Introduction
Presbycusis or age-related hearing loss (AHL) is a
common sensory functional disturbance (1,2),
which negatively affects the quality of life of affected
individuals (3). AHL decreases a
person's ability to communicate with a spoken language and has been
implicated in various somatopsychic illnesses, including
depression, social isolation and a lower self-esteem. Despite
extensive research efforts, our understanding of the mechanisms
responsible for AHL remains limited, and there are currenlty no
available effective medications to prevent or treat this condition.
Environmental and other factors, such as endocrine disease and
ototoxic drugs may contribute to the development of AHL. In the
United States, approximately two-thirds of all adults older than 70
years have clinically significant hearing loss (4). Globally, the incidence of AHL has
continued to increase due to the aging population, and it has
become a major burden to families and society.
Sensorineural hearing loss is characterized by a
difficulty in hearing high frequencies and is caused by the
degeneration of hair cells and spiral ganglion neurons (SGNs). The
organ of Corti is located in the cochlea. It is composed of
mechanosensory cells known as hair cells, and is responsible for
the sensations of sound. SGNs establish connections between hair
cells and central auditory system neurons in the brainstem.
Unfortunately, hair cells and SGNs are non-renewable; hence, their
impairment results in decreased hearing sensitivity (5,27).
The normal lifespan and fecundity of NOD/LtJ mice
provide a complementary model to facilitate the genetic basis of
human presbycusis. Inbred strains of NOD/LtJ mice are susceptible
to early-onset AHL (6). This
phenotype has been traced to the hypomorphic Cdh23 (753A)
allele in the cadherin 23 gene (Cdh23). In addition, the
corresponding cadherin 23 (CDH23) is a component of the tip link in
hair-cell stereocilia (7). The
major contributor to the difference in hearing loss onset time
between NOD/LtJ and C57BL/6J mice is the Ahl2 locus on mouse
chromosome (Chr)5 in NOD/LtJ mice (6). NOD/LtJ mice exhibit hearing loss
before 3 months, and this is much earlier than C57BL/6J mice, which
do not exhibit hearing loss until 10 months of age or older
(6). Thus, these features
established an appropriate therapeutic time frame for
AHL-associated genetic mechanisms, and may be used to provide new
insight as to the treatment of AHL.
Calcium channel blockers has been demonstrated to
improve hearing thresholds in females during aging, suggesting that
calcium disorders are an important factor underlying human AHL
(8). Furthermore, Ca2+
ions can activate the apoptotic signaling pathway. Apoptosis plays
a key role in the age-related decline of physiological function in
multiple organs, including the cochlea (9–11).
Thus, in this study, we selected ethosuximide, which is used as a
first-line treatment for the absence seizures, and which its
primary mechanism is to selectively inhibit T-type Ca2+
channels in the neuronal cell membrane (12). This study aimed to evaluate the
otoprotective effects of ethosuximide as a Ca2+ channel
blocker in NOD/LtJ mice with AHL.
The pan-caspase inhibitor, Z-Val-Ala-Asp
(OMe)-fluoromethyl ketone (Z-VAD-FMK), can attenuate the
progression of AHL in
Cdh23erl/erl mice and reduce
age-related outer hair cell (OHC) degeneration, and prevent
apoptotic cell death by inhibiting the calpain signaling pathway
(13). Calpain is a cysteine
protease, and alterations in intracellular Ca2+ can
activate calpain and the caspase family (14,15). In addition, erythropoietin (EPO)
can protect against hearing loss in
Cdh23erl/erl mice through
apoptosis-related pathways (16).
In the present study, NOD/LtJ mice were treated with
ethosuximide from post-natal day 7 (P7). We hypothesized that
ethosuximide could block T-type Ca2+ channels, suppress
apoptotic factors, and thus prevent hair cell degeneration and
preserve auditory function.
Materials and methods
Animals
NOD/LtJ mice (age range, P7 to 5 months) obtained
from the Jackson Laboratory (Bar Harbor, ME, USA), were housed at
24°C with a 12-h light/dark cycle in a standard animal house. A
total of 95 NOD/LtJ mice were used in this study. Food and water
were available ad libitum. The Animal Care and Use Committee
of Binzhou Medical University, Yantai, China approved the care and
use of mice for this study.
Allocation of mice for use in
experiments
In the experiment shown in Fig. 1, we tested ABR and DPOAE at 3, 4,
6, 8, 10 and 14 weeks of age for the NOD/Shi LtJ mice (n=5/time
point) and the mice were anesthetized and sacrificed for phalloidin
staining after testing. In order to determine the tendency for cell
loss, the mice were anesthetized at 18 weeks (n=5) and sacrificed
for phalloidin staining after testing. The key time points are
shown. As mouse hearing can be tested at 3 weeks of age by ABR and
we did not observe any loss of hair cells, this was not shown
(total mice used = 35). In the experiment shown in Fig. 2, the ABR and DPOAE of the
untreated mice and ethosuximide-treated mice were assessed at 4, 6
and 8 weeks of age (n=5/group). After testing at 4 weeks, the mice
were then allowed to grow 6 weeks for testing and then to 8 weeks
for testing (total mice used = 10). In the experiment shown in
Fig. 3, the mice in the
ethosuximide-treated and untreated groups were sacrificed at 4 and
8 weeks of age (n=5/group/time point), and the Corti were isolated,
OHC and IHC hair bundles were visualized with phalloidin staining
(total mice used = 20, 10 at each time point in both groups). In
the experiment shown in Fig. 4,
at 4 and 8 weeks of age, the mice in the ethosuximide-treated and
untreated groups were sacrificed (n=5/group/time point) (total mice
used = 20, 10 at each time point in both groups). In the experiment
shown in Fig. 5, we examined
expression differences between the ethosuximide-treated and
untreated mice (n=5/group) at the age of 8 weeks (total mice used =
10).
