Introduction
Hair is one of the unique characteristics of mammals
and is a marker of individual health as it serves multiple
physiological functions, including protecting the body from
environmental insults and providing thermal regulation (1). Alopecia, or spot baldness, is a
common and incurable disease that can appear early in life, for
which there are limited treatment options (2). Traditional Chinese herbal medicines,
including Scutellaria baicalensis have been used as
treatments for hair loss and may be advantageous as they are safe,
have minimal toxicity and fewer side effects, and are economical.
Baicalin is an active ingredient that is found in several species
in the genus Scutellaria, including Scutellaria
baicalensis (3). It has also
been reported that Baicalin exerts potent biological activities,
including antiviral (4) and
antitumor effects (5). A study by
Shin et al (6) revealed
that topical use of baicalin promoted anagen induction in C57BL/6
mice by increasing the activity of dermal papilla cells. Another
study by a Chinese research group demonstrated that baicalin
promotes the growth of cultured human scalp hair follicles in
vitro, likely by inducing the secretion of vascular endothelial
growth factor (VEGF) from dermal papilla cells (7). Baicalin has been reported to
activate the Wnt/β-catenin signaling pathway and increase the
activity of alkaline phosphatase (ALP) in dermal papillar cells
(DPCs), which facilitates their differentiation (6,8).
In the present study, a novel model was devised by dissecting the
dermis and epidermis of neonatal mice, obtaining single cells from
each and then grafting the mixture of cells onto the dorsa of
immunodeficient mice in specified proportions (9). This reconstituted model of mouse
hair follicle growth accurately simulates the induction,
organogenesis and cell differentiation stages of hair follicle
morphogenesis (10,11). In order to elucidate the molecular
mechanism(s) underlying baicalin-induced hair growth, baicalin was
applied topically to the reconstituted hair follicles and its
effects on canonical Wnt signaling and dermal papilla cell activity
was evaluated.
Materials and methods
Animal experiments
A total of 45 6-week-old female BALB/c-nu mice
(weight, 18–20 g) were purchased from Vital River Corporation
(Beijing, China) and housed at a constant temperature (23°C) and
humidity (55%) with a 12 h light/dark cycle and free access to food
and water. A total of 45 1-day-old female C57BL/6 mice (mean
weight, 1.3±0.21 g) were purchased from the Centre of Disease
Control of Hubei Province (Wuhan, China) and housed as above.
Dermal and epidermal cells were isolated from skin of C57BL/6 mice
grafted to excision wounds on the dorsa of BALB/c-nu mice in a 1:1
ratio, on which a silicon chamber (cylindrical shape, 20 mm inner
diameter and 19.07 mm height, with a 2.5 mm hole bored through the
top; Cole Equipment Co., Ormond Beach, FL, USA) was implanted as
described previously (9,10). The number of dermal and epidermal
cells injected into a silicon chamber was 2.5×106 each.
All procedures followed the protocols described previously
(9,10). Following surgery, BALB/c-nu mice
were divided into five groups: The negative control group, treated
with vehicle; the positive control group, treated with 100
μmol of Minoxidil (Wuhan Galaxy Pharmaceutical Chemical Raw
Materials Co., Ltd., Wuhan, China); the B50 group, treated with 50
μmol of baicalin (Chengdu Must Bio-Technology Co., Ltd.,
Chengdu, China); the B100 group, treated with 100 μmol of
baicalin; and the B + IWR-1 group, treated with 100 μmol
baicalin + 1 μmol IWR-1 (Sigma Aldrich; Merck KGaA,
Darmstadt, Germany). All reagents were dissolved in 50% ethanol in
PBS. An equal amount of the aqueous solution (50% ethanol in PBS)
was used as the vehicle control. At 1 week post-surgery, the domes
of the silicon chambers were removed. At 2 weeks post-surgery, when
black hair began to emerge on the dorsa of the mice, the treatments
were initiated by applying 100 μl of respective treatment
solutions or vehicle to the wound site once daily for 2 weeks.
Images were captured 0, 14 and 28 days following grafting. On day
28 the mice were sacrificed and the dorsal skins were harvested and
flash-frozen in liquid nitrogen or fixed in 4% formaldehyde at room
temperature for 48 h. The number of hair follicles in the 10×10 mm
wound site was quantified using ImageJ densitometry software ImageJ
(Version 1.46r; National Institutes of Health, Bethesda, MD, USA).
All experiments were performed according to the guidelines of the
National Institutes of Health and the protocol was approved by the
Institutional Animal Care and Use Committee of Wuhan University
Laboratory Animal Research Center (Wuhan, China; permit number:
11203A).
Histology
Formaldehyde-fixed paraffin-embedded dorsal skins
were hydrated with ethanol and 4 μm sections were cut
longitudinally along the hair follicles, which were subsequently
stained with hematoxylin and eosin (H&E) for 5 and 2 min at
room temperature, respectively. Digital images were captured using
a Nikon E100 light microscope (Nikon, Tokyo, Japan) at ×100 and
×200 magnification.
