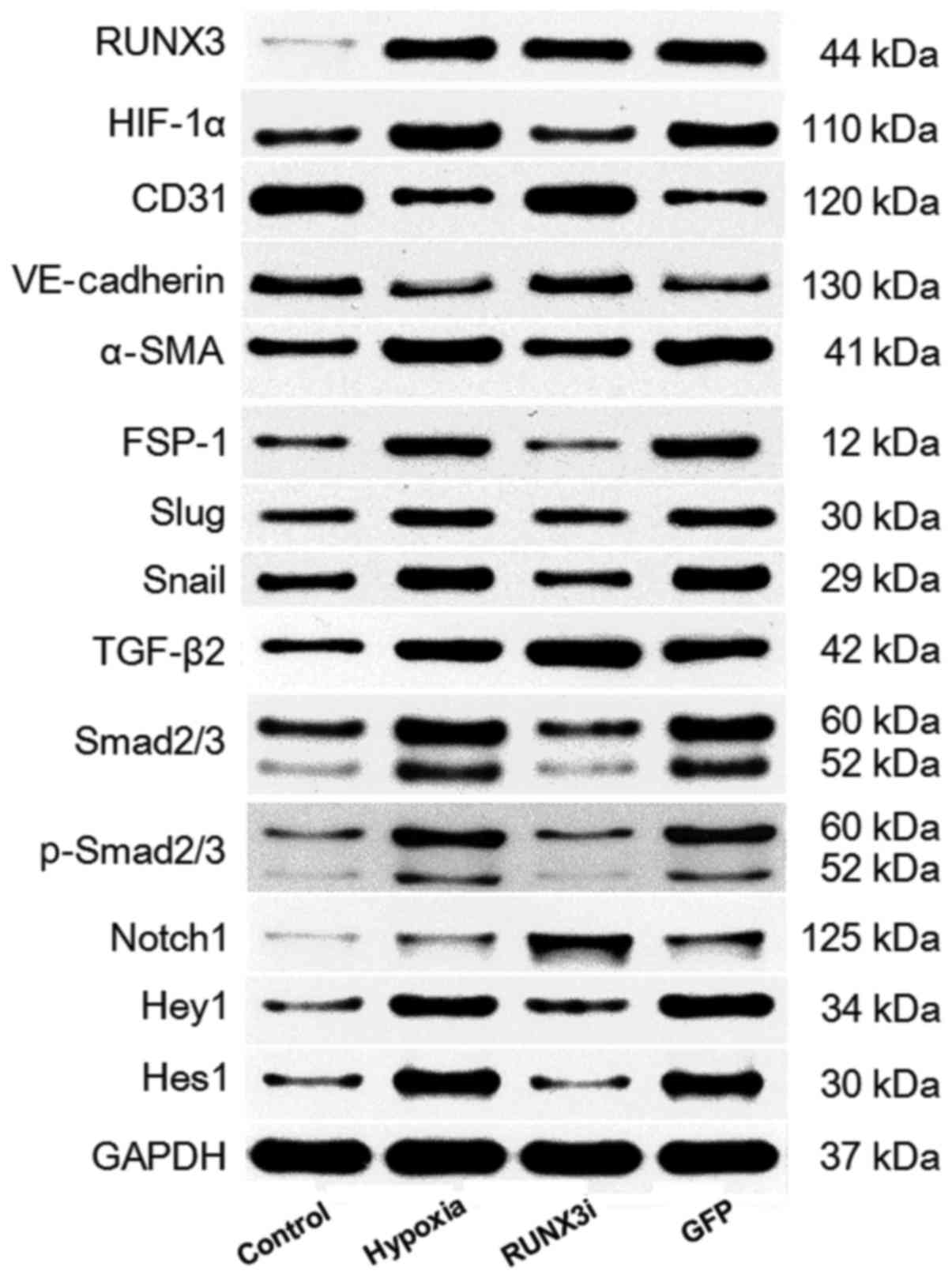
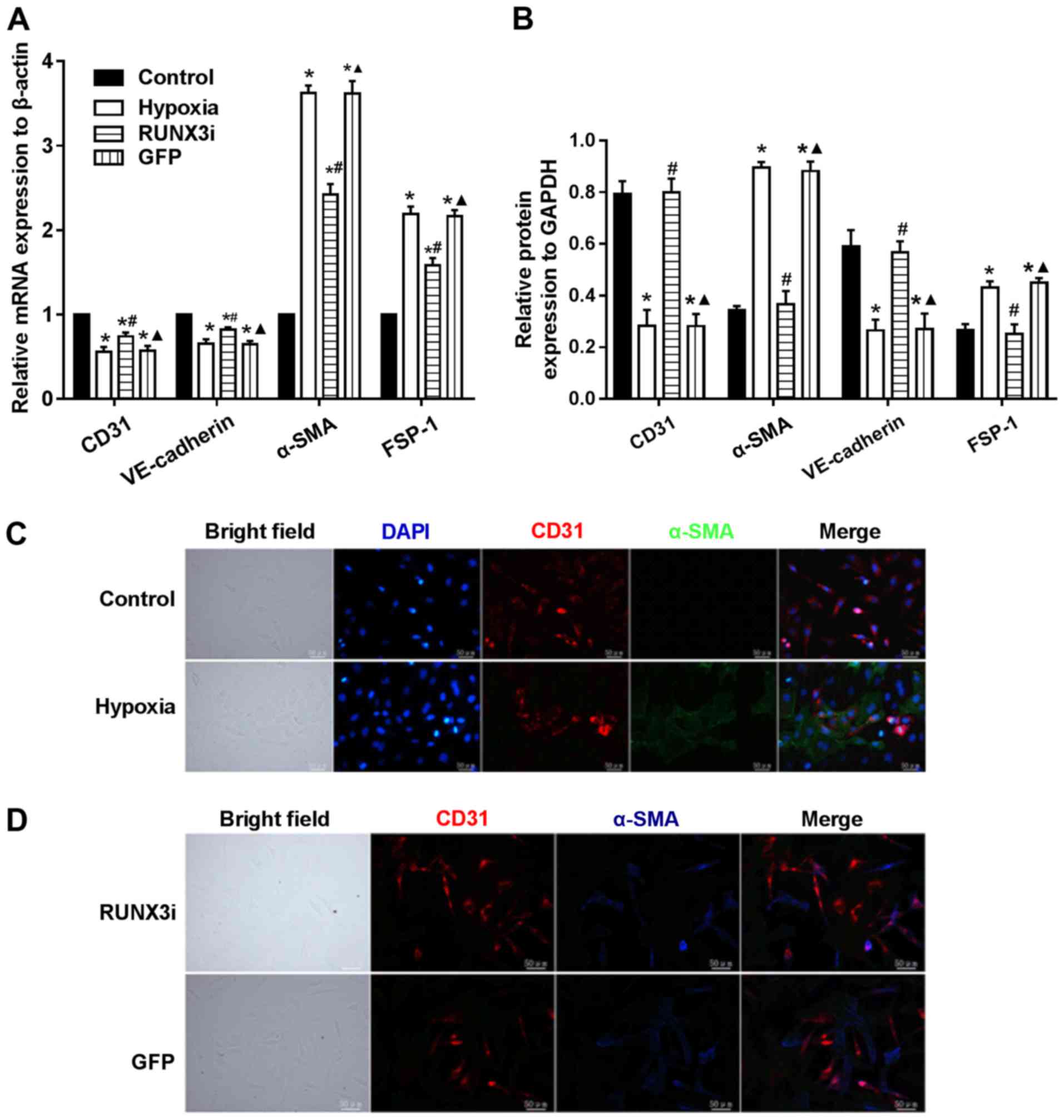
