Introduction
Chondrocytes, cells in articular cartilage, can
support mechanical loads and regulate their metabolic activities in
response to mechanical loading. Chondrocytes are the only cells in
cartilage and are responsible for maintaining and modeling
cartilage through a homeostatic balance of anabolic and catabolic
activities (1). Under abnormal
loading conditions, such as obesity, trauma, or joint instability,
mechanical factors play a critical role in the onset and
progression of osteoarthritis (OA) (2). However, the mechanisms determining
how OA-derived chondrocytes sense and transduce mechanical signals
inducing their apoptosis remain unclear.
Recently, a novel mechanically activated (MA) cation
channel named 'Piezo' was identified by Coste et al
(3,4), which is an evolutionarily conserved
ion channel family of cation-permeable proteins involved in
mechano-transduction. In Drosophila, the dPiezo protein was
found to be a mechano-transducer in mechanical nociception
(5). The hPiezo protein has also
been reported to be a key player in cellular response to mechanical
stimuli in human erythrocyte membranes and bladder urothelium, and
the mutation of the hPiezo protein was found to be related to human
anemia, hereditary xerocytosis and distal arthrogryposis type 5
(6–12). hPiezo1 and hPiezo2 were identified
as proteins involved in mechanosensation ion channels which have
the ability to sense mechanical signals and maintain cell volume
homeostasis. Studies have also shown that mechanical stimuli such
as fluid shear stress, which causes lower expression of Bcl-2,
leads to the apoptosis of OA-derived chondrocytes (13–15). Chondrocytes are mechanosensation
cells, thus Piezo1 may play an important role in the apoptosis of
human OA-derived chondrocytes.
The endoplasmic reticulum (ER) is one type of
organelle which plays an essential role in multiple cellular
processes that are required for cell adaptation, apoptosis, and
other cellular functions (16).
Notwithstanding, ER stress-induced apoptosis of OA-derived
chondrocytes in patients with OA still remains incompletely
understood. Caspases are cysteinyl aspartate-specific proteases
that play a pivotal role not only in the inflammatory responses
against microbial infection but also in the induction of apoptotic
cell death. During these processes, caspase-12 can dampen the
responses to bacterial infection, inhibit IL-1 and trigger
pyroptosis. However, evidence is limited to prove that caspase-12
can induce the apoptosis of OA-derived chondrocytes in OA patients
by mechanic stress.
B cell lymphoma/leukemia-2 (Bcl-2), Bcl-associated X
protein (Bax) and Bcl-2-associated death promoter (BAD) serve as
the apoptosis cascade, which is closely related to the apoptosis of
cells (17–21). Bcl-2 is an anti-apoptosis
signaling factor, which promotes cell proliferation and inhibits
apoptosis through many complex pathways (17). However, BAD is an important
apopotosis factor, whose homology with Bcl-2 is restricted by BH1
and BH2 domains (21). BAD can be
activated by Bcl-xL leading to cell apoptosis by suppressing the
Bcl-2 family, which acts in the function of Bax. In this study, the
expression levels of Bcl-2, Bax and BAD were detected by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR), in
order to explore the connection between mechanical stress-induced
apoptosis and the Piezo1 protein.
Materials and methods
OA-derived chondrocyte culture
Human articular cartilage tissue was isolated from
the knee of 20 patients suffering from OA (mean age, 40±12.5 years;
12 females and 8 males) during total knee arthroplasty from
October, 2014 to December, 2015, without infections or blood
diseases. The study protocol was approved by the Ethics Committee
of the Affiliated Hospital of Qingdao University, China. All
patients provided informed consent according to the 2013 Helsinki
Declaration (22). Osteochondral
specimens were harvested from the femoral trochlea without
macroscopical fibrillation, briefly washed in phosphate-buffered
saline (PBS), mixed with 400 U/ml penicillin and 0.4 mg/ml
streptomycin under aseptic condition and cut into small pieces
(1×1×1 mm3). Then the specimens were added to 0.25%
pancreatic enzymes and 0.2% collagenase II for 30 min and 4 h
respectively. After that, the appropriate 10% α-minimum essential
medium (α-MEM) was added to the mixture. Trypan blue staining was
used to detect the viability of the OA-derived chondrocytes. The
OA-derived chondrocytes were plated in 50 cm2 cell
culture flasks (Nunc, Roskilde, Denmark) at a density of
5×104/cm2 containing human OA-derived
chondrocyte culture media comprised of α-MEM supplemented with 12%
fetal bovine serum (FBS) (both from Hyclone, Logan, UT, USA) and 1%
penicillin-streptomycin (P/S) (Invitrogen, Carlsbad, CA, USA). The
cells were cultured at 37°C with 5% CO2, and the medium
was changed twice a week. When the cells reached 70–80% confluency,
the adherent OA-derived chondrocytes were harvested using 0.25%
Trypsin-EDTA (HyClone), at 37°C for 3 min. Following passages, the
cells were plated (1×106 cells/185 cm2) in
Nunclon Delta Solo flasks (Sigma-Aldrich, Darmstadt, Germany).
Viability of the OA-derived
chondrocytes
The mixture containing 0.04% trypan blue in final
concentration was added to the cells, and was observed under a
light microscope. The viability of the OA-derived chondrocytes was
calculated based on the formula: Viability (%) = living
cells/(living cells + dead cells) ×100%.
Application of cyclic stretch
The primary OA-derived chondrocytes were seeded in
growth medium [(GM) containing 15% heat-inactivated FBS; 100 U/ml
of penicillin and 100 μg/ml streptomycin (Pen Strep); as
well as L-glutamine (all from Life Technologies, Carlsbad, CA,
USA)] at 3×106 cells/well on 6-well collagen-coated
BioFlex plates containing a flexible silicone elastomer substratum
and grown to 80% confluence under non-stretch conditions for 3–5
days. BioFlex plates were then mounted in a Flexercell Strain Unit
(both from Flexercell International, McKeesport, PA, USA) and
subjected to 20% surface elongation at a frequency of 6 cycles/min,
each cycle consisting of a 3-sec stretch alternating with 3 sec of
relaxation with a computer-controlled vacuum stretch apparatus
(FX-4000T Tension Plus System; Flexcell International). Cells were
harvested after 2, 12, 24 and 48 h, respectively. Control cells
(0%) were cultured on similar plates and kept in the same incubator
without mechanical strain.
Analysis of dead cells
The lactate dehydrogenase (LDH) detection kit (Roche
Diagnostics, Indianapolis, IN, USA) was used to monitor the
activity of LDH in the OA-derived chondrocytes after 2, 12 and 48
h. One hundred microliters of the medium was discarded from each
well, and then 50 μl of 2% Triton X-100 solution was added
to lyse the cells. The samples were incubated in the dark for 30
min at room temperature, and then were detected by fluorescence
(490 nm) using a BioTek spectrofluorometer plate reader with KC4
analysis software (BioTek, Winooski, VT, USA).
