Introduction
Osteoarthritis (OA) is a prevalent degenerative
joint disease associated with aging, obesity and trauma and is the
most common form of arthritis characterized by the progressive
destruction of articular cartilage, with >270 million cases
reported worldwide (1).
Approximately 80% of OA cases diagnosed by radiography occur in
patients over 65 years old (2).
OA typically causes joint instability, pain, loss of function,
stiffness and mobility difficulties (3), altogether leading to a deterioration
in quality of life and increasing the cost of health care for the
aging population (4). The
etiology and pathogenesis of OA are not well understood due to a
combination of various risk factors and initiating mechanisms
(5). Furthermore, a significant
and positive correlation between the degeneration of articular
cartilage (6), which is the root
cause of OA, and bioactive compounds, including pro-inflammatory
cytokines and a dipokines (7),
has been demonstrated.
Although the pathogenesis of OA has multiple
underlying mechanisms that are not well understood, it has been
reported that decreased chondrocyte proliferation and apoptosis
dysregulation are significantly associated with OA pathogenesis and
are observed in OA cartilage more frequently compared with normal
cartilage (8). Furthermore,
chondrocyte apoptosis is positively correlated with cartilage
degradation during the development and progression of OA (9). Chondrocytes are required to maintain
cartilage structure and function via the production of
extracellular matrix components (10), which are responsible for
maintaining the cartilaginous matrix. Consequently, the coordinated
regulation of chondrocyte proliferation and apoptosis is of great
importance in cartilage function and cartilage injury repair due to
OA. Pro-inflammatory cytokines, including interleukin-1β (IL-1β),
are important for dysregulated chondrocyte apoptosis and, together
with matrix metalloproteinases (MMPs), trigger a vigorous
pro-inflammatory response (11,12). In addition to MMP-1, MMP-13 is
also important in the pathological process of OA, inducing matrix
degradation, further chondrocyte senescence and aging changes
(13). However, cellular
responses to upregulation and downregulation of IL-1β do not
dominate the overall gene expression signature in osteoarthritic
chondrocytes (14). Therefore,
the mechanism of IL-1β regulation in osteoarthritic cartilage
degeneration remains unclear.
A previous study reported that the expression of
collagen triple helix repeat containing 1 (CTHRC1) was upregulated
in OA (15), indicating a central
role of CTHRC1 in OA progression. However, the molecular mechanisms
of CTHRC1 associated with the development and progression of OA are
not well understood. The aim of the present study was to
investigate the function of CTHRC1 in an IL-1β-induced OA model in
rat chondrocytes in vitro. The results suggest that CTHRC1
downregulation inhibits IL-1β-induced chondrocyte apoptosis via
inactivating JNK1/2 signaling.
Materials and methods
Tissue specimens
OA joint fluid samples (n=50) were collected from
patients (67.9±7.2 years; male: female, 11:39) with OA who
underwent knee arthroplasty at the First Affiliated Hospital of
Anhui Medical University (Hefei, China) between June 2012 and April
2016. Human normal joint fluid samples were collected from 30
patients (62.8±11.2 years old; male: female, 1:4) with trauma and
no history of OA or other joint diseases at The First Affiliated
Hospital of Anhui Medical University. Patients who presented with
obvious joint injury or with generalized OA were excluded from the
study. The present study was approved by the Ethics Committee of
Anhui Medical University. Written informed consent was obtained
from all participants of this study and all investigations were
performed in accordance with the Declaration of Helsinki. All
patients agreed to the use of their samples in scientific
research.
Cell culture
Articular chondrocytes were harvested from 20 male
4-week-old Sprague Dawley rats (250–300 g; Shanghai BK Experimental
Animal Center, Shanghai, China), which were provided with free
access to food and water and kept under a 12 h light/dark cycle at
a constant temperature of 25°C in a humidified atmosphere conaining
5% CO2. Chondrocytes were treated with 75% ethanol for
10 min, washed with PBS and digested with 4 ml collagen II (EMD
Millipore, Billerica, MA, USA) at 37°C for 5 h. The chondrocytes
were collected by centrifugation at 400 × g for 5 min at 37°C and
resuspended with Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 15%
fetal bovine serum (FBS; HyClone; Thermo Fisher Scientific, Inc.,
Logan, UT, USA) and cultured at 37°C in an atmosphere containing 5%
CO2. Immunohistochemistry was performed when cells
reached 50–60% confluence and cells were subsequently cultured in
DMEM supplemented with 10% FBS and 1% penicillin/streptomycin at
37°C in an atmosphere containing 5% CO2. The present
study was approved by the Ethics Committee of Anhui Medical
University.
Construction of pLKO.1-CTHRC1-short
hairpin (sh)RNA lentiviral vector and transfection
The specific shRNA sequence that targets the CTHRC1
coding sequence was used, with a scramble shRNA sequence used as
negative control. shRNAs (40 nM) were cloned into the pLKO.1
lentiviral vector (Addgene, Inc., Cambridge, MA, USA). The sequence
of target and scramble shRNA were as follows: 5′-ATC CCA AGT ATA
ATG GGA T-3′; 5′-ATC TGG AGA GAT CCA ATA T-3′. A total of 1,000 ng
pLKO.1, 900 ng psPAX2 and 100 ng pMD2G (all Addgene, Inc.) were
then co-transfected into 293T cells (American Type Culture
Collection, Manassas, VA, USA). Chondrocytes were transfected with
pLKO.1-CTHRC1-shRNA (shNRA-CTHRC1) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The cells were analyzed 48 h following
transfection.
Construction of pLVX-Puro-CTHRC1
lentiviral vector and transfection
cDNA encoding CTHRC1 was obtained using GENEWIZ
(Suzhou, China) and cloned into pLVX-Puro to generate a CTHRC1
expression vector. The forward primer was 5′-GCG AAT TCA TGC ACC
CCC AAG GCC GCG-3′ and the reverse primer was 5′-CGG GAT CCT TAT
TTT GGT AGT TCT TCA AT-3′. A total of 1,000 ng pLVX-Puro 900 ng
psPAX2 and 100 ng pMD2G were then co-transfected into 293T cells.
Chondrocytes were transfected with pLVX-Puro-CTHRC1 using
Lipofectamine 2000 according to the manufacturer's protocol. The
empty pLVX-Puro vector was used as control. The cells were analyzed
48 h following transfection.
