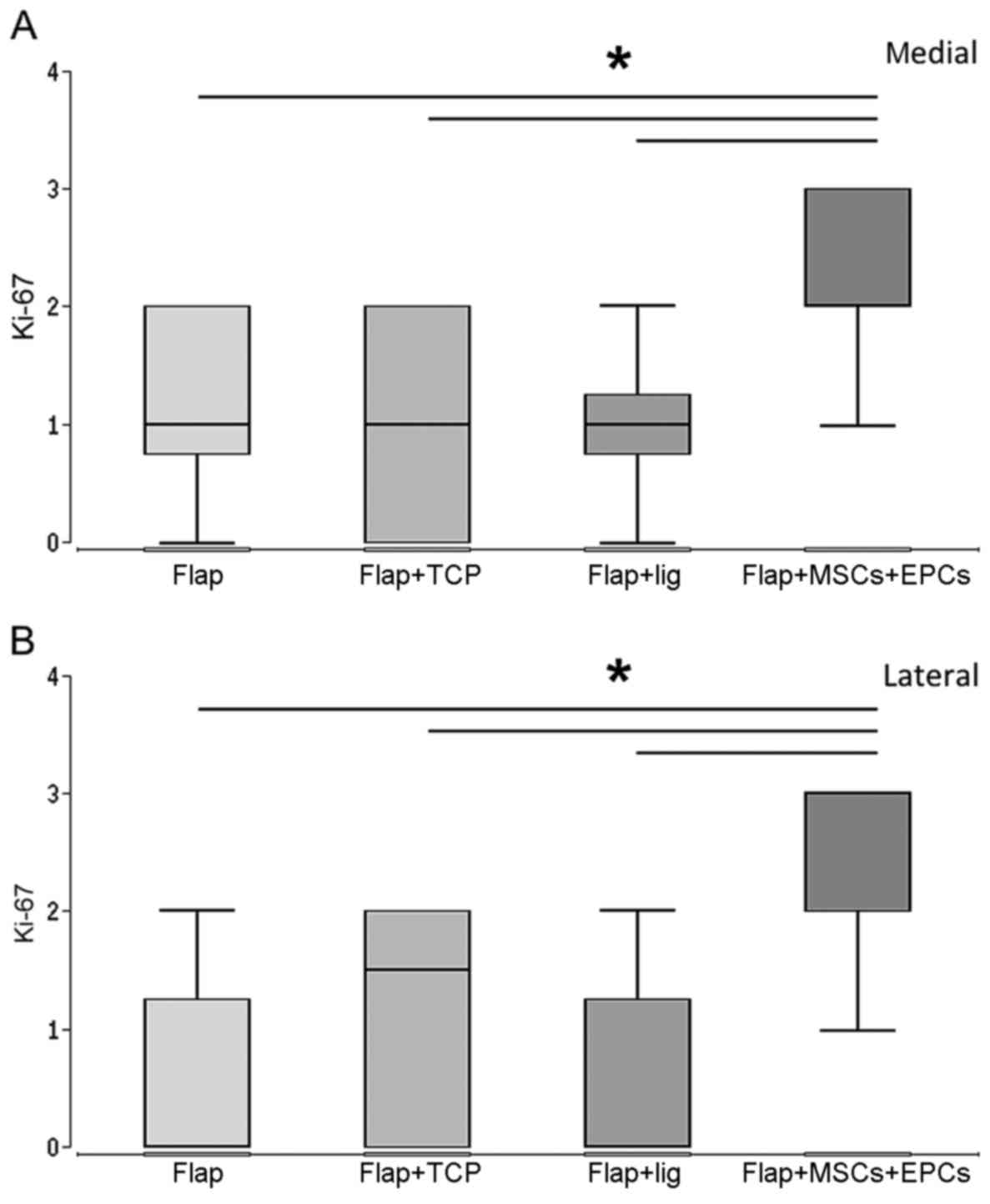
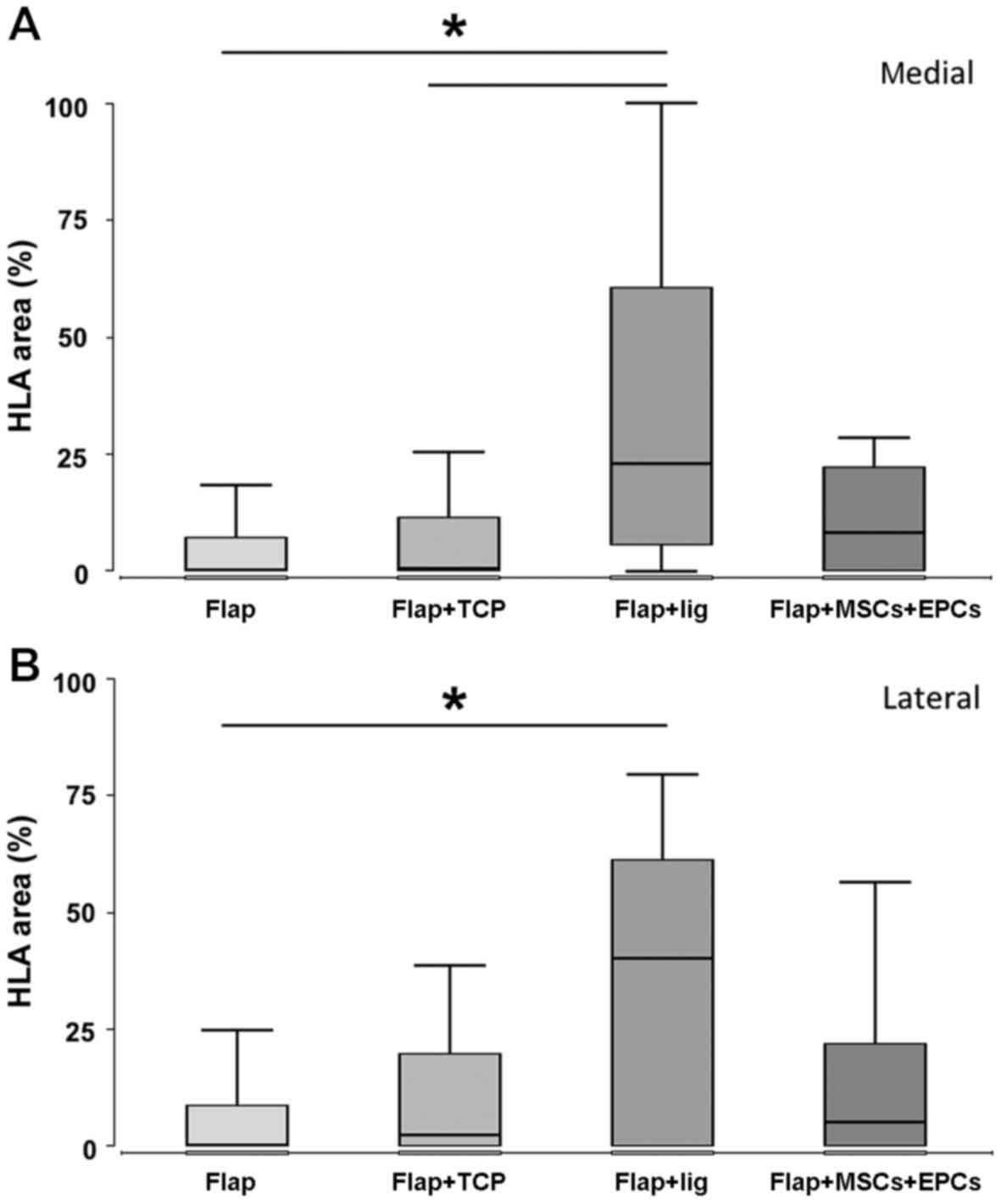
|
1
|
Bauer TW and Muschler GF: Bone graft
materials. An overview of the basic science. Clin Orthop Relat Res.