RT-qPCR
Total RNAs were extracted with RNAiso kit (Takara,
Tokyo, Japan) after 2, 12, 24 and 48 h of compressive stress,
respectively. The concentration and purity of the total RNA were
evaluated with a spectrophotometer. RT-qPCR was performed and
analyzed to assess the expression of Piezo1 and caspase-12, using
the SYBR Premix Ex Taq II kit (Perfect Real-Time; Takara) on a
FTC-3000 RT-qPCR system (Funglyn Biotech Inc., Toronto, ON, Canada)
according to the manufacturer's instructions. The PCR primers
(synthesized by Sangon Biotech, Shanghai, China) were used to
amplify the genes (Table I). The
levels of the housekeeping gene GADPH were normalized to the
threshold cycle of the target genes. To evaluate Piezo1 and
caspase-12 expression, the relative expression was analyzed by the
comparative 2−ΔΔCT method.
 | Table IThe oligo sequences of the target
genes. |
Table I
The oligo sequences of the target
genes.
| Oligo name | Oligo sequence |
|---|
| Piezo1 | F:
5′-CATCTTGGTGGTCTCCTCTGTCT-3′ |
| R:
5′-CTGGCATCCACATCCCTCTCATC-3′ |
| Caspase-12 | F:
5′-AATGGAATCTGTGGGACCAA-3′ |
| R:
5′-GAACCAAACAATCCCAGCAC-3′ |
| hBAD | F:
5′-CCGGAGGATGAGTGACGAGT-3′ |
| R:
5′-CCGATCCCACCAGGACTG-3′ |
| hBcl-2 | F:
5′-TGGGATGCCTTTGTGGAACT-3′ |
| R:
5′-GAGACAGCCAGGAGAAATCAAAC-3′ |
| hBax | F:
5′-CCTTTTGCTTCAGGGTTTCAT-3′ |
| R:
5′-GAGACACTCGCTCAGCTTCTTG-3′ |
| hGAPDH | F:
5′-GCACCGTCAAGGCTGAGAAC-3′ |
| R:
5′-TGGTGAAGACGCCAGTGGA-3′ |
Immunofluorescence
After mechanical stimulation for 2, 12, 24 and 48 h,
respectively, the cells were seeded into a 24-well plate with
circle slices added. After rinsing with PBS twice, the cells were
fixed with 4% paraformaldehyde (HyClone) and then permeabilized
with 0.2% Triton X-100 (MP Biomedicals, Santa Ana, CA, USA) for 10
min at room temperature. BSA (5%) in PBS was used as a blocking
solution to prevent nonspecific binding for 1 h at room
temperature. Then, the slices were incubated with the primary
antibody for Piezo1 (Cat. no. NBP1-78537; Novus Biologicals,
Littleton, CO, USA) at 4°C overnight. Alexa Fluor 488 goat
anti-rabbit IgG (Cat. no. CW0105; diluted 1:2,000; CwBio, Beijing,
China.) was used as the secondary antibody. Then the slices were
stained with Hoechst 33342 to visualize nuclei (Thermo Scientific,
Shanghai, China). A laser-scanning confocal microscope (LSCM) was
used to observe the location of the Piezo1 protein.
Staining with Fluo3-AM, an indicator of fluorescent
Ca2+, was used to detect the intracellular
Ca2+ concentration, and mixed with 44.2 μl DMSO
to form 1 mmol/l Fluo3-AM fluid. The Pluronic F-127 was then added
into the dye solution. The mixture was diluted to 1 μmol/l
before the experiment in order to keep the activity of the
Fluo3-AM. The cells were harvested from the 6-well plates after 2,
12, 24 and 48 h. After being treated with GsMTx4, the specific
inhibitor of Piezo1, cells were then implanted into a 24-well plate
containing the appropriate size of glass-made slices. Following
washing with HBSS twice, the Fluo3-AM mixture was added to the
slices and incubated at 37°C in a cell incubator for 60 min. Then
the cells were washed with HBSS for 3 times and incubated with HBSS
for 20 min at 37°C in a cell incubator. Laser-scanning confocal
microscope was used to detect the OA-derived chondrocyte calcium
transients under different mechanical stretch forces. The results
of the expression of Ca2+ were assessed by Image J2X
(Rawak Software, Stuttgart, Germany), a software that can analyze
the light intensity level of the fluorochrome of
Ca2+.
Analysis of apoptosis
Annexin V binding and propidium iodide staining were
used to analyze the apoptosis of the OA-derived chondrocytes. The
cells were harvested and centrifuged after continuous stretching
for 2, 12, 24 and 48 h. The same condition was applied to the
GsMTx4 group, which was the inhibitor of Piezo1, and then stained
with FITC-conjugated Annexin V and propidium iodide (PI) following
the manufacturer's instructions of the Apoptosis Detection kit
(R&D Systems, Minneapolis, MN, USA). Extra binding buffer was
added to the control group. Flow cytometry (Epics XL;
Beckman-Coulter, Krefeld, Germany) was used to collect the data.
GraphPad software (GraphPad Software, Inc., La Jolla, CA, USA) was
used to analyze the results of the apoptosis in the early stage,
late stage and total apoptosis.
Statistical analysis
Data are expressed as mean ± standard deviation (SD)
of separate experiments. The unpaired t-test was used to analyze
the difference between groups. Statistical significance was set at
P<0.05. Analysis was performed using SPSS version 13 (SPSS Inc.,
Chicago, IL, USA).
Results
Culture of the OA-derived
chondrocytes
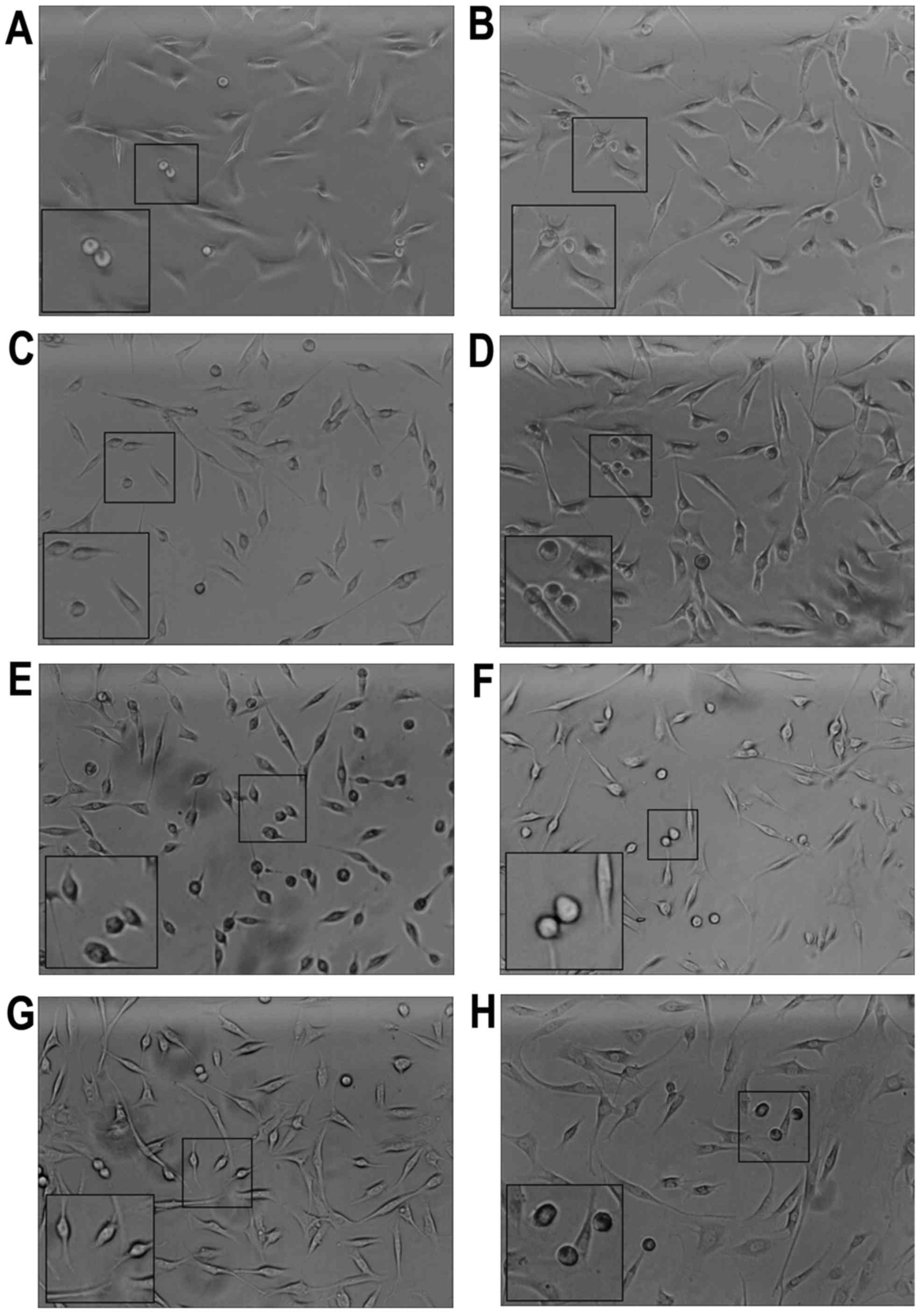
The OA-derived chondrocytes grew into a polygonal
shape and could be stained by toluidine blue. After application of
the mechanical stretch, the OA-derived chondrocytes had a tendency
to arrange in a line (Fig. 1).