Cell proliferation assay
The proliferation of chondrocytes was measured using
a Cell Counting Kit-8 (CCK-8) assay. Briefly, the chondrocytes
(3×103 cells/well) were cultured with at 37°C overnight
in an atmosphere containing 5% CO2, following which they
were treated with 0, 5, 10 or 20 ng/ml IL-1β for 0, 24, 48 and 72 h
and incubated with 10 µl CCK-8 solution at 37°C for 1 h in
an atmosphere containing 5% CO2. Cell proliferation was
calculated using a microplate reader (ELX 800; Bio-Tek Instruments,
Inc., Winooski, VT, USA) at a wavelength of 450 nm.
Cell apoptosis assay
The apoptotic rate was evaluated using an Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit
(Beyotime Institute of Biotechnology, Haimen, China). Briefly, the
chondrocytes (5×104 cells/well) were centrifuged at
1,000 × g for 5 min at 4°C. Pellets were fixed overnight in 70%
cold ethanol. Following fixation, cells were washed twice with PBS
and incubated in PBS containing RNase (1 mg/ml) for 10 min at room
temperature. Finally, samples were mixed with 195 µl Annexin
V FITC and 5 µl propidium iodide and incubated for 15 min at
4°C. The apoptotic cells were analyzed by flow cytometry (BD Accuri
C6; software version 1.0.264.21; BD Biosciences, San Jose, CA,
USA).
Assay of caspase-3 activity
The caspase-3 colorimetric assay kit (KGA203; Kaiji
Biological Engineering Materials Co., Ltd., Nanjing, China) was
used according to the manufacturer's protocol to examine the
activity of caspase-3. Chondrocytes (5×106 cells/ml)
were collected by centrifugation at 1,000 × g for 5 min at 4°C,
resuspended in 150 µl of chilled cell lysis buffer (KGA203;
Kaiji Biological Engineering Materials Co., Ltd.) and incubated on
ice for 1 h, during which cells were shocked (220 V) 3-4 times
every 10 sec. Following centrifugation at 400 × g for 1 min at 4°C,
the supernatant was transferred to a fresh tube and 50 µl of
cell lysis buffer containing 100-200 µg protein was added
with 50 µl 2X Reaction Buffer and 5 µl caspase-3
substrate and incubated at 37°C for 4 h in the dark. Samples were
read at 405 nm using a Multiskan EX microplate reader (Labsystems,
Helsinki, Finland).
RT-quantitative PCR
Total RNA was extracted using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. cDNA was synthesized from isolated RNA using a
PrimeScript RT reagents kit (Takara, Dalian, China). The conditions
were as follows: 37°C for 60 min, 85°C for 5 min and 4°C for 5 min.
PCR was performed using a DyNAmo Flash SYBR Green qPCR kit
(Finnzymes Oy; Thermo Fisher Scientific, Inc.). The PCR cycling
conditions were as follows: 95°C for 10 min, followed by 40 cycles
at 95°C for 15 sec and 60°C for 45 sec, a final extension step of
95°C for 15 sec, 60°C for 1 min, 95°C for 15 sec and 60°C for 15
sec. Data collection was performed using an Applied Biosystems 7300
Fast Real-Time PCR System (Thermo Fisher Scientific, Inc.) and
relative quantification of gene expression was calculated using the
2−ΔΔCq method (16)
with GAPDH as a reference gene. To compare relative mRNA expression
levels, the expression of CTHRC1, B-cell lymphoma (Bcl)-2,
Bcl-2-associated X protein (Bax), cleaved caspase-3, poly ADP
ribose polymerase (PARP)-1 and matrix metalloproteinase (MMP)-13
were given as ratios to GAPDH. The primers were designed using
Primer Express software (v3.0.1; Thermo Fisher Scientific, Inc.)
and were as follows: CTHRC1, forward 5′-CTC GCT TCG GCT CAA ATG-3′
and reverse 5′-GCA CCA ATC CCT TCA CAG-3′; MMP-13, forward 5′-CAG
ACA GCA AGA ATA AAG AC-3′ and reverse 5′-CAA CAT AAG CAC AGT GTA
AC-3′; Bcl-2, forward 5′-GGG ATG CCT TTG TGG AAC-3′ and reverse
5′-GTC TGC TGA CCT CAC TTG-3′; Bax, forward 5′-GGA CGC ATC CAC CAA
GAA G-3′ and reverse 5′-CTG CCA CAC GGA AGA AGA C-3′; caspase-3,
forward 5′-GGC ATC TCC TGT GAT TGG-3′ and reverse 5′-CTC AGC ACT
CTG GGA AAG-3′; GAPDH, forward 5′-GGA GTC TAC TGG CGT CTT CAC-3′
and reverse 5′-ATG AGC CCT TCC ACG ATG C-3′.
Western blotting
Total protein was extracted in lysis buffer
supplemented with protease inhibitors (Beyotime Institute of
Biotechnology). A total of 15 µl/lane protein was separated
by 10-15% SDS-PAGE and electrophoretically transferred onto
polyvinylidene fluoride membranes. The membranes were incubated
with primary antibodies: Anti-CTHR1 (1:1,000 dilution; cat. no.
16534-1-AP; ProteinTech Group, Inc., Chicago, IL, USA),
anti-p-JNK1/2 (1:2,000 dilution; cat. no. 9255; Cell Signaling
Technology, Inc., Danvers, MA, USA), anti-JNK1/2 (1:1,000 dilution;
cat. no. 9252; Cell Signaling Technology, Inc.), anti-Bax (1:300
dilution; cat. no. Sc-493; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), anti-Bcl-2 (1:300 dilution; cat. no. Sc-492; Santa Cruz
Biotechnology, Inc.), anti-Caspase-3 (1:500 dilution; cat. no.
ab44976, Abcam, Cambridge, MA, USA), anti-PARP1 (cat. no. ab32064,
Abcam), anti-MMP-13 (1:3,000 dilution; cat. no. ab39012; Abcam),
and anti-GAPDH (1:200 dilution; cat. no. 5174; Cell Signaling
Technology, Inc.) overnight at 4°C, and subsequently incubated with
a horseradish peroxidase-coupled secondary antibody, following
which they were detected using enhanced chemiluminescence (cat. no.
WBKLS0100; EMD Millipore) according to the manufacturer's
instructions (Pierce; Thermo Fisher Scientific, Inc.).