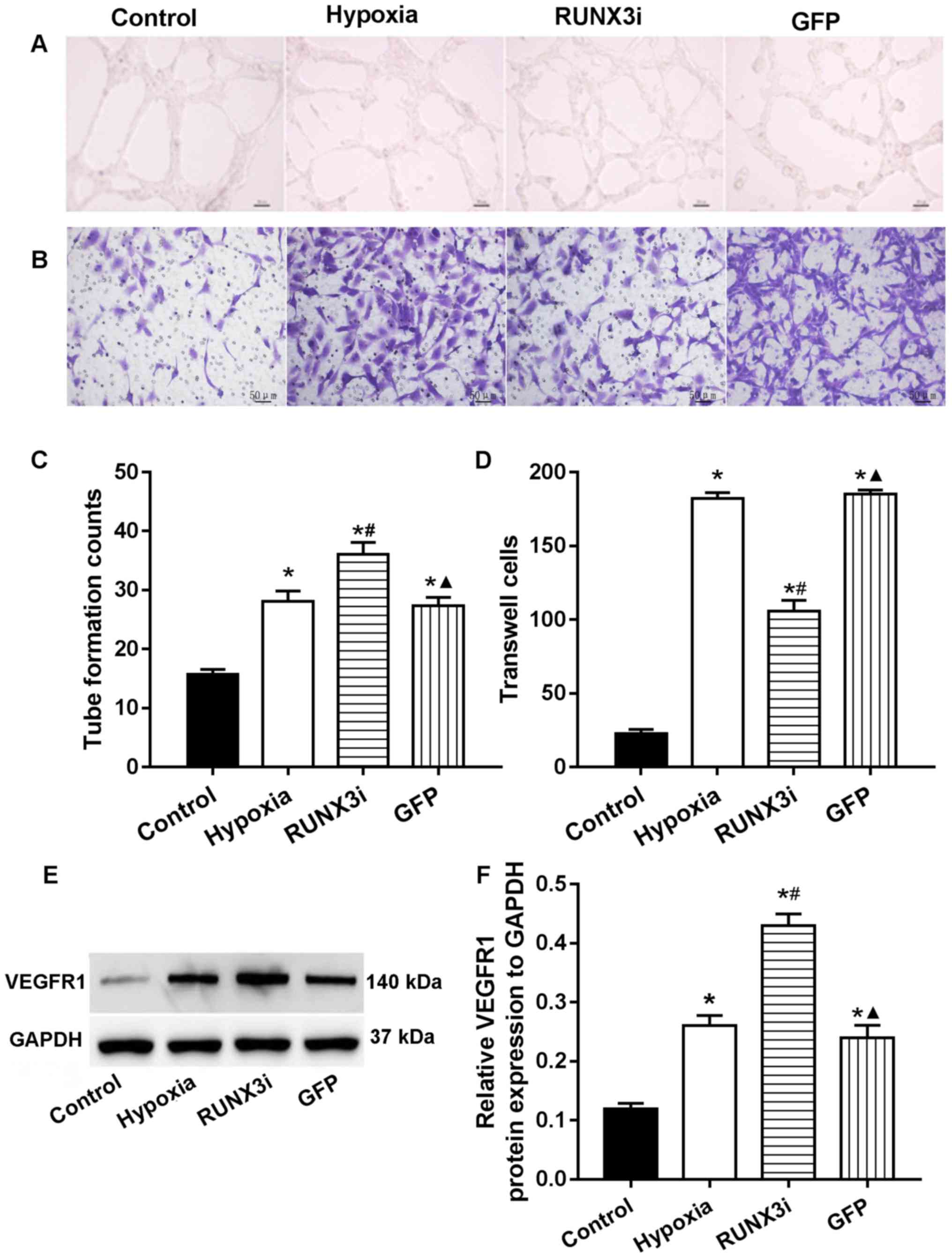
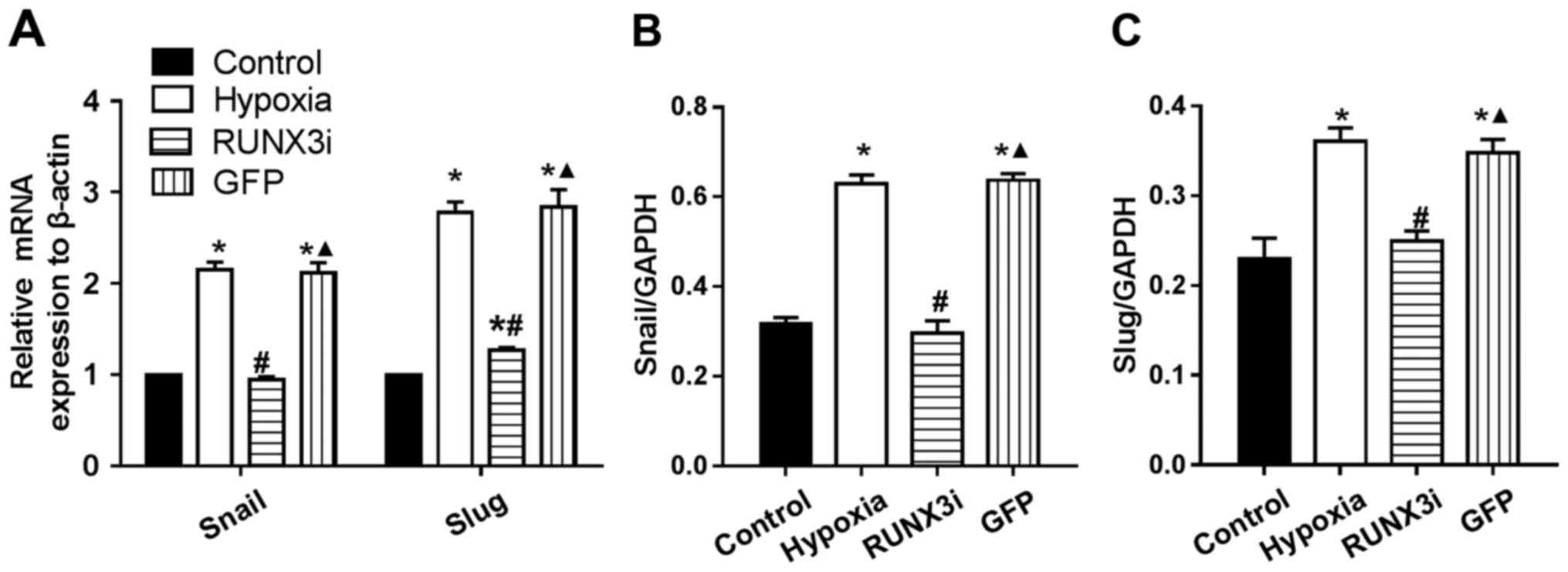