371:10–27. 2000. View Article : Google Scholar
|
|
2
|
Horner EA, Kirkham J, Wood D, Curran S,
Smith M, Thomson B and Yang XB: Long bone defect models for tissue
engineering applications: Criteria for choice. Tissue Eng Part B
Rev. 16:263–271. 2010. View Article : Google Scholar
|
|
3
|
Giannoudis PV, Dinopoulos H and Tsiridis
E: Bone substitutes: An update. Injury. 36(Suppl 3): S20–S27. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nandi SK, Roy S, Mukherjee P, Kundu B, De
DK and Basu D: Orthopaedic applications of bone graft & graft
substitutes: A review. Indian J Med Res. 132:15–30. 2010.PubMed/NCBI
|
|
5
|
Finkemeier CG: Bone-grafting and
bone-graft substitutes. J Bone Joint Surg Am. 84-A:454–464. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iacobellis C, Berizzi A and Aldegheri R:
Bone transport using the Ilizarov method: A review of complications
in 100 consecutive cases. Strateg Trauma Limb Reconstr. 5:17–22.
2010. View Article : Google Scholar
|
|
7
|
Doi K and Sakai K: Vascularized periosteal
bone graft from the supracondylar region of the femur.
Microsurgery. 15:305–315. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soldado F, Garcia Fontecha C, Haddad S,
Hernandez-Fernandez A, Corona P and Guerra-Farfan E: Treatment of
congenital pseudarthrosis of the tibia with vascularized fibular
periosteal transplant. Microsurgery. 32:397–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsumura G, Hibino N, Ikada Y, Kurosawa H
and Shin'oka T: Successful application of tissue engineered
vascular autografts: Clinical experience. Biomaterials.
24:2303–2308. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Doi M, Nagano A and Nakamura Y:
Genome-wide screening by cDNA microarray of genes associated with
matrix mineralization by human mesenchymal stem cells in vitro.
Biochem Biophys Res Commun. 290:381–390. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marcacci M, Kon E, Moukhachev V, Lavroukov
A, Kutepov S, Quarto R, Mastrogiacomo M and Cancedda R: Stem cells
associated with macroporous bioceramics for long bone repair: 6- to
7-year outcome of a pilot clinical study. Tissue Eng. 13:947–955.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vögelin E, Jones NF, Huang JI, Brekke JH
and Lieberman JR: Healing of a critical-sized defect in the rat
femur with use of a vascularized periosteal flap, a biodegradable
matrix, and bone morphogenetic protein. J Bone Joint Surg Am.
87:1323–1331. 2005.PubMed/NCBI
|
|
13
|
Mastrogiacomo M, Scaglione S, Martinetti
R, Dolcini L, Beltrame F, Cancedda R and Quarto R: Role of scaffold
internal structure on in vivo bone formation in macroporous calcium
phosphate bioceramics. Biomaterials. 27:3230–3237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nauth A, Giannoudis PV, Einhorn TA,
Hankenson KD, Friedlaender GE, Li R and Schemitsch EH: Growth
factors: Beyond bone morphogenetic proteins. J Orthop Trauma.
24:543–546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang Y, Tang W, Lin Y, Long J, Wang H, Liu
L and Tian W: Combination of bone tissue engineering and BMP-2 gene
transfection promotes bone healing in osteoporotic rats. Cell Biol
Int. 32:1150–1157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Madeddu P: Therapeutic angiogenesis and
vasculogenesis for tissue regeneration. Exp Physiol. 90:315–326.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Henrich D, Seebach C, Kaehling C, Scherzed
A, Wilhelm K, Tewksbury R, Powerski M and Marzi I: Simultaneous
cultivation of human endothelial-like differentiated precursor
cells and human marrow stromal cells on beta-tricalcium phosphate.
Tissue Eng Part C Methods. 15:551–560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seebach C, Henrich D, Kähling C, Wilhelm
K, Tami AE, Alini M and Marzi I: Endothelial progenitor cells and
mesenchymal stem cells seeded onto beta-TCP granules enhance early
vascularization and bone healing in a critical-sized bone defect in
rats. Tissue Eng Part A. 16:1961–1970. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seebach C, Henrich D, Wilhelm K, Barker JH
and Marzi I: Endothelial progenitor cells improve directly and
indirectly early vascularization of mesenchymal stem cell-driven
bone regeneration in a critical bone defect in rats. Cell
Transplant. 21:1667–1677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seebach C, Schultheiss J, Wilhelm K, Frank
J and Henrich D: Comparison of six bone-graft substitutes regarding
to cell seeding efficiency, metabolism and growth behaviour of
human mesenchymal stem cells (MSC) in vitro. Injury. 41:731–738.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gamradt SC and Lieberman JR: Genetic
modification of stem cells to enhance bone repair. Ann Biomed Eng.
