and
C). However, co-transfection of the cells with miR-196a mimic
and the ANXA1 overexpression plasmid reversed the inhibitory
effects of miR-196a on cell migration and invasion (99 vs. 56,
p=0.015; 38 vs. 24, p=0.04, respectively) (Fig. 5B and C). These results confirmed
that miR-196a decreases ANXA1 expression, thereby inhibiting the
proliferation, migration and invasion of Eca109 cells.
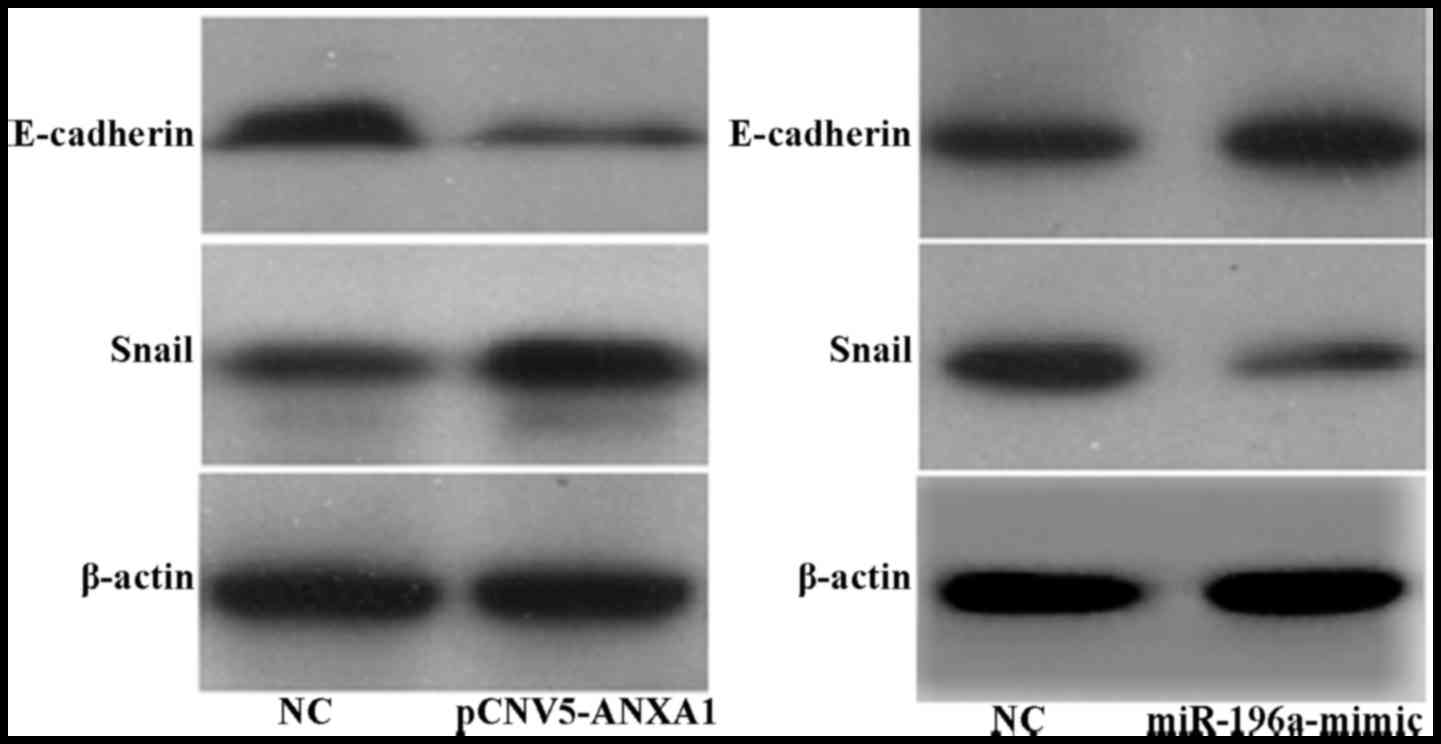
Association between the expression of
ANXA1, Snail and E-cadherin
Our results revealed that following the
overexpression of ANXA1, the expression of E-cadherin in the Eca109
cells was decreased, while that of the transcription factor Snail
was upregulated (Fig. 6A).