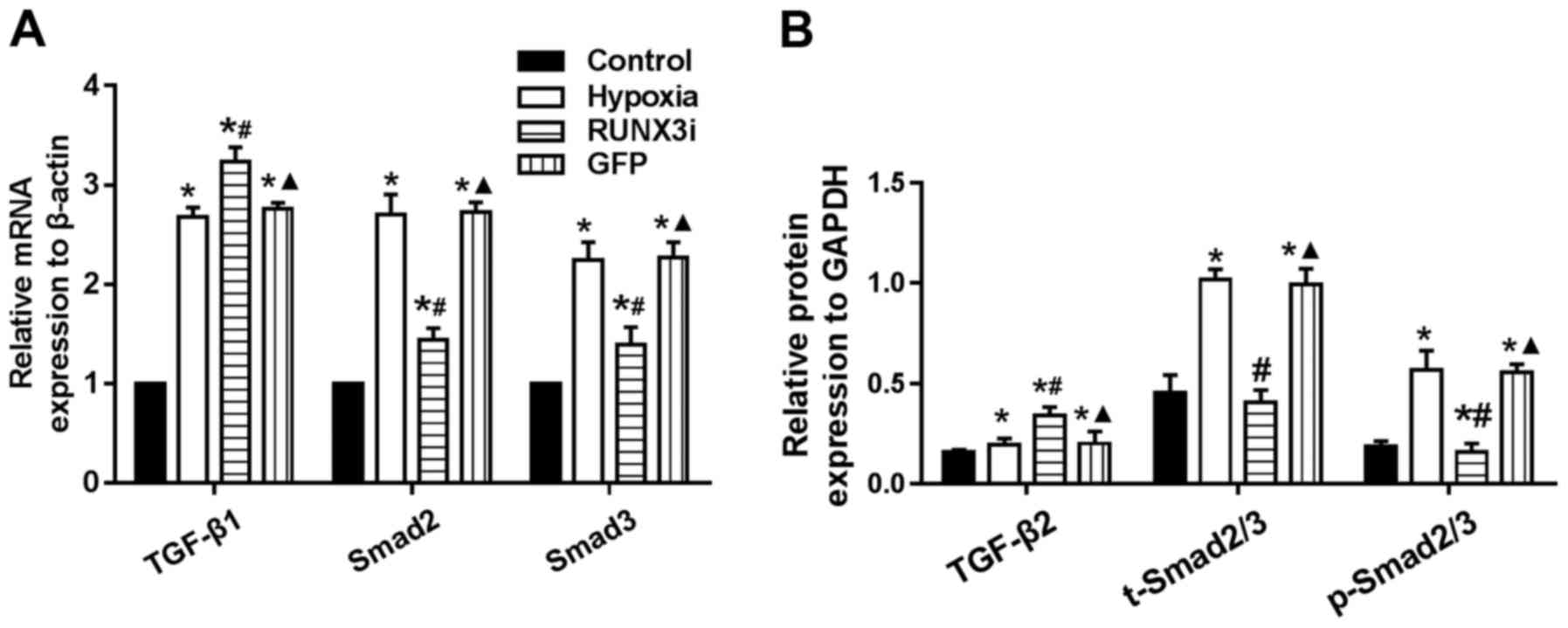
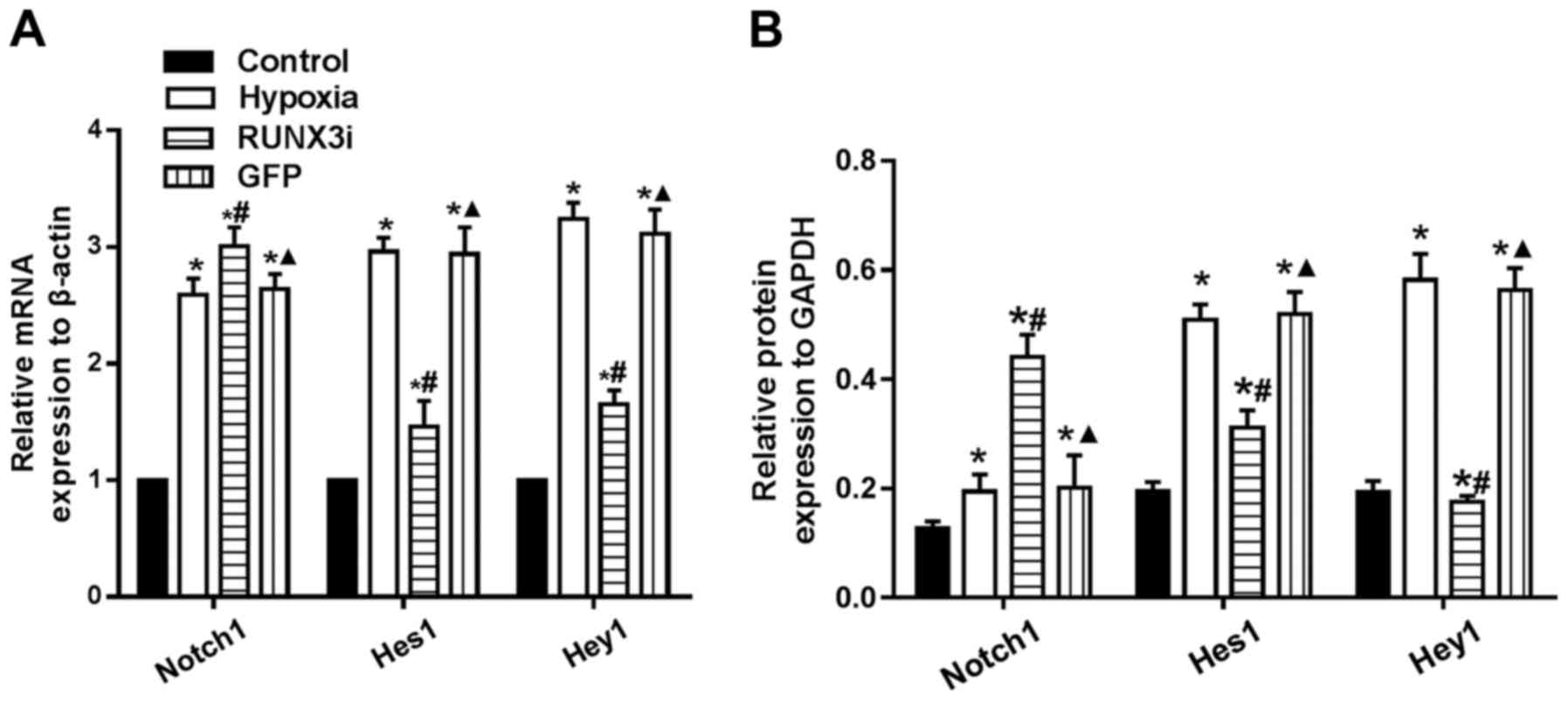