Histochemistry
Cryosections (6 μm) were placed in deionized
water for 5 min at 20°C, and stained using the BCIP/NBT kit
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) at room temperature for 30 min according to the
manufacturer’s protocol. Digital images captured using an Olympus
BX53 light microscope (Olympus Corp., Tokyo, Japan) at ×40 and ×100
magnification.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the skin tissues using
TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer’s protocol. Total RNA (2.5 μg)
was reverse-transcribed (25°C for 5 min, 50°C 15 for min, 85°C for
5 min and 4°C for 10 min) using the HiScript Reverse Transcriptase
(RNase H) kit (Vazyme, Piscataway, NJ, USA) according to the
manufacturer’s protocol. In order to determine the internal control
(β-actin), the semi-quantitative RT-PCR was performed using the
following temperature protocol: Denaturation at 94°C for 30 sec,
annealing at 56°C for 30 sec, extension at 72°C for 25 sec for 30
cycles, and a final extension at 72°C for 4 min. RT-qPCR was
performed using SYBR-Green Master Mix (Vazyme). Thermoycling
conditions were as follows: 2 min at 50°C and 10 min at 95°C
followed by 40 cycles of 95°C for 30 sec and 60°C for 30 sec. The
following primers were used: β-actin, forward
5′-CACGATGGAGGGGCCGGACTCATC-3′ and reverse
5′-TAAAGACCTCTATGCCAACACAGT-3′; Wnt3a, forward
5′-GGGGATTTCCTCAAGGACAAGT-3′ and reverse
5′-TTAGGTTCGCAGAAGTTGGGTG-3′; Wnt5a, forward
5′-AAAGGGAACGAATCCACGCTAA-3′ and reverse
5′-CCAGACACTCCATGACACTTACAG-3′; frizzled 7, forward
5′-GGCGTCTTCAGCGTGCTC TAC-3′ and reverse
5′-CCCACGATCATGGTCATCAGGTAC-3′; disheveled 2, forward
5′-CCATCCCAAACGCCTTTCTAG-3′ and reverse
5′-AGTAATCTTGTTGACGGTGTGC-3′; glycogen synthase kinase (GSK)3β,
forward 5′-ATCCTTATCCCTCCACATGCTCG-3′ and reverse
5′-CGTTATTGGTCTGTCCACGGTCT-3′; β-catenin, forward
5′-GTGCTGGTGACAGGGAAGACA-3′ and reverse
5′-GGATGGTGGGTGCAGGAGTTT-3′; lymphoid enhancing binding factor 1
(LEF1), forward 5′-ATCAAATAAAGTGCCCGTGGTGC-3′ and reverse
5′-CTGGACATGCCTTGCTTGGAGTT-3′; and alkaline phosphatase (ALP),
forward 5′-GCAAGGACATCGCATATCAGCTAA-3′ and reverse
5′-TTCAGTGCGGTTCCAGACATAG-3′. Differences between the samples and
controls were analyzed by RT-qPCR using amplification curve. The
relative amount of mRNA was calculated using the 2−ΔΔCq
method with β-actin as the reference gene (12).
Western blotting
The nuclear and cytoplasmic reagent kit (KGP150;
Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) was used to
extract nuclear and cytoplasmic proteins according to the
manufacturer’s protocol. A total of 40 μg per lane was
separated by % SDS-PAGE and transferred onto a polyvinylidene
fluoride membrane. After blocking with 5% nonfat dry milk in TBST
for 2 h at room temperature, the membrane was incubated with
primary antibodies against the following: β-actin (BM0627; 1:200),
lamin (BA1228; 1:200), Wnt3a (BA2628-2; 1:300), Wnt5a (155184-1-AP;
1:1,000) frizzled 7 (16974-1-AP; 1:2,000), disheveled 2
(12037-1-AP; 1:2,000), GSK3β 1 (22104-1-AP; 1:2,000), β-catenin
(51067-2-AP; 1:5,000) LEF1 (14972-1-AP; 1:500) and ALP (11400-1-AP;
1:1,000; all Wuhan Proteintech Co., Ltd., Wuhan, China) overnight
at 4°C. Horseradish peroxidase-conjugated goat anti-mouse
antibodies (BA1051; 1:50,000; Wuhan Boster Biological Technology
Co., Ltd., Wuhan, China) were used as the secondary antibody and
were incubate with the membrane at room temperature for 2 h. The
membranes were washed five times for 5 min each time with TBST, and
developed using an ECL Plus kit (NCI5079; Thermo Fisher Scientific,
Inc.) according to the manufacturer’s protocol. The relative band
densities were determined using a BandScan system version 4.30
(Thermo Fisher Scientific, Inc.).
Statistical analysis
Statistical analyses were performed with SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard deviation. Groups were compared using a one-way
analysis of variance with a post hoc Student-Newman-Keul test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of baicalin on the morphology of
developing hair follicles
The progression of hair follicle development in mice
was evaluated by images captured on the day of grafting and 14 and
28 days following engraftment. The hair shaft emerged on the mice
dorsa at 14 days post-engraftment (Fig. 1A). This point represents the
initiation of the anagen stage of the hair follicle cycle, at which
point the treatment regime was begun. On day 28, following 14 days
of treatment, the content of the hair shafts was significantly
increased in the B50, B100 and Minoxidil-treated groups compared
with the control group (Fig. 1B and
C). The baicalin + IWR-1 group exhibited significantly fewer
hair shafts compared with the control (Fig. 1B and C), which was expected as Wnt
signaling is inhibited by IWR-1 (13). To further assess the number and
size of hair follicles, skin biopsy sections were obtained from the
mice and stained with H&E. The skin biopsy analysis confirmed
that the number and size of hair follicles were markedly increased
in the B50, b100 and Minoxidil-treated group compared with the
control group, whereas the B + IWR-1 group had the fewest and
smallest hair follicles (Fig.
1D). No apparent differences in the histology were observed
between the B50, B100 and Minoxidil-treated groups, although both
were distinguishable from the control and B + IWR-1 groups
(Fig. 1D).