 | Figure 2Effects of ethosuximide treatment in
different frequencies at different time points (n=5/group). (A–E
and G–I) In 4-week-old mice, auditory-evoked brainstem response
(ABR) thresholds in the ethosuximide-treated group (red line) were
significantly lower compared to the untreated group (blue line) at
a stimulus frequency of 8 kHz (P<0.01). In 6-week-old mice, ABR
thresholds in the ethosuximide-treated group (red line) were
significantly lower compared to the untreated group (blue line) at
a stimulus frequency of 16 kHz (P<0.01). In 8-week-old mice, ABR
thresholds in the ethosuximide-treated group (red 2 groups during
the period (4–8 weeks) of drug application. (F) There were no
significant differences in body weight (8-week-old) in
ethosuximide-treated mice compared to untreated mice after drug
application. Comparison of distortion product oto-acoustic emission
(DPOAE) amplitudes between the 2 mouse groups in 4-week-old mice
(G), 6-week-old mice (H) and 8-week-old (I) mice. (G) In 4-week-old
mice, mean DPOAE amplitudes in the ethosuximide-treated group (red
line) at 8,844 and 12,503 Hz were significantly higher compared to
the untreated group (blue line). (H) In 6-week-old mice, mean DPOAE
amplitudes in the ethosuximide-treated group (red line) at 4,422,
17,672 and 24,990 Hz were significantly higher compared to the
untreated group (blue line). (I) In 8-week-old mice, mean DPOAE
amplitudes in the ethosuximide-treated group (red line) at 8,844,
12,503 and 35,344 Hz were significantly higher compared to the
untreated group (blue line). Error bars indicate the standard error
of the mean. *P<0.05 and **P<0.01. |
Assessment of auditory function
Auditory-evoked brainstem response (ABR) was tested
to identify the lowest level at which an ABR pattern can be
recognized. Distortion product oto-acoustic emission (DPOAE)
represented the function of OHCs. They were measured at various
intervals at 3, 4, 6, 8, 10 and 14 weeks of age for the NOD/Shi LtJ
mice (n=5/time point). The computer-aided evoked potential system
(Intelligent Hearing Systems, Miami, FL, USA) was used to test for
ABR thresholds (17,18). Briefly, the mice were anesthetized
with 3% chloral hydrate (10 mg/kg) and body temperature was
maintained at 37±1°C. Platinum needle electrodes were inserted
subcutaneously at the vertex (active), and ventrolateral to the
right ear (reference) and left ear (ground). ABR thresholds were
detected by reducing the sound pressure level (SPL) at 10-dB steps,
and subsequently at 5-dB steps. Following the ABR test, we measured
DPOAE using the IHS Smart EP 3.30 and USB ez Software (Intelligent
Hearing Systems, Miami, FL, USA) (19). Stimuli (ranging from 4,422 to
35,344 Hz) were presented from lowest to highest frequency.
Amplitudes were recorded automatically.
Morphometric analysis
For morphometric observation, at 4, 8, 10, 14 and 18
weeks, the mice were anesthetized and sacrificed (n=5/time-point).
The inner ears were removed, fixed in 4% paraformaldehyde, and
decalcified in Cal-Ex solution. Cochleae were isolated and divided
into 3 turns (apex, middle turn and base turn). Finally, the organ
of Corti was exposed in each turn and specimens were mounted on
glass slides. OHC and inner hair cell (IHC) hair bundles were
visualized with Alexa Fluor® 488-conjugated phalloidin
staining and a confocal laser scanning microscope (Leica TCS SPE;
Leica Microsystems GmbH, Wetzlar Germany). The number of missing
hair cells was counted per view using a x40 oil immersion objective
lens (13); counts of continuous
views for each turn were recorded for statistical analyses.
Cytocochleograms were generated.
Drug application
Seven-day-old mice were divided into 2 groups as
follows: ethosuximide-treated group and untreated group
(n=5/group). Ethosuximide (Sigma Chemical Co., St. Louis, MO, USA)
was dissolved in ddH2O and stored at 4°C in dark bottles
until use. Ethosuximide was administered by intraperitoneal
injection (10 mg/kg of body weight) every other day from P7 to 8
weeks of age. A higher dosage 14 mg/kg injection of ethosuximide
every other day resulted in the death of 2 litters of mice (not the
mice used in these experiments) after 2 weeks initial treatment. We
measured body weight prior to injection. Body weight was also used
for monitoring the animal health status.
Evaluation of the protection of auditory
function
The ABR and DPOAE of the untreated mice and
ethosuximide-treated mice were assessed at 4, 6 and 8 weeks of age
(n=5/group/time point).
