Introduction
In developed countries, thromboembolic diseases are
a leading cause of mortality and disability (1). Blood platelet activation and the
plasma coagulation system play a crucial role in thrombus
formation. Despite the fact that established antiplatelet agents,
such as aspirin, clopidogrel and tirofiban, and anticoagulant
agents, such as heparin and warfarin have been reported to be
beneficial in the treatment of thromboembolic disease, they all
have considerable limitations (2). Therefore, there is an urgent need
from the clinical aspect for the development of more effective and
safer antithrombotic agents. Although aspirin is the most economic
and effective antiplatelet drug prescribed for the treatment of
cardiovascular and cerebrovascular diseases, it nevertheless has
untoward drawbacks, such as gastrointestinal bleeding and
hemorrhagic stroke in patients with low cardiovascular risk
(2).
Currently, the use of anticoagulants, such as
warfarin and heparin is restricted due to their modest therapeutic
benefits, limited clinical applications, and increased risk of
bleeding and drug-induced thrombophilia. A number of available
antiplatelet and anticoagulant agents have limited use in
antithrombotic therapy (3).
However, in recent years considerable progress has been made in the
synthesis of oligosaccharides with the anticoagulant properties of
heparin (4,5). Unfortunately, the chemical synthesis
of oligosaccharides remains a major challenge. As such, the
development of efficient strategies for oligosaccharide synthesis
stands out as a demanding area of research.
Various substituted derivatives of benzimidazole
exhibit marked biological activity, such as
antitumor/antiproliferative/anticancer activity, antimicrobial
activity (including anti-HIV activity), antioxidant and cysticidal
activities. Compounds with the chemical structure of a
benzimidazole backbone have shown antiplatelet activities (6) or anticoagulant activities through
their inhibitory effects on coagulation factors (7). The antiplatelet (anti-aggregation
and anti-coagulation) effects of RU-891, a compound of
9-[2-(3,4-dioxyphenyl)-2-oxoethyl]-2,3-dihydro
imidazo[1,2-a]benzimidazole hydrobromide has been reported both
in vitro and in vivo (8). It has been reported that the ability
of heparin and related compounds that induce thrombocytopenia is
closely associated with the structure of the polysaccharides, and
particularly to its negative charge and to the length of the
molecules (9). Therefore, this
study was designed to investigate the effects of the newly
synthesized benzimidazole-based saccharides, M3BIM, Malto-BIM and
Melibio-BIM, on platelet activation. The molecular basis of their
effects was also confirmed by assessing the roles of adenosine
triphosphate (ATP) release, Ca2+ mobilization, p38
mitogen-activated protein kinase (MAPK), and p47 in the
collagen-mediated responses in platelets.
Materials and methods
Materials
Collagen (type I), thrombin, luciferin-luciferase,
maltotriose, maltose and melibiose were all purchased from Sigma
(St. Louis, MO, USA). Fura 2-AM and fluorescein isothiocyanate
(FITC) were from Molecular Probe (Eugene, OR, USA);
anti-phospho-(Ser) PKC substrate (cat. no. 2261), anti-phospho-p38
MAPK (Ser180/Tyr182) polyclonal antibody
(pAb) (cat. no. 9211) and anti-p38 MAPK (5F11) monoclonal antibody
(mAb) (cat. no. 9217) were from Cell Signaling Technology (Beverly,
MA, USA); the pleckstrin pAb (cat. no. GTX17020) was from GeneTex
(Irvine, CA, USA). Ortho-phenyl diamine (cat. no. 523121), iodine
(cat. no. 104761), ethyl acetate (cat. no. 100789) and acetic acid
(cat. no. 100063) were purchased from Merck Millipore (Billerica,
MA, USA). Hybond-P polyvinylidene difluoride membrane, enhanced
chemiluminescence (ECL), western blot detection reagent and
analysis system, horseradish peroxidase (HRP)-conjugated donkey
anti-rabbit immunoglobulin G (IgG) (cat. no. NA934), and sheep
anti-mouse IgG (cat. no. NA931) were from Amersham
(Buckinghamshire, UK).
Synthesis and purification of M3BIM,
Malto-BIM and Melibio-BIM
According to the method previously described by Lin
et al (10), the compound
M3BIM was synthesized and purified by the oxidative condensation of
maltotriose. Briefly, a mixture of D-maltotriose monohydrate (50.4
mg, 0.1 mM) and o-phenylenediamine (21.6 mg, 0.2 mM) was
stirred with iodine (25 mg, 0.1 mM) in 3 ml of AcOH for 30 h at
room temperature. The reaction was completed in 6 h as indicated by
the following thin layer chromatography (TLC) analysis:
(acetone/ethyl acetate/water/acetic acid, 60:30:20:1) Rf
0.44; [R] 25 D + 55.40 (c 1.0, H2O). The reaction
mixture was triturated with ethyl acetate to yield precipitates,
which were collected by filtration using a nylon membrane filter.
The aldonaphthimidazole products prepared as such were practically
pure for characterization. The crude product was purified by C18
reversed-phase silica gel column chromatography (methanol/water,
1–30% as gradient) to yield M3BIM (30 mg, 51% yield,
C24H36N2O15, yellowish
foam).
The compounds Malto-BIM and Melibio-BIM were
prepared and purified by the oxidative condensation of respective
maltose and melibiose (10). To
this, a mixture of each maltose and melibiose (34.2 mg, 0.1 mM) and
o-phenylenediamine (21.6 mg, 0.2 mM) was stirred with iodine
(25 mg, 0.1 mM) in 3 ml of AcOH for 24 h at room temperature. The
reaction was completed in 12 h. The reaction mixture was triturated
with EtOAc to yiled precipitates, which were collected by
filtration using a nylon membrane filter. The crude product was
purified by C18 reversed-phase silica gel column chromatography
(MeOH/H2O, 1–30% as gradient) to afford the desired
product of Malto-BIM (44.2 mg, 92% yield,
C18H26N2O10; brownish
solid) and Melibio-BIM (38.4 mg, 80% yield,
C18H26N2O10; brownish
solid). All compounds were dissolved in DMSO and stored at 4°C.
Platelet preparation
Blood was collected from healthy human volunteers
(following informed consent) who did not take medication during the
preceding 2 weeks and was mixed with acid-citrate-dextrose solution
(1:9). Human platelet suspensions were prepared following the
methods described by Sheu et al (11). Briefly, the blood samples were
subjected to centrifugation at 120 × g for 10 min, and
platelet-rich plasma (PRP) was collected. PRP was supplemented with
prostaglandin E1 (PGE1; 0.5 μM) and
heparin (6.4 IU/ml) and then incubated for 10 min at 37°C.