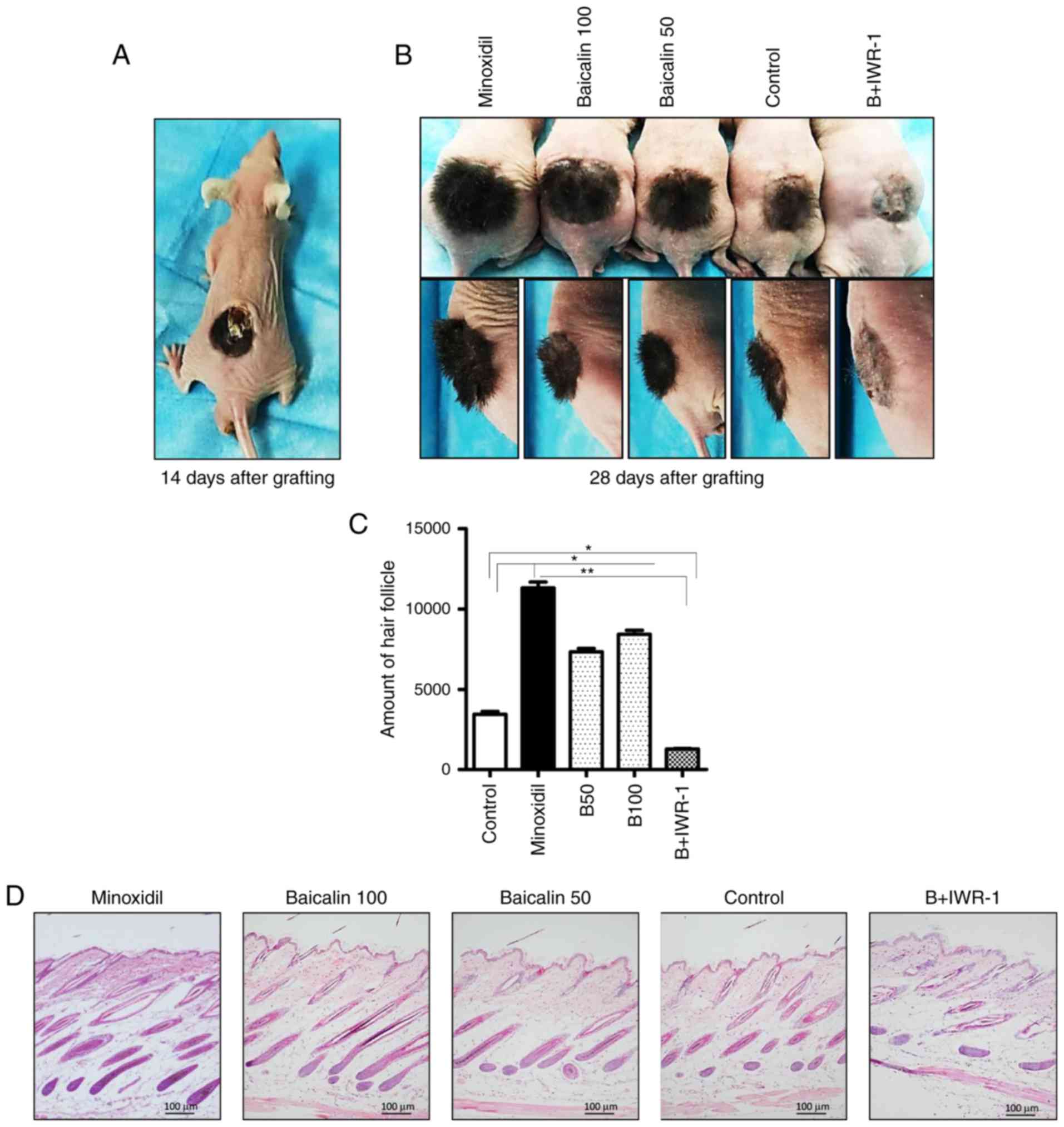 | Figure 1Morphological features of hair
follicles on mice dorsa. Female BALB/c-nu mice were grafted with
isolated dermal and epidermal cells from the skin of C57BL/6 mice.
A 2 weeks post-grafting, the appropriate topical treatments were
applied once daily to mice in each group. (A) Representative image
of a mouse on day 14 following engraftment, pre-treatment. (B)
Representative frontal and lateral images of mice in the Minoxidil,
B100, B50, control and B + IWR-1 groups on day 28 following
engraftment. (C) The number of hair follicles in each group. (D)
Representative histological images of hematoxylin and eosin stained
dorsal skin sections (magnification, ×100). Data are presented as
the mean ± standard error of the mean from 3 independent
experiments. Control mice were treated with the vehicle only.
*P<0.05 and **P<0.01. Minoxidil, 100
μmol Minoxidil treatment; B50, 50 μmol baicalin
treatment; B100, 100 μmol baicalin treatment; B + IWR-1, 100
μmol baicalin and 1 μmol IWR-1 treatment. |
Baicalin modulates the Wnt/β-catenin
pathway in mice
To determine whether baicalin modulates the
Wnt/β-catenin pathway in developing hair follicles, the expression
of pathway components were measured at the mRNA and protein levels.
The mRNA levels of Wnt3a, Wnt5a, frizzled 7, disheveled 2,
β-catenin and LEF1 were significantly increased in the B50, B100
and Minoxidil-treated groups compared with the control and B +
IWR-1 groups, whereas the mRNA level of GSK3β was significantly
decreased in the B50, B100 and Minoxidil-treated groups compared
with the control and B + IWR-1 groups (Fig. 2). Western blotting results were
similar to the mRNA levels, as protein levels of Wnt3a, Wnt5a,
frizzled 7, disheveled 2, β-catenin (cytoplasmic and nuclear) and
LEF1 were significantly increased in the B50, B100 and
Minoxidil-treated groups compared with the control and B + IWR-1
groups, whereas the level of GSK3β protein was significantly
decreased in the B50, B100 and Minoxidil-treated groups compared
with the control and B + IWR-1 groups (Fig. 3).