The mice in the ethosuximide-treated and untreated
groups were sacrificed at 4 and 8 weeks of age (n=5/group/time
point), and the Corti were isolated, OHC and IHC hair bundles were
visualized with phalloidin staining as described previously. Hair
cells in the ethosuximide-treated and untreated groups were
observed and counted, and these were reported as the percentage of
missing OHCs and IHCs.
At 4 and 8 weeks of age, the mice in the
ethosuximide-treated and untreated groups were sacrificed
(n=5/group/time point) and the bullae (including both the middle
and inner ears) were isolated, and immersed in 4% paraformaldehyde,
decalcified with Cal-Ex solution, and embedded in paraffin.
Subsequently, 5-mm-thick sections of cochleae were cut, mounted on
glass slides, counterstained with hematoxylin and eosin (H&E),
and observed under a microscope using a 40× objective lens and 100×
oil immersion objective lens (Leica DM4000 B; Leica Microsystems
GmbH).
Reverse transcription-quantitative PCR
(RT-qPCR)
The experiment were assessed between the
ethosuximide-treated and untreated mice (n=5/group) at the age of 8
weeks. The entire inner ears were dissected, and total RNA was
extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). For
each sample, a reverse transcriptase reaction was performed with 1
μg of total RNA using the ReverTra Ace qPCR RT kit (Toyobo,
Osaka, Japan). The resulting cDNA was diluted for use as template
for the real-time PCR reaction. Primer3 was used to design the
primers (http://primer3.sourceforge.net/) (Table I). The PCR reaction was carried
out using the FastStart Universal SYBR-Green Master kit (Roche,
Mannheim, Germany) and specific primers in a Bio-Rad iCycler iQ5
peltier thermal cycler. The process was as follows: one cycle at
95°C for 10 min; 40 cycles at 95°C for 15 sec, 58°C for 30 sec and
72°C for 10 sec; and 71 cycles at 60–95°C. PCR cycles were
continued until the fluorescence intensity exceeded a predetermined
threshold, and he cycle number was measured automatically. The
relative quantification of the initial amount of template for each
sample was achieved by determining the number of cycles. The ΔCt
value was calculated using the comparative Ct threshold method. The
difference in the initial amount of total RNA among samples was
normalized using the housekeeping gene, glyceraldehyde 3-phosphate
dehydrogenase (GAPDH). The relative levels of target gene mRNA were
analyzed using the 2-ΔΔCt method (20).
 | Table IPrimers used for RT-qPCR for the
detection of apoptosis- related genes. |
Table I
Primers used for RT-qPCR for the
detection of apoptosis- related genes.
| ID | Primer
sequences | Product size
(bp) |
|---|
| GAPDH-F |
5′-CTTCCGTGTTCCTACCCCCAATGT-3′ | 132 |
| GAPDH-R |
5′-GCCTGCTTCACCACCTTCTTGATG-3′ | |
| α1G-F |
5′-GGGCTGTATTCCCCTCCATCGT-3′ | 97 |
| α1G-R |
5′-CTTCTGACCCATTCCCACCATCAC-3′ | |
| α1H-F |
5′-GCTATGTTGCCCTGGATTTTGAGC-3′ | 130 |
| α1H-R |
5′-GGAAGGAAGGCTGGAAGAGTGC-3′ | |
| α1I-F |
5′-CAAGTTCTCTTTGGTTGGCATCG-3′ | 123 |
| α1I-R |
5′-GGGTAAGTGTTGCGTTCCCTTCAT-3′ | |
| m-calpain-F |
5′-GTTTGTGACCGCCAAGAAAAATGG-3′ | 107 |
| m-calpain-R |
5′-GAAGAGTTCCGAGTCCCCTGCTGT-3′ | |
|
μ-calpain-F |
5′-TCCTGGTCAATACCCTCAGC-3′ | 95 |
|
μ-calpain-R |
5′-AGGCTGGTGAAGACGATGTT-3′ | |
| Caspase-3-F |
5′-TGAATCCACTGAGGTTTTGTTG-3′ | 93 |
| Caspase-3-R |
5′-TGCTGGTGGGATCAAAGC-3′ | |
| Caspase-9-F |
5′-CATATCTGCATGTCCCCTGA-3′ | 104 |
| Caspase-9-R |
5′-AGCCAGAGGTTCTCAGACCA-3′ | |
| Caspase-12-F |
5′-AAAAATCCTGGGATCTTGGA-3′ | 97 |
| Caspase-12-R |
5′-GGGAATTAGCACAGGCAACT-3′ | |
Statistical analysis
Statistical comparisons of differences between
groups were conducted using the Student's t-test. A P-value
<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using the
statistical package SPSS for Windows (version 16.0; SPSS, Inc.,
Chicago, IL, USA).
Results
NOD/LtJ mice are characterized by
increased hearing impairment with age
To investigate hearing loss during the lifespan of
NOD/LtJ mice, we detected ABR and DPOAE at 6 time points (in mice
aged between 3 and 14 weeks, n=5/group/time point) (Fig. 1A). The 3-week-old mice exhibited
hearing loss with average ABR thresholds >60 dB above normal,
which is considered to be a profound impairment (6). The ABR thresholds increased after 8
weeks of age, particularly at high frequencies (16 and 32 kHz).