Immunohistochemistry
Cells were washed with 0.02 M PBS, fixed with 4%
methanol for 30 min at room temperature, incubated with 3%
H2O2 for 10 min and washed three times with
0.02 M PBS. Slides were incubated with anti-Collagen II (cat. no.
ab34712; 1:200 dilution; Abcam) or anti-Sry-type high mobility
group-box (SOX)9 (cat. no. ab185230; 1:200 dilution; Abcam)
antibody at 4°C overnight and subsequently washed three times with
0.02 M PBS. The slides were stained with horseradish
peroxidase-labeled goat anti-rat immunoglobulin G (cat. no. D-3004;
1:500 dilution; Shanghai Long Island Biotec Co., Ltd., Shanghai,
China) for 30 min at 37°C and washed three times in PBS for 3 min
each time. Subsequently, the sections were stained with
diaminobenzidine for 5 min at room temperature, counterstained with
hematoxylin for 3 min at room temperature and washed in water. For
the negative controls, the primary antibody was omitted. Images
were captured using a light microscope (Olympus Corporation, Tokyo,
Japan; magnification, ×200).
ELISA
IL-1β and CTHRC1 expression in the joint fluid of
patients with OA was determined using CTHRC1 (cat. no.
CSB-EL006162HU; Cusabio Biotech Co., Ltd., College Park, MD, USA)
and IL-1β (cat. no. 583311; Cayman Chemical Company, Ann Arbor, MI,
USA) ELISA kits according to the manufacturer's protocol.
Statistical analysis
Results are presented as the mean ± standard
deviation. All data were analyzed using SPSS 18.0 software (SPSS,
Inc., Chicago, IL, USA). Comparisons were made using t-test,
analysis of variance and post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
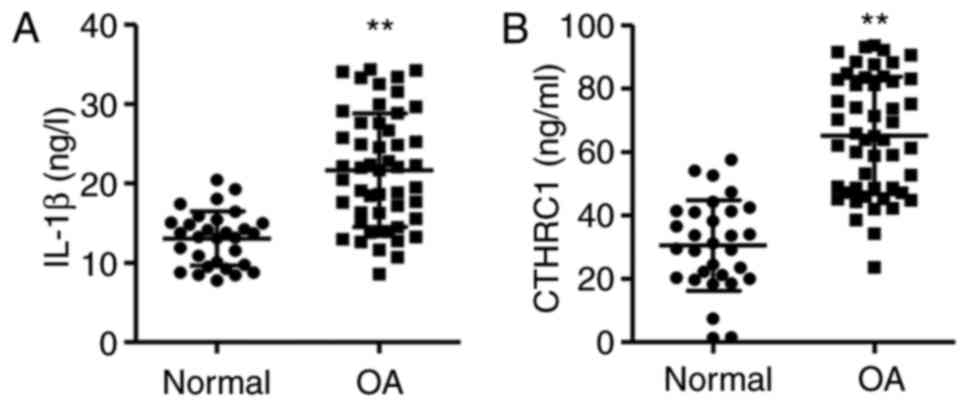
IL-1β and CTHRC1 are upregulated in
patients with OA
Joint fluid from patients with OA was assessed using
ELISA kits. The results demonstrated that the levels of IL-1β and
CTHRC1 were significantly higher in the joint fluid of patients
with OA compared with the normal controls, with IL-1β and CTHRC1
expression 65.7 and 113.6% higher, respectively (P<0.01;
Fig. 1).
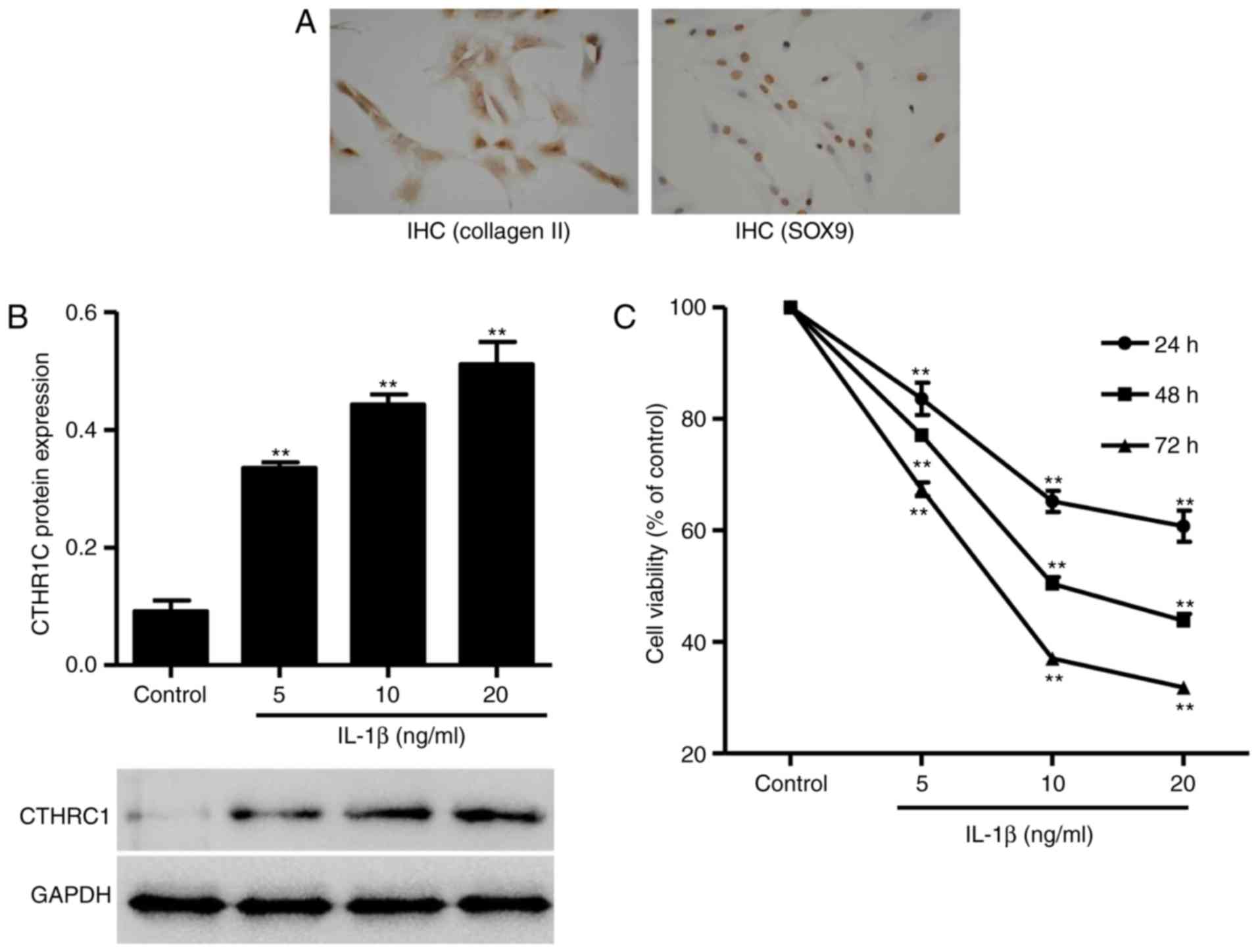
CTHRC1 expression is increased in
IL-1β-induced rat chondrocytes
To investigate the role of CTHRC1 in osteoarthritic
chondrocytes in vitro, primary rat chondrocytes were
collected and chondrocyte-asociated genes were assessed using
immunohistochemistry. SOX9 is a member of the Sox gene family,
which are predominantly expressed in cartilage and activate
Collagen II (17).