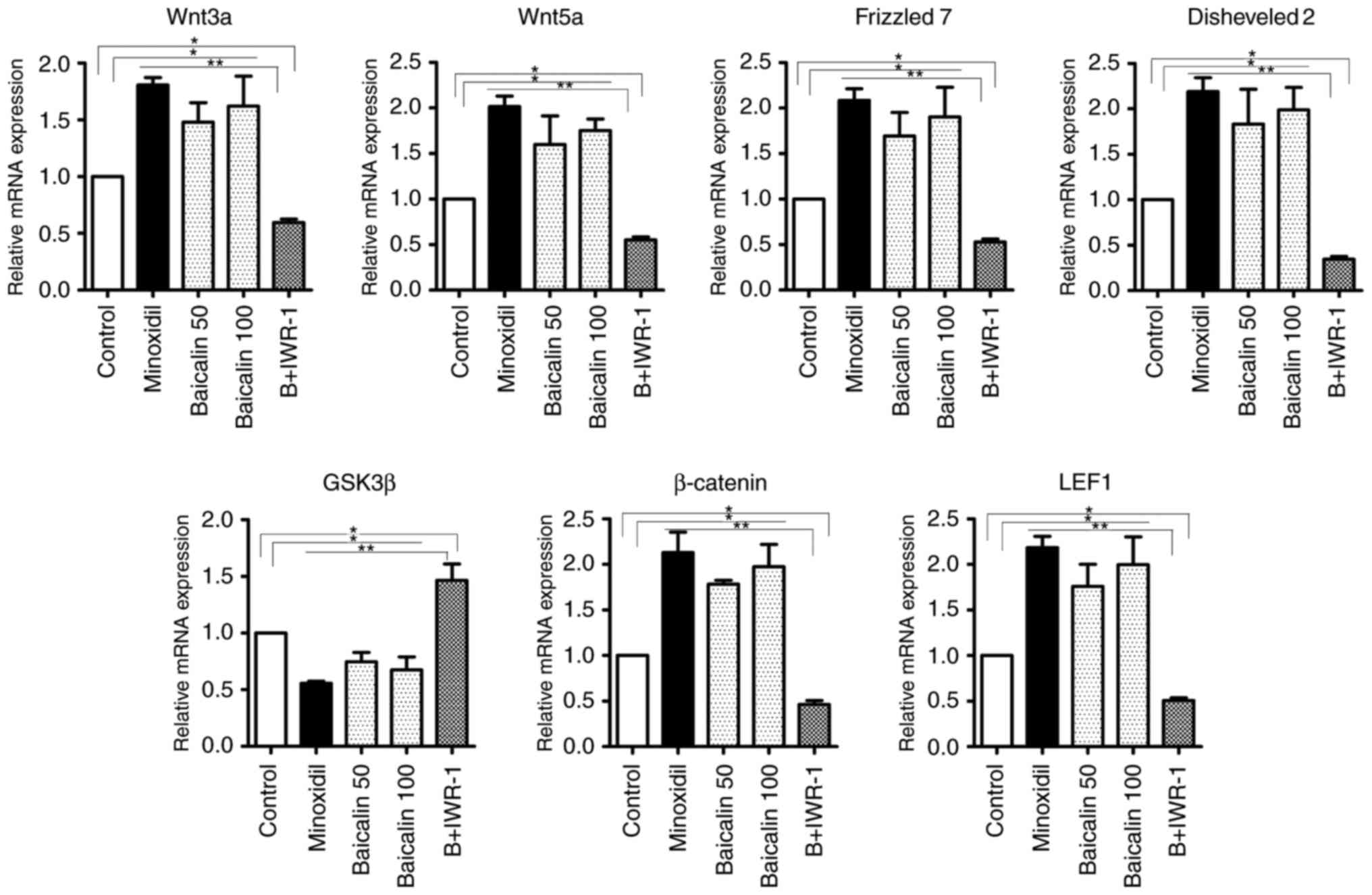 | Figure 2Effects of baicalin on the expression
of genes associated with hair growth in reconstituted mouse skin
tissue. Levels of Wnt3a, Wnt5a, frizzled 7, disheveled 2, GSK3β,
β-catenin and LEF1 mRNA in reconstituted mouse skin tissue as
measured using reverse transcription polymerase chain reaction.
Control mice were treated with the vehicle only.