Following centrifugation at 500 × g for 10 min, the platelet
pellets were suspended in Tyrode's solution containing 3.5 mg/ml
bovine serum albumin (BSA), pH 7.3 (NaCl 11.9 mM, KCl 2.7 mM,
MgCl2 2.1 mM, NaH2PO4 0.4 mM,
NaHCO3 11.9 mM and glucose 11.1 mM). Subsequently,
PGE1 (0.5 μM), apyrase (1.0 U/ml), and heparin
(6.4 IU/ml) were added, and the mixture was incubated for 10 min at
37°C. The mixtures were centrifuged at 500 × g for 10 min and
subjected for the repeated washing procedure. Finally, the platelet
pellets were resuspended in Tyrode's solution, and then calcium
chloride was added to platelet suspensions in which the
concentration of Ca2+ was 1 mM. This study was approved
by the Institutional Review Board of Taipei Medical University and
conformed to the directives of the Helsinki Declaration.
Platelet aggregation
Platelet aggregation was examined as previously
described (11) and monitored by
measuring light transmission via a Lumi-Aggregometer (Payton
Associates, Scarborough, ON, Canada). Prior to the addition of
agonists, such as collagen (1 μg/ml) to induce platelet
aggregation, the platelet suspensions (3.6×108 cells/ml)
were pre-treated with various concentrations (20–60 μM) of
M3BIM, Malto-BIM and Melibio-BIM or an isovolumetric solvent
control (0.5% DMSO) for 3 min. Moreover, the platelets were
pre-treated with 40–80 μM of M3BIM, Malto-BIM and
Melibio-BIM for thrombin (0.01 U/ml)-induced aggregation. A
light-transmission unit was used to present the extent of platelet
aggregation.
ATP release assay
The washed platelets were pre-incubated with 60
μM of each M3BIM, Malto-BIM, and Melibio-BIM for 3 min at
37°C and then stimulated with collagen. The reaction was
terminated, the samples were centrifuged and supernatants were used
for the assay. For the measurement of ATP release, a 20 μl
of luciferin-luciferase mixture was added 1 min before adding the
testing compounds or agonists, and the relative amount of ATP
release was compared to the solvent control.
Measurement of relative Ca2+
mobilization
Citrated blood was centrifuged at 120 × g for 10
min. The supernatant (PRP) was incubated with 5 μM Fura 2-AM
for 1 h. The platelets were then prepared as described above.
Finally, the external Ca2+ concentration of the platelet
suspensions was adjusted to 1 mM. The relative Ca2+
mobilization was measured as previously described (11).
Immunoblotting
The washed platelets (1.2×109 cells/ml)
were pre-incubated with M3BIM, Malto-BIM and Melibio-BIM (60
μM) or a solvent control for 3 min, followed by the addition
of 1 μg/ml collagen to trigger platelet activation. The
platelets were immediately resuspended in lysis buffer. Samples
containing protein (80 μg) were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12%);
proteins were electrotransferred using semidry transfer (Bio-Rad,
Hercules, CA, USA). The blots were blocked with Tris-buffered
saline with Tween-20 (TBST; 10 mM Tris-base, 100 mM NaCl, and 0.01%
Tween-20) and then probed with p-p47 and p-p38 MAPK primary
antibodies for 1 h. The membranes were incubated with HRP-linked
