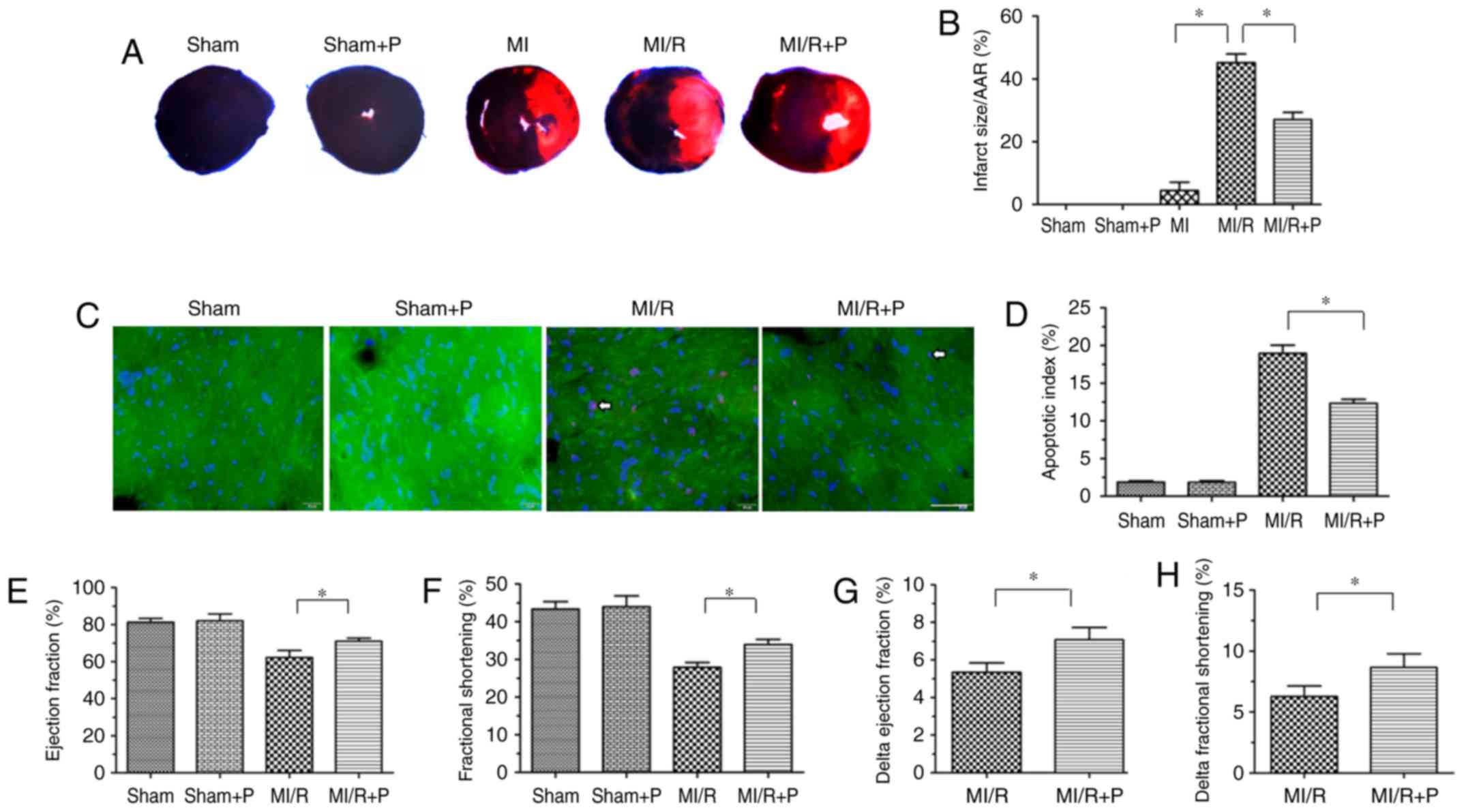
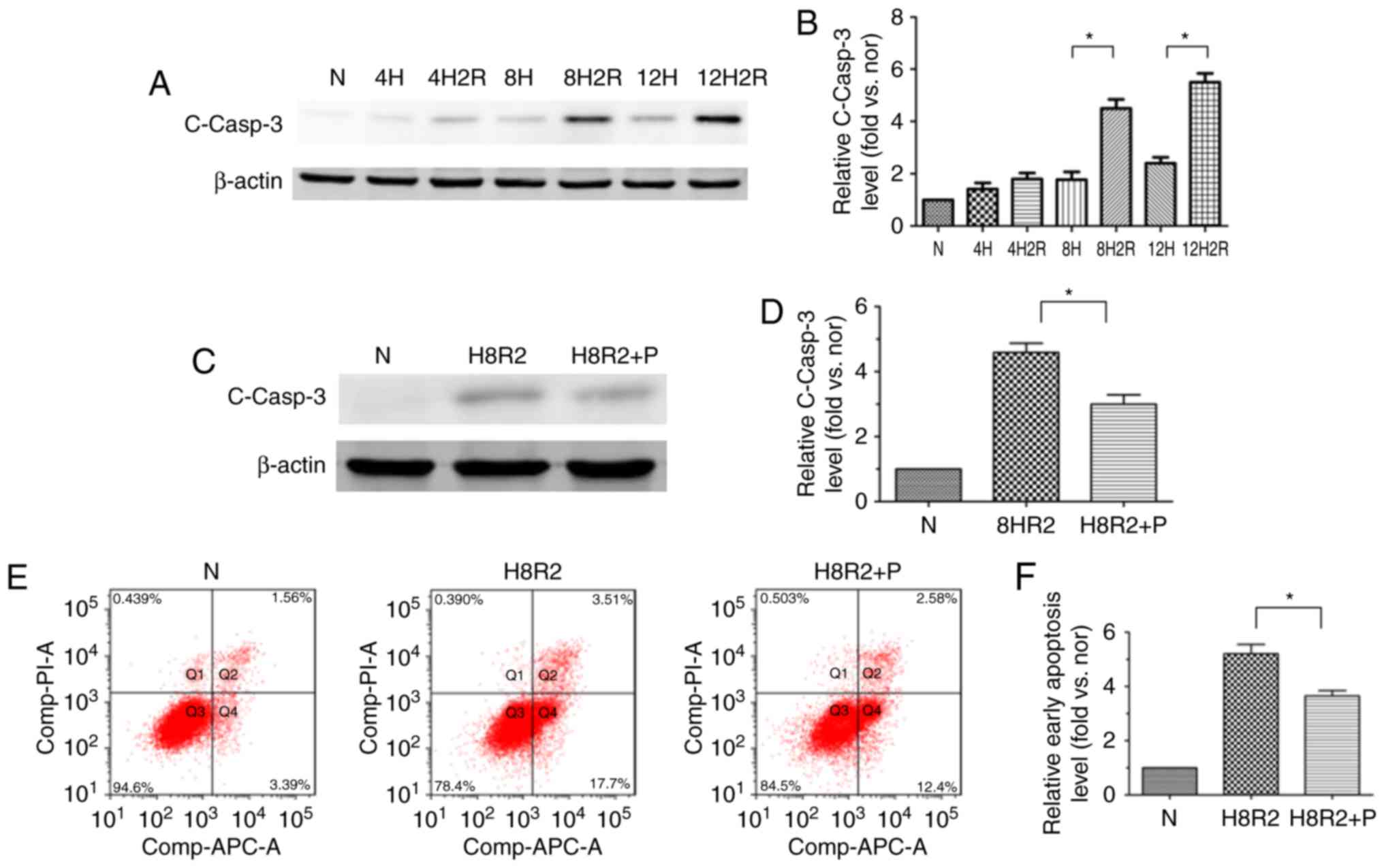
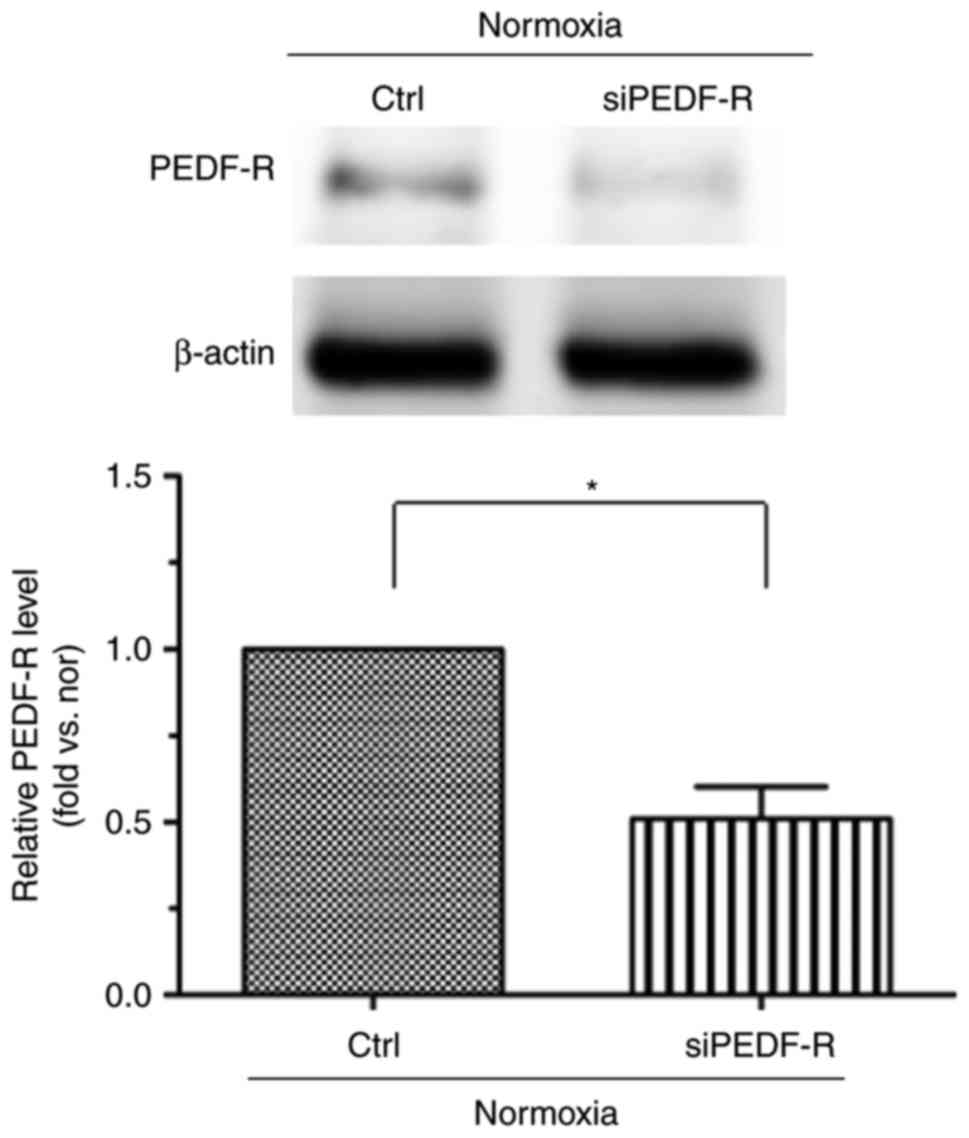
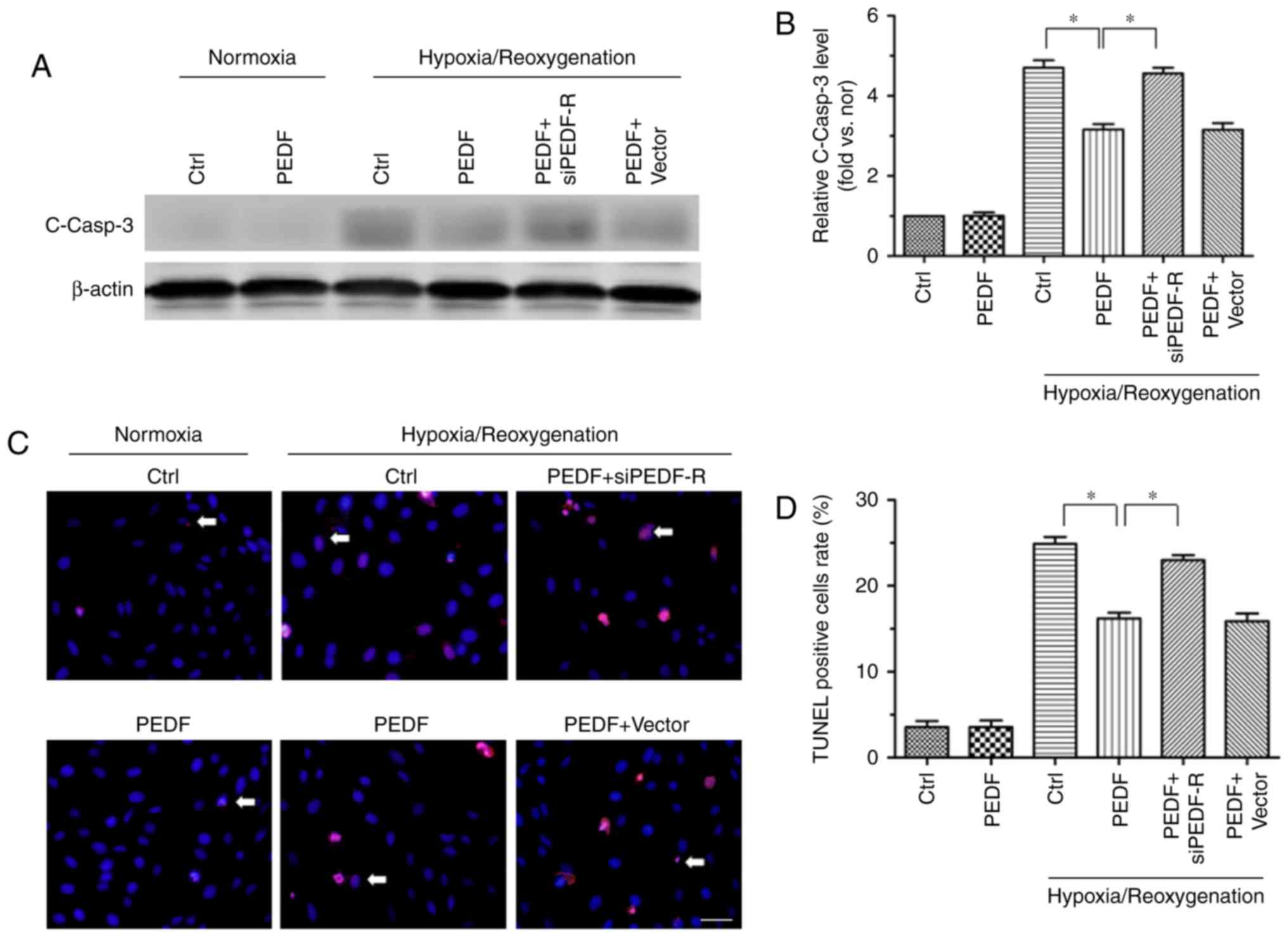