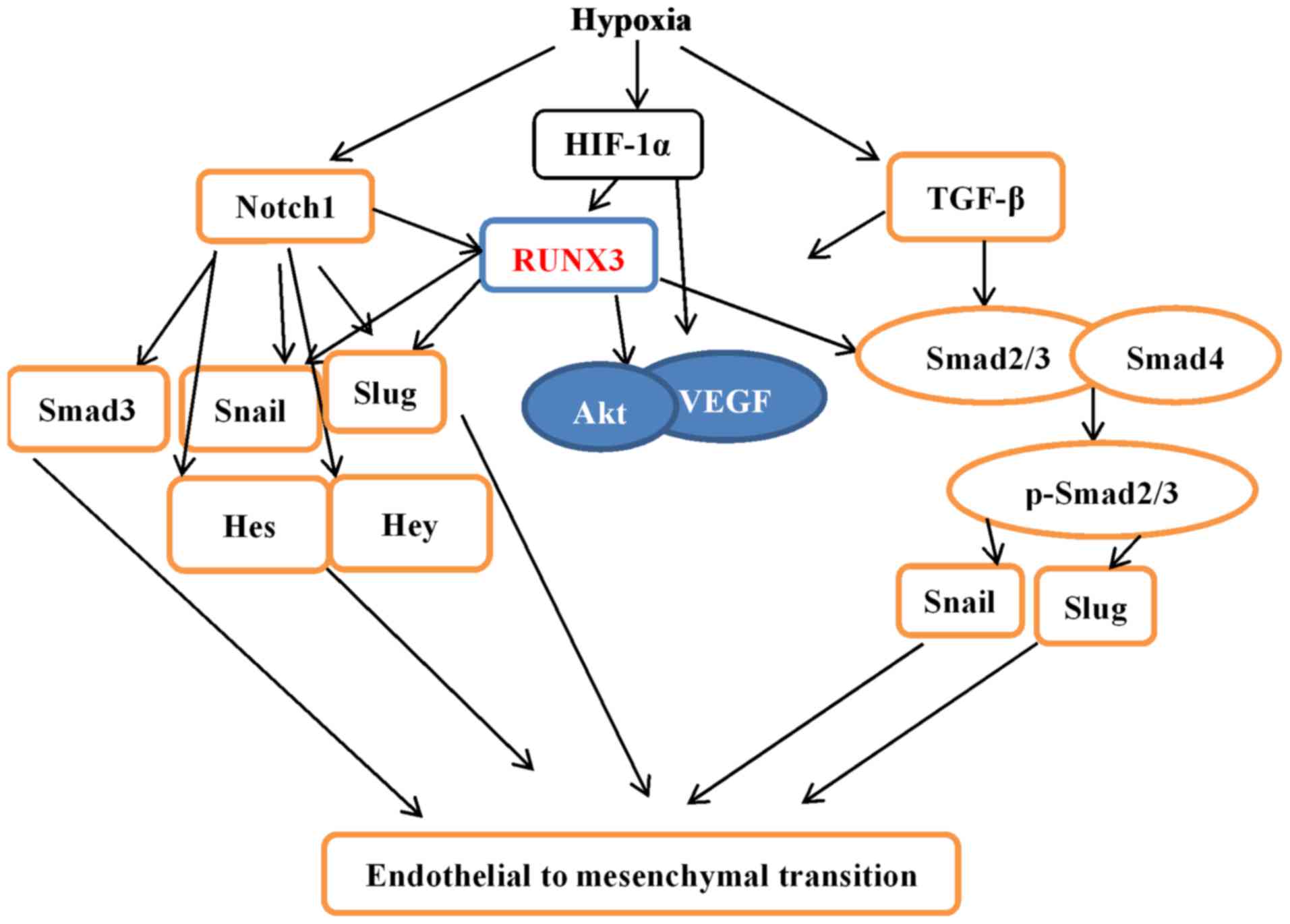
|
1
|
Chen W, Gao R, Liu L, Zhu M, Wang W, Wang
Y, Wu Z, Li H, Zheng Z, Jiang L and Hu S: Outline of the report on
cardiovascular disease in China 2014. Chin Circ J. 30:617–622.