secondary antibodies for 1 h in TBST. Subsequently, the
immunoreactive bands were detected by an ECL system. The bar graph
depicts the ratios of semiquantitative results obtained by scanning
reactive bands and quantifying the optical density using video
densitometry (Bio-profil; Biolight Windows Application V2000.01;
Vilber Lourmat, Marne la Vallée, France).
Zebrafish genotoxicity assay
The toxic effects of imidazole-derived saccharides
were tested in zebrafish-based assays. Zebrafish (Danio
rerio) were obtained from the Zebrafish Core Facility of Taipei
Medical University and maintained at 28°C on a 14 h light/10 h dark
cycle. The embryos were incubated at 28°C, and different
developmental stages after fertilization (the zygote, cleavage,
blastula, gastrula, segmentation, pharyngula, and hatching periods)
were determined as previously described (12). Fifteen wild-type embryos each were
treated with various concentrations of M3BIM, Malto-BIM and
Melibio-BIM (25, 50, 100 and 200 μM) or a solvent control
(0.5% DMSO) in a 24-well chamber. At 3 days post-fertilization
(dpf), the percentage of embryos exhibiting developmental
abnormalities and the survival rate were determined. The embryos
were observed using an Olympus IX70-FL inverted fluorescence
microscope (Olympus, Tokyo, Japan). Images were acquired using a
SPOT digital camera system (Diagnostic Instruments, Sterling
Heights, MI, USA) and assembled with ImageJ software.
Statistical analysis
The experimental results are expressed as the means
± SEM and are accompanied by the number of observations. The
experiments were assessed by the method of analysis of variance
(ANOVA). If this analysis indicated significant differences among
the group means, then each group was compared using the
Newman-Keuls method. A P-value of <0.05 was considered to
indicate a statistically significant difference.
Results
Synthesis of benzimidazole
derivatives
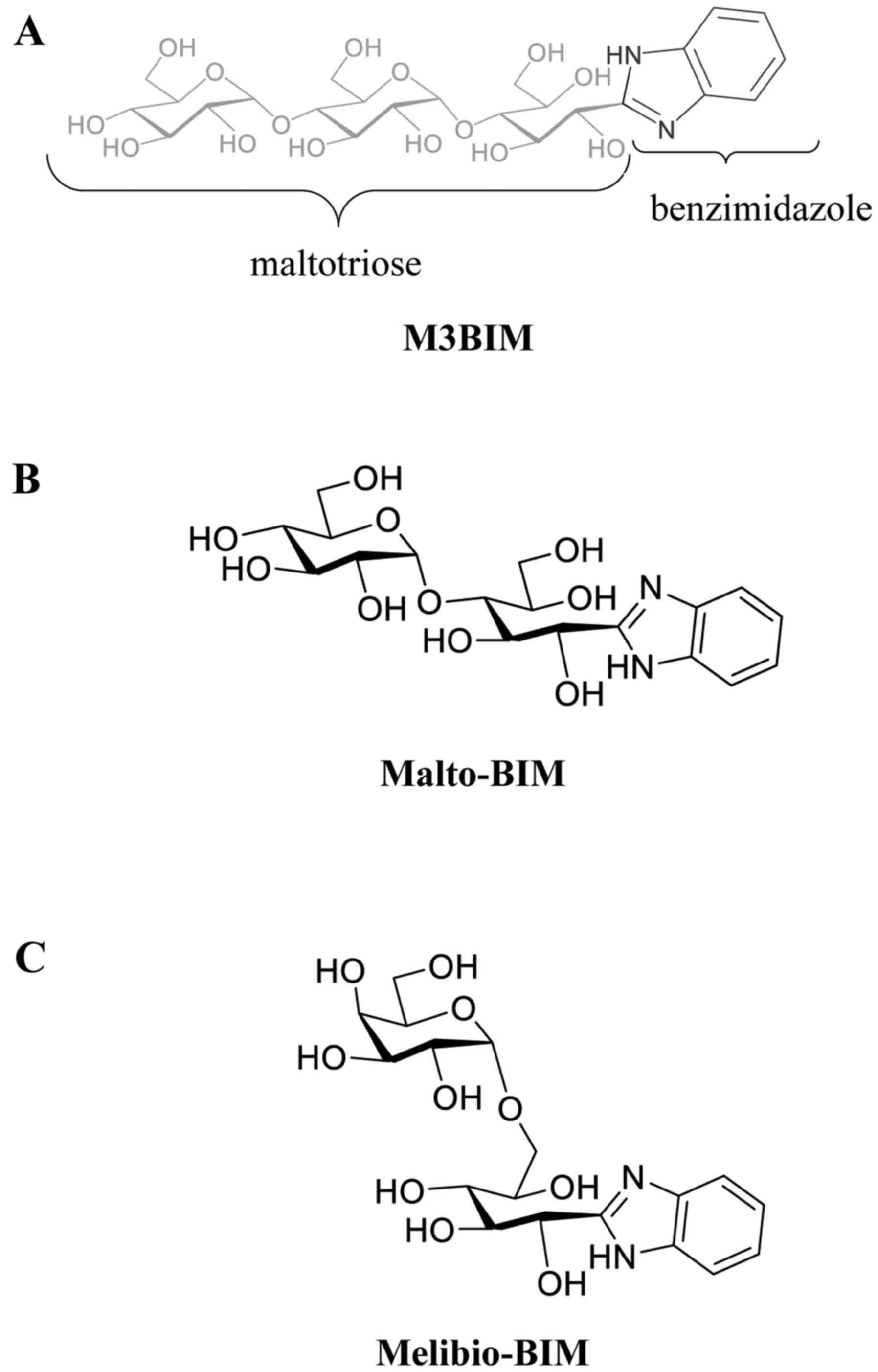
The novel benzimidazole derivatives of M3BIM
[(1R,2R,3R)-1-(1H-benzo[d]imidazol-2-yl)-3-(2R,3R,4R,5S,6R)-3,4-dihydroxy-6-(hydroxymethyl)-5-2R,
3R,4S,5S,6R)-3,4,5-trihydroxy-6-hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)
pentane-1,2,4,5-tetraol], Malto-BIM
(1′S,2′R,3′R,4′R-2)-[1,2,4,5-tetrahydoxy-3-O-(2,3,4,5-tetrahydroxy-α-D-glucopyranosyl)]pentyl-1H-benzimidazole]
and Melibio-BIM
[(1′S,2′R,3′S,4′R)-2-[1,2,3,4-tetrahydoxy-5-O-(2,3,4,5-tetrahydroxy-α-D-glucopyranosyl)]pentyl-1H-benzimidazole]
were prepared by the oxidative condensation of maltotriose, maltose
and melibiose, respectively as previously described by Lin et
al (10). The chemical
structures of the synthesized compounds M3BIM, Malto-BIM and
Melibio-BIM are shown in Fig.
1.
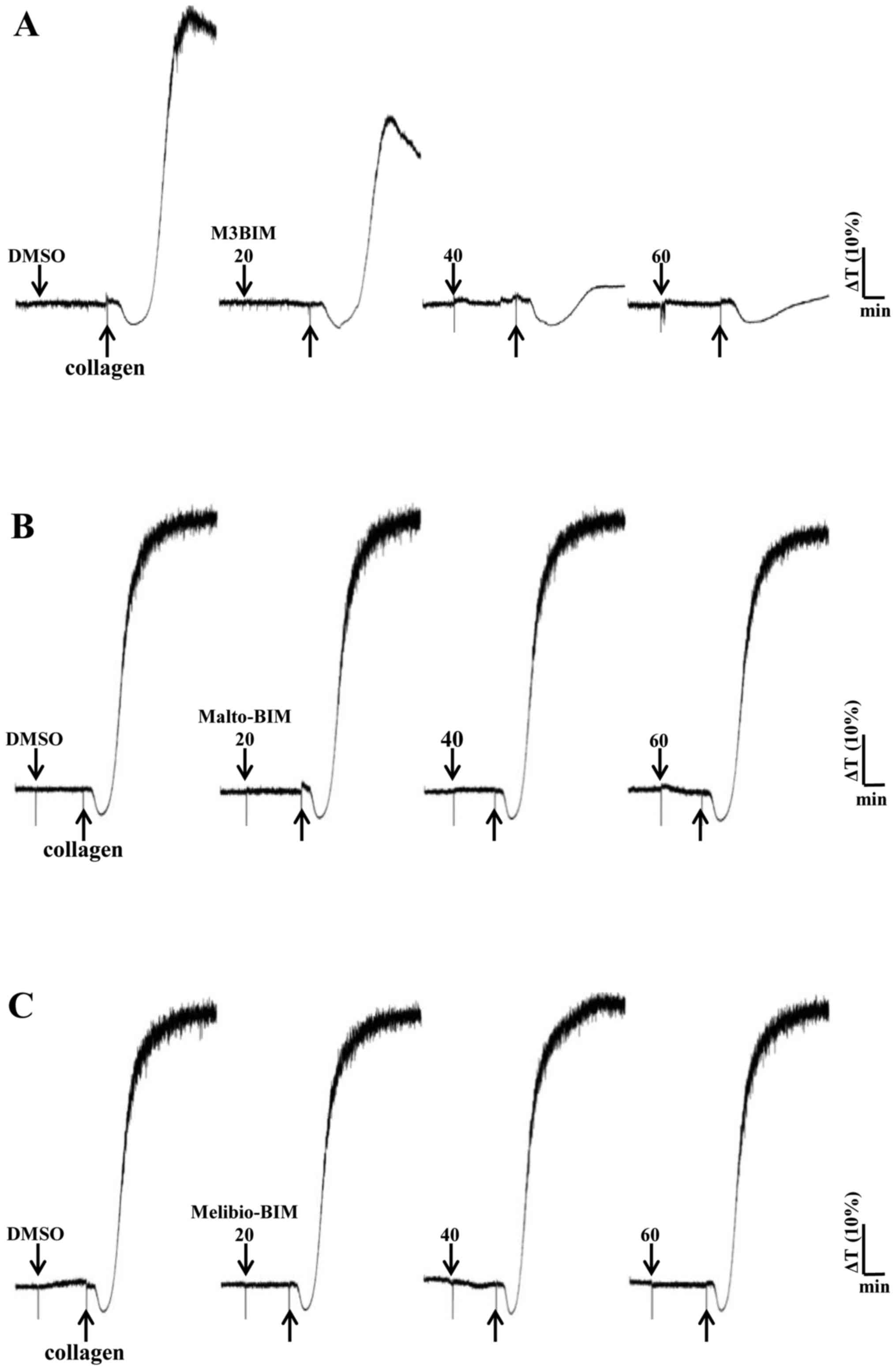
M3BIM inhibits platelet aggregation
induced by collagen and thrombin
We previously determined that collagen (1
μg/ml) and thrombin (0.01 U/ml) induced complete platelet
aggregation (28); hence, the
present study employed these agonists to stimulate platelets and
evaluate the effects of the test compounds on platelet aggregation.