Within 2 h, apoptosis of the OA-derived chondrocytes was observed,
and apoptotic bodies were apparent under a optical microscope
(Fig. 1A). Maximum apoptotic
bodies appeared in the 24 h group (Fig. 1E). However, after 48 h, there were
less apoptotic bodies compared with that noted in the 24 h group
(Fig. 1G). The OA-derived
chondrocytes were protected by GsMTX4 from mechanical-induced
apoptosis (Fig. 1B, D, F and
H).
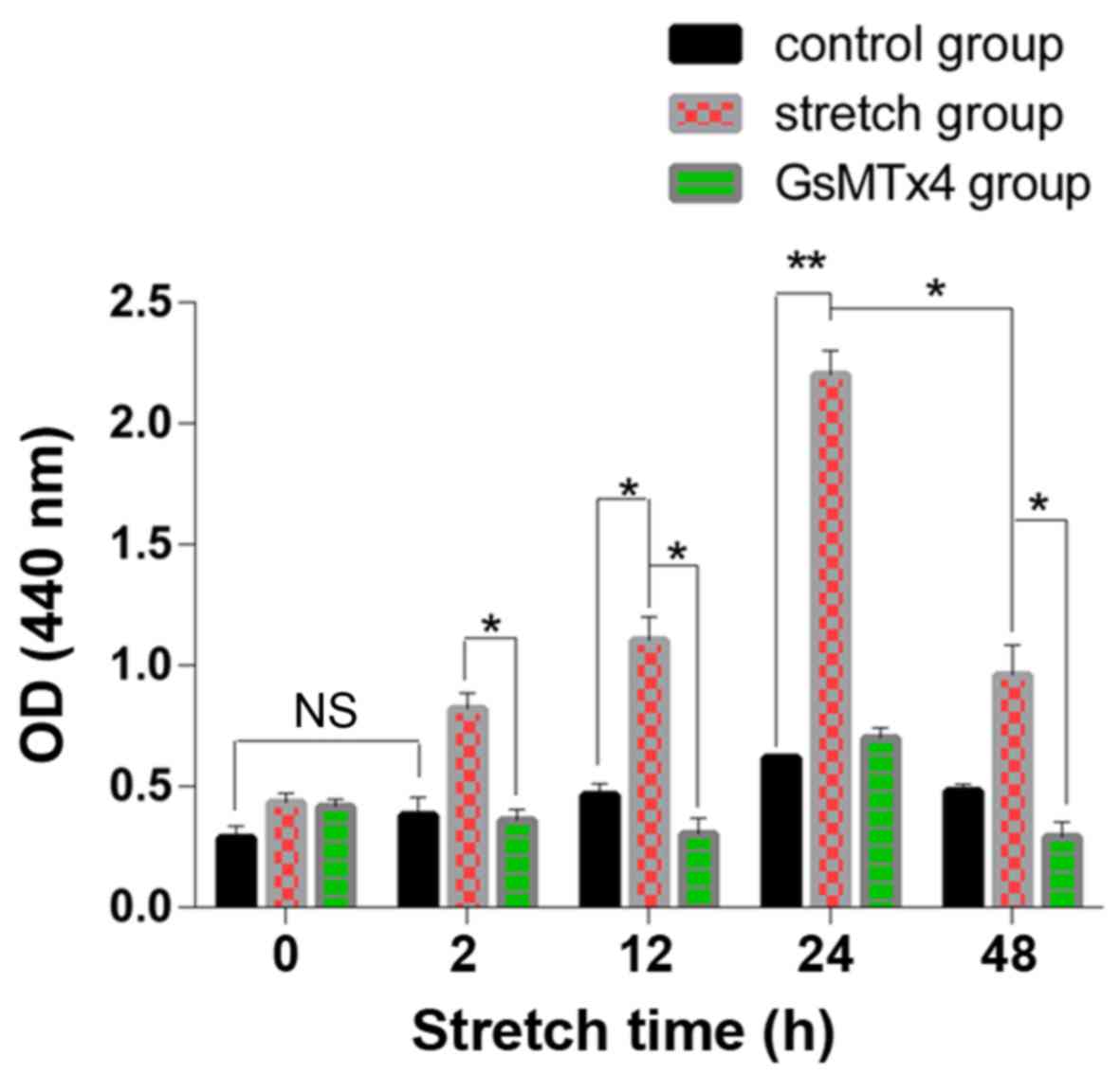
Cell death during the stretch
process
The LDH in the cells of the control groups increased
slowly without statistical significance (P>0.05) and in the
mechanical stretch group, the LDH release was significantly higher
than that in the control group (P<0.05) (Fig. 2). However, in the 48 h stretch
group, the LDH level was lower than that in the 24 h stretch group
(P<0.05). The LDH level was decreased by GsMTx4.
RT-qPCR
Piezo1, which is encoded by FAM38A, and the
apoptotic-associated genes, Bcl-2, Bax and BAD, were detected using
RT-qPCR, as well as caspase-12 (Fig.
3). As shown in Fig. 3A and
B, the expression of Piezo1 (encoded by FAM38A) in the 0 and 2
h group was at a low level, while the expression of Piezo1 in the
12 h group was significantly increased compared with the 0 h group
(P<0.05). Under mechanical stretch for 24 h, the expression of
FAM38A reached the highest level. After 48 h, the expression of
Piezo1 was lower than that of the 24 h group (P<0.05),
indicating that the expression of Piezo1 was a time-dependent
biomarker associated with the apoptosis of OA-derived
chondrocytes.