However, following the overexpression of miR-196a, the expression
of E-cadherin was upregulated, while that of Snail was
downregulated (Fig. 6B).
Discussion
ANXA1 (also known as lipocortin 1, phospholipase A2
inhibitory protein) is a member of the Annexin family, and belongs
to calcium-dependent phospholipid binding protein. It was
previously found that ANXA1 was an intracellular
inflammation-related factor that regulates the anti-inflammatory
effects of glucocorticoids, and it has also been shown to be
involved in the regulation of cell proliferation, differentiation
and apoptosis, as well as endocytosis and secretion, and to be
closely related to the occurrence or progression of tumors (12 and
refs. therein). Certain conflicting results have been found in the
published studies on the association between ANXA1 and malignant
tumors: firstly, ANXA1 is differently expressed in different
malignancies; in gastric cancer, pancreatic cancer, liver cancer,
esophageal adenocarcinoma and glioma, ANXA1 has been shown to be
upregulated (13–16); however, it has been found to be
downregu-lated or even absent in prostate cancer, ESCC, breast
cancer and B-cell lymphoma (17–20). Secondly, there are differences in
the understanding of the roles of ANXA1 in tumorigenesis. In some
tumors, ANXA1 has been shown to stimulate cell proliferation and
promote the invasion and metastasis of tumors (14,21–24), whereas in other tumors, ANXA1 has
been shown to exhibit the characteristics of a tumor suppressor
gene, such as inhibiting cell proliferation and inducing apoptosis
(11,25–28). Thus, the expression of ANXA1 in
malignant tumors seems to be tumor-specific, and plays
multi-factorial roles.
Our results confirmed that ANXA1 promoted the
proliferation of ESCC cells; however, but the exact mechanisms
involved remain unclear. The possible mechanisms responsible for
the promoting effects of ANXA1 on cell proliferation may include:
i) ANXA1 has multiple phosphorylation sites, and can act as the
receptor substrate of EGF/HGF and other growth signals, when
proliferated by protein kinase C (PKC) or tyrosine kinase (TK), and
it may then activate IKK, NF-κB and other downstream molecules,
thus promoting cell proliferation (29–31); ii) combined with formyl peptide
receptors (FPRs) on the cell surface, it participates in the
induction of mitogen-activated proliferation signals (32,33); iii) it acts as the transcriptional
target of forkhead transcription factor FoxM1, thus promoting the
proliferation, invasion, metastasis and angiogenesis of tumor cells
(34) and iv) it inhibits tumor
necrosis factor-induced apoptosis (35). However, there is evidence to
indicate that ANXA1 can inhibit cell proliferation, as demonstrated
in the study by Alldridge and Bryant, who reported that ANXA1
activated the ERK1/2 and MAPK signal transduction pathways, thus
destroying the cytoskeleton, inhibiting the expression of cyclin D1
and inhibiting cell proliferation (36). By inhibiting COX-2, ANXA1 has been
shown to inhibit the proliferation of gastric cancer cells
(11). It is unclear as to why
ANXA1 can promote cell proliferation in some tumors, while it
inhibits it in others; this may be due to the different phenotypes
of ANXA1. Hu et al analyzed the mutations in the promoter
region and the coding region of the whole ANXA1 gene, and did not
find any mutation or polymorphism (37) so as to support this hypothesis.
Thus, further studies are warranted to elucidate the mechanisms
through which ANXA1 affects the proliferation of ESCC cells.
This study also found that the overexpression of
ANXA1 promoted the migration and invasion of ESCC Eca109 cells; the
enhanced cell migration, invasion and growth are closely related to
clinical metastasis and progression. Thus, this study suggested
that ANXA1 promotes the progression and metastasis of ESCC,
consistent with other studies in which ANXA1 has been reported to
be able to promote the invasion and metastasis of gastric cancer,
pancreatic cancer, breast cancer, lung cancer and colorectal cancer
(14,38–42). However, other studies have found
opposite results, demonstrating that ANXA1 inhibits the growth,
invasion and metastasis of nasopharyngeal carcinoma (43), and that the knockdown of ANXA1
promoted cell proliferation and invasion (44).