Immunohistochemical staining demonstrated that Collagen II and SOX9
were highly expressed in normal primary cultured chondrocytes
(Fig. 2A), which was indicative
of a well-established chondrocyte system.
The exact cause of OA is not known; however, the
degradation of extracellular matrix components is associated with
elevated levels of the pro-inflammatory cytokine IL-1β (18). In the present study, IL-1β was
introduced in rat chondrocytes to establish an in vitro
osteoarthritis model. CTHRC1 protein expression was increased in
chondrocytes in a dose-dependent manner in response to IL-1β (5, 10
and 20 ng/ml; P<0.01; Fig.
2B). Furthermore, chondrocyte proliferation was suppressed by
IL-1β in a dose-dependent manner (P<0.01; Fig. 2C).
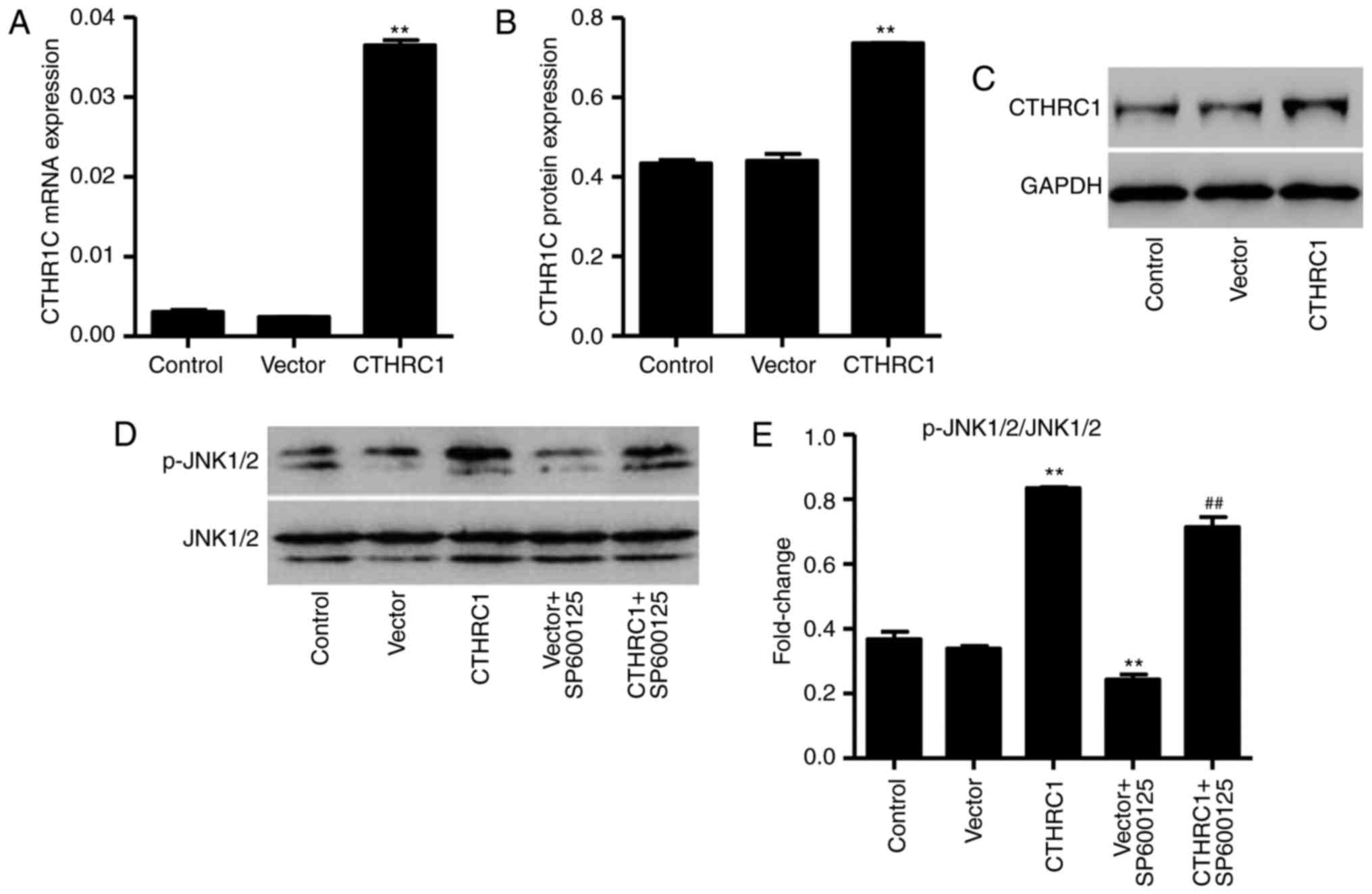
CTHRC1 upregulation activates the JNK1/2
pathway in chondrocytes
The pLVX-Puro-CTHRC1 vector was constructed to and
transfected into chondrocytes to induce overexpression of CTHRC1.
Levels of CTHRC1 mRNA and protein were studied using RT-qPCR and
western blot analysis, respectively. The expression of CTHRC1 mRNA
and protein was significantly increased in chondrocytes transfected
with the pLVX-Puro-CTHRC1 vector compared with chondrocytes
transfected with the empty pLVX-Puro vector (P<0.01; Fig. 3A–C). Furthermore, CTHRC1
upregulation significantly activated JNK1/2, and this activation
was markedly reduced by the inhibitor, SP600125 (both P<00.01;
Fig. 3D and E).