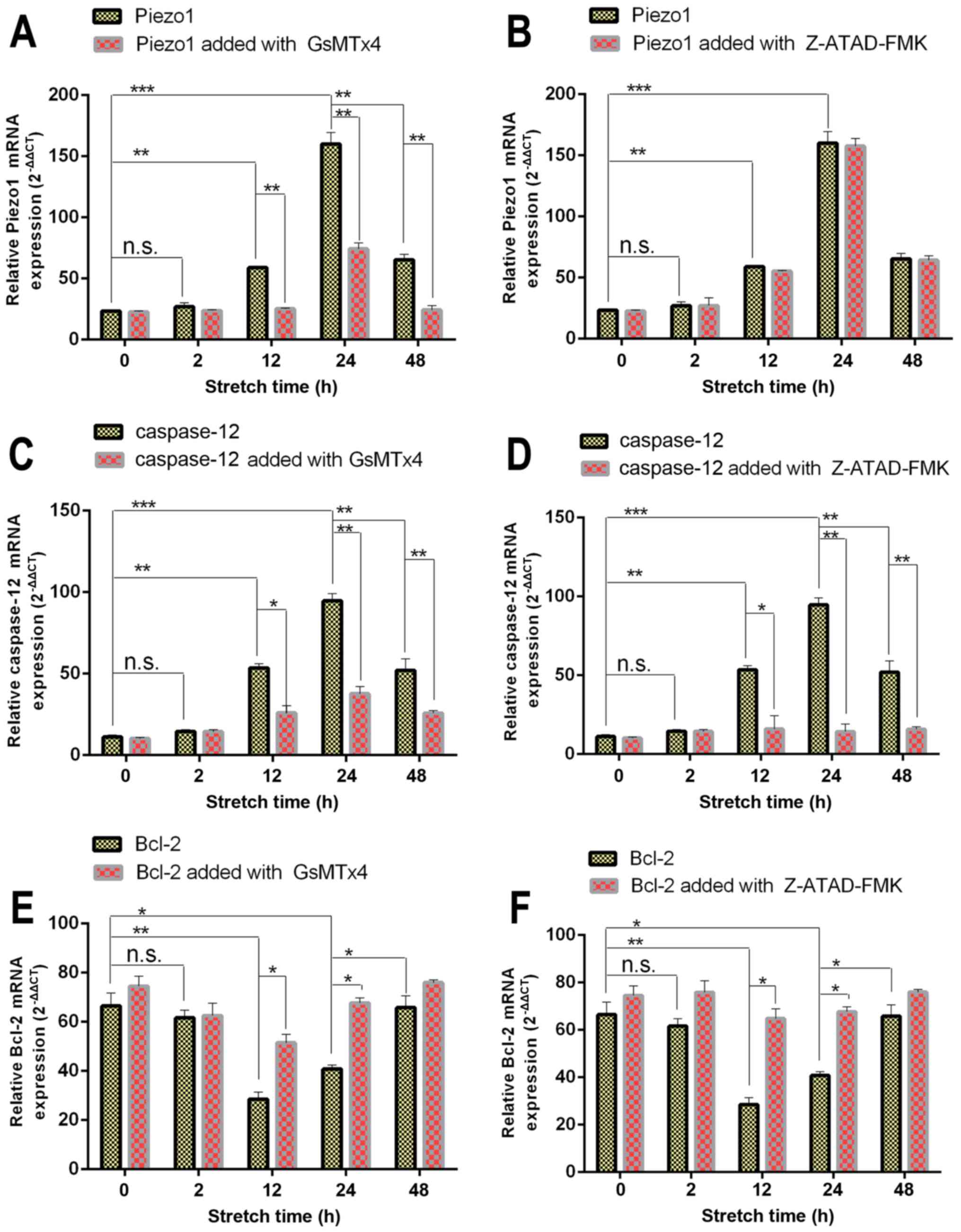 | Figure 3RT-qPCR results of Piezo1, caspase-12,
hBcl-2, hBAD and hBax expression in osteoarthritis (OA)
chondrocytes treated with (A, C, E, G and I) Piezo1 inhibitor,
GsMTx4, or (B, D, F, H and J) caspase-12 inhibitor, Z-ATAD-FMK
under increasing stretch time. Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was used as a housekeeping gene for
normalization. Expression of Piezo1, caspase-12, hBAD and hBax in
the stretch group was increasing under mechanical force in a
time-dependent manner, while expression of the anti-apoptotic gene
Bcl-2 was decreased. Results represent mean ± SE. NS, not
significant at P>0.05, the mechanical stretch group vs. the
blank group; *P<0.05 and **P<0.01, the
mechanical stretch group vs. the blank group. |
Meanwhile, expression of caspase-12, the signaling
marker of ER stress, presented a similar trend (Fig. 3C). The expression of caspase-12
was blocked by the caspase-12 inhibitor Z-ATAD-FMK (Fig. 3D).
As shown in Fig.
3G–J, the expression of the apoptosis-activated genes Bax and
BAD increased from 2 h (P<0.05), with the highest level in the
24 h group, especially compared with the 0 h group (P<0.05). The
expression level of Bax and BAD in the 48 h group was less than
that noted in the 24 h group (P<0.05). However, the expression
of Bcl-2, a type of anti-apoptotic gene, which promotes cell
proliferation was decreased in the 2 h group (P<0.05), and
reached the lowest level at 24 h compared with the 0 h group
(P<0.05) (Fig. 3E and F). In
addition, the 48 h group had higher expression than the 24 h group
(P<0.05). Thus, it was evident that the 48 h group had the trend
of cell proliferation.
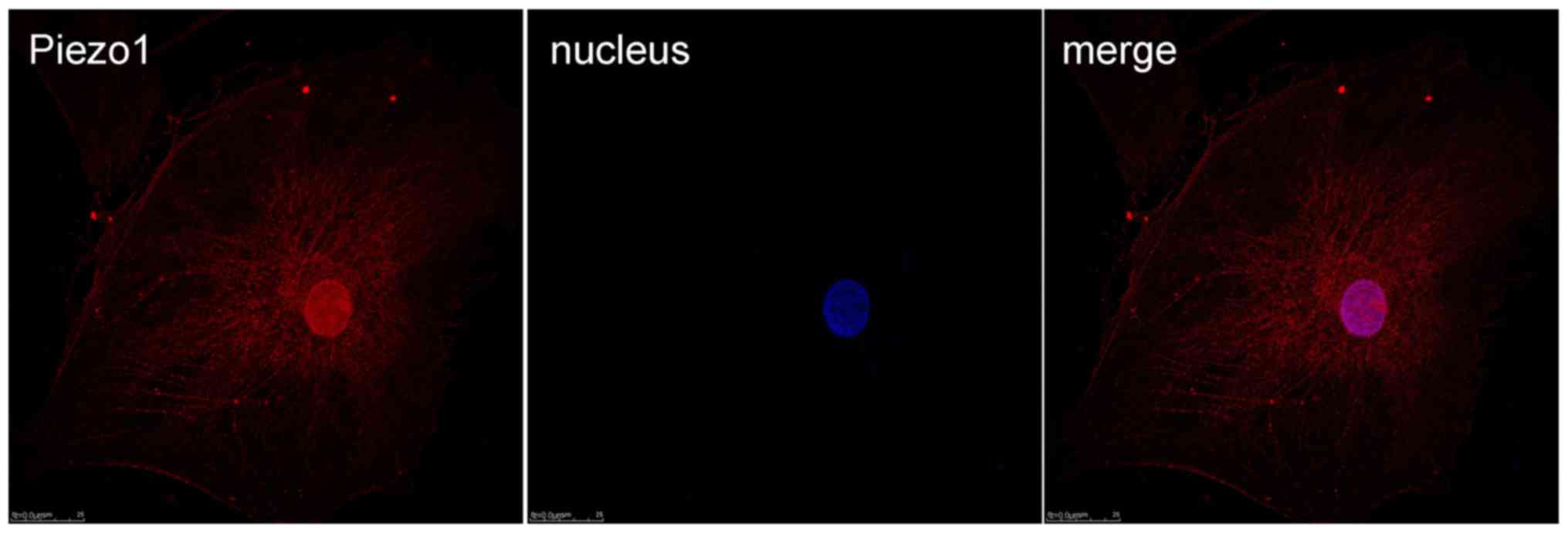
Immunofluorescence of Piezo1 in
OA-derived chondrocytes
Immunofluorescence was used to test the expression
and location of the MA ion channel Piezo1 protein (Fig. 4). From the results, it was shown
that Piezo1 could be detected in the OA-derived chondrocytes, and
the Piezo1 protein was located in the cell membrane and nucleus of
the OA-derived chondrocytes.