From 3 to 8 weeks of age, the average shift in the ABR threshold
was approximately 15 dB; while from 8 to 14 weeks of age, the
average shift in the ABR threshold was >25 dB (Fig. 1A). In the 12-week-old mice, the
average ABR thresholds were >90 dB SPL, and the 14-week-old mice
exhibited almost complete deafness. DPOAE was measured to determine
OHC function over time (Fig. 1B).
The 3-week-old mice exhibited decreased DPOAE amplitudes, which
deteriorated with age.
NOD/LtJ mice are characterized by the
abnormal morphology of the cochleae
To investigate changes in cochleae morphology during
the course of hearing loss to the status of complete deafness in
NOD/LtJ mice, the organ of Corti was examined in all 3 turns of the
cochleae (Fig. 1C–G). To quantify
hair cell loss, cytocochleograms were generated from mice aged
between 4 and 14 weeks (Fig. 1H).
From post-natal day zero (P0) to 3 weeks of age, the mice exhibited
no evidence of OHC loss. However, in the 4-week-old mice, evidence
of OHC degeneration began at the basal turn and OHC loss was 6% in
all 3 turns totally (Fig. 1C and
H). At 8 and 14 weeks of age, OHC loss was 25 and 45%,
respectively, in all 3 turns (Fig. 1D
and H). The loss of OHCs coincided with increased ABR
thresholds. In the 18-week-old mice, no normal OHCs were visible
(Fig. 1G). In the 10-week-old
mice, evidence of IHC degeneration was observed (Fig. 1H). IHC loss was 9% in the
10-week-old mice, which increased with age.
Ethosuximide treatment protects against
decreased auditory function in NOD/LtJ mice
To investigate whether ethosuximide protects against
hearing loss in NOD/LtJ mice, we conducted hearing tests in 4-, 6-
and 8-week-old ethosuximide-treated and untreated mice
(n=5/group).
ABR thresholds were lower in the
ethosuximide-treated group than in the untreated group at stimulus
frequencies of 8 kHz in the 4-week-old mice (P<0.01), 16 kHz in
the 6-week-old mice (P<0.01), and at click, 8 and 32 kHz in the
8-week-old mice (P<0.05) (Fig.
2A–D). ABR threshold shifts in the 2 groups during the period
(4–8 weeks) of drug application were also measured to determine
whether ethosuximide can alter or attenuate the progression of
hearing loss. The ethosuximide-treated group displayed lower ABR
threshold shifts compared to the untreated group (Fig. 2E).
The mean amplitude of DPOAE was significantly higher
in the ethosuximide-treated group than in the untreated group at
8,844 and 12,503 Hz in the 4-week-old mice (P<0.01), at 4,422,
17,672 and 24,990 Hz in the 6-week-old mice (P<0.05), and at
8,844, 35,344 Hz (P<0.01) and 12,503 Hz (P<0.05) in the
8-week-old mice (Fig. 2G–I). As
DPOAE reflects OHC function, these data suggest that ethosuximide
protects auditory function in NOD/LtJ mice.
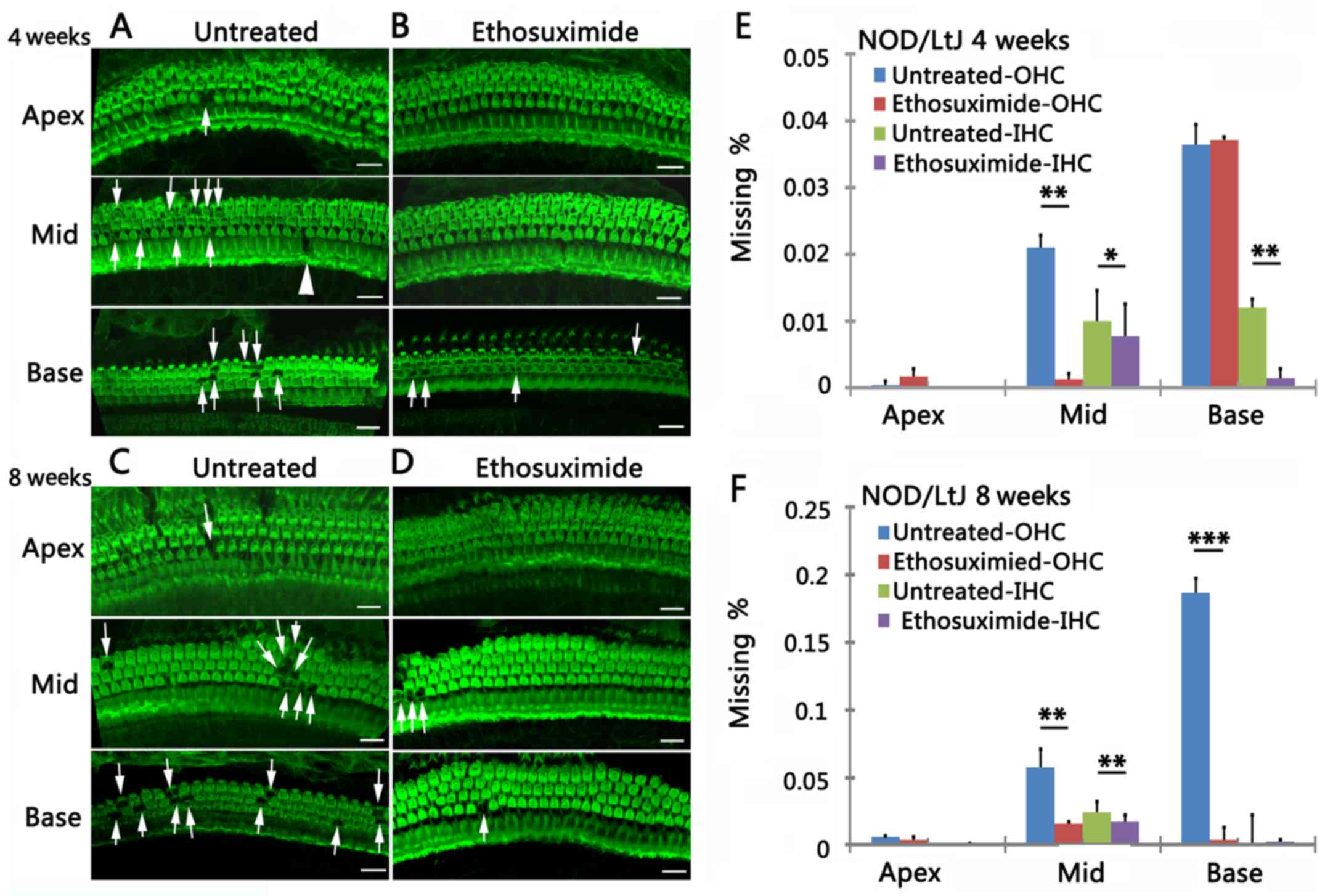
Fluorescence images of the OHCs and IHCs
characterized the progression of hair cell degeneration in the
cochleae in the 4- and 8-week-old untreated mice (Fig. 3A and C). OHC survival was
increased in the ethosuximide-treated group compared to the
untreated group (Fig. 3B and D).
Cytocochleograms indicated that the mean percentage of OHC loss was
significantly lower in the ethosuximide-treated group than in the
untreated group at all 3 cochlear turns. IHC loss was not
substantially altered by ethosuximide treatment (Fig. 3E and F) (n=5/group/time
point).
H&E staining demonstrated that the
ethosuximide-treated group (Fig. 4B
and D) experienced minor SGN and hair cell degeneration in the