*P<0.05 and **P<0.01. GSK, glycogen
synthase kinase; LEF, lymphoid enhancing binding factor; Minoxidil,
100 μmol Minoxidil treatment; B50, 50 μmol baicalin
treatment; B100, 100 μmol baicalin treatment; B+IWR-1, 100
μmol baicalin and 1 μmol IWR-1 treatment. |
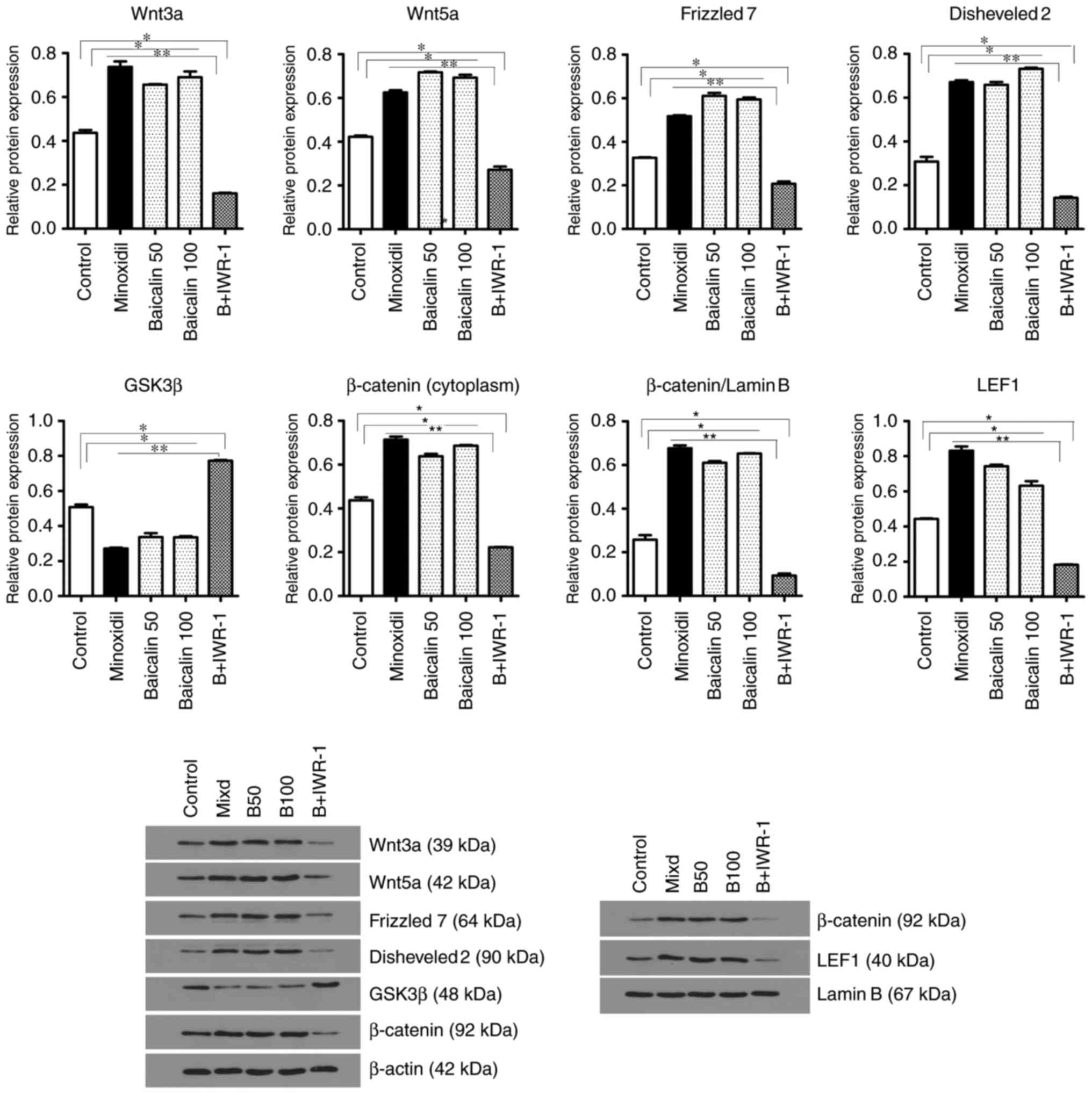 | Figure 3Effects of baicalin on the expression
of genes associated with hair growth in reconstituted mouse skin
tissue. Levels of Wnt3a, Wnt5a, frizzled 7, disheveled 2, GSK3β,
β-catenin and LEF1 protein in reconstituted mouse skin tissue as
measured using western blotting. Control mice were treated with the
vehicle only. *P<0.05 and **P<0.01.
GSK, glycogen synthase kinase; LEF, lymphoid enhancing binding
factor; Minoxidil, 100 μmol Minoxidil treatment; B50, 50
μmol baicalin treatment; B100, 100 μmol baicalin
treatment; B+IWR-1, 100 μmol baicalin and 1 μmol
IWR-1 treatment. |
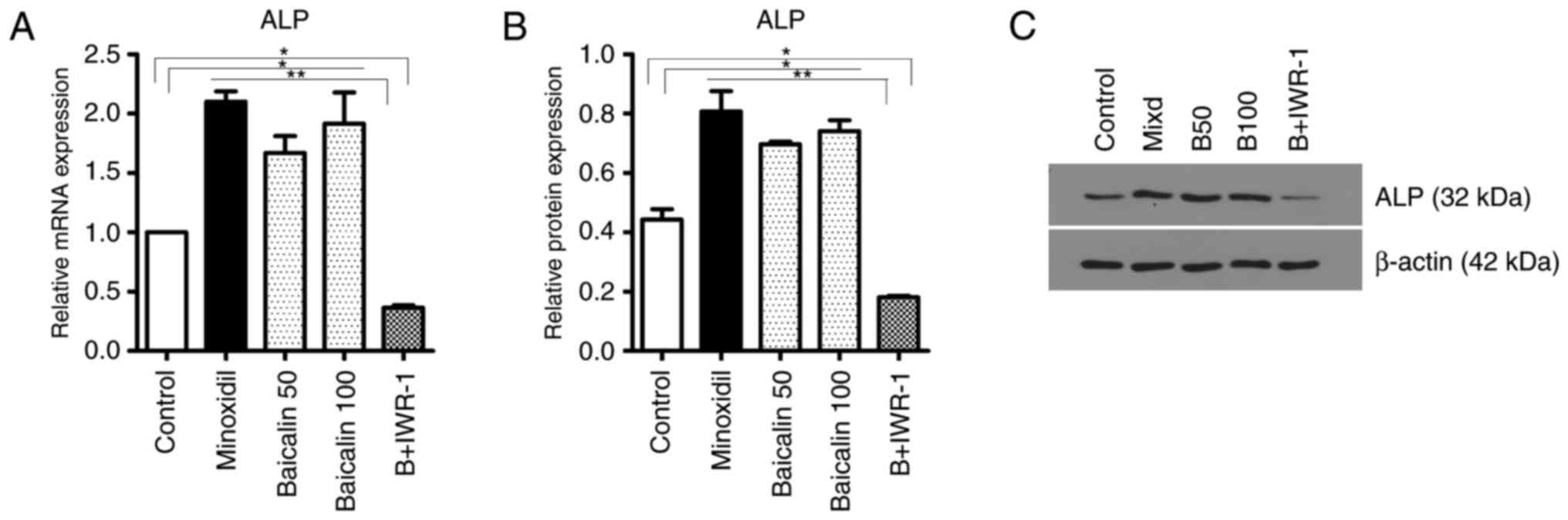
Baicalin upregulates the activity of DPCs
in mice
ALP is a marker of DPCs and its expression level is
representative of the activity of DPCs. The mRNA levels of ALP were
significantly increased in the skin tissues of mice in the B50,
B100 and Minoxidil-treated groups compared with the control and B +
IWR-1 groups (Fig. 4A). The level
of ALP was also significantly increased in the skin tissues of the
B50, B100 and Minoxidil-treated groups compared with the control
and B + IWR-1 groups (Fig. 4B and
C). Furthermore, and consistent with the prior results, ALP
activity in hair follicles as detected by staining was markedly
increased in the B100 compared with the control group (Fig. 4D).