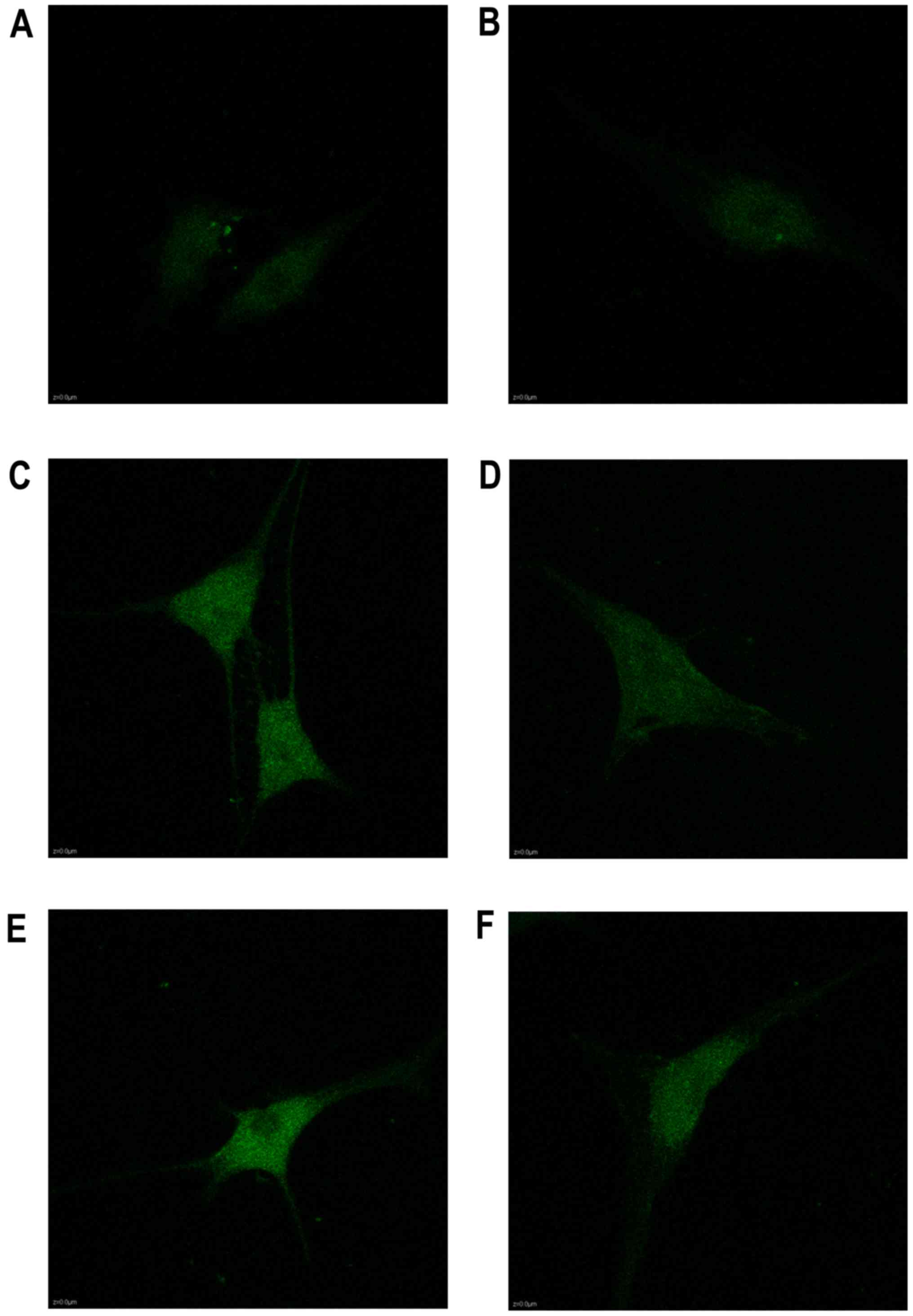
Analysis of the Ca2+ influx
under mechanical stretch
The calcium in the cytoplasm increased from 2 to 24
h as shown in the Fluo3-AM staining, as well as the expression of
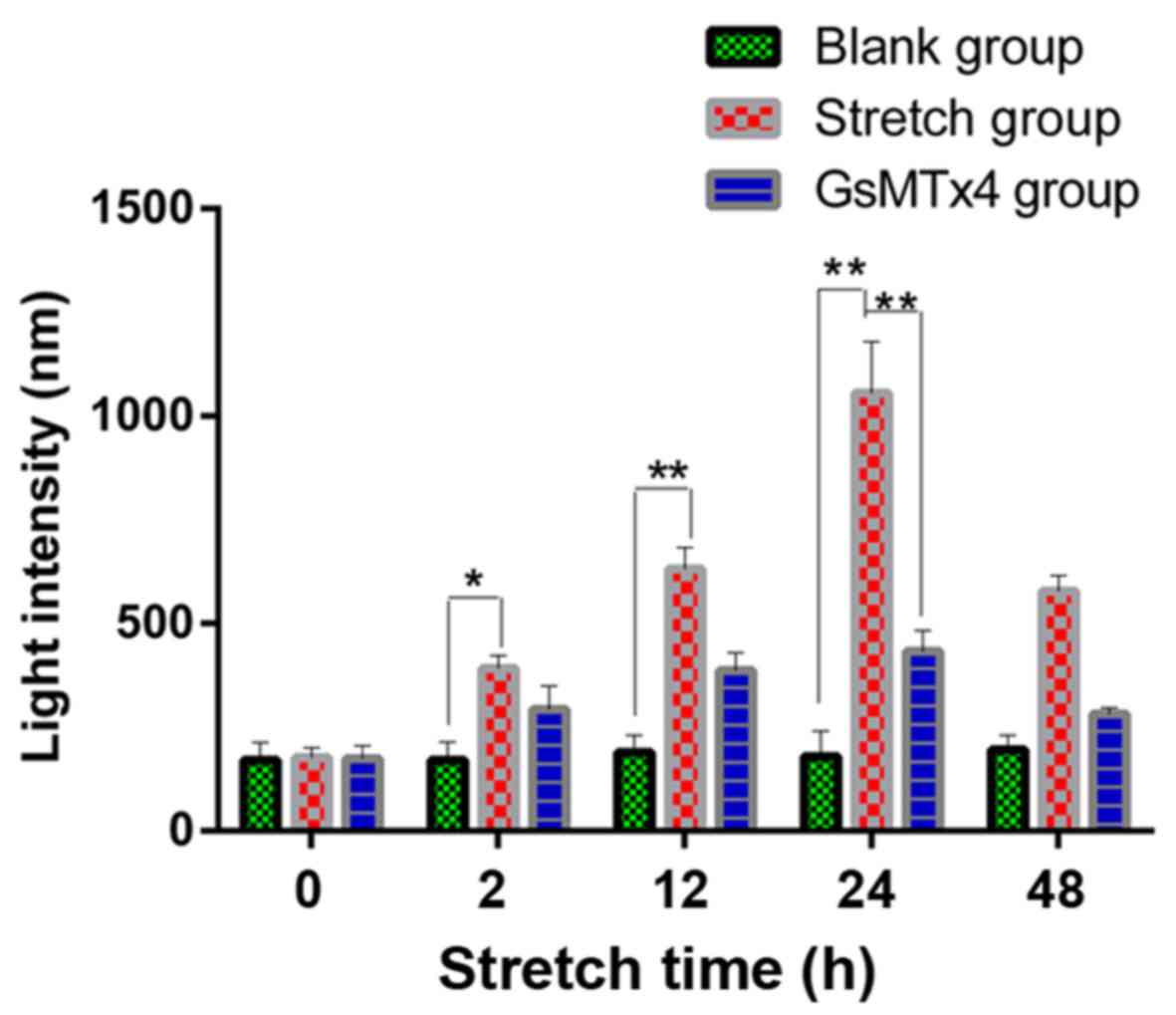
Piezo1 and caspase-12 (Fig. 5).
During the stretch period, the light intensity of the fluorochrome
of Ca2+ was increased in a time-dependent trend,
indicating that Ca2+ acted as a second messenger between
the activated Piezo1 protein and ER stress, as well as the
apoptosis of the OA-derived chondrocytes (Fig. 6).