32:136–147. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keramaris NC, Calori GM, Nikolaou VS,
Schemitsch EH and Giannoudis PV: Fracture vascularity and bone
healing: A systematic review of the role of VEGF. Injury. 39(Suppl
2): S45–S57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo X, Zheng Q, Kulbatski I, Yuan Q, Yang
S, Shao Z, Wang H, Xiao B, Pan Z and Tang S: Bone regeneration with
active angiogenesis by basic fibroblast growth factor gene
transfected mesenchymal stem cells seeded on porous beta-TCP
ceramic scaffolds. Biomed Mater. 1:93–99. 2006. View Article : Google Scholar
|
|
24
|
Peng H, Wright V, Usas A, Gearhart B, Shen
HC, Cummins J and Huard J: Synergistic enhancement of bone
formation and healing by stem cell-expressed VEGF and bone
morphogenetic protein-4. J Clin Invest. 110:751–759. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumar S, Wan C, Ramaswamy G, Clemens TL
and Ponnazhagan S: Mesenchymal stem cells expressing osteogenic and
angiogenic factors synergistically enhance bone formation in a
mouse model of segmental bone defect. Mol Ther. 18:1026–1034. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Asahara T, Masuda H, Takahashi T, Kalka C,
Pastore C, Silver M, Kearne M, Magner M and Isner JM: Bone marrow
origin of endothelial progenitor cells responsible for postnatal
vasculogenesis in physiological and pathological
neovascularization. Circ Res. 85:221–228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Atesok K, Li R, Stewart DJ and Schemitsch
EH: Endothelial progenitor cells promote fracture healing in a
segmental bone defect model. J Orthop Res. 28:1007–1014.
2010.PubMed/NCBI
|
|
28
|
Rozen N, Bick T, Bajayo A, Shamian B,
Schrift-Tzadok M, Gabet Y, Yayon A, Bab I, Soudry M and Lewinson D:
Transplanted blood-derived endothelial progenitor cells (EPC)
enhance bridging of sheep tibia critical size defects. Bone.
45:918–924. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jones AL, Bucholz RW, Bosse MJ, Mirza SK,
Lyon TR, Webb LX, Pollak AN, Golden JD and Valentin-Opran A; BMP-2
Evaluation in Surgery for Tibial Trauma-Allgraft (BESTT-ALL) Study
Group: Recombinant human BMP-2 and allograft compared with
autogenous bone graft for reconstruction of diaphyseal tibial
fractures with cortical defects. A randomized, controlled trial. J
Bone Joint Surg Am. 88:1431–1441. 2006.PubMed/NCBI
|
|
30
|
Daniels T, DiGiovanni C, Lau JT, Wing K
and Younger A: Prospective clinical pilot trial in a single cohort
group of rhPDGF in foot arthrodeses. Foot Ankle Int. 31:473–479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O'Driscoll SW and Fitzsimmons JS: The role
of periosteum in cartilage repair. Clin Orthop Relat Res. (Suppl):
S190–S207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
O'Driscoll SW, Saris DB, Ito Y and
Fitzimmons JS: The chondrogenic potential of periosteum decreases
with age. J Orthop Res. 19:95–103. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jaffe HL: Metabolic, Degenerative and
Inflammatory Diseases of Bone and Joints. 1st edition. Urban and
Schwarzenberg; München-Berlin-Wien: 1972
|
|
34
|
Dwek JR: The periosteum: What is it, where
is it, and what mimics it in its absence? Skeletal Radiol.
39:319–323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Allen MR, Hock JM and Burr DB: Periosteum:
Biology, regulation, and response to osteoporosis therapies. Bone.
35:1003–1012. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nau C, Henrich D, Seebach C, Schröder K,
Fitzsimmons SJ, Hankel S, Barker JH, Marzi I and Frank J: Treatment
of large bone defects with a vascularized periosteal flap in
combination with biodegradable scaffold seeded with bone
marrow-derived mononuclear cells: An experimental study in rats.
Tissue Eng Part A. 22:133–141. 2016. View Article : Google Scholar
|
|
37
|
Arthur A, Zannettino A and Gronthos S: The
therapeutic applications of multipotential mesenchymal/stromal stem
cells in skeletal tissue repair. J Cell Physiol. 218:237–245. 2009.