Discussion
Alopecia is a common and incurable disease that
affects ~2% of all people worldwide at some point in their
lifetime, for which there is currently no effective treatment
(14). Based on its
pharmaceutical properties and hair follicle growth promoting
activity, baicalin, a primary active constituent of the traditional
Chinese herbal medicine Scutellaria baicalensis may be an
effective alopecia treatment. In the present study, baicalin was
topically applied to reconstituted hair follicles on mice dorsa to
investigate its effects on canonical Wnt/β-catenin signaling as
well as its effects on DPCs. The dosages of baicalin selected 50
and 100 μmol were based on a previous study (6), in which dosages of 5 to 100
μmol baicalin were found to be nontoxic to DPCs as assessed
using an MTT cell viability assay. The administration of Minoxidil
and IWR-1, which is an antagonist of Wnt signaling, was also
performed in accordance with prior reports (15,16).
The results of the present study demonstrate that
baicalin promotes the growth of hair follicles likely via
activation of Wnt/β-catenin signaling and increasing the ALP
activity of DPCs in mice. Furthermore, baicalin was not able to
overcome the Wnt/β-catenin signaling antagonism or ameliorate the
inhibition of hair follicle growth caused by IWR-1 in mice. This
supports the hypothesis that baicalin promotes hair follicle
development by activating the Wnt pathway.
Wnt pathway activity is essential for hair follicle
development and the hair cycle (17–20). The canonical Wnt pathway depends
on intracellular β-catenin, whose signal transduction cascade
components in the hair follicle include Wnt proteins, frizzled
receptors and the co-receptor low-density lipoprotein receptor
(LRP)5/6, disheveled proteins, the multicomponent degradation
complex [comprising Axin, casein kinase (CK)1α, adenomatous
polyposis coli (APC) and GSK3β], β-catenin and transcription factor
(TCF)/LEF (21). In the absence
of Wnt ligands, the Axin/CK1α/APC/GSK3β degradation complex
phosphorylates cytoplasmic β-catenin, leading to
ubiquitin-dependent degradation of β-catenin (Fig. 5A). When extracellular Wnt ligands
bind to the transmembrane frizzled receptors and co-receptors
LRP5/6, disheveled proteins bind to the intracellular domains of
the receptors, sequestering and inducing LRP5/6-mediated
phosphorylation of the multicomponent degradation complex and
leading to β-catenin stabilization (Fig. 5B). Once β-catenin accumulates in
the cytoplasm, it translocates into the nucleus where it acts as a
transcriptional co-activator for the TCF/LEF transcription factors,
inducing the expression of Wnt pathway target genes (22,23).
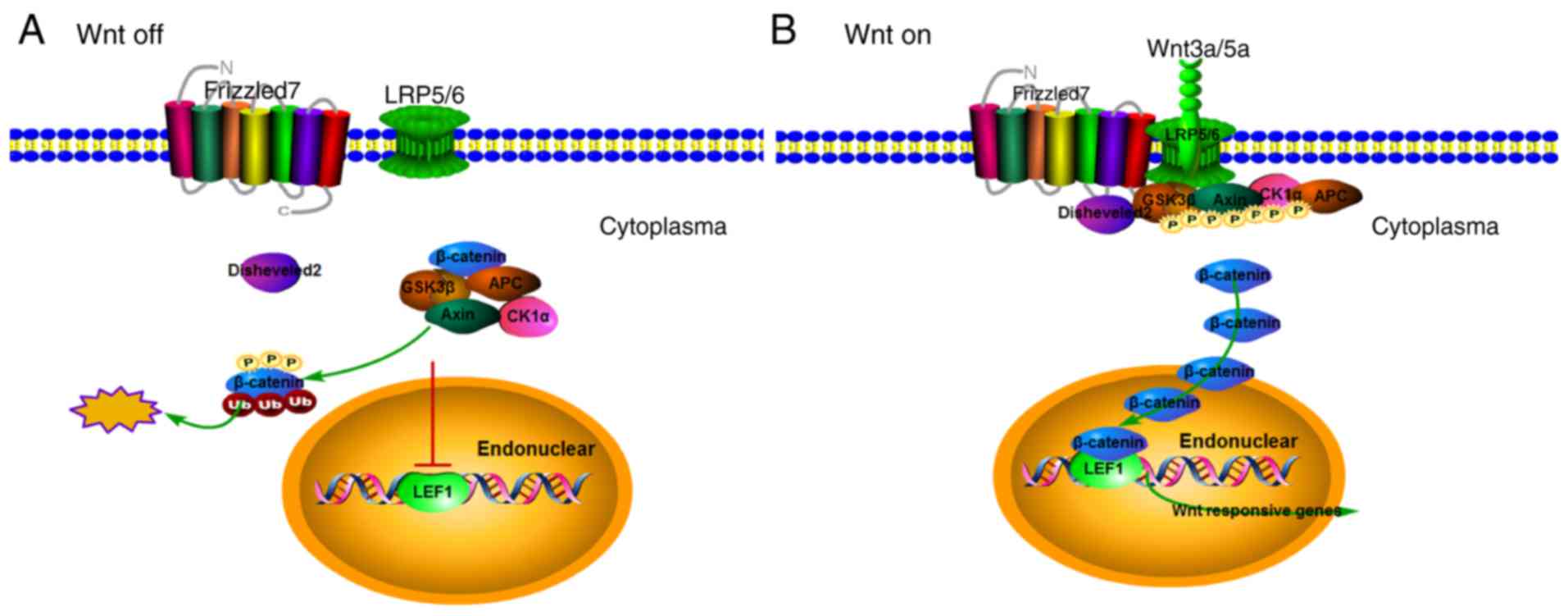 | Figure 5The canonical Wnt pathway regulates
gene expression by modulating β-catenin levels. (A) In the absence
of Wnt ligands, the Axin/CK1α/APC/GSK3β degradation complex binds
and phosphorylates cytoplasmic β-catenin, leading to
ubiquitin-dependent degradation of β-catenin. This prevents the
accumulation of β-catenin and keeps Wnt pathway target gene
expression switched off. (B) Upon binding of extracellular Wnt
ligands to transmembrane frizzled receptors and the co-receptors
LRP5/6, the Axin/CK1α/APC/GSK3β degradation complex is diverted to
the plasma membrane and binds to LRP5/6. Wnt ligand binding also
causes disheveled to interact with frizzled receptors and LRP5/6.