Apoptosis of the OA-derived
chondrocytes
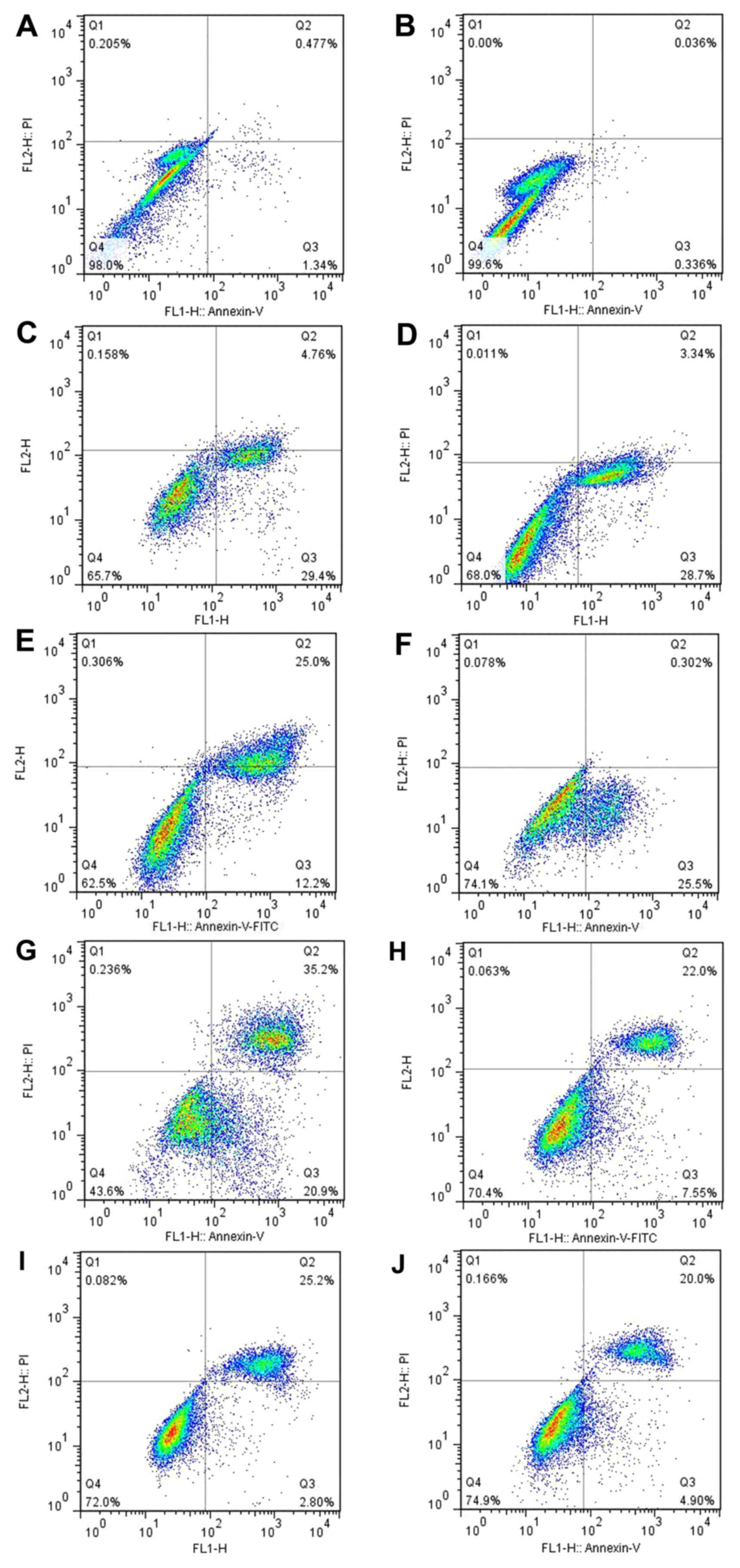
Annexin V binding, PI staining and flow cytometry
were used to analyze the apoptosis of the human OA-derived
chondrocytes. The results showed a time-dependent apoptosis shift
in response to the mechanical stretch. At 48 h after stretch force
was initiated, the rate of the apoptosis of the OA-derived
chondrocytes was lower than that at 24 h (Fig. 7G–J). A time-dependent apoptosis
shift could be found. The apoptosis of the OA-derived chondrocytes
was not blocked by GsMTx4, a Piezo1 inhibitor, indicating that the
Piezo1 pathway was not the only route causing the
mechanical-induced apoptosis of the OA-derived chondrocytes. The
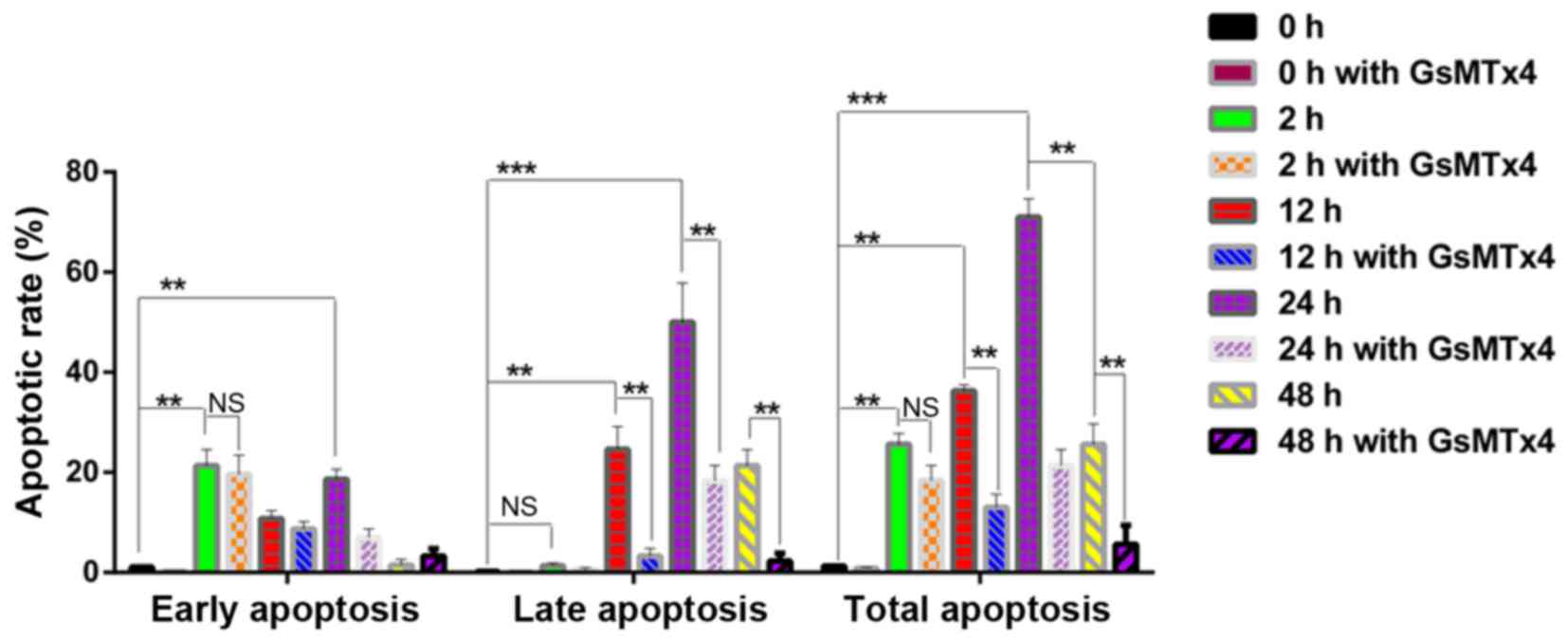
GraphPad primer 5.0 was used to analyze the apoptosis data. Results
showed that the 2 h group was characterized by the early stage of
apoptotic rate with little late apoptosis (P<0.05) (Fig. 8). The highest rate of apoptosis
appeared in the 24 h group (P<0.05). Meanwhile, the late stage
of apoptosis was inhibited by GsMTx4, as well as in the 12 h group
(P<0.05), indicating that the activated Piezo1 protein could
lead to the mechanical-induced late-stage apoptosis of the
OA-derived chondrocytes, and could be inhibited by GsMTx4.