M3BIM potently inhibited collagen-induced platelet aggregation in a
concentration-dependent manner (Fig.
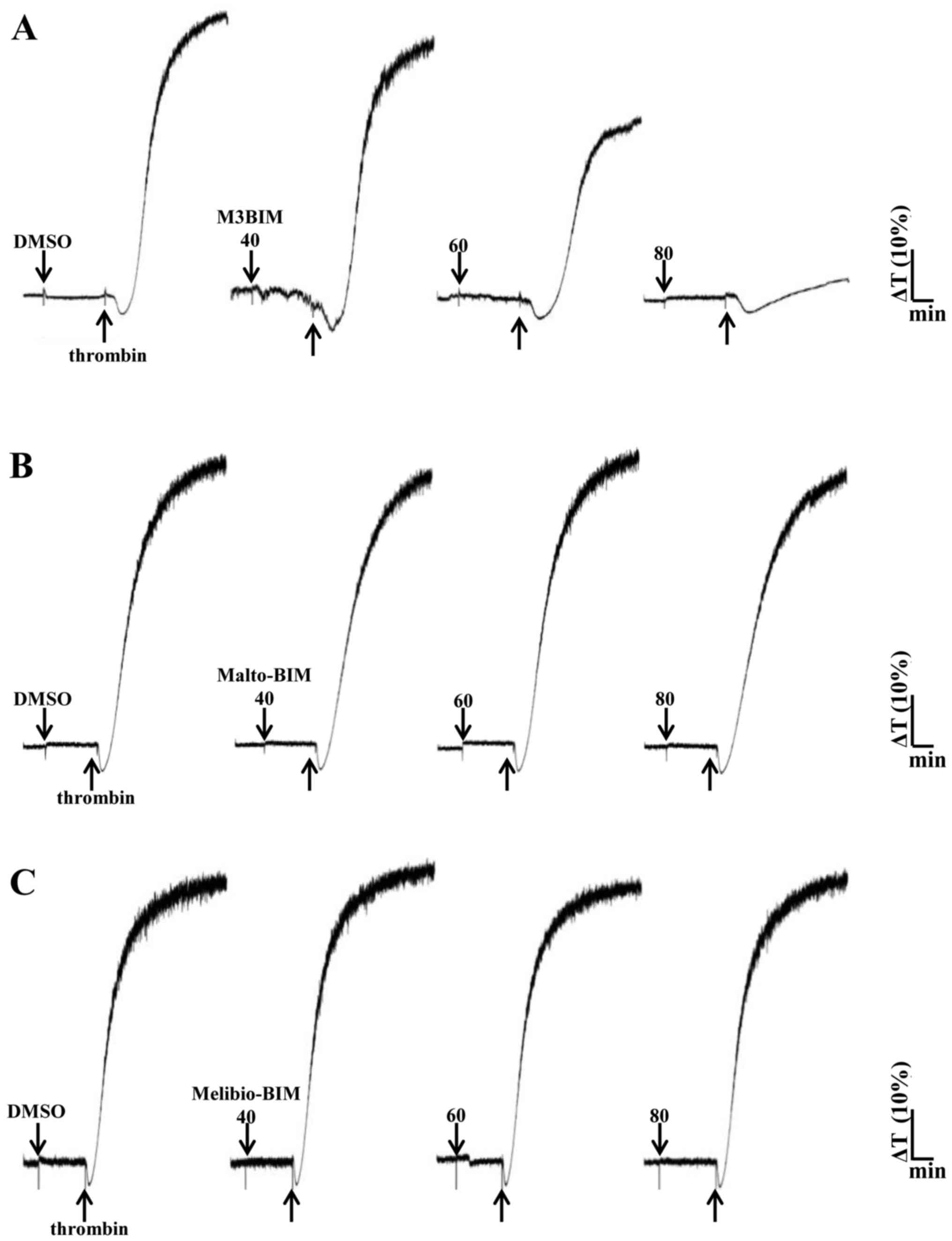
2A). We also tested the compound M3BIM against thrombin (0.01
U/ml)-induced platelet aggregation, and the results revealed that
M3BIM (40–80 μM) attenuated thrombin-induced platelet
aggregation (Fig. 3A) and
exhibiting similar pattern as collagen-induced aggregation
(Fig. 2A). However, the compounds
Malto-BIM and Melibio-BIM did not inhibit collagen (Fig. 2B and C) or thrombin-induced
platelet aggregation (Fig. 3B and
C). The observed inhibitory effects of M3BIM on agonist-induced
platelet aggregation may be attributed to its chemical structure;
namley the occurrence of a longer chain length (increased number of
sugar moieties) than that of the other two compounds.