View Article : Google Scholar
|
|
38
|
Eldesoqi K, Henrich D, El-Kady AM, Arbid
MS, Abd El-Hady BM, Marzi I and Seebach C: Safety evaluation of a
bioglass-polylactic acid composite scaffold seeded with progenitor
cells in a rat skull critical-size bone defect. PLoS One.
9:e876422014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eldesoqi K, Seebach C, Nguyen Ngoc C,
Meier S, Nau C, Schaible A, Marzi I and Henrich D: High calcium
bioglass enhances differentiation and survival of endothelial
progenitor cells, inducing early vascularization in critical size
bone defects. PLoS One. 8:e790582013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Henrich D, Hahn P, Wahl M, Wilhelm K,
Dernbach E, Dimmeler S and Marzi I: Serum derived from multiple
trauma patients promotes the differentiation of endothelial
progenitor cells in vitro: Possible role of transforming growth
factor-beta1 and vascular endothelial growth factor165. Shock.
21:13–16. 2004. View Article : Google Scholar
|
|
41
|
Henrich D, Seebach C, Wilhelm K and Marzi
I: High dosage of simvastatin reduces TNF-alpha-induced apoptosis
of endothelial progenitor cells but fails to prevent apoptosis
induced by IL-1beta in vitro. J Surg Res. 142:13–19. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Del Piñal F, García-Bernal FJ, Regalado J,
Ayala H, Cagigal L and Studer A: Vascularised corticoperiosteal
grafts from the medial femoral condyle for difficult non-unions of
the upper limb. J Hand Surg Eur Vol. 32:135–142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fuchs B, Steinmann SP and Bishop AT: Free
vascularized corticoperiosteal bone graft for the treatment of
persistent nonunion of the clavicle. J Shoulder Elbow Surg.
14:264–268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Camilli JA and Penteado CV: Bone formation
by vascularized periosteal and osteoperiosteal grafts. An
experimental study in rats. Arch Orthop Trauma Surg. 114:18–24.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ghanaati S, Barbeck M, Orth C,
Willershausen I, Thimm BW, Hoffmann C, Rasic A, Sader RA, Unger RE
and Peters F: Influence of β-tricalcium phosphate granule size and
morphology on tissue reaction in vivo. Acta Biomater. 6:4476–4487.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Faour O, Dimitriou R, Cousins CA and
Giannoudis PV: The use of bone graft substitutes in large
cancellous voids: Any specific needs? Injury. 42(Suppl 2): S87–S90.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Schultheiss J, Seebach C, Henrich D,
Wilhelm K, Barker JH and Frank J: Mesenchymal stem cell (MSC) and
endothelial progenitor cell (EPC) growth and adhesion in six
different bone graft substitutes. Eur J Trauma Emerg Surg.
37:635–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee DE, Ayoub N and Agrawal DK:
Mesenchymal stem cells and cutaneous wound healing: Novel methods
to increase cell delivery and therapeutic efficacy. Stem Cell Res
Ther. 7:372016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao L, Liu X, Zhang Y, Liang X, Ding Y,
Xu Y, Fang Z and Zhang F: Enhanced cell survival and paracrine
effects of mesenchymal stem cells overexpressing hepatocyte growth
factor promote cardioprotection in myocardial infarction. Exp Cell
Res. 344:30–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Merino-González C, Zuñiga FA, Escudero C,
Ormazabal V, Reyes C, Nova-Lamperti E and Salomón C: Aguayo C.
Mesenchymal stem cell-derived extracellular vesicles promote
angiogenesis: Potencial clinical application. Front Physiol.
7:242016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Potapova IA, Gaudette GR, Brink PR,
Robinson RB, Rosen MR, Cohen IS and Doronin SV: Mesenchymal stem
cells support migration, extracellular matrix invasion,
proliferation, and survival of endothelial cells in vitro. Stem
Cells. 25:1761–1768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hoch AI, Binder BY, Genetos DC and Leach
JK: Differentiation-dependent secretion of proangiogenic factors by
mesenchymal stem cells. PLoS One. 7:e355792012. View Article : Google Scholar : PubMed/NCBI
|