Phosphorylated LRP5/6 and disheveled then sequester and induce
phosphorylation of the Axin/CK1α/APC/GSK3β degradation complex,
which inhibits its ability to phosphorylate β-catenin, leading to
β-catenin stabilization and accumulation in the cytoplasm.
Accumulated β-catenin is then able to translocate into the nucleus
and bind to TCF/LEF, activating the expression of the Wnt pathway
target genes. CK, casein kinase; APC, adenomatous polyposis coli;
GSK, glycogen synthase kinase; LRP, low-density lipoprotein
receptor; TCF, transcription factor; LEF, lymphoid enhancing
binding factor. |
In the present study, Wnt3a, Wnt5a, frizzled 7,
disheveled 2, GSK3β, β-catenin and LEF1 were selected for analysis
as they are known to be associated with hair follicle development
(24). The data herein
demonstrates that baicalin treatment increases canonical Wnt
pathway signaling in mouse skin tissues by increasing the levels of
Wnt3a, Wnt5a, frizzled 7, disheveled 2, β-catenin and LEF1, while
decreasing the expression of the β-catenin degradation complex
member GSK3β. The resulting activation of canonical Wnt pathway
signaling is the likely mechanism by which baicalin stimulates hair
follicle growth, as treatment with the Wnt pathway inhibitor IWR-1
blocked baicalin-mediated Wnt pathway activation and hair follicle
growth enhancement. IWR-1 blocks canonical Wnt pathway signaling by
stabilizing Axin proteins and thereby increasing the activity of
the β-catenin destruction complex (25). As co-treatment with IWR-1
prevented baicalin from decreasing the level of GSK3β and
activating Wnt signaling, baicalin must act upstream from the
destruction complex to activate the canonical Wnt pathway and
stimulate hair follicle development.
The hair follicle is comprised of epidermal and
dermal compartments, whose reciprocal interactions are considered
to be key regulators of hair follicle development and the hair
cycle (19). Although the
crosstalk between these compartments is complex, DPCs are thought
to be the inducers and epidermal cells to be the responders in hair
follicle formation and the hair cycle (26–28). As a marker of DPCs, ALP is
believed to indicate the induction of hair follicle growth and its
activity changes coincide with the hair cycle (29,30). Therefore, it is noteworthy that
baicalin also increased the expression of ALP and induced the
proliferation of DPCs, which may explain its ability to promote the
growth of hair follicles, although the exact mechanism by which
baicalin promotes ALP expression is unknown.
In summary, the results of the present study
indicate that topical administration of baicalin at daily doses of
50 and 100 μmol facilitates the growth of reconstituted hair
follicles in mice. The ability of baicalin to activate the
Wnt/β-catenin signaling pathway and increase ALP activity in DPCs
in mice likely contributes to the induction of hair follicle
growth. It is possible that activating Wnt/β-catenin signaling may
be more important than increasing ALP activity in DPCs for
baicalin-mediated hair growth, as blocking Wnt/β-catenin signaling
with IWR-1 inhibited the effect if baicalin treatment. Therefore,
ALP may be induced downstream from Wnt signaling, at least in the
context of baicalin-induced Wnt pathway activation and hair growth
stimulation. Future studies should extend the observation period to
investigate the effects of baicalin on the catagen and telogen
phases of the hair cycle and test for any potential adverse events.
It may also be of interest to test the effect of baicalin on human
dermal and epidermal cells grafted onto immunodeficient mice
(31) to determine whether the
same effects of baicalin on Wnt/β-catenin signaling and DPC
activity are observed in human-derived cells.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81371717
and 81573028). The authors would like to thank Dr Wei-Zheng Wei for
his help with this manuscript.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee J and Tumbar T: Hairy tale of
signaling in hair follicle development and cycling. Semin Cell Dev
Biol. 23:906–916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Santos Z, Avci P and Hamblin MR: Drug
discovery for alopecia: Gone today, hair tomorrow. Expert Opin Drug
Dis. 10:269–292. 2015. View Article : Google Scholar
|
|
3
|
Liang YY, Jiang QE and Li G: The clinical
effects of Chinese Medicine Hair Restorer on androgenetic alopecia.