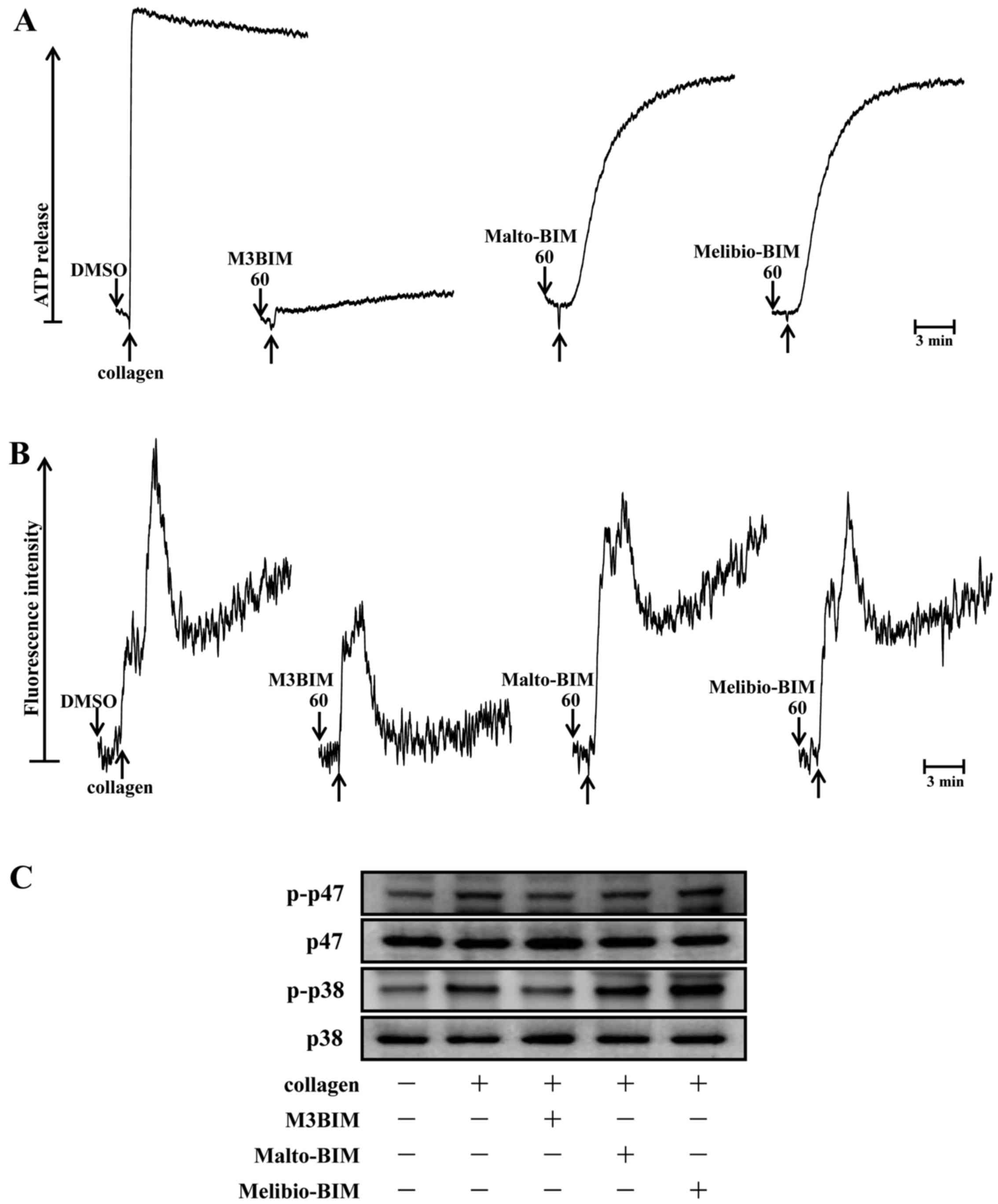
M3BIM suppresses collagen-induced ATP
release and intracellular calcium levels
Since a previous study reported that granule
secretions are critical markers of platelet activation prior to
aggregation (13), the present
study examined whether the test compounds M3BIM, Malto-BIM and
Melibio-BIM affect collagen-induced-platelet granule secretion.
Consistent with the aggregation experiment, only the compound M3BIM
(60 μM) significantly inhibited collagen-induced ATP release
in washed human platelets (Fig.
4A). This suggests that M3BIM attenuates platelet dense-granule
secretion.
It is well known that intracellular calcium ion
[Ca2+]i plays a critical role in
agonist-induced platelet aggregation (14). That is,
[Ca2+]i activates downstream signaling
molecules, and thus, it is the prerequisite for the full activation
of platelets. Therefore, we analyzed the inhibitory effects of the
test compounds on Ca2+ mobilization in human platelets.
When the platelets were stimulated with collagen, the level of
[Ca2+]i was significantly increased. However,
this was markedly diminished only by pre-treatment with M3BIM, but
not by Malto-BIM and Melibio-BIM (Fig. 4B). This result suggested that the
antiplatelet activity of M3BIM may be mediated by the inhibition of
cytoplasmic calcium increase.
M3BIM suppresses the phosphorylation of
p47 and p38 MAPK
To investigate the effects of newly synthesiszed
imidazole compounds on the downstream pathway of collagen-induced
PKC activation, the phosphorylation of p47 was measured in
collagen-stimulated platelets. The stimulation of platelets with a
number of different agonists induces the activation of PKC, which
then phosphorylates p47 protein (pleckstrin) (15). When collagen (1 μg/ml) was
added to human platelets, p47 protein was predominately
phosphorylated compared with resting platelets. Of the three
compounds tested, only compound M3BIM (60 μM) inhibited
collagen-induced p47 phosphorylation (Fig. 4C), whereas Malto-BIM (60
μM) and Melibio-BIM (60 μM) did not affect this
phosphorylation with the same concentration.
It has been well established that MAPKs, p38 MAPK,
JNK and ERK, are present in platelets and that they are activated
by various agonists (16). The
activation of MAPKs plays an important role in the secretion of
platelet granules. In this study, we determined whether
collagen-induced platelet p38 MAPK phosphorylation may be modulated
as a signaling pathway by the test compounds. Consistent with the
inhibitory effect of M3BIM on collagen-induced p47 phosphorylation,
this compound significantly abolished p38 MAPK phosphorylation as
shown in Fig. 4C. The other
compounds Malto-BIM and Melibio-BIM did not seem to be effective;
the fact that they were not effective may be attributed to the fact
that these compounds have a lower number (two) of sugar moieties in
the imidazole ring than M3BIM (three). These results suggest that
the antiplatelet effect of M3BIM may be mediated via the inhibition
of the activation of hte PKC/p38 MAPK signaling pathway.
Toxicity of M3BIM, Malto-BIM and
Melibio-BIM
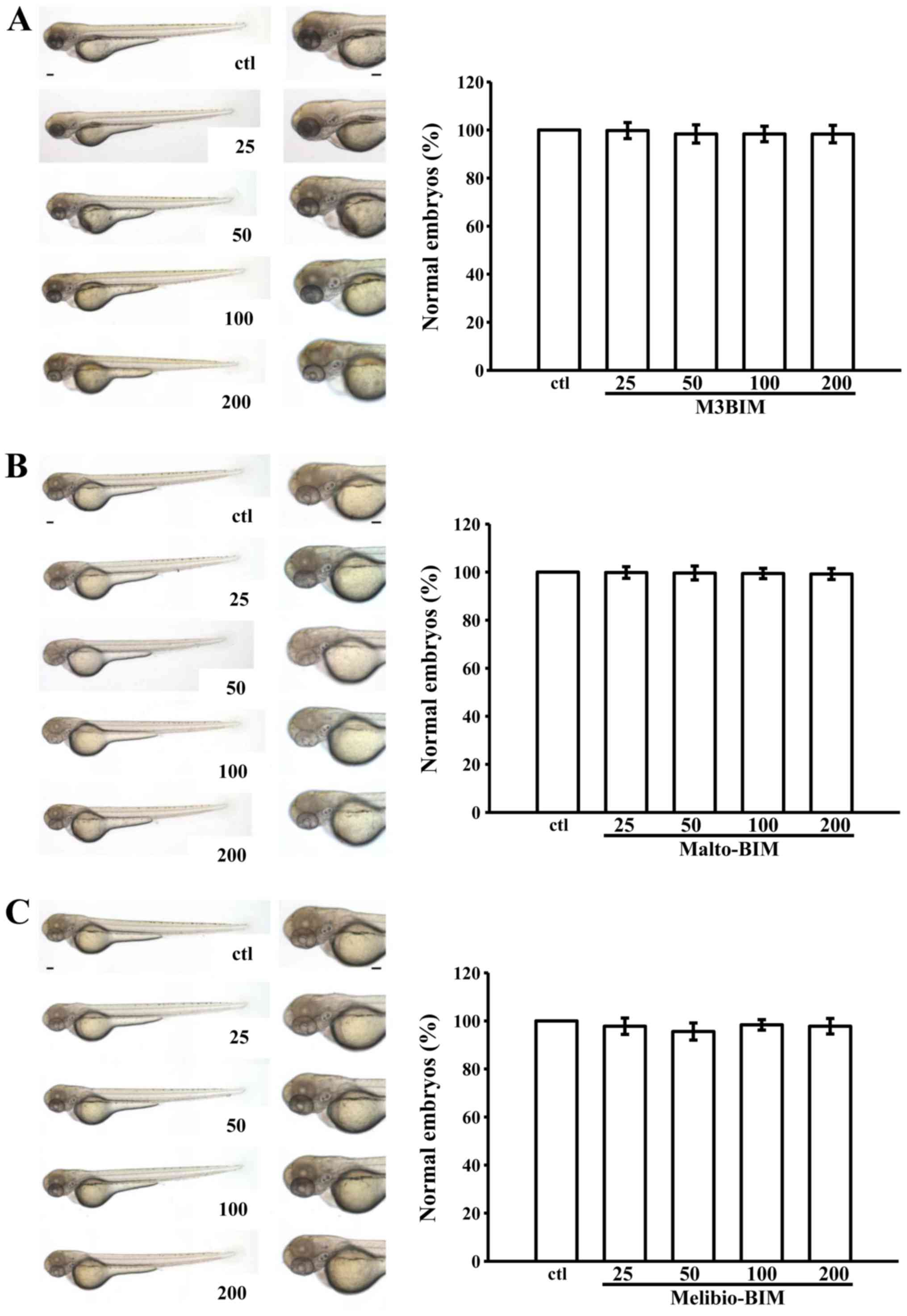
We assessed the toxic effects of M3BIM, Malto-BIM
and Melibio-BIM in wild-type zebrafish embryos treated for 3 days
post-fertilization with a range of doses of (25–200 μM). The
results revealed no significant phenotypic differences between the
solvent control (0.5% DMSO)- and test compound-treated zebrafish
embryos throughout the experiment (n=15) (Fig. 5). It is noteworthy that no
developmental defects or decreases in viability were observed in
the presence of M3BIM, Malto-BIM and Melibio-BIM, even at the
highest concentration of 200 μM. This indicates that M3BIM
exhibits antiplatelet activity without causing genotoxicity.
Discussion
Benzimidazole derivatives belong to selected
molecules that can be used to synthesize new substances with
various biological properties (17). The most noteworthy finding from
the present study was the direct antiplatelet potential of newly
derived imidazole-labeled saccharide compounds M3BIM, Malto-BIM and
Melibio-BIM in vitro. As collagen and thrombin are
considered to be potent agonists for platelet and a component of
subendothelial matrix in blood vessel, they were used to induce