basal turn compared to the untreated group (Fig. 4A and C) in the 8-week-old mice
(n=5/group/time point). In the untreated group, the SGNs did not
exhibit apoptotic features in the basal turn until the mice were 8
weeks old (Fig. 4A). At this time
poinr, auditory nerve fibers became loosened and the neurons
exhibited a shrunken cytoplasm, condensed chromatin and pyknosis
(Fig. 4A and E). In the basal
turn, the neuronal population was decreased in the untreated group
compared to the ethosuximide-treated group (Fig. 4G). However, the SGN in the apical
turn was normal at this time point. Cell counting demonstrated that
ethosuximide treatment increased SGN survival (Fig. 4G) in the 8-week-old mice, and
increased OHC survival in both the 4- and 8-week-old mice (Fig. 3E and F).
Ethosuximide treatment was not associated with any
noticeable side-effects in the 4- and 8-week-old mice. There were
no significant differences in body weight in the
ethosuximide-treated mice compared to the untreated mice (Fig. 2F).
Ethosuximide treatment decreases
calpain-mediated apoptosis-related gene expression
We then determined whether the blocking of T-type
Ca2+ channels can downregulate cochlear cell gene
expression. The family of T-type Ca2+ channels is
comprised of 3 main pore-forming α subunits: α1G, α1H and α1I
(21). The mean mRNA expression
levels of the α1G, α1H and α1I subunits were decreased in the
cochleae of the ethosuximide-treated 8-week-old NOD/LtJ mice; a
significant difference was observed between the
ethosuximide-treated group and the untreated group for the α1H and
α1I genes (P<0.01) (Fig.
5A).
There are 2 major intracellular apoptotic pathways.
Extracellular stimuli can initiate an extrinsic pathway (caspase-8)
that activates the caspase family. Alternatively, the intrinsic
pathway involves intracellular calpain, which ultimately triggers
apoptosis by activating caspase-3 (Fig. 6) (22,23). Calpains (m-calpain and μ-calpain)
are activated by Ca2+(24). Our results suggested that
ethosuximide treatment downregulated the intrinsic apoptotic
pathway (Fig. 5B and C).
Discussion
This study included NOD/LtJ mice with a Cdh23
and Ahl2 background, and this strain experienced progressive
hearing loss starting at approximately 3 weeks after birth. AHL
began in the high frequency region and progressed to the low
frequency region with increasing age. The phenotype is similar to
that observed in some humans with presbycusis, in which the basal
turn of the cochlea first degenerates (25). These functional deficits were
accompanied by the loss of hair cells and SGNs that began at the
base of the cochlea, which spread toward the apex. NOD/LtJ mice
were deaf by 14 weeks of age.
AHL is characterized by the widespread structural
and functional degeneration of hair cells and SGNs in the cochleae
(26). Hair cells are responsible
for collecting acoustic signals and transducing them to SGNs. SGNs
transform acoustic signals into nerve impulses that are received by
the auditory cortex. Finally, information is interpreted in the
brain (27). Hair cells promote
neurotrophin release; and 93% of SGNs innervate IHCs (28), which suggests that the
degeneration of SGNs may be a secondary phenomenon associated with
the loss of IHCs. However, in NOD/LtJ mice, SGN degeneration
occurred earlier than the loss of IHCs, suggesting the occurrence
of early-stage SGN degeneration, which is independent of the
age-related loss of hair cells.