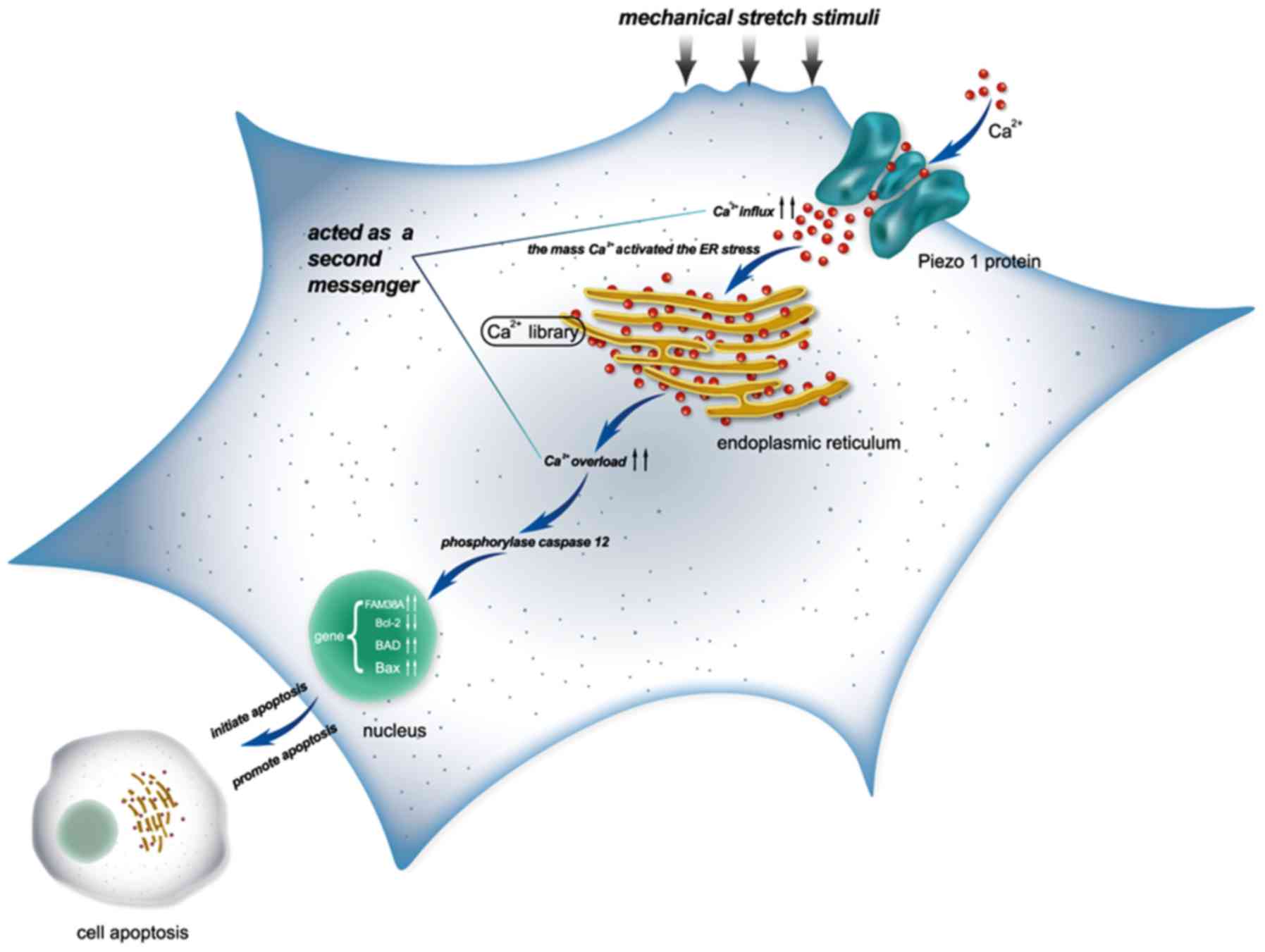
Briefly, the model of the findings of the present
study are shown in Fig. 9.
Discussion
The novel stretch-activated ion channel (SACs),
Piezo1, is expressed extensively in mammals (23). Notwithstanding, the function of
Piezo1 is still not known completely. OA is related to abnormal
mechanical stress altering joint loading, such as obesity, trauma
and joint instability, which lead to joint degeneration (2). Consequently, it is meaningful that
the selective mechanosensory pathway, such as TRPV4, is related
with OA, and it is potentially beneficial to find a novel
mechanically activated signaling pathway, such as Piezo1, for the
therapy of OA (24). It is also
helpful to discover new mechanically sensitive ion channels related
with the pathogenesis of OA-derived chondrocytes. In this study, we
explored the role of Piezo1 in the apoptosis of OA-derived
chondrocytes. Our findings found that Piezo1 plays an important
role in the process of apoptosis of OA-derived chondrocytes, and
the rate of OA-derived chondrocyte apoptosis was inhibited by
GsMTx4, an inhibitor of Piezo1.
A previous study exploring the connection between
mechanical forces and the apoptosis of myoblast cells, found that
the stretching pattern could induce the apoptosis of the cells
(25), but to date the mechanism
of stretch-induced apoptosis of OA-derived chondrocytes remains
unclear. In the present study, we hypothesized that the mechanical
force could activate Piezo1, further resulting in the apoptosis of
OA-derived chondrocytes during the progression of OA.
In this study, we monitored the expression levels of
Piezo1 and apoptosis-associated genes, including Bcl-2, Bax and
BAD, using RT-qPCR after mechanical-induced apoptosis of human
OA-derived chondrocytes from OA patients. The apoptotic rate in the
48 h group was lower than that in the 24 h group, as well as the
expression of Piezo1, caspase-12, Bax and BAD. However, the
expression of Bcl-2, an anti-apoptosis and cell proliferation gene
(26), was higher in the 48 h
group than that noted in the 24 h group (P<0.05), indicating
that appropriate mechanical stretch increased the expression of
Bcl-2 gene at least for 48 h, which aided cell proliferation.
Nevertheless, the exact mechanism of these findings still needs
elucidation. We also found that the expression of Piezo1 and the
apoptosis of the OA-derived chondrocytes in the 24 h group were
both higher than that of the 0 h group, which indicated that Piezo1
plays an important role in the mechanical-induced apoptosis of
OA-derived chondrocytes, and may serve as a possible target for the
treatment of OA, especially for patients suffereing traumatic
arthritis.
A previous study found that the divalent ion
Ca2+ was the main influx ion which could get through
human Piezo1 channels (27).
There is evidence that Ca2+ influx can be influenced by
L-type Ca2+ voltage-gated channels after mechanical
staining (1). In this study, we
found that the level of the calcium load in the cytoplasm of the
OA-derived chondrocytes was increased with the higher rate of
apoptosis of the OA-derived chondrocytes; the level of calcium load
in the 24 h group was higher than that in the 0 h group
(P<0.05). It is meaningful to speculate that Ca2+ can
act as a second messenger between activating Piezo1 and the
apoptosis of OA-derived chondrocytes. Recent research reported that
the L-type calcium channel blocker could protect cartilage from
apoptosis in OA patients (28).
The function of Piezo1 is similar to that of L-type calcium
channel, so that excessive Ca2+ loading in OA-derived
chondrocytes impacts the apoptotic equilibrium through the Piezo1
channel.
Some studies have shown that ER stress is associated
with apoptosis of chondrocytes in patients with OA (29,30). The caspase family of proteins can
be activated by ER stress, especially caspase-12, a murine protein
associated with the ER membrane. However, it is controversial
whether caspase-12 plays an important role in ER stress-induced
apoptosis in humans (31–33). Results of this study confirmed
that caspase-12 was activated by ER stress, resulting in induced
apoptosis of human OA-derived chondrocytes. We also found that
Piezo1 induced the apoptosis of the OA-derived chondrocytes through
ER stress. In this way, Piezo1 protein could be regarded as a
potential therapeutic target for helping to inhibit the apoptosis
of chondrocytes, especially for OA patients. A specific blocker for
Piezo1 may be useful for articular degeneration.
Although the exact mechanism of the Piezo protein
and the specific blocker are not clear, the architecture of the
mammalian mechanosensitive Piezo1 channel has been clarified
(34). Cryo-electron microscopy
has been used to determine the structure of the mouse Piezo1 and
explore the trimeric propeller-like chemical compound. A compound
named Yoda1 was found to act as an agonist for human Piezo1
(35). Novel specific inhibitors
for Piezo1 which are not harmful to humans warrant further
study.
Acknowledgments
The authors thank Ying-Zhen Wang for assistance in
providing experimental material. This study was supported by the
National Natural Science Foundation of China (nos. 81171774 and
81272056).
References
|
1
|
Lee W, Leddy HA, Chen Y, Lee SH, Zelenski
NA, McNulty AL, Wu J, Beicker KN, Coles J, Zauscher S, et al:
Synergy between Piezo1 and Piezo2 channels confers high-strain
mechanosensitivity to articular cartilage. Proc Natl Acad Sci USA.
111:E5114–E5122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guilak F: Biomechanical factors in
osteoarthritis. Best Pract Res Clin Rheumatol. 25:815–823. 2011.