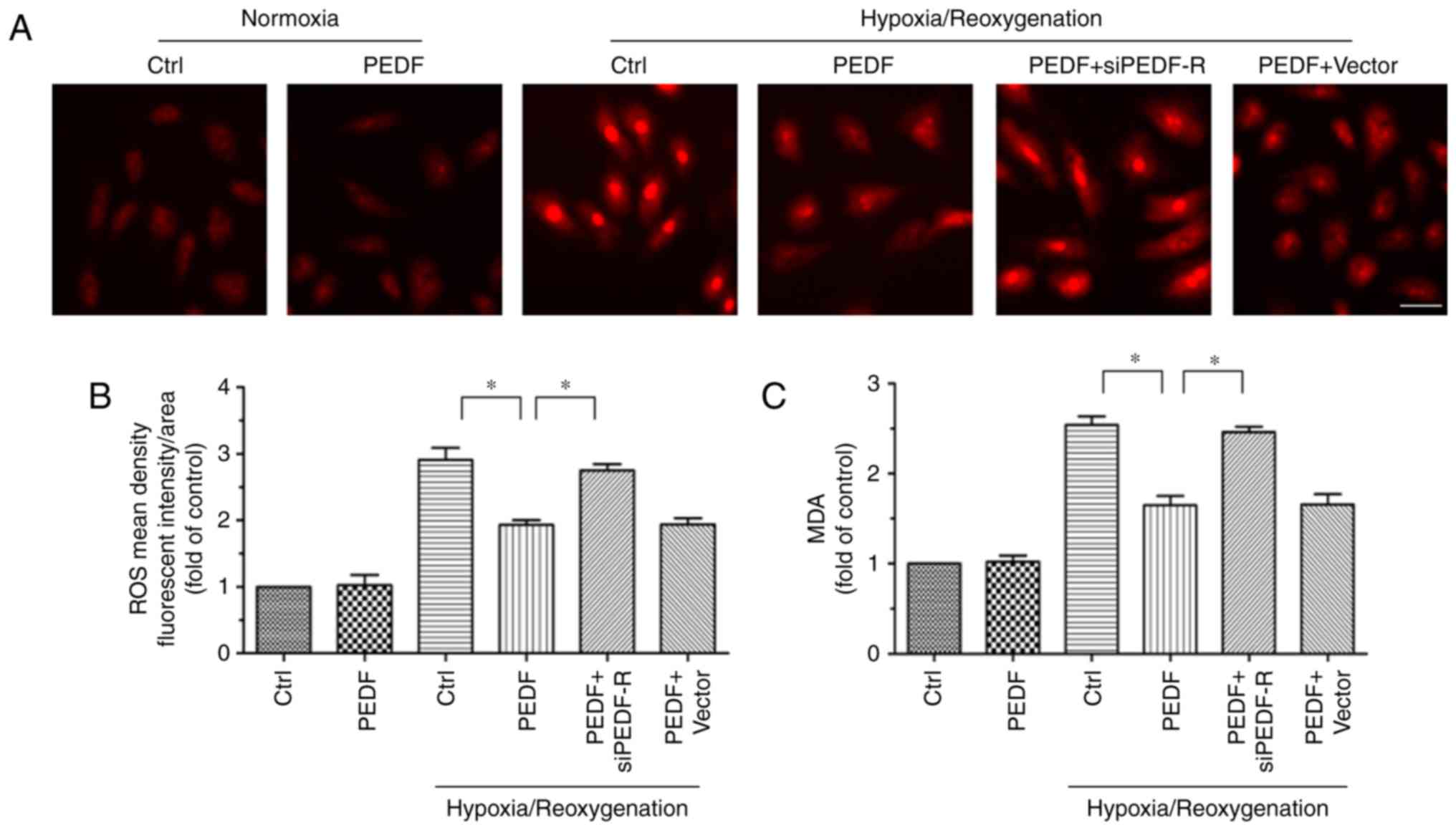
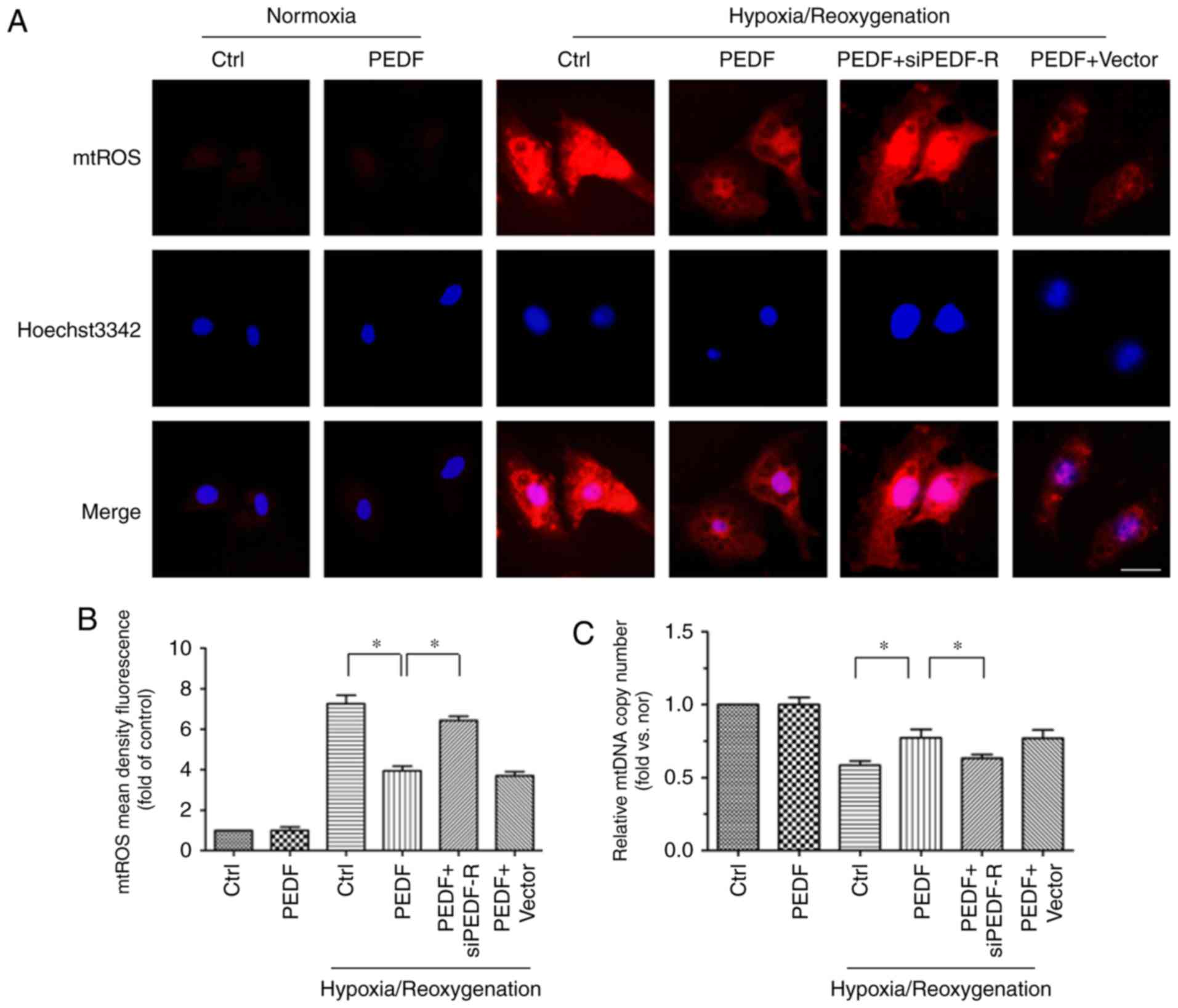
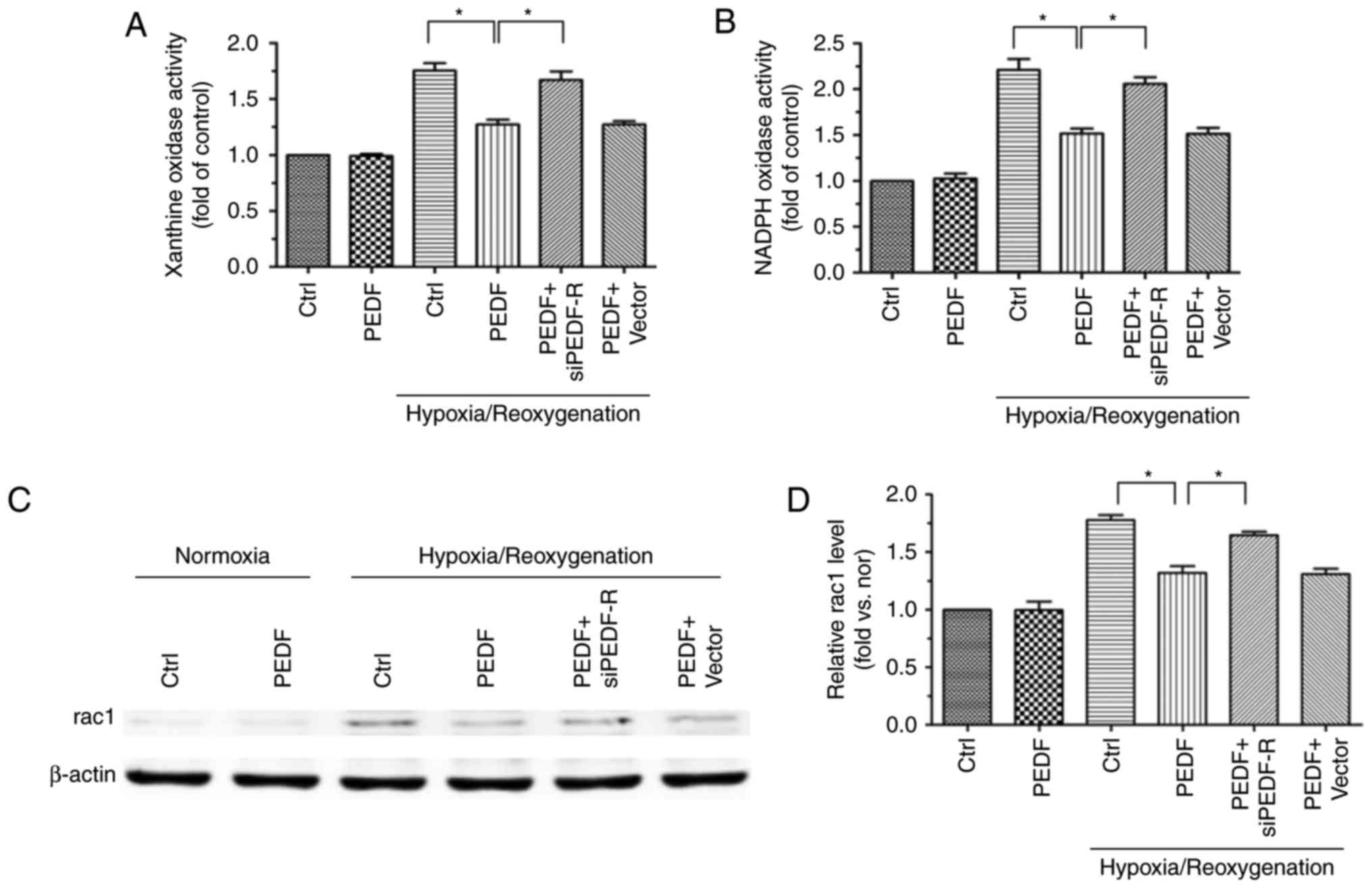
|
1
|
Damiani G, Salvatori E, Silvestrini G,
Ivanova I, Bojovic L, Iodice L and Ricciardi W: Influence of
socioeconomic factors on hospital readmissions for heart failure
and acute myocardial infarction in patients 65 years and older:
Evidence from a systematic review. Clin Interv Aging. 10:237–245.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crea F, Battipaglia I and Andreotti F: Sex
differences in mechanisms, presentation and management of ischaemic
heart disease. Atherosclerosis. 241:157–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hearse DJ: Myocardial protection during
ischemia and reperfusion. Mol Cell Biochem. 186:177–184. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hearse DJ and Bolli R: Reperfusion induced
injury: Manifestations, mechanisms, and clinical relevance.