platelet aggregation. Of the three compounds tested, only compound
M3BIM significantly and concentration-dependently impaired collagen
and thrombin-induced platelet aggregation. Moreover, Malto-BIM and
Melibio-BIM did not affect the responses stimulated either by
collagen or thrombin in human platelets. To elucidate the molecular
aspects of the antiplatelet effects of M3BIM, we further analyzed
downstream signaling using the intracellular Ca2+
concentration, dense granule secretions and protein
phosphorylations (e.g., p38 MAPK and p47).
Numerous studies have defined the role of dense
granules in platelets. A platelet contains approximately 2–7 dense
granules. The activation of platelets leads to the secretion of
dense granule components, such as ATP, ADP, calcium and serotonin
(18). These materials play a
crucial role in constricting damaged blood vessels and aggregating
platelets. Calcium is an important second messenger in the platelet
activation cascade. A previous study evidenced that collagen
activation in human platelets requires an increase in the levels of
[Ca2+]i (19). Another study confirmed that in
response to a moderate dose of collagen (10 μg/ml),
approximately 70% of the increase in [Ca2+]i
levels was due to the influx of Ca2+ from the
extracellular milieu, with the remainder as a function of
Ca2+ release from the dense tubular system (20). Our previous studies demonstrated
that the inhibition of relative intracellular [Ca2+]
mobilization and ATP-release reaction contributed to the
antiaggregant effects of natural substances against agonist induced
platelets aggregation (21–23). Rakesh et al (24) demonstrated that synthesized
ibuprofen derivatives exert antiplatelet effects by inhibiting
Ca2+. In this study, we found that M3BIM significantly
blocked collagen-activated calcium-ion mobilization and ATP
secretion to a lesser extent in human platelets (Fig. 4). This indicated that M3BIM
impaired dense granule secretion, which is an earlier phase of
platelet activation process.
It is well established that MAPKs, including ERKs,
JNKs, and p38 MAPK, are found in platelets (25) where they are activated by collagen
and thrombin, and are involved in thrombosis. ERK and p38 MAPK play
important roles in stimulating the secretion of granules and
facilitating clot retraction. During platelet activation, the
arachidonic acid (AA) metabolism may deal a positive feedback
amplifier to activate p38 MAPK, followed by the stimulation of
cytosolic phospholipase A2, which promotes thromboxane
A2 (TxA2) formation (26). In this study, we demonstrated that
the collagen-induced activation of p38 MAPK was inhibited by M3BIM,
but not by Malto-BIM and Melibio-BIM, suggesting that M3BIM
attenuated platelet activation, at least in part, through the p38
MAPK signaling pathway. PKC is rapidly activated in
agonist-stimulated platelets. It comprises a large family of
closely-related serine/threonine protein kinases, classed according
to their co-factor requirements. Conventional (α, βI, βII and γ)
family members are dependent on calcium, phospholipid and
diacylglycerol for activation, whereas non-conventional isoforms
[δ, ε, η (L), θ and μ] are insensitive to calcium. An often used
measure of PKC activation in agonist stimulated cells is the
phosphorylation of defined substrate proteins. In platelets and
other cells of hematopoietic origin, the major PKC substrate is the
p47 phosphoprotein, pleckstrin (platelet and leukocyte C kinase
substrate), which is rapidly phosphorylated in response to a
variety of agonists, including thrombin, thrombin receptor
activating peptide (TRAP), as well as phorbol ester (27). Previous studies have demonstrated
that natural compounds such as CME-1, a polysaccharide (23,28), sulforaphane, a naturally occurring
isothiocyanate (22), hinokitiol,
a tropolone derivatives (29),
and andrographolide, a labdane diterpene lactone (21), exert their antiplatelet effects
against various agonist-induced human platelets via the suppression
of p38 MAPK or p47 phosphoproteins. These results support the
findings of the present study in that the imidazole-derived
saccharide compound, M3BIM, inhibited collagen-induced platelet
activation and that this effect may be at least partly mediated via
the suppression of p38 MAPK and p47 protein phosphorylation.