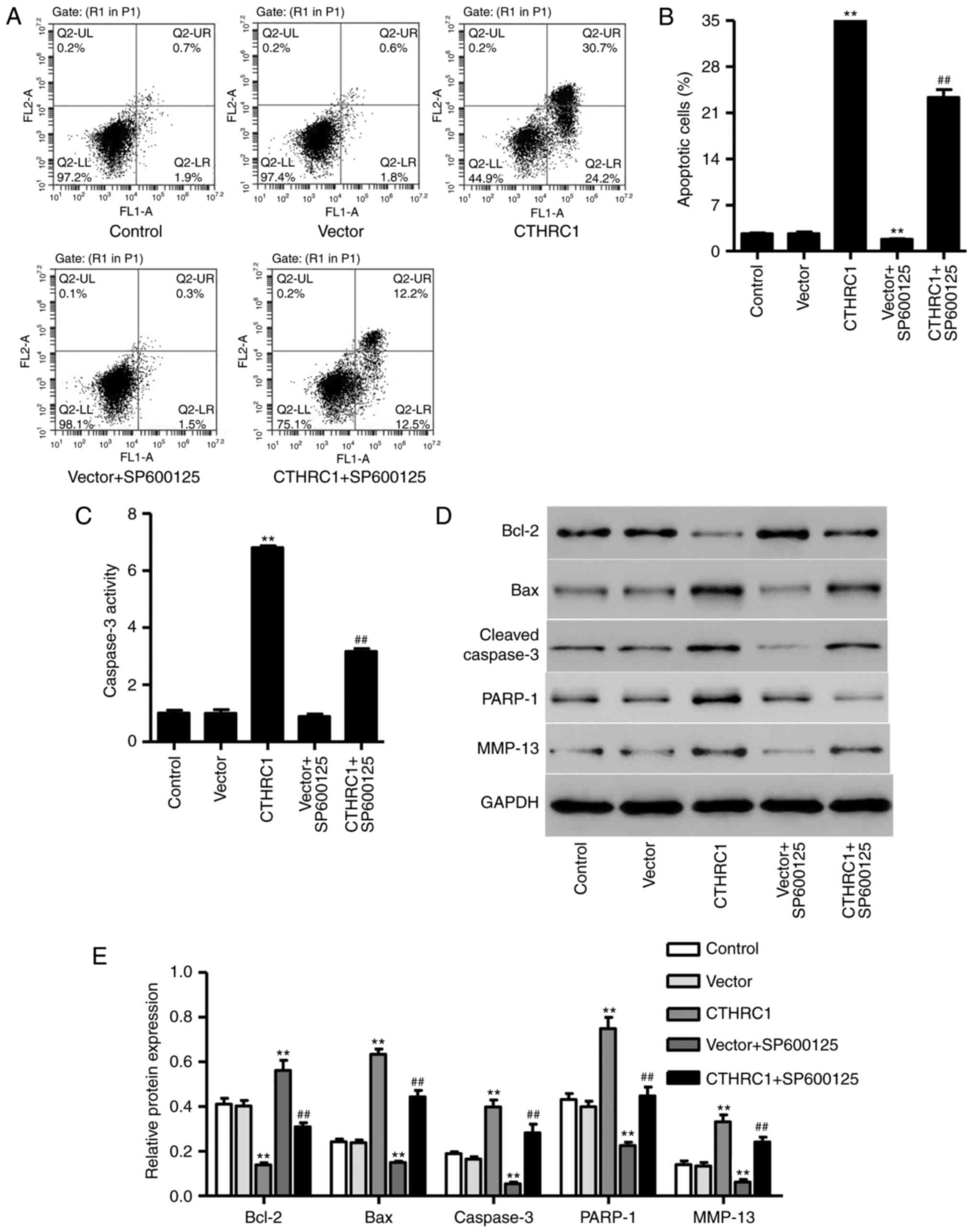
CTHRC1 upregulation induces chondrocyte
apoptosis
Following transfection with pLVX-Puro-CTHRC1 for 24
h, the percentage of apoptotic cells and caspase-3 activity were
significantly increased in chondrocytes (P<0.01; Fig. 4A–C); however, apoptosis and
caspase-3 activity were significantly decreased following SP600125
treatment (P<0.01; Fig. 4B and
C). The expression of MMP-13, Bcl-2, Bax, PARP-1 and cleaved
caspase-3 was also measured using western blotting (Fig. 4D). The results revealed that
CTHRC1 upregulation significantly increased the expression of
MMP-13, Bax, PARP-1 and cleaved caspase-3 (P<0.01; Fig. 4E), whereas it significantly
decreased Bcl-2 expression (P<0.01; Fig. 4E) compared with cells transfected
with the empty vector. However, SP600125 treatment significantly
decreased the expression of MMP-13, Bax, PARP-1 and cleaved
caspase-3 and increased Bcl-2 expression in chondrocytes with
pLVX-Puro-CTHRC1 transfection (P<0.01; Fig. 4E), suggesting that JNK1/2
signaling is associated with the mechanism of CTHRC1 upregulation
in chondrocyte apoptosis.
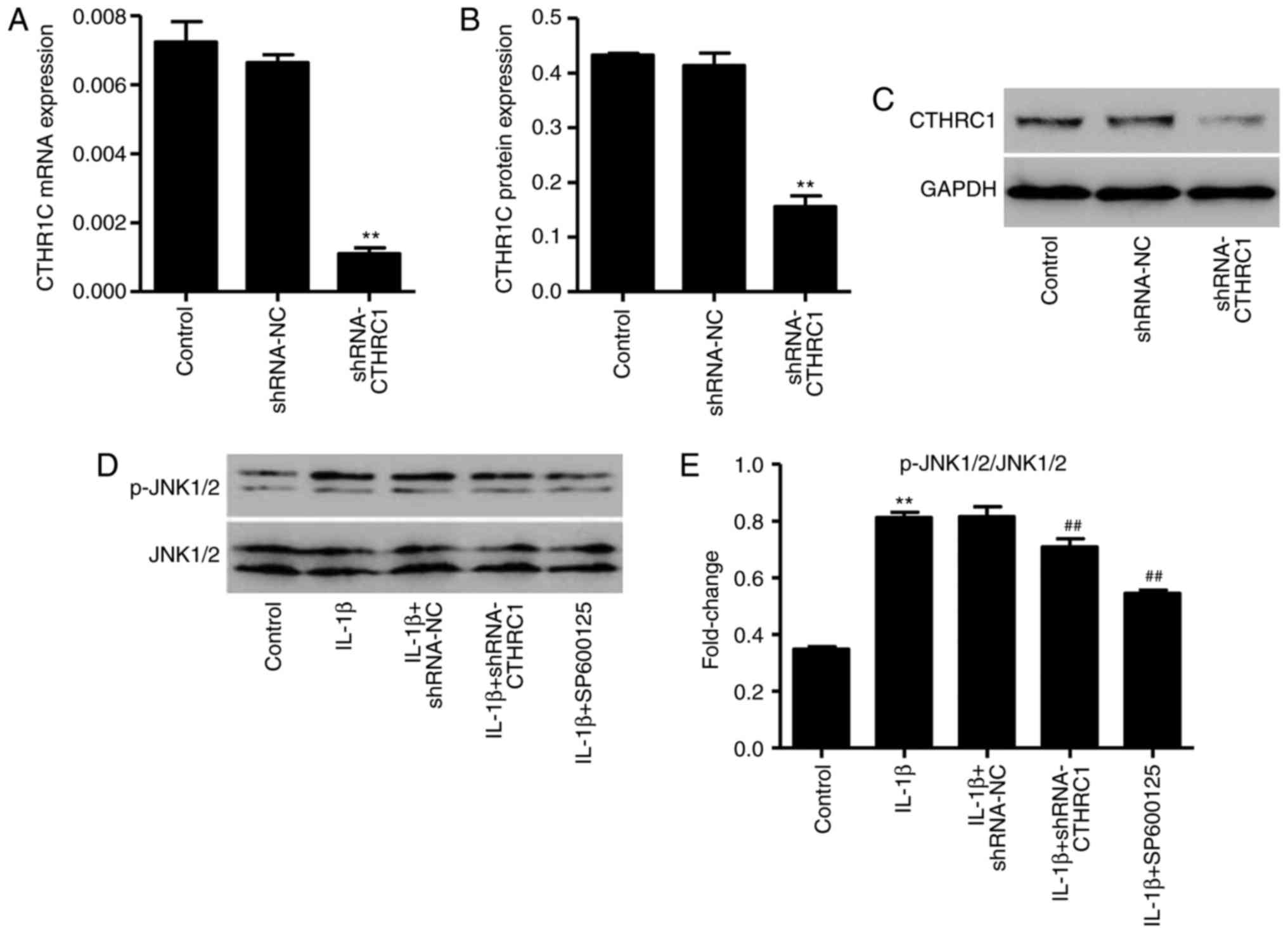
CTHRC1 downregulation suppresses
IL-1β-induced JNK1/2 activation
As CTHRC1 protein expression is significantly
increased in patients with OA and in osteoarthritic chondrocytes,
it was hypothesized that CTHRC1 may be correlated with OA
development and chondrocyte apoptosis. The pLKO.1-CTHRC1-shRNA