Cardiovasc Res. 26:101–108. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Turer AT and Hill JA: Pathogenesis of
myocardial ischemia-reperfusion injury and rationale for therapy.
Am J Cardiol. 106:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murphy E and Steenbergen C: Mechanisms
underlying acute protection from cardiac ischemia-reperfusion
injury. Physiol Rev. 88:581–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Minamino T: Cardioprotection from
ischemia/reperfusion injury: Basic and translational research. Circ
J. 76:1074–1082. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Braunersreuther V and Jaquet V: Reactive
oxygen species in myocardial reperfusion injury: From
physiopathology to therapeutic approaches. Curr Pharm Biotechnol.
13:97–114. 2012. View Article : Google Scholar
|
|
9
|
Radak Z, Zhao Z, Goto S and Koltai E:
Age-associated neuro-degeneration and oxidative damage to lipids,
proteins and DNA. Mol Aspects Med. 32:305–315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Campos JC, Bozi LH, Bechara LR, Lima VM
and Ferreira JC: Mitochondrial quality control in cardiac diseases.
Front Physiol. 7:4792016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Afanas'ev I: ROS and RNS signaling in
heart disorders: Could antioxidant treatment be successful? Oxid
Med Cell Longev. 2011:2937692011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Becerra SP, Sagasti A, Spinella P and
Notario V: Pigment epithelium-derived factor behaves like a
noninhibitory serpin. Neurotrophic activity does not require the
serpin reactive loop. J Biol Chem. 270:25992–25999. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rychli K, Kaun C, Hohensinner PJ, Dorfner
AJ, Pfaffenberger S, Niessner A, Bauer M, Dietl W, Podesser BK,
Maurer G, et al: The anti-angiogenic factor PEDF is present in the
human heart and is regulated by anoxia in cardiac myocytes and
fibroblasts. J Cell Mol Med. 14:198–205. 2010. View Article : Google Scholar :
|
|
14
|
Gao X, Zhang H, Zhuang W, Yuan G, Sun T,
Jiang X, Zhou Z, Yuan H, Zhang Z and Dong H: PEDF and PEDF-derived
peptide 44mer protect cardiomyocytes against hypoxia-induced
apoptosis and necroptosis via anti-oxidative effect. Sci Rep.
4:56372014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Sun T, Jiang X, Yu H, Wang M, Wei
T, Cui H, Zhuang W, Liu Z, Zhang Z and Dong H: PEDF and
PEDF-derived peptide 44mer stimulate cardiac triglyceride
degradation via ATGL. J Transl Med. 13:682015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Wang Z, Feng SJ, Xu L, Shi HX,
Chen LL, Yuan GD, Yan W, Zhuang W, Zhang YQ, et al: PEDF improves
cardiac function in rats with acute myocardial infarction via
inhibiting vascular permeability and cardiomyocyte apoptosis. Int J
Mol Sci. 16:5618–5634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Notari L, Baladron V, Aroca-Aguilar JD,
Balko N, Heredia R, Meyer C, Notario PM, Saravanamuthu S, Nueda ML,
Sanchez-Sanchez F, et al: Identification of a lipase-linked cell
membrane receptor for pigment epithelium-derived factor. J Biol
Chem. 281:38022–38037. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bernard A, Gao-Li J, Franco CA, Bouceba T,
Huet A and Li Z: Laminin receptor involvement in the
anti-angiogenic activity of pigment epithelium-derived factor. J
Biol Chem. 284:10480–10490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zimmermann R, Strauss JG, Haemmerle G,
Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger
G, Eisenhaber F, Hermetter A and Zechner R: Fat mobilization in
adipose tissue is promoted by adipose triglyceride lipase. Science.