As NOD/LtJ mice exhibit rapid and progressive
hearing loss, associated genetic mechanisms may provide new insight
into the development of AHL and may aid in prevention and
treatment. AHL is a genetically complex quantitative trait in
common inbred mouse strains, including NOD/LtJ mice. Based on our
observations, auditory functional deficiencies occurred before
cochlear cell degeneration in the NOD/LtJ mice. We hypothesize that
synergistic interactions of Cdh23 and Ahl2 variants
are responsible for the AHL phenotype. Interactions between
Cdh23 and Ahl2 have not been characterized, as
Ahl2 has not been identified at the gene level. In NOD/LtJ
mice, exon 7 in Cdh23 has a single nucleotide polymorphism
that displays an important association with AHL. AHL may be caused
by homozygosity in Cdh23 (753A) in association with the
effects of heterogeneous secondary factors (7). Cadherins mediate
Ca2+-dependent cell-cell adhesion, migration and
compaction (29). CDH23 is
encoded by the Cdh23 gene, which is a transmembrane protein
and a component of the tip-link in hair cells in the organ of Corti
(29–31). CDH23 is involved in regulating the
activity of mechanically gated ion channels in hair cells (6,32,33). Evidence suggests that
Ca2+-dependent cell-cell adhesion deficiency
significantly influences cochlear cell integrity (34).
Population-based studies have linked the use of
Ca2+ channel blockers to improved hearing thresholds in
elderly females. T-type Ca2+ channels are voltage-gated
Ca2+ channels (VGCCs) that can regulate intracellular
Ca2+ levels (35–38). In this study, we demonstrated that
a blocker of T-type Ca2+ channels, ethosuximide,
significantly reduced the increase in age-related ABR thresholds,
as well as OHC and SGN loss, in NOD/LtJ mice; this most likely
mediated through the effects of the downstream apoptotic pathway.
Ethosuximide is a relatively selective inhibitor of T-type
Ca2+ channels. In accordance with our findings, it has
been demonstrated that the blocking of T-type Ca2+
channels with ethosuximide can prevent epileptogenesis (39). In a study on glutamate-induced
injury in stroke, the blocking of Ca2+ channels
effectively prevented Ca2+ entry and delayed neuronal
death (40). In addition, as
shown in another study, the protective effect of hydrogen sulfide
on colonic mucosa relied on T-type Ca2+
channel-dependent neuronal excitation in rats (41). In a noise-induced C57BL/6J mouse
model, ethosuximide delayed age-related SGN loss and preserved ABR
thresholds (42). However,
Ca2+ was not the sole mediator in ferutinin-mediated
eryptosis/erythroptosis, as the wastage of external Ca2+
did not prevent the apoptotic effect of ferutinin (43).
The mechanisms responsible for AHL in NOD/LtJ mice
include structural alterations of the tip-links in hair cells and
cochlear cell apoptosis. CDH23 protein is located near the
Ca2+-binding site. We speculate that Ca2+ may
influence the efficiency of CDH23 interactions with proteins,
affect Ca2+-dependent cell adhesions, and ultimately
lead to apoptosis in NOD/LtJ mice. Thus we suggest that blocking
the Ca2+ channel with ethosuximide may inhibit the
calpain-mediated (intrinsic) apoptotic pathway that activates the
caspase family and a cascade that leads to cell death. This is
supported by data indicating that leupeptin, which is a calpain
inhibitor, protects the cochlear and vestibular hair cells against
gentamicin (GM) ototoxicity (44). Furthermore, conventional
Ca2+ entry antagonists prevent neuronal death when
administered before and during the injury phase of glutamate
excitotoxicity (45,46). However, following excitotoxic
insults, these Ca2+ entry antagonists are no longer
effective. Thus, our results demonstrate that the prophylactic use
of ethosuximide may prevent neuronal death.
The debate of determining which component of the
cochlea is involved in the aging process and which part of the
inner ear is involved if affected by ethosuximide continues. It
should be noted that the elevation of hearing thresholds in AHL
detected by traditional audiograms is primarily based on OHC and
not SGNs. Further studies are required in order to further
understand the subtle structural changes in AHL and also following
the application of ethosuximide.
In conclusion, the NOD/LtJ mouse provides a valuable
model for the study of AHL-associated genetic mechanisms. In
particular, the role of cadherins in the tip-link of hair cells can
be investigated. The effects of mutation in Ahl2 remain
unclear, and warrant further investigation. This study confirmed
that blocking T-type Ca2+ channels plays a crucial role
in the maintenance of cochlear cells. Additional research is
required in order to explore the clinical significance of
ethosuximide in AHL in humans.