vector was synthesized to downregulate the expression of CTHRC1,
and the results indicated that, in chondrocytes transfected with
the pLKO.1-CTHRC1-shRNA, CTHRC1 was significantly downregulated
compared with control cells (P<0.01; Fig. 5A–C). In addition, treatment with
10 ng/ml of IL-1β for 6 h significantly increased the expression of
p-JNK1/2/JNK1/2 (P<0.01; Fig. 5D
and E); however, this was significantly decreased following
pLKO.1-CTHRC1-shRNA transfection (P<0.01; Fig. 5D and E). These results suggest
that CTHRC1 downregulation inhibits JNK1/2 activation in
IL-1β-induced rat chondrocytes.
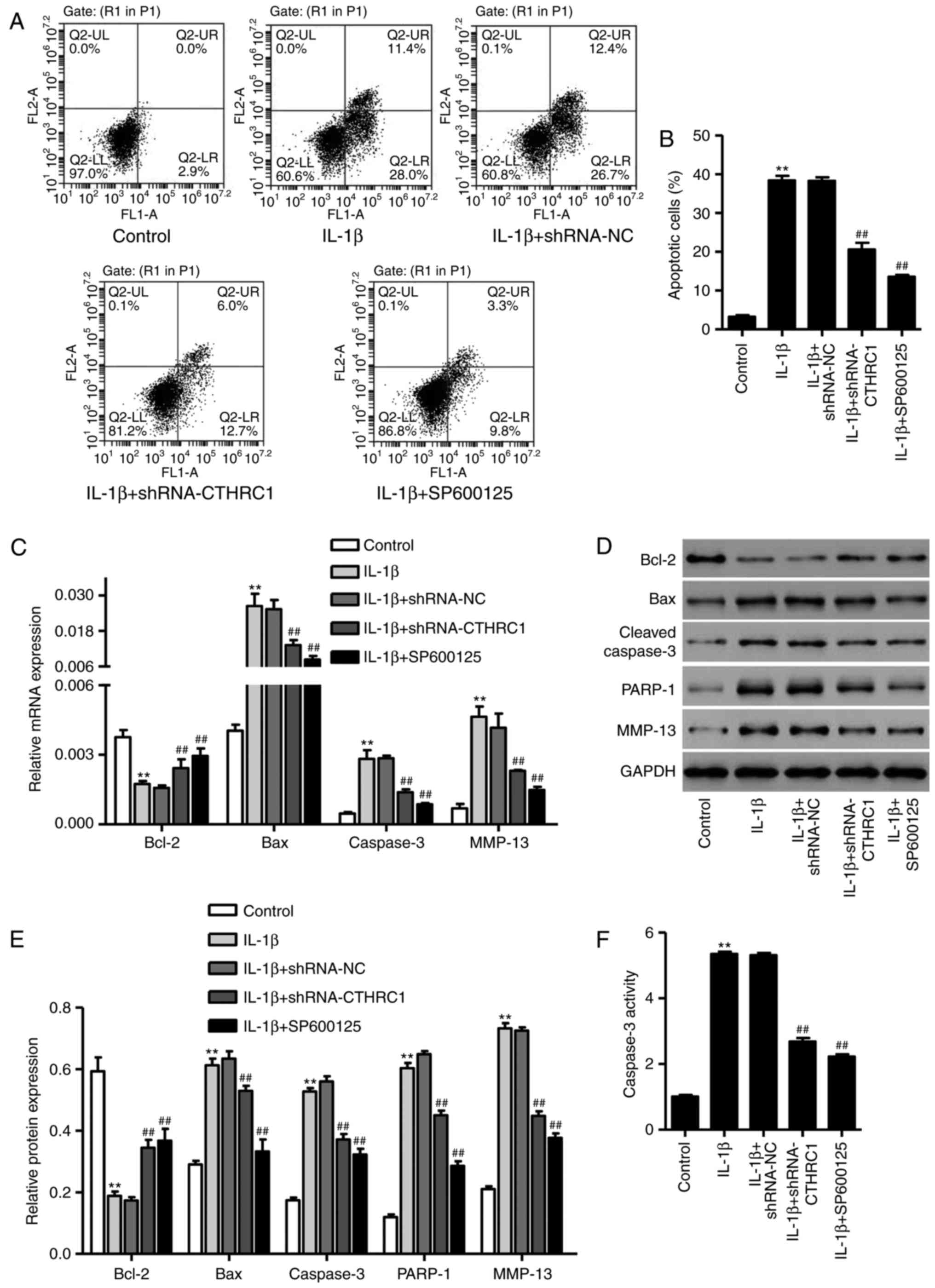
CTHRC1 downregulation suppresses the
IL-1β-induced apoptosis of chondrocytes
To investigate the role of CTHRC1 downregulation in
chondrocytes apoptosis, apoptotic cells were assessed using flow
cytometry following treatment with 10 ng/ml of IL-1β for 24 h. The
percentage of apoptotic cells was significantly increased compared
with the control group (P<0.01; Fig. 6A and B). However, transfection
with pLKO.1-CTHRC1-shRNA significantly attenuated this increase
(P<0.05; Fig. 6A and B). To
further explore the effect of JNK1/2 signaling in CTHRC1
downregulation-mediated protection against IL-1β-induced
chondrocyte apoptosis, the JNK1/2 inhibitor SP600125 (10 µM)
was added 30 min prior to IL-1β. SP600125 also markedly inhibited
chondrocyte apoptosis in response to IL-1β (Fig. 6A and B; P<0.01vs. IL-1β
treatment alone).