Moreover, in the present study, the toxicity of
imidazole-derived saccharide compounds was tested using a zebrafish
toxicity method, as previously described (30). It is notable that no developmental
defects or decreases in viability were observed in the presence of
the test compounds, indicating that compared to the other two
compounds, M3BIM exerted its antiplatelet activity without causing
any genotoxicity. Recent studies have also used zebrafish assay to
examine the toxic effects of synthetic compounds, such as thiazole
compounds (31),
quinoline-derived trifluoromethyl alcohols (32) and brominated compounds (33) for the evaluation of their
respective biological effects.
A stable TxA2 metabolite, thromboxane
B2 (TxB2), is widely used as a prognostic
risk marker of platelet activation in cardiovascular disease, which
is closely related to cyclooxygenase (COX-1) and thromboxane
synthetase (TXS) activity (34).
A recent study found that a series of thioureas derivatives reduced
TxB2 production in human platelets via the direct
inhibition of COX-1 for their antiplatelet effects (35). Currently, the basic structural
requirements for the selective inhibition of TXS are a 1-imidazolyl
or a 3-pyridyl moiety at one end of the molecule (36). In addition, the increases of chain
length of bioactive molecules for improved lipophilicity have been
reported as a relevant parameter to inhibit human platelet
aggregation (37). Some
conceivable reasons may be drawn from the above-mentioned evidence
regarding the association between chemical structure and
antiplatelet activity of the tested compounds. It is clear that
among the three compounds tested, only compound M3BIM exerted a
greater inhibitory effect against collagen-induced platelet
aggregation. A plausible explanation of this effect that M3BIM
possesses 1-imidazolyl moiety at one end and has longer chain
length (three sugar moieties attached at one end in imidazole ring;
whereas Malto-BIM and Melibio-BIM hold only two sugar moieties).
This suggests that the number of sugar moiety in imidazole ring of
the test compounds is important for their antiplatelet activity.
Considering the only structural differences among the test
compounds, M3BIM has a longer chain length with an increased number
of free OH groups. Consistent with this structure, a previous study
stated that compounds with more free OH groups are critical for its
antiplatelet function (38).
However, additional mechanistic studies are warranted in order to
validate whether M3BIM directly inhibits TxA2 via
suppressing COX-1 and TXS or its OH group play role to exert its
antiplatelet effect.
In conclusion, our study indicates that among the
three newly synthesized imidazole-based saccharide compounds, only
compound M3BIM exerted a potent inhibitory effect against induced
in vitro platelet aggregation. A noteworthy finding of this
study was that the antiplatelet activity of M3BIM may originally
obstruct the phosphorylation of p38 MAPK and p47, then inhibit
intracellular [Ca2+] mobilization and ATP release
reaction, and eventually, inhibit platelet activation. As compounds
with anti-platelet property stand better and receive much attention
by the medical practitioners in order to treat thrombolytic
disorders, the results of the present study may contribute to a
better understanding of benzimidazole derivatives for their
platelet protective properties.
Acknowledgments
The present study was supported by grants (nos.
MOST103-2320-B-038-017, MOST104-2622-B-038-003, and MOST
104-2320-B-038-045-MY2) from the Ministry of Science and Technology
of Taiwan, the Wan-Fang Hospital-Taipei Medical University (no.
102TMU-WFH-02-1), and Shin Kong Wu Memorial Hospital (no.
SKH-8302-103-DR-28).
References
|
1
|
Murray CJ and Lopez AD: Mortality by cause
for eight regions of the world: Global Burden of Disease Study.
Lancet. 349:1269–1276. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tantry US, Bliden KP and Gurbel PA:
Resistance to anti-platelet drugs: Current status and future
research. Expert Opin Pharmacother. 6:2027–2045. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cox D: Oral GPIIb/IIIa antagonists: What
went wrong? Curr Pharm Des. 10:1587–1596. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herbert JM, Hérault JP, Bernat A, Savi P,
Schaeffer P, Driguez PA, Duchaussoy P and Petitou M: SR123781A, a
synthetic heparin mimetic. Thromb Haemost. 85:852–860.
2001.PubMed/NCBI
|
|
5
|
Petitou M, Hérault JP, Bernat A, Driguez
PA, Duchaussoy P, Lormeau JC and Herbert JM: Synthesis of
thrombin-inhibiting heparin mimetics without side effects. Nature.
398:417–422. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dogné JM, Hanson J, de Leval X, Masereel
B, Kolh P and Pirotte B: New developments on thromboxane
modulators. Mini Rev Med Chem. 4:649–657. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Z, Arnaiz DO, Griedel B, Sakata S,
Dallas JL, Whitlow M, Trinh L, Post J, Liang A, Morrissey MM and
Shaw KJ: Design, synthesis, and in vitro biological activity of
benzimidazole based factor Xa inhibitors. Bioorg Med Chem Lett.
10:963–966. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kucheryavenko AF, Spasov AA, Petrov VI and
Anisimova VA: Antiaggregant activity of a new benzimidazole
derivative. Bull Exp Biol Med. 156:796–798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Greinacher A, Alban S, Dummel V, Franz G
and Mueller-Eckhardt C: Characterization of the structural
requirements for a carbohydrate based anticoagulant with a reduced
risk of inducing the immunological type of heparin-associated
thrombocytopenia. Thromb Haemost. 74:886–892. 1995.PubMed/NCBI
|
|
10
|
Lin C, Lai PT, Liao SK, Hung WT, Yang WB
and Fang JM: Using molecular iodine in direct oxidative
condensation of aldoses with diamines: An improved synthesis of
aldo-benzimidazoles and aldo-naphthimidazoles for carbohydrate
analysis. J Org Chem. 73:3848–3853. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sheu JR, Lee CR, Lin CH, Hsiao G, Ko WC,
Chen YC and Yen MH: Mechanisms involved in the antiplatelet
activity of Staphylococcus aureus lipoteichoic acid in human
platelets. Thromb Haemost. 83:777–784. 2000.PubMed/NCBI
|
|
12
|
Westerfield M: The Zebrafish Book: A Guide
for the Laboratory Use of Zebrafish (Brachydanio rerio). University
of Oregon Press; Eugene, OR: 1993
|
|
13
|
Mackman N: Triggers, targets and
treatments for thrombosis. Nature. 451:914–918. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang WS, Chung KH, Chung JH, Lee JY, Park
JB, Zhang YH, Yoo HS and Yun YP: Antiplatelet activity of green tea
catechins is mediated by inhibition of cytoplasmic calcium
increase. J Cardiovasc Pharmacol. 38:875–884. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tyers M, Rachubinski RA, Stewart MI,
Varrichio AM, Shorr RG, Haslam RJ and Harley CB: Molecular cloning
and expression of the major protein kinase C substrate of
platelets. Nature. 333:470–473. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toth-Zsamboki E, Oury C, Cornelissen H, De
Vos R, Vermylen J and Hoylaerts MF: P2X1-mediated ERK2 activation
amplifies the collagen-induced platelet secretion by enhancing
myosin light chain kinase activation. J Biol Chem. 278:46661–46667.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nofal ZM, Soliman EA, Abd El-Karim SS, El
Zahar MI, Srour AM, Sethumadhavan S and Maher TJ: Novel
benzimidazole derivatives as expected anticancer agents. Acta Pol
Pharm. 68:519–534. 2011.PubMed/NCBI
|
|
18
|
Unsworth AJ, Smith H, Gissen P, Watson SP
and Pears CJ: Submaximal inhibition of protein kinase C restores
ADP-induced dense granule secretion in platelets in the presence of
Ca2+. J Biol Chem. 286:21073–21082. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roberts DE, McNicol A and Bose R:
Mechanism of collagen activation in human platelets. J Biol Chem.