Tumor invasion and metastasis is a complex process,
with tumor cells shedding from the primary sites as the first step,
which is related to the reduced intercellular adhesion functions
and epithelial-mesenchymal transition (EMT). EMT refers to the
process that polar, mutually adhered epithelial cells transform
into stromal cell-like substances that are active and can move
freely within the intercellular matrix. EMT occurs in the early
stage of the cascade of tumor invasion and metastasis, during which
the downregulation of the adhesion-associated protein, E-cadherin,
is the most important molecular event (45,46). E-cadherin is a member of the
cadherin family, and mediates the adhesion of cells, thus playing
important roles in maintaining cell polarity and the structural
integrity of the tissues. The transcription factor Snail binds with
E-box in the promoter region of E-cadherin, thus downregulating
E-cadherin and promoting cell shedding and migration (47–49); thus, it is closely related to the
in situ invasion and remote metastasis of tumor cells. The
results of this study demonstrated that the overexpression of ANXA1
upregulated Snail expression, while it downregulated that of
E-cadherin. However, when the expression of ANXA1 was decreased by
transfection with miR-196a mimic, Snail expression was then
downregulated, while E-cadherin expression was upregulated,
confirming that the ANXA1 gene exerts its effects on the invasion
and metastasis of ESCC cells through the Snail/E-cadherin
pathway.
It had been reported that the 3′-UTR sequences of
the ANXA1 gene mRNA and 5′-end sequence of miR-196a are highly
consistent, and ANXA1 is the target gene of miR-196a. When ANXA1
mRNA and miR-196a are combined with each other, this can lead to
the formation of a gene-silencing complex, thus blocking the
protein translation of ANXA1 (10,50). This study found that miR-196a
downregulated the expression of ANXA1, thus inhibiting the
expression of Snail, while promoting the expression of E-cadherin,
as well as inhibiting the proliferation, invasion and metastasis of
ESCC cells, consistent with the findings of other studies (51,52). Based on the oncogeneic effects of
ANXA1 (promoting the proliferation, invasion and metastasis of ESCC
cells), it may be conceivable to use miR-196a in the treatment of
patients with ESCC who exhibit a high ANXA1 expression, as well as
with a high risk of metastasis/recurrence. As miR-196a is only
approximately 21 bases, it can be stably expressed long-term in
vivo, and it would not interfere with host DNA replication and
transcription; thus, it would not lead to iatrogenic mutation. The
use of miR-196a may thus be a promising new approach for the
treatment of ESCC.
In conclusion, we demonstrate that the
overexpression of ANXA1 promotes the proliferation of ESCC cells,
and promotes the invasion and metastasis of ESCC cells by
increasing the expression of the transcription factor Snail, while
inhibiting that of E-cadherin. miR-196a may downregulate ANXA1,
thus negatively regulating ANXA1 and inhibiting the
Snail/E-cadherin pathway, followed by the inhibition of EMT, and
ultimately the inhibition of the proliferation, invasion and
metastasis of ESCC cells. The results of this study may be used to
screen patients with EC who are at risk of recurrence/metastasis.
Our findings may prove useful in the development of drugs targeting
ANXA1 and miR-196a, thus providing new treatment methods, apart
from conventional radiotherapy, for the treatment of high-risk
patients with ESCC who exhibit a high expression of ANXA1.
Acknowledgments
This study was supported by the Jiangsu Province
Ministry of Health, China (grant. no. H201260) and the Taizhou
Committee of Science and Technology, China (grant. no.
TS201346).
References
|
1
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yan W, Wistuba II, Emmert-Buck MR and
Erickson HS: Squamous cell carcinoma - similarities and differences
among anatomical sites. Am J Cancer Res. 1:275–300. 2011.PubMed/NCBI
|
|
3
|
Matsuda S, Takeuchi H, Kawakubo H, Ando N
and Kitagawa Y: Current advancement in multidisciplinary treatment
for resectable cStage II/III esophageal squamous cell carcinoma in
Japan. Ann Thorac Cardiovasc Surg. 22:275–283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim LH and Pervaiz S: Annexin 1: the new
face of an old molecule. FASEB J. 21:968–975. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo C, Liu S and Sun MZ: Potential role of
Anxa1 in cancer. Future Oncol. 9:1773–1793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang KL, Wu TT, Resetkova E, Wang H,
Correa AM, Hofstetter WL, Swisher SG, Ajani JA, Rashid A, Hamilton
SR and Albarracin C: Expression of Annexin A1 in esophageal and
esophagogastric junction adenocarcinomas: association with poor
outcome. Clin Cancer Res. 12:4598–4604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhardwaj A, Ganesan N, Tachibana K,
Rajapakshe K, Albarracin CT, Gunaratne PH, Coarfa C and Bedrosian
I: Annexin A1 preferentially predicts poor prognosis of basal-like
breast cancer patients by activating mTOR-S6 signaling. PLoS One.