2015.In Chinese.
|
|
2
|
Zeisberg EM, Tarnavski O, Zeisberg M,
Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT,
Roberts AB, et al: Endothelial-to-mesenchymal transition
contributes to cardiac fibrosis. Nat Med. 13:952–961. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeisberg EM and Kalluri R: Origins of
cardiac fibroblasts. Circ Res. 107:1304–1312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oka T, Akazawa H, Naito AT and Komuro I:
Angiogenesis and cardiac hypertrophy: maintenance of cardiac
function and causative roles in heart failure. Circ Res.
114:565–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kovacic JC, Mercader N, Torres M, Boehm M
and Fuster V: Epithelial-to-mesenchymal and
endothelial-to-mesenchymal transition: from cardiovascular
development to disease. Circulation. 125:1795–1808. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ranchoux B, Antigny F, Rucker-Martin C,
Hautefort A, Péchoux C, Bogaard HJ, Dorfmüller P, Remy S, Lecerf F,
Planté S, et al: Endothelial-to-mesenchymal transition in pulmonary
hypertension. Circulation. 131:1006–1018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Richards J, El-Hamamsy I, Chen S, Sarang
Z, Sarathchandra P, Yacoub MH, Chester AH and Butcher JT:
Side-specific endothelial-dependent regulation of aortic valve
calcification: interplay of hemodynamics and nitric oxide
signaling. Am J Pathol. 182:1922–1931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao Y, Jumabay M, Ly A, Radparvar M,
Cubberly MR and Boström KI: A role for the endothelium in vascular
calcification. Circ Res. 113:495–504. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hashimoto N, Phan SH, Imaizumi K, Matsuo
M, Nakashima H, Kawabe T, Shimokata K and Hasegawa Y:
Endothelial-mesenchymal transition in bleomycin-induced pulmonary
fibrosis. Am J Respir Cell Mol Biol. 43:161–172. 2010. View Article : Google Scholar
|
|
10
|
Liu J, Dong F, Jeong J, Masuda T and Lobe
CG: Constitutively active Notch1 signaling promotes endothelial
mesenchymal transition in a conditional transgenic mouse model. Int
J Mol Med. 34:669–676. 2014.PubMed/NCBI
|
|
11
|
Aisagbonhi O, Rai M, Ryzhov S, Atria N,
Feoktistov I and Hatzopoulos AK: Experimental myocardial infarction
triggers canonical Wnt signaling and endothelial-to-mesenchymal
transition. Dis Model Mech. 4:469–483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu X, Tan X, Tampe B, Sanchez E, Zeisberg
M and Zeisberg EM: Snail is a direct target of hypoxia-inducible
factor 1α (HIF1α) in hypoxia-induced endothelial to mesenchymal
transition of human coronary endothelial cells. J Biol Chem.
290:16653–16664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng Z, Wei D, Wang L, Tang H, Zhang J, Le
X, Jia Z, Li Q and Xie K: RUNX3 inhibits the expression of vascular
endothelial growth factor and reduces the angiogenesis, growth, and
metastasis of human gastric cancer. Clin Cancer Res. 12:6386–6394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu Y, Chang AC, Fournier M, Chang L,
Niessen K and Karsan A: RUNX3 maintains the mesenchymal phenotype
after termination of the Notch signal. J Biol Chem.