Hebei Chinese Med. 1156–1157. 2012.In Chinese.
|
|
4
|
Moghaddam E, Teoh BT, Sam SS, Lani R,
Hassandarvish P, Chik Z, Yueh A, Abubakar S and Zandi K: Baicalin,
a metabolite of baicalein with antiviral activity against dengue
virus. Sci Rep. 4:54522014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takahashi H, Chen MC, Pham H, Angst E,
King JC, Park J, Brovman EY, Ishiguro H, Harris DM, Reber HA, et
al: Baicalein, a component of Scutellaria baicalensis, induces
apoptosis by Mcl-1 down-regulation in human pancreatic cancer
cells. Biochim Biophys Acta. 1813:1465–1474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shin SH, Bak S, Kim MK, Sung YK and Kim
JC: Baicalin, a flavonoid, affects the activity of human dermal
papilla cells and promotes anagen induction in mice. Naunyn
Schmiedebergs Arch Pharmacol. 388:583–586. 2015. View Article : Google Scholar
|
|
7
|
Zhu HQ, Fan WX and Zhang H: In vitro
effects of baicalin on the growth of human hair follicles and
secretion of vascular endothelial growth factor by human dermal
papilla cells. Chin J Dermatol. 40:416–418. 2007.
|
|
8
|
Guo AJ, Choi RC, Cheung AW, Chen VP, Xu
SL, Dong TT, Chen JJ and Tsim KW: Baicalin, a flavone, induces the
differentiation of cultured osteoblasts: An action via the
Wnt/beta-catenin signaling pathway. J Biol Chem. 286:27882–27893.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohyama M, Zheng Y, Paus R and Stenn KS:
The mesenchymal component of hair follicle neogenesis: Background,
methods and molecular characterization. Exp Dermatol. 19:89–99.
2010. View Article : Google Scholar
|
|
10
|
Lichti U, Anders J and Yuspa SH: Isolation
and short-term culture of primary keratinocytes, hair follicle
populations and dermal cells from newborn mice and keratinocytes
from adult mice for in vitro analysis and for grafting to
immunodeficient mice. Nat Protoc. 3:799–810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ehama R, Ishimatsu-Tsuji Y, Iriyama S,
Ideta R, Soma T, Yano K, Kawasaki C, Suzuki S, Shirakata Y,
Hashimoto K and Kishimoto J: Hair follicle using grafted rodent and
human cells. J Invest Dermatol. 127:2106–2115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rao X, Huang X, Zhou Z and Lin X: An
improvement of the 2^(-delta delta CT) method for quantitative
real-time polymerase chain reaction data analysis. Biostat
Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
13
|
Gupta PS, Folger JK, Rajput SK, Lv L, Yao
J, Ireland J and Smith GW: Regulation and regulatory role of WNT
signaling in potentiating FSH action during bovine dominant
follicle selection. PLoS One. 9:e1002012014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qi J and Garza LA: An overview of
alopecias. Cold Spring Harb Perspect Med. 4:pii:a013615. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shirai A, Ikeda J, Kawashima S, Tamaoki T
and Kamiya T: KF19418, a new compound for hair growth promotion in
vitro and in vivo mouse models. J Dermatol Sci. 25:213–218. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Zhu Y, Sun C, Wang T, Shen Y, Cai
W, Sun J, Chi L, Wang H, Song N, et al: Feedback activation of
basic fibroblast growth factor signaling via the Wnt/β-catenin
pathway in skin fibroblasts. Front Pharmacol. 8:322017.
|
|
17
|
Andl T, Reddy ST, Gaddapara T and Millar
SE: WNT signals are required for the initiation of hair follicle
development. Dev Cell. 2:643–653. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rishikaysh P, Dev K, Diaz D, Qureshi W,
Filip S and Mokry J: Signaling involved in hair follicle
morphogenesis and development. Int J Mol Sci. 15:1647–1670. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schneider MR, Schmidt-Ullrich R and Paus
R: The hair follicle as a dynamic miniorgan. Curr Biol.
19:R132–R142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang CC and Cotsarelis G: Review of hair
follicle dermal cells. J Dermatol Sci. 57:2–11. 2010. View Article : Google Scholar :
|
|
21
|
Van Camp JK, Beckers S, Zegers D and Van
Hul W: Wnt signaling and the control of human stem cell fate. Stem
Cell Rev Rep. 10:207–229. 2014. View Article : Google Scholar
|
|
22
|
Schmidt-Ullrich R and Paus R: Molecular
principles of hair follicle induction and morphogenesis. Bioessays.
27:247–261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kishimoto J, Burgeson RE and Morgan BA:
Wnt signaling maintains the hair-inducing activity of the dermal
papilla. Genes Dev. 14:1181–1185. 2000.PubMed/NCBI
|
|
25
|
Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan
CW, Wei S, Hao W, Kilgore J, Williams NS, et al: Small
molecule-mediated disruption of Wnt-dependent signaling in tissue
regeneration and cancer. Nat Chem Biol. 5:100–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hardy MH: The secret life of the hair
follicle. Trends Genet. 8:55–61. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Millar SE: Molecular mechanisms regulating
hair follicle development. J Invest Dermatol. 118:216–225. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paus R and Cotsarelis G: The biology of
hair follicles. N Engl J Med. 341:491–497. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iida M, Ihara S and Matsuzaki T: Hair
cycle-dependent changes of alkaline phosphatase activity in the
mesenchyme and epithelium in mouse. Dev Growth Differ. 49:185–195.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Handjiski BK, Eichmüller S, Hofmann U,
Czarnetzki BM and Paus R: Alkaline phosphatase activity and
localization during the murine hair cycle. Br J Dermatol.
131:303–310. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu X, Scott L Jr, Washenik K and Stenn K:
Full-thickness skin with mature hair follicles generated from
tissue culture expanded human cells. Tissue Eng Part A.
20:3314–3321. 2014. View Article : Google Scholar : PubMed/NCBI
|