10:e01276782015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang AX, Lu FQ, Yang YP, Ren XY, Li ZF
and Zhang W: MicroRNA-217 overexpression induces drug resistance
and invasion of breast cancer cells by targeting PTEN signaling.
Cell Biol Int. Jun 24–2015.Epub ahead of print. View Article : Google Scholar
|
|
9
|
Lynam-Lennon N, Reynolds JV, Marignol L,
Sheils OM, Pidgeon GP and Maher SG: MicroRNA-31 modulates tumour
sensitivity to radiation in oesophageal adenocarcinoma. J Mol Med
(Berl). 90:1449–1458. 2012. View Article : Google Scholar
|
|
10
|
Luthra R, Singh RR, Luthra MG, Li YX,
Hannah C, Romans AM, Barkoh BA, Chen SS, Ensor J, Maru DM, et al:
MicroRNA-196a targets Annexin A1: a microRNA-mediated mechanism of
Annexin A1 downregulation in cancers. Oncogene. 27:6667–6678. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao Y, Chen Y, Xu D, Wang J and Yu G:
Differential expression of ANXA1 in benign human gastrointestinal
tissues and cancers. BMC Cancer. 14:5202014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mussunoor S and Murray GI: The role of
Annexins in tumour development and progression. J Pathol.
216:131–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bai XF, Ni XG, Zhao P, Liu SM, Wang HX,
Guo B, Zhou LP, Liu F, Zhang JS, Wang K, et al: Overexpression of
Annexin 1 in pancreatic cancer and its clinical significance. World
J Gastroenterol. 10:1466–1470. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin Y, Lin G, Fang W, Zhu H and Chu K:
Increased expression of Annexin A1 predicts poor prognosis in human
hepatocellular carcinoma and enhances cell malignant phenotype. Med
Oncol. 31:3272014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue LY, Teng LH, Zou SM, Ren LQ, Zheng S,
Luo W, Bi R and Lü N: Expression of Annexin I in different
histological types of carcinomas. Zhonghua Zhong Liu Za Zhi.
29:444–448. 2007.PubMed/NCBI
|
|
16
|
Zhang ZQ, Li XJ, Liu GT, Xia Y, Zhang XY
and Wen H: Identification of Annexin A1 protein expression in human
gastric adenocarcinoma using proteomics and tissue microarray.
World J Gastroenterol. 19:7795–7803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang JS, Calvo BF, Maygarden SJ, Caskey
LS, Mohler JL and Ornstein DK: Dysregulation of Annexin I protein
expression in high-grade prostatic intraepithelial neoplasia and
prostate cancer. Clin Cancer Res. 8:117–123. 2002.PubMed/NCBI
|
|
18
|
Shen D, Nooraie F, Elshimali Y, Lonsberry
V, He J, Bose S, Chia D, Seligson D, Chang HR and Goodglick L:
Decreased expression of Annexin A1 is correlated with breast cancer
development and progression as determined by a tissue microarray
analysis. Hum Pathol. 37:1583–1591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vishwanatha JK, Salazar E and
Gopalakrishnan VK: Absence of Annexin I expression in B-cell
non-Hodgkin's lymphomas and cell lines. BMC Cancer. 4:82004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia SH, Hu H, Hu LP, Xu X, Cai Y, Han YL,
Chen BS, Wei F, Ying WT, Qian XH, et al: Analysis of proteins with
altered expression in human esophageal squamous cell carcinomas. Ai
Zheng. 21:11–15. 2002.In Chinese. PubMed/NCBI
|
|
21
|
Sato Y, Kumamoto K, Saito K, Okayama H,
Hayase S, Kofunato Y, Miyamoto K, Nakamura I, Ohki S, Koyama Y, et
al: Up-regulated Annexin A1 expression in gastrointestinal cancer
is associated with cancer invasion and lymph node metastasis. Exp
Ther Med. 2:239–243. 2011.PubMed/NCBI
|
|
22
|
Woś M and Bandorowicz-Pikuła J:
Participation of Annexins in endocytosis and EGFR-mediated signal
transduction. Postepy Biochem. 60:55–61. 2014.In Polish.