286:11803–11813. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen F, Liu X, Bai J, Pei D and Zheng J:
The emerging role of RUNX3 in cancer metastasis (Review). Oncol
Rep. 35:1227–1236. 2016.
|
|
16
|
Xu Q, Meng S, Liu B, Li MQ, Li Y, Fang L
and Li YG: Micro-RNA-130a regulates autophagy of endothelial
progenitor cells through Runx3. Clin Exp Pharmacol Physiol.
41:351–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meng S, Cao J, Zhang X, Fan Y, Fang L,
Wang C, Lv Z, Fu D and Li Y: Downregulation of microRNA-130a
contributes to endothelial progenitor cell dysfunction in diabetic
patients via its target Runx3. PLoS One. 8:e686112013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Li B, Zheng Z, Kang T, Zeng M,
Liu Y and Xia B: Protective effects of Notch1 signaling activation
against high glucose-induced myocardial cell injury: analysis of
its mechanisms of action. Int J Mol Med. 36:897–903.
2015.PubMed/NCBI
|
|
19
|
Ma FX, Zhou B, Chen Z, Ren Q, Lu SH,
Sawamura T and Han ZC: Oxidized low density lipoprotein impairs
endothelial progenitor cells by regulation of endothelial nitric
oxide synthase. J Lipid Res. 47:1227–1237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu X, Tan X, Hulshoff MS, Wilhelmi T,
Zeisberg M and Zeisberg EM: Hypoxia-induced endothelial-mesenchymal
transition is associated with RASAL1 promoter hypermethylation in
human coronary endothelial cells. FEBS Lett. 590:1222–1233. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SW, Won JY, Kim WJ, Lee J, Kim KH,
Youn SW, Kim JY, Lee EJ, Kim YJ, Kim KW, et al: Snail as a
potential target molecule in cardiac fibrosis: paracrine action of
endothelial cells on fibroblasts through snail and CTGF axis. Mol
Ther. 21:1767–1777. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Frías A, Lambies G, Viñas-Castells R,
Martínez-Guillamon C, Dave N, García de Herreros A and Díaz VM: A
switch in Akt isoforms is required for Notch-induced Snail1
expression and protection from cell death. Mol Cell Biol.
36:923–940. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watson CJ, Collier P, Tea I, Neary R,
Watson JA, Robinson C, Phelan D, Ledwidge MT, McDonald KM, McCann
A, et al: Hypoxia-induced epigenetic modifications are associated
with cardiac tissue fibrosis and the development of a
myofibroblast-like phenotype. Hum Mol Genet. 23:2176–2188. 2014.
View Article : Google Scholar
|
|
25
|
Zheng Z, Zhu L, Zhang X, Li L, Moon S, Roh
MR and Jin Z: RUNX3 expression is associated with sensitivity to
pheophorbide a-based photodynamic therapy in keloids. Lasers Med
Sci. 30:67–75. 2015. View Article : Google Scholar
|
|
26
|
Tang RN, Lv LL, Zhang JD, Dai HY, Li Q,
Zheng M, Ni J, Ma KL and Liu BC: Effects of angiotensin II receptor
blocker on myocardial endothelial-to-mesenchymal transition in
diabetic rats. Int J Cardiol. 162:92–99. 2013. View Article : Google Scholar
|
|
27
|
Zhou X, Chen X, Cai JJ, Chen LZ, Gong YS,
Wang LX, Gao Z, Zhang HQ, Huang WJ and Zhou H: Relaxin inhibits
cardiac fibrosis and endothelial-mesenchymal transition via the
Notch pathway. Drug Des Devel Ther. 9:4599–4611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vasconcelos RC, Costa AL, Freitas RA,
Bezerra BA, Santos BR, Pinto LP and Gurgel BC: Immunoexpression of
HIF-1α and VEGF in periodontal disease and healthy gingival
tissues. Braz Dent J. 27:117–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garside VC, Chang AC, Karsan A and
Hoodless PA: Co-ordinating Notch, BMP, and TGF-β signaling during
heart valve development. Cell Mol Life Sci. 70:2899–2917. 2013.
View Article : Google Scholar
|
|
30
|
Niessen K and Karsan A: Notch signaling in
cardiac development. Circ Res. 102:1169–1181. 2008. View Article : Google Scholar : PubMed/NCBI
|