306:1383–1386. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirsch J, Johnson CL, Nelius T, Kennedy R,
Riese Wd and Filleur S: PEDF inhibits IL8 production in prostate
cancer cells through PEDF receptor/phospholipase A2 and regulation
of NFκB and PPARγ. Cytokine. 55:202–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang XY, Zhang YQ, Lu P, Zhang H, Li Y,
Dong H and Zhang Z: PEDF attenuates hypoxia-induced apoptosis and
necrosis in H9c2 cells by inhibiting p53 mitochondrial
translocation via PEDF-R. Biochem Biophys Res Commun. 465:394–401.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu P, Zhang YQ, Zhang H, Li YF, Wang XY,
Xu H, Liu ZW, Li L, Dong HY and Zhang ZM: Pigment
epithelium-derived factor (PEDF) improves ischemic cardiac
functional reserve through decreasing hypoxic cardiomyocyte
contractility through PEDF receptor (PEDF-R). J Am Heart Assoc.
5:e0031792016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reim DF and Speicher DW: N-terminal
sequence analysis of proteins and peptides. Curr Protoc Protein
Sci. Chapter 11: Unit 11.10. 2001.
|
|
24
|
Chen L, Zhao X, Liang G, Sun J, Lin Z, Hu
R, Chen P, Zhang Z, Zhou L and Li Y: Recombinant SFRP5 protein
significantly alleviated intrahepatic inflammation of nonalcoholic
steatohepatitis. Nutr Metab (Lond). 14:562017. View Article : Google Scholar
|
|
25
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. 8th edition. The National
Academies Press; Washington, DC: 2011
|
|
26
|
European Commission: Directive 2010/63/EU
of the European Parliament and of the council of 22 September 2010
on the protection of animals used for scientific purposes. Off J
Eur Union. L276:33–79. 2010.
|
|
27
|
Zhang Y, Li Y, Wang X, Zhao Q, Lu P, Zhang
H, Dong H and Zhang Z: A closed-chest rat model of myocardial
isch-aemia/reperfusion supports direct intramyocardial gene
delivery. Exp Ther Med. In press.
|
|
28
|
Black SC and Rodger IW: Methods for
studying experimental myocardial ischemic and reperfusion injury. J
Pharmacol Toxicol Methods. 35:179–190. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peshavariya HM, Dusting GJ and Selemidis
S: Analysis of dihydroethidium fluorescence for the detection of
intracellular and extracellular superoxide produced by NADPH
oxidase. Free Radic Res. 41:699–712. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
31
|
Tsutsui H, Kinugawa S and Matsushima S:
Oxidative stress and mitochondrial DNA damage in heart failure.
Circ J. 72(Suppl A): A31–A37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li JM, Zhu HQ, Shen E, Wan L, Arnold JMO
and Peng TQ: Deficiency of Rac1 blocks NADPH oxidase activation,
inhibits endoplasmic reticulum stress, and reduces myocardial
remodeling in a mouse model of type 1 diabetes. Diabetes.
59:2033–2042. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garcia-Dorado D, Andres-Villarreal M,
Ruiz-Meana M, Inserte J and Barba I: Myocardial edema: A
translational view. J Mol Cell Cardiol. 52:931–939. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gustafsson AB and Gottlieb RA: Heart
mitochondria: Gates of life and death. Cardiovasc Res. 77:334–343.
2008. View Article : Google Scholar
|
|
35
|
Zorov DB, Filburn CR, Klotz LO, Zweier JL
and Sollott SJ: Reactive oxygen species (ROS)-induced ROS release:
A new phenomenon accompanying induction of the mitochondrial
permeability transition in cardiac myocytes. J Exp Med 1.
92:1001–1014. 2000. View Article : Google Scholar
|
|
36
|
Zhou ZX, Wang Z, Guan QH, Qiu F, Li Y, Liu
Z, Zhang H, Dong H and Zhang Z: PEDF inhibits the activation of
NLRP3 inflammasome in hypoxia cardiomyocytes through PEDF
receptor/phospholipase A2. Int J Mol Sci. 17:E20642016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kuo HF, Liu PL, Chong IW, Liu YP, Chen YH,
Ku PM, Li CY, Chen HH, Chiang HC, Wang CL, et al: Pigment
epithelium-derived factor mediates autophagy and apoptosis in
myocardial hypoxia/reoxygenation injury. PLoS One. 11:e01560592016.
View Article : Google Scholar : PubMed/NCBI
|