|
|
23
|
Sheu MJ, Li CF, Lin CY, Lee SW, Lin LC,
Chen TJ and Ma LJ: Overexpression of ANXA1 confers independent
negative prognostic impact in rectal cancers receiving concurrent
chemoradiotherapy. Tumour Biol. 35:7755–7763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yi M and Schnitzer JE: Impaired tumor
growth, metastasis, angiogenesis and wound healing in Annexin
A1-null mice. Proc Natl Acad Sci USA. 106:17886–17891. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsiang CH, Tunoda T, Whang YE, Tyson DR
and Ornstein DK: The impact of altered Annexin I protein levels on
apoptosis and signal transduction pathways in prostate cancer
cells. Prostate. 66:1413–1424. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang LP, Bi J, Yao C, Xu XD, Li XX, Wang
SM, Li ZL, Zhang DY, Wang M and Chang GQ: Annexin A1 expression and
its prognostic significance in human breast cancer. Neoplasma.
57:253–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ang EZ, Nguyen HT, Sim HL, Putti TC and
Lim LH: Annexin-1 regulates growth arrest induced by high levels of
estrogen in MCF-7 breast cancer cells. Mol Cancer Res. 7:266–274.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mu D, Gao Z, Guo H, Zhou G and Sun B:
Sodium butyrate induces growth inhibition and apoptosis in human
prostate cancer DU145 cells by up-regulation of the expression of
Annexin A1. PLoS One. 8:e749222013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
D'Acunto CW, Gbelcova H, Festa M and Ruml
T: The complex understanding of Annexin A1 phosphorylation. Cell
Signal. 26:173–178. 2014. View Article : Google Scholar
|
|
30
|
Bist P, Leow SC, Phua QH, Shu S, Zhuang Q,
Loh WT, Nguyen TH, Zhou JB, Hooi SC and Lim LH: Annexin-1 interacts
with NEMO and RIP1 to constitutively activate IKK complex and
NF-κB: implication in breast cancer metastasis. Oncogene.
30:3174–3185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoque M, Rentero C, Cairns R, Tebar F,
Enrich C and Grewal T: Annexins - scaffolds modulating PKC
localization and signaling. Cell Signal. 26:1213–1225. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khau T, Langenbach SY, Schuliga M, Harris
T, Johnstone CN, Anderson RL and Stewart AG: Annexin-1 signals
mitogen-stimulated breast tumor cell proliferation by activation of
the formyl peptide receptors (FPRs) 1 and 2. FASEB J. 25:483–496.
2011. View Article : Google Scholar
|
|
33
|
Gastardelo TS, Cunha BR, Raposo LS,
Maniglia JV, Cury PM, Lisoni FC, Tajara EH and Oliani SM:
Inflammation and cancer: role of Annexin A1 and FPR2/ALX in
proliferation and metastasis in human laryngeal squamous cell
carcinoma. PLoS One. 9:e1113172014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng SX, Tu Y and Zhang S: FoxM1 promotes
glioma cells progression by up-regulating Anxa1 expression. PLoS
One. 8:e723762013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu YL, Jiang XR, Lillington DM, Newland AC
and Kelsey SM: Upregulation of lipocortin 1 inhibits tumour
necrosis factor-induced apoptosis in human leukaemic cells: a
possible mechanism of resistance to immune surveillance. Br J
Haematol. 111:807–816. 2000.PubMed/NCBI
|
|
36
|
Alldridge LC and Bryant CE: Annexin 1
regulates cell proliferation by disruption of cell morphology and
inhibition of cyclin D1 expression through sustained activation of
the ERK1/2 MAPK signal. Exp Cell Res. 290:93–107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu N, Flaig MJ, Su H, Shou JZ, Roth MJ, Li
WJ, Wang C, Goldstein AM, Li G, Emmert-Buck MR and Taylor PR:
Comprehensive characterization of Annexin I alterations in
esophageal squamous cell carcinoma. Clin Cancer Res. 10:6013–6022.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Okano M, Kumamoto K, Saito M, Onozawa H,
Saito K, Abe N, Ohtake T and Takenoshita S: Upregulated Annexin A1
promotes cellular invasion in triple-negative breast cancer. Oncol
Rep. 33:1064–1070. 2015.PubMed/NCBI
|
|
39
|
Babbin BA, Lee WY, Parkos CA, Winfree LM,
Akyildiz A, Perretti M and Nusrat A: Annexin I regulates SKCO-15
cell invasion by signaling through formyl peptide receptors. J Biol
Chem. 281:19588–19599. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim SW, Rhee HJ, Ko J, Kim YJ, Kim HG,
Yang JM, Choi EC and Na DS: Inhibition of cytosolic phospholipase
A2 by Annexin I. Specific interaction model and mapping of the
interaction site. J Biol Chem. 276:15712–15719. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Belvedere R, Bizzarro V, Popolo A, Dal
Piaz F, Vasaturo M, Picardi P, Parente L and Petrella A: Role of
intracellular and extracellular Annexin A1 in migration and
invasion of human pancreatic carcinoma cells. BMC Cancer.