279:19421–19430. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roberts DE and Bose R: Reverse mode
Na+/Ca2+ exchange in the collagen activation of human
platelets. Ann NY Acad Sci. 976:345–349. 2002. View Article : Google Scholar
|
|
21
|
Lu WJ, Lee JJ, Chou DS, Jayakumar T, Fong
TH, Hsiao G and Sheu JR: A novel role of andrographolide, an
NF-kappa B inhibitor, on inhibition of platelet activation: The
pivotal mechanisms of endothelial nitric oxide synthase/cyclic GMP.
J Mol Med (Berl). 89:1261–1273. 2011. View Article : Google Scholar
|
|
22
|
Jayakumar T, Chen WF, Lu WJ, Chou DS,
Hsiao G, Hsu CY, Sheu JR and Hsieh CY: A novel antithrombotic
effect of sulforaphane via activation of platelet adenylate
cyclase: Ex vivo and in vivo studies. J Nutr Biochem. 24:1086–1095.
2013. View Article : Google Scholar
|
|
23
|
Chang Y, Hsu WH, Lu WJ, Jayakumar T, Liao
JC, Lin MJ, Wang SH, Geraldine P, Lin KH and Sheu JR: Inhibitory
mechanisms of CME-1, a novel polysaccharide from the mycelia of
Cordyceps sinensis, in platelet activation. Curr Pharm Biotechnol.
16:451–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rakesh KS, Jagadish S, Vinayaka AC,
Hemshekhar M, Paul M, Thushara RM, Sundaram MS, Swaroop TR, Mohan
CD, Basappa, et al: A new ibuprofen derivative inhibits platelet
aggregation and ROS mediated platelet apoptosis. PLoS One.
9:e1071822014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bugaud F, Nadal-Wollbold F, Lévy-Toledano
S, Rosa JP and Bryckaert M: Regulation of c-jun-NH2 terminal kinase
and extracellular-signal regulated kinase in human platelets.
Blood. 94:3800–3805. 1999.PubMed/NCBI
|
|
26
|
Coulon L, Calzada C, Moulin P, Véricel E
and Lagarde M: Activation of p38 mitogen-activated protein
kinase/cytosolic phospholipase A2 cascade in hydroperoxide-stressed
platelets. Free Radic Biol Med. 35:616–625. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tyers M, Haslam RJ, Rachubinski RA and
Harley CB: Molecular analysis of pleckstrin: The major protein
kinase C substrate of platelets. J Cell Biochem. 40:133–145. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu WJ, Chang NC, Jayakumar T, Liao JC, Lin
MJ, Wang SH, Chou DS, Thomas PA and Sheu JR: Ex vivo and in vivo
studies of CME-1, a novel polysaccharide purified from the mycelia
of Cordyceps sinensis that inhibits human platelet activation by
activating adenylate cyclase/cyclic AMP. Thromb Res. 134:1301–1310.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin KH, Kuo JR, Lu WJ, Chung CL, Chou DS,
Huang SY, Lee HC and Sheu JR: Hinokitiol inhibits platelet
activation ex vivo and thrombus formation in vivo. Biochem
Pharmacol. 85:1478–1485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He N, Li X, Feng D, Wu M, Chen R, Chen T,
Chen D and Feng X: Exploring the toxicity of a bismuth-asparagine
coordination polymer on the early development of zebrafish embryos.
Chem Res Toxicol. 26:89–95. 2013. View Article : Google Scholar
|
|
31
|
Chen MC, Zhou B, Zhang K, Yuan YC, Un F,
Hu S, Chou CM, Chen CH, Wu J, Wang Y, et al: The novel
ribonucleotide reductase inhibitor COH29 inhibits DNA repair in
vitro. Mol Pharmacol. 87:996–1005. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sittaramane V, Padgett J, Salter P,
Williams A, Luke S, McCall R, Arambula JF, Graves VB, Blocker M,
Van Leuven D, et al: Discovery of quinoline-derived trifluoromethyl
alcohols, determination of their in vivo toxicity and anticancer
activity in a zebrafish embryo model. ChemMedChem. 10:1802–1807.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang J and Chan KM: Evaluation of the
toxic effects of brominated compounds (BDE-47, 99, 209, TBBPA) and
bisphenol A (BPA) using a zebrafish liver cell line, ZFL. Aquat
Toxicol. 159:138–147. 2015. View Article : Google Scholar
|
|
34
|
Sathler PC, Santana M, Lourenço AL,
Rodrigues CR, Abreu P, Cabral LM and Castro HC: Human thromboxane
synthase: Comparative modeling and docking evaluation with the
competitive inhibitors Dazoxiben and Ozagrel. J Enzyme Inhib Med
Chem. 29:527–531. 2014. View Article : Google Scholar
|
|
35
|
Lourenço AL, Saito MS, Dorneles LE, Viana
GM, Sathler PC, Aguiar LC, de Pádula M, Domingos TF, Fraga AG,
Rodrigues CR, et al: Synthesis and antiplatelet activity of
antithrombotic thiourea compounds: Biological and
structure-activity relationship studies. Molecules. 20:7174–7200.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liedtke AJ, Crews BC, Daniel CM, Blobaum
AL, Kingsley PJ, Ghebreselasie K and Marnett LJ:
Cyclooxygenase-1-selective inhibitors based on the
(E)-2′-des-methyl-sulindac sulfide scaffold. J Med Chem.
55:2287–2300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reyes JJ, De La Cruz JP, Muñoz-Marin J,
Guerrero A, Lopez-Villodres JA, Madrona A, Espartero JL and
Gonzalez-Correa JA: Antiplatelet effect of new lipophilic
hydroxytyrosol alkyl ether derivatives in human blood. Eur J Nutr.
52:591–599. 2013. View Article : Google Scholar
|
|
38
|
Guo C, Liu S, Guo Y, Yin Y, Lin J, Chen X
and Sun MZ: Comparative function-structural analysis of
antiplatelet and antiradical activities of flavonoid
phytochemicals. J Anim Plant Sci. 24:926–935. 2014.
|