Abbreviations:
|
AHL
|
age-related hearing loss
|
|
ABR
|
auditory-evoked brainstem response
|
|
DPOAE
|
distortion product oto-acoustic
emission
|
|
OHC
|
outer hair cell
|
|
IHC
|
inner hair cell
|
|
SGN
|
spiral ganglion neuron
|
Acknowledgments
We would like to thank Fengchan Han, Qingzhu Wang
and Hui Wang for breeding the NOD/LtJ mice. The present study was
supported by grants from National Natural Science Foundation of
China (no. 81271085, 81530030 and 81500797), the Natural Science
Foundation of Shandong Province (ZR2012HL30, ZR2012HL31 and
ZR2014HL050), the Foundation of Taishan Scholar.
References
|
1
|
Gorlin RJ, Toriello HV and Cohen M:
Hereditary Hearing Loss and Its Syndrome. Oxford University Press;
1995
|
|
2
|
Morton NE: Genetic epidemiology of hearing
impairment. Ann NY Acad Sci. 630:16–31. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mulrow CD, Aguilar C, Endicott JE, Velez
R, Tuley MR, Charlip WS, Hill Mulrow CD, Aguilar C, Endicott JE,
Velez R, Tuley MR, Charlip WS and Hill JA: JA: Association between
hearing impairment and the quality of life of elderly individuals.
J Am Geriatr Soc. 38:45–50. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin FR, Niparko JK and Ferrucci L: Hearing
loss prevalence in the United States. Arch Intern Med.
171:1851–1852. 2001. View Article : Google Scholar
|
|
5
|
Appler JM and Goodrich LV: Connecting the
ear to the brain: Molecular mechanisms of auditory circuit
assembly. Prog Neurobiol. 93:488–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson KR and Zheng QY: Ahl2, a second
locus affecting age-related hearing loss in mice. Genomics.
80:461–464. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noben-Trauth K, Zheng QY and Johnson KR:
Association of cadherin 23 with polygenic inheritance and genetic
modification of sensorineural hearing loss. Nat Genet. 35:21–23.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mills JH, Matthews LJ, Lee FS, Dubno JR,
Schulte BA and Weber PC: Gender-specific effects of drugs on
hearing levels of older persons. Ann NY Acad Sci. 884:381–388.
1999. View Article : Google Scholar
|
|
9
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar
|
|
10
|
Someya S, Yamasoba T, Weindruch R, Prolla
TA and Tanokura M: Caloric restriction suppresses apoptotic cell
death in the mammalian cochlea and leads to prevention of
presbycusis. Neurobiol Aging. 28:1613–1622. 2007. View Article : Google Scholar
|
|
11
|
Someya S, Yamasoba T, Kujoth GC, Pugh TD,
Weindruch R, Tanokura M and Prolla TA: The role of mtDNA mutations
in the pathogenesis of age-related hearing loss in mice carrying a
mutator DNA polymerase gamma. Neurobiol Aging. 29:1080–1092. 2008.
View Article : Google Scholar
|
|
12
|
Rogawski MA and Löscher W: The
neurobiology of antiepileptic drugs for the treatment of
nonepileptic conditions. Nat Med. 10:685–692. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han F, Yu H, Tian C, Chen HE,
Benedict-Alderfer C, Zheng Y, Wang Q, Han X and Zheng QY: A new
mouse mutant of the Cdh23 gene with early-onset hearing loss
facilitates evaluation of otoprotection drugs. Pharmacogenomics J.
12:30–44. 2012. View Article : Google Scholar
|
|
14
|
Kusakawa G, Saito T, Onuki R, Ishiguro K,
Kishimoto T and Hisanaga S: Calpain-dependent proteolytic cleavage
of the p35 cyclin-dependent kinase 5 activator to p25. J Biol Chem.
275:17166–17172. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakagawa T and Yuan J: Cross-talk between
two cysteine protease families. Activation of caspase-12 by calpain
in apoptosis. J Cell Biol. 150:887–894. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han F, Yu H, Zheng T, Ma X, Zhao X, Li P,
Le L, Su Y and Zheng QY: Otoprotective effects of erythropoietin on
Cdh23erl/erl mice. Neuroscience. 237:1–6. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnson KR, Erway LC, Cook SA, Willott JF
and Zheng QY: A major gene affecting age-related hearing loss in
C57BL/6J mice. Hear Res. 114:83–92. 1997. View Article : Google Scholar
|
|
18
|
Zheng QY, Johnson KR and Erway LC:
Assessment of hearing in 80 inbred strains of mice by ABR threshold
analyses. Hear Res. 130:94–107. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Polak M, Eshraghi AA, Nehme O, Ahsan S,
Guzman J, Delgado RE, He J, Telischi FF, Balkany TJ and Van De
Water TR: Evaluation of hearing and auditory nerve function by
combining ABR, DPOAE and eABR tests into a single recording
session. J Neurosci Methods. 134:141–149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
21
|
Perez-Reyes E, Cribbs LL, Daud A, Lacerda
AE, Barclay J, Williamson MP, Fox M, Rees M and Lee JH: Molecular
characterization of a neuronal low-voltage-activated T-type calcium
channel. Nature. 391:896–900. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Creagh EM, Conroy H and Martin SJ:
Caspase-activation pathways in apoptosis and immunity. Immunol Rev.
193:10–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin XM: Signal transduction mediated by
Bid, a pro-death Bcl-2 family proteins, connects the death receptor
and mitochondria apoptosis pathways. Cell Res. 10:161–167. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Azam M, Andrabi SS, Sahr KE, Kamath L,
Kuliopulos A and Chishti AH: Disruption of the mouse mu-calpain
gene reveals an essential role in platelet function. Mol Cell Biol.