To explore the role of CTHRC1
downregulation-mediated protection against IL-1β-induced
chondrocyte apoptosis, the expressions of MMP-13, Bcl-2, Bax and
cleaved caspase-3 were detected via RT-qPCR and western blot-ting.
Compared with control cells, expression of Bcl-2 was significantly
reduced (P<0.01; Fig. 6–E),
whereas the expression of Bax, PARP-1, cleaved caspase-3 and MMP-13
was significantly increased in the IL-1β group compared with the
control (P<0.01; Fig. 6C–E).
When chondrocytes were transfected with pLKO.1-CTHRC1-shRNA or
SP600125 was added prior to IL-1β, Bcl-2 expression was
significantly higher compared with the IL-1β group (P<0.01;
Fig. 6C–E); however, MMP-13, Bax,
PARP-1 and cleaved caspase-3 expression were significantly lower
(P<0.01; Fig. 6C–E).
Furthermore, pLKO.1-CTHRC1-shRNA or SP600125 treatment also
significantly inhibited IL-1β-induced caspase-3 activation
(P<0.01; Fig. 6F), suggesting
that it may have a protective effect on chondrocyte apoptosis.
Discussion
OA is a degenerative joint disorder with
multifactorial risk factors, including genetic and epigenetic
factors, age, sex, ethnicity and obesity (7,19).
CTHRC1 protein is expressed in a number of embryonic and neonatal
tissues, including developing cartilage and bone (20). In a previous study, CTHRC1 was
reported to stimulate bone formation in vitro and it was
suggested that the endogenous expression of CTHRC1 contributes to
effective osteogenic differentiation by affecting cell
proliferation and increasing the expression of osteogenic marker
genes (21). CTHRC1 is associated
with the severity of murine collagen antibody-induced arthritis
(22) and inhibition of
osteoclast differentiation (23).
CTHRC1 is upregulated in patients with OA (15), suggesting a correlation between
CTHRC1 and arthritis progression. The results of the present study
demonstrate that CTHRC1 is more highly expressed in the joint fluid
of patients with OA compared with normal joint fluid samples.
However, the molecular mechanisms of and the role of CTHRC1 in
human chondrocytes and OA progression remain to be elucidated.
IL-1β has been implicated in chondrocyte apoptosis
and the degeneration of articular cartilage (24) and is of great importance for the
mechanisms of degeneration and degradation of articular cartilage
in OA (25); as such, IL-1β was
used in the present study to determine the function of CTHRC1 in
OA. It has previously been reported that IL-1β levels are increased
in the synovial fluid of patients with OA and this is associated
with chondrocyte apoptosis, resulting in cartilage destruction and
pain (26,27), which is consistent with the
findings of the present study. Karaliotas et al (28) reported that the ratio of Bax/Bcl-2
was increased in patients with OA compared with a normal cartilage
control group, suggesting apoptosis induction. Furthermore,
caspase-3 and MMP-13, which is commonly used as a marker of
chondrocyte apoptosis and matrix degradation (29), were downregulated when CTHRC1
levels were reduced. This indicates the potential role of CTHRC1
downregulation in IL-1β-induced apoptosis inhibition, suggesting
that the suppression of chondrocyte apoptosis may, in part, result
in increased chondrocyte proliferation.
To investigate the underlying mechanisms of CTHRC1
downregulation, its effect on JNK1/2 activation in chondrocytes was
assessed. A previous study also provided evidence that the JNK1/2
signaling pathway is of great of importance for regulating cell
apoptotic signals in a number of cells (30) and is activated following IL-1β
stimulation in osteoarthritic cartilage but not in normal cartilage
(31). These studies serve to
increase our understanding of the molecular mechanisms of
chondrocyte proliferation, differentiation and apoptosis, and are
in agreement with the in vitro experiments performed in the
present study. Furthermore, JNK1/2 inhibitor SP600125 treatment and
CTHRC1 downregulation were demonstrated to inhibit IL-1β-induced
JNK1/2 activation, whereas CTHRC1 upregulation mimics the effect of
IL-1β on chondrocyte apoptosis and JNK1/2 activation, suggesting
that JNK1/2 signaling is associated with CTHRC1-mediated
chondrocyte proliferation and apoptosis.
The results of the present study suggest that CTHRC1
downregulation promotes chondrocyte proliferation and inhibits
apoptosis by directly regulating the JNK1/2 signaling pathway in
IL-1β-induced primary rat chondrocytes. CTHRC1 upregulation may
strengthen disease progression in an in vitro rat model of
OA. CTHRC1 may serve as a novel therapeutic target for the
regeneration of cartilage in patients with OA.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Vos T, Flaxman AD, Naghavi M, Lozano R,
Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V,
et al: Years lived with disability (YLDs) for 1160 sequelae of 289
diseases and injuries 1990-2010: A systematic analysis for the
Global Burden of Disease Study 2010. Lancet. 380:2163–2196. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karsdal MA, Michaelis M, Ladel C, Siebuhr
AS, Bihlet AR, Andersen JR, Guehring H, Christiansen C, Bay-Jensen
AC and Kraus VB: Disease-modifying treatments for osteoarthritis
(DMOADs) of the knee and hip: Lessons learned from failures and
opportunities for the future. Osteoarthritis Cartilage.