View Article : Google Scholar
|
|
3
|
Coste B, Mathur J, Schmidt M, Earley TJ,
Ranade S, Petrus MJ, Dubin AE and Patapoutian A: Piezo1 and Piezo2
are essential components of distinct mechanically activated cation
channels. Science. 330:55–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coste B, Xiao B, Santos JS, Syeda R,
Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, et al:
Piezo proteins are pore-forming subunits of mechanically activated
channels. Nature. 483:176–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SE, Coste B, Chadha A, Cook B and
Patapoutian A: The role of Drosophila Piezo in mechanical
nociception. Nature. 483:209–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zarychanski R, Schulz VP, Houston BL,
Maksimova Y, Houston DS, Smith B, Rinehart J and Gallagher PG:
Mutations in the mechanotransduction protein PIEZO1 are associated
with hereditary xerocytosis. Blood. 120:1908–1915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyamoto TM, Nakagomi H, Kira S, Mochizuki
T, Koizumi S, Tominaga M, et al: Piezo1, a novel mechanosensor in
the bladder urothelium, transmits signals of bladder sensation. Eur
Urol. 31:1015–1017. 2012.
|
|
8
|
Gottlieb PA, Bae C, Gnanasambandam R,
Nicolai C, Nicolai C, Nicolai C and Sachs F: Piezo1 mutations
identified in xerocytosi-salter the inactivation rate. Biophys J.
104:467A2013. View Article : Google Scholar
|
|
9
|
Demolombe S, Duprat F, Honoré E and Patel
A: Slower Piezo1 inactivation in dehydrated hereditary
stomatocytosis (xerocytosis). Biophys J. 105:833–834. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andolfo I, Alper SL, De Franceschi L,
Auriemma C, Russo R, De Falco L, Vallefuoco F, Esposito MR,
Vandorpe DH, Shmukler BE, et al: Multiple clinical forms of
dehydrated hereditary stomatocytosis arise from mutations in
PIEZO1. Blood. 121:3925–3935. S1–S12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coste B, Houge G, Murray MF, Stitziel N,
Bandell M, Giovanni MA, Philippakis A, Hoischen A, Riemer G, Steen
U, et al: Gain-of-function mutations in the mechanically activated
ion channel PIEZO2 cause a subtype of Distal Arthrogryposis. Proc
Natl Acad Sci USA. 110:4667–4672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McMillin MJ, Beck AE, Chong JX, Shively
KM, Buckingham KJ, Gildersleeve HI, Aracena MI, Aylsworth AS,
Bitoun P, Carey JC, et al: University of Washington Center for
Mendelian Genomics: Mutations in PIEZO2 cause Gordon syndrome,
Marden-Walker syndrome, and distal arthrogryposis type 5. Am J Hum
Genet. 94:734–744. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee MS, Trindade MC, Ikenoue T, Schurman
DJ, Goodman SB and Smith RL: Effects of shear stress on nitric
oxide and matrix protein gene expression in human osteoarthritic
chondrocytes in vitro. J Orthop Res. 20:556–561. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martin JA and Buckwalter JA:
Post-traumatic osteoarthritis: The role of stress induced
chondrocyte damage. Biorheology. 43:517–521. 2006.PubMed/NCBI
|
|
15
|
Rennier K and Ji JY: Effect of shear
stress and substrate on endothelial DAPK expression, caspase
activity, and apoptosis. BMC Res Notes. 6:102013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anelli T and Sitia R: Protein quality
control in the early secretory pathway. EMBO J. 27:315–327. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X and Zhu XZ: Roles of p53, c-Myc,
Bcl-2, Bax and caspases in serum deprivation-induced neuronal
apoptosis: A possible neuroprotective mechanism of basic fibroblast
growth factor. Neuroreport. 10:3087–3091. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mocetti P, Silvestrini G, Ballanti P,
Patacchioli FR, Di Grezia R, Angelucci L and Bonucci E: Bcl-2 and
Bax expression in cartilage and bone cells after high-dose
corticosterone treatment in rats. Tissue Cell. 33:1–7. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wiren KM, Toombs AR, Semirale AA and Zhang
X: Osteoblast and osteocyte apoptosis associated with androgen
action in bone: Requirement of increased Bax/Bcl-2 ratio. Bone.
38:637–651. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sattler M, Liang H, Nettesheim D, Meadows
RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ,
et al: Structure of Bcl-xL-Bak peptide complex: Recognition between
regulators of apoptosis. Science. 275:983–986. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang E, Zha J, Jockel J, Boise LH,
Thompson CB and Korsmeyer SJ: Bad, a heterodimeric partner for
Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell.
80:285–291. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mastroleo L: Post-trial obligations in the
Declaration of Helsinki 2013: Classification, reconstruction and
interpretation. Dev World Bioeth. 16:80–90. 2016. View Article : Google Scholar
|
|
23
|
Bagriantsev SN, Gracheva EO and Gallagher
PG: Piezo proteins: Regulators of mechanosensation and other
cellular processes. J Biol Chem. 289:31673–31681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Drexler S, Wann A and Vincent TL: Are
cellular mechanosensors potential therapeutic targets in
osteoarthritis? Int J Clin Rheumatol. 9:155–167. 2014. View Article : Google Scholar
|
|
25
|
Liu J, Liu J, Mao J, Yuan X, Lin Z and Li
Y: Caspase-3-mediated cyclic stretch-induced myoblast apoptosis via
a Fas/FasL-independent signaling pathway during myogenesis. J Cell
Biochem. 107:834–844. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fröhlich M, Jaeger A, Weiss DG and
Kriehuber R: Inhibition of BCL-2 leads to increased apoptosis and
delayed neuronal differentiation in human ReNcell VM cells in
vitro. Int J Dev Neurosci. 48:9–17. 2016. View Article : Google Scholar
|
|
27
|
Gnanasambandam R, Bae C, Gottlieb PA and
Sachs F: Ionic selectivity and permeation properties of human
PIEZO1 channels. PLoS One. 10:e01255032015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takamatsu A, Ohkawara B, Ito M, Masuda A,
Sakai T, Ishiguro N and Ohno K: Verapamil protects against
cartilage degradation in osteoarthritis by inhibiting Wnt/β-catenin
signaling. PLoS One. 9:e926992014. View Article : Google Scholar
|
|
29
|
Liu C, Cao Y, Yang X, Shan P and Liu H:
Tauroursodeoxycholic acid suppresses endoplasmic reticulum stress
in the chondrocytes of patients with osteoarthritis. Int J Mol Med.
36:1081–1087. 2015.PubMed/NCBI
|
|
30
|
Boyce M and Yuan J: Cellular response to
endoplasmic reticulum stress: A matter of life or death. Cell Death
Differ. 13:363–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brostrom MA and Brostrom CO: Calcium
dynamics and endoplasmic reticular function in the regulation of
protein synthesis: Implications for cell growth and adaptability.
Cell Calcium. 34:345–363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saleh M, Vaillancourt JP, Graham RK, Huyck
M, Srinivasula SM, Alnemri ES, Steinberg MH, Nolan V, Baldwin CT,
Hotchkiss RS, et al: Differential modulation of endotoxin
responsiveness by human caspase-12 polymorphisms. Nature.
429:75–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pannaccione A, Secondo A, Molinaro P,
D'Avanzo C, Cantile M, Esposito A, Boscia F, Scorziello A,
Sirabella R, Sokolow S, et al: A new concept: Aβ1-42 generates a
hyperfunctional proteolytic NCX3 fragment that delays caspase-12
activation and neuronal death. J Neurosci. 32:10609–10617. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P,
Li R, Gao N, Xiao B and Yang M: Architecture of the mammalian
mechanosensitive Piezo1 channel. Nature. 527:64–69. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Syeda R, Xu J, Dubin AE, Coste B, Mathur
J, Huynh T, Matzen J, Lao J, Tully DC, Engels IH, et al: Chemical
activation of the mechanotransduction channel Piezo1. eLife.
4:e073692015. View Article : Google Scholar :
|