21:2213–2220. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schuknecht HF and Gacek MR: Cochlear
pathology in presbycusis. Ann Otol Rhinol Laryngol. 102:1–16. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Keithley EM, Canto C, Zheng QY,
Fischel-Ghodsian N and Johnson KR: Age-related hearing loss and the
ahl locus in mice. Hear Res. 188:21–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hudspeth AJ: How hearing happens. Neuron.
19:947–950. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ehret G: Quantitative analysis of nerve
fibre densities in the cochlea of the house mouse (Mus musculus). J
Comp Neurol. 183:73–88. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Di Palma F, Pellegrino R and Noben-Trauth
K: Genomic structure, alternative splice forms and normal and
mutant alleles of cadherin 23 (Cdh23). Gene. 281:31–41. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Di Palma F, Holme RH, Bryda EC,
Belyantseva IA, Pellegrino R, Kachar B, Steel KP and Noben-Trauth
K: Mutations in Cdh23, encoding a new type of cadherin, cause
stereocilia disorganization in waltzer, the mouse model for Usher
syndrome type 1D. Nat Genet. 27:103–107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siemens J, Lillo C, Dumont RA, Reynolds A,
Williams DS, Gillespie PG and Müller U: Cadherin 23 is a component
of the tip link in hair-cell stereocilia. Nature. 428:950–955.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Howard J and Hudspeth AJ: Compliance of
the hair bundle associated with gating of mechanoelectrical
transduction channels in the bullfrog's saccular hair cell. Neuron.
1:189–199. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jia S, Dallos P and He DZ: Mechanoelectric
transduction of adult inner hair cells. J Neurosci. 27:1006–1014.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chan DK, Lieberman DM, Musatov S, Goldfein
JA, Selesnick SH and Kaplitt MG: Protection against
cisplatin-induced ototoxicity by adeno-associated virus-mediated
delivery of the X-linked inhibitor of apoptosis protein is not
dependent on caspase inhibition. Otol Neurotol. 28:417–425. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee S, Briklin O, Hiel H and Fuchs P:
Calcium-dependent inactivation of calcium channels in cochlear hair
cells of the chicken. J Physiol. 583:909–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lopez I, Ishiyama G, Acuna D, Ishiyama A
and Baloh RW: Immunolocalization of voltage-gated calcium channel
alpha1 subunits in the chinchilla cochlea. Cell Tissue Res.
313:177–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nie L, Zhu J, Gratton MA, Liao A, Mu KJ,
Nonner W, Richardson GP and Yamoah EN: Molecular identity and
functional properties of a novel T-type Ca2+ channel
cloned from the sensory epithelia of the mouse inner ear. J
Neurophysiol. 100:2287–2299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shen H, Zhang B, Shin JH, Lei D, Du Y, Gao
X, Wang Q, Ohlemiller KK, Piccirillo J and Bao J: Prophylactic and
therapeutic functions of T-type calcium blockers against
noise-induced hearing loss. Hear Res. 226:52–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Becker AJ, Pitsch J, Sochivko D, Opitz T,
Staniek M, Chen CC, Campbell KP, Schoch S, Yaari Y and Beck H:
Transcriptional upregulation of Cav3.2 mediates epileptogenesis in
the pilocarpine model of epilepsy. J Neurosci. 28:13341–13353.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deshpande LS, Limbrick DD Jr, Sombati S
and DeLorenzo RJ: Activation of a novel injury-induced
calcium-permeable channel that plays a key role in causing extended
neuronal depolarization and initiating neuronal death in
excitotoxic neuronal injury. J Pharmacol Exp Ther. 322:443–452.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Matsunami M, Kirishi S, Okui T and
Kawabata A: Hydrogen sulfide-induced colonic mucosal cytoprotection
involves T-type calcium channel-dependent neuronal excitation in
rats. J Physiol Pharmacol. 63:61–68. 2012.PubMed/NCBI
|
|
42
|
Lei D, Gao X, Perez P, Ohlemiller KK, Chen
CC, Campbell KP, Hood AY and Bao J: Anti-epileptic drugs delay
age-related loss of spiral ganglion neurons via T-type calcium
channel. Hear Res. 278:106–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gao M, Wong SY, Lau PM and Kong SK:
Ferutinin induces in vitro eryptosis/erythroptosis in human
erythrocytes through membrane permeabilization and calcium influx.
Chem Res Toxicol. 26:1218–1228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ding D, Stracher A and Salvi RJ: Leupeptin
protects cochlear and vestibular hair cells from gentamicin
ototoxicity. Hear Res. 164:115–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Coulter DA, Sombati S and DeLorenzo RJ:
Electrophysiology of glutamate neurotoxicity in vitro: Induction of
a calcium-dependent extended neuronal depolarization. J
Neurophysiol. 68:362–373. 1992.PubMed/NCBI
|
|
46
|
Limbrick DD Jr, Pal S and DeLorenzo RJ:
Hippocampal neurons exhibit both persistent Ca2+ influx
and impairment of Ca2+ sequestration/extrusion
mechanisms following excitotoxic glutamate exposure. Brain Res.
894:56–67. 2001. View Article : Google Scholar : PubMed/NCBI
|