14:9612014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng TY, Wu MS, Lin JT, Lin MT, Shun CT,
Huang HY, Hua KT and Kuo ML: Annexin A1 is associated with gastric
cancer survival and promotes gastric cancer cell invasiveness
through the formyl peptide receptor/extracellular signal-regulated
kinase/integrin beta-1-binding protein 1 pathway. Cancer.
118:5757–5767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu A, Huang W, Zeng G, Ma X, Zhou X, Wang
Y, Ouyang C and Cheng A: Expression of the Annexin A1 gene is
associated with suppression of growth, invasion and metastasis of
nasopharyngeal carcinoma. Mol Med Rep. 10:3059–3067.
2014.PubMed/NCBI
|
|
44
|
Suh YE, Raulf N, Gäken J, Lawler K, Urbano
TG, Bullenkamp J, Gobeil S, Huot J, Odell E and Tavassoli M:
MicroRNA-196a promotes an oncogenic effect in head and neck cancer
cells by suppressing Annexin A1 and enhancing radioresistance. Int
J Cancer. 137:1021–1034. 2015. View Article : Google Scholar
|
|
45
|
Buda A and Pignatelli M: E-cadherin and
the cytoskeletal network in colorectal cancer development and
metastasis. Cell Commun Adhes. 18:133–143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gheldof A and Berx G: Cadherins and
epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci.
116:317–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Peinado H, Ballestar E, Esteller M and
Cano A: Snail mediates E-cadherin repression by the recruitment of
the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell
Biol. 24:306–319. 2004. View Article : Google Scholar :
|
|
48
|
Wang YL, Zhao XM, Shuai ZF, Li CY, Bai QY,
Yu XW and Wen QT: Snail promotes epithelial-mesenchymal transition
and invasiveness in human ovarian cancer cells. Int J Clin Exp Med.
8:7388–7393. 2015.PubMed/NCBI
|
|
49
|
Montserrat N, Gallardo A, Escuin D,
Catasus L, Prat J, Gutiérrez-Avignó FJ, Peiró G, Barnadas A and
Lerma E: Repression of E-cadherin by SNAIL, ZEB1, and TWIST in
invasive ductal carcinomas of the breast: a cooperative effort? Hum
Pathol. 42:103–110. 2011. View Article : Google Scholar
|
|
50
|
Engels BM and Hutvagner G: Principles and
effects of microRNA-mediated post-transcriptional gene regulation.
Oncogene. 25:6163–6169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pin AL, Houle F, Fournier P, Guillonneau
M, Paquet ÉR, Simard MJ, Royal I and Huot J: Annexin-1-mediated
endothelial cell migration and angiogenesis are regulated by
vascular endothelial growth factor (VEGF)-induced inhibition of
miR-196a expression. J Biol Chem. 287:30541–30551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Swa HL, Blackstock WP, Lim LH and
Gunaratne J: Quantitative proteomics profiling of murine mammary
gland cells unravels impact of Annexin-1 on DNA damage response,
cell adhesion, and migration. Mol Cell Proteomics. 11:381–393.
2012. View Article : Google Scholar : PubMed/NCBI
|