24:2013–2021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Findlay DM and Atkins GJ:
Osteoblast-chondrocyte interactionsin osteoarthritis. Curr
Osteoporos Rep. 12:127–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gore M, Tai KS, Sadosky A, Leslie D and
Stacey BR: Clinical comorbidities, treatment patterns, and direct
medical costs of patients with osteoarthritis inusual care: A
retrospective claims database analysis. J Med Econ. 14:497–507.
2011. View Article : Google Scholar
|
|
5
|
Portal-Núñez S, Esbrit P, Alcaraz MJ and
Largo R: Oxidative stress, autophagy, epigenetic changes and
regulation by miRNAs as potential therapeutic targets in
osteoarthritis. Biochem Pharmacol. 108:1–10. 2016. View Article : Google Scholar
|
|
6
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Charlier E, Relic B, Deroyer C, Malaise O,
Neuville S, Collée J, Malaise MG and De Seny D: Insights on
molecular mechanisms of chondrocytes death in osteoarthritis. Int J
Mol Sci. 17:E21462016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan S, Wang M, Zhao J, Zhang H, Zhou C,
Jin L, Zhang Y, Qiu X, Ma B and Fan Q: MicroRNA-34a affects
chondrocyte apoptosis and proliferation by targeting the SIRT1/p53
signaling pathway during the pathogenesis of osteoarthritis. Int J
Mol Med. 38:201–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu XX, Zhang XH, Diao Y and Huang YX:
Achyranthes bidentate saponins protect rat articular chondrocytes
against interleukin-1β-induced inflammation and apoptosis in
Svitro. Kaohsiung J Med Sci. 33:62–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wuelling M and Vortkamp A: Chondrocyte
proliferation and differentiation. Endocr Dev. 21:1–11. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shakibaei M, Allaway D, Nebrich S and
Mobasheri A: Botanical extracts from Rosehip (Rosa canina), Willow
Bark (Salix alba), and Nettle Leaf (Urtica dioica) suppress
IL-1β-induced NF-κB activation in canine articular chondrocytes.
Evid Based Complement Alternat Med. 2012:5093832012. View Article : Google Scholar
|
|
12
|
Kong D, Zheng T, Zhang M, Wang D, Du S, Li
X, Fang J and Cao X: Static mechanical stress induces apoptosis in
rat end plate chondrocytes through MAPK and mitochondria-dependent
caspase activation signaling pathways. PLoS One. 8:e694032013.
View Article : Google Scholar
|
|
13
|
Boileau C, Pelletier JP, Tardif G, Fahmi
H, Laufer S, Lavigne M and Martel-Pelletier J: The regulation of
human MMP-13 by licofelone, an inhibitor of cyclooxygenases
and5-lipoxygenase, in human osteoarthritic chondrocytes is mediated
by the inhibition of the p38MAP kinase signalling pathway. Ann
Rheum Dis. 64:891–898. 2005. View Article : Google Scholar
|
|
14
|
Aigner T, McKenna L, Zien A, Fan Z,
Gebhard PM and Zimmer R: Gene expression profiling of serum-and
interleukin-1beta-stimulated primary human adult articular
chondrocytes-a molecular analysis based on chondrocytes isolated
from one donor. Cytokine. 31:227–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rao ZT, Wang SQ and Wang JQ: Exploring the
osteoarthritis-related genes by gene expression analysis. Eur Rev
Med Pharmacol Sci. 18:3056–3062. 2014.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Yasuda H, Oh CD, Chen D, de Crombrugghe B
and Kim JH: A novel regulatory mechanism of type II collagen
expression via a SOX9-dependent enhancer in intron 6. J Biol Chem.
292:528–538. 2017. View Article : Google Scholar :
|
|
18
|
Aigner T, Soeder S and Haag J: IL-1beta
and BMPs-interactive players of cartilage matrix degradation and
regeneration. Eur Cell Mater. 12:49–56. 2006. View Article : Google Scholar
|
|
19
|
Sellam J and Berenbaum F: Osteoarthritis
and obesity. Rev Prat. 62:621–624. 2012.In French. PubMed/NCBI
|
|
20
|
Durmus T, LeClair RJ, Park KS, Terzic A,
Yoon JK and Lindner V: Expression analysis of the novel gene
collagen triple helix repeat containing-1 (Cthrc1). Gene Expr
Patterns. 6:935–940. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kimura H, Kwan KM, Zhang Z, Deng JM,
Darnay BG, Behringer RR, Nakamura T, de Crombrugghe B and Akiyama
H: Cthrc1 is a positive regulator of osteoblastic bone formation.
PLoS One. 3:e31742008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kudryavtseva E, Forde TS, Pucker AD and
Adarichev VA: Wnt signaling genes of murine chromosome 15 are
involved in sex-affected pathways of inflammatory arthritis.
Arthritis Rheum. 64:1057–1068. 2012. View Article : Google Scholar
|
|
23
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar
|
|
24
|
Kapoor M, Martelpelletier J, Lajeunesse D,
Pelletier JP and Fahmi H: Role of proinflammatory cytokines in the
pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar
|
|
25
|
Bian Q, Wang YJ, Liu SF and Li YP:
Osteoarthritis: Genetic factors, animal models, mechanisms, and
therapies. Front Biosci. 4:74–100. 2012. View Article : Google Scholar
|
|
26
|
Jotanovic Z, Mihelic R, Sestan B and
Dembic Z: Role of interleukin-1 inhibitors in osteoarthritis: An
evidence-based review. Drugs Aging. 29:343–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fernandes JC, Martel-Pelletier J and
Pelletier JP: The role of cytokines in osteoarthritis
pathophysiology. Biorheology. 39:237–246. 2002.PubMed/NCBI
|
|
28
|
Karaliotas GI, Mavridis K, Scorilas A and
Babis GC: Quantitative analysis of the Mrna expression levels of
BCL2 and BAX genes in human osteoarthritis and normal articular
cartilage: An investigation into their differential expression. Mol
Med Rep. 12:4514–4521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng W, Wu D, Zuo Q, Wang Z and Fan W:
Ginsenoside Rb1 prevents interleukin-1beta induced inflammation and
apoptosis in human articular chondrocytes. Int Orthop.
37:2065–2070. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang D, Guo S, Zhang T and Li H:
Hypothermia attenuatesis chemia/reperfusion-induced endothelial
cell apoptosis via alterations in apoptotic pathways and JNK
signaling. FEBS Lett. 583:2500–2506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan Z, Söder S, Oehler S, Fundel K and
Aigner T: Activation of interleukin-1 signaling cascades in normal
and osteoarthritic articular cartilage. Am J Pathol. 171:938–946.
2007. View Article : Google Scholar : PubMed/NCBI
|