Introduction
Gastric cancer is the fifth most common cancer
worldwide, with an estimated 951,000 new cases and 745,000 deaths
reported in 2012. Nearly 70% of the cases occurred in less
developed regions, with the highest in eastern Asia especially in
China (1). A survey of the
incidence of malignant tumors and mortality in China (2015) showed
that the incidence of gastric cancer was 67.91/million, which was
the 2nd highest, just after the lung cancer incidence rate of
73.33/million. In addition, the mortality of 49.80% was also ranked
second (2). Gastric cancer remains
a common disease that threatens people's health and the median
survival time for these patients is only 6–9 months (3). Fortunately, a large number of
tumor-suppressor genes and oncogenes have been reported in recent
years. However, the molecular mechanisms underlying the development
of gastric carcinomas remain poorly understood (4).
Ubiquitination is an important cellular mechanism
for the targeted degradation of proteins, and is instrumental for a
variety of cellular processes including, but not limited to, cell
cycle progression, antigen presentation, transcription, and
programmed cell death (5). In
eukaryotes, particularly, the ubiquitin proteasome system (UPS)
precisely regulates the cell cycle at key checkpoints by targeting
cell cycle regulators for proteasome-mediated degradation (6). This regulation requires
ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme
(E2), and ubiquitin ligases (E3) to work in concert to facilitate
the ubiquitination of target proteins (7,8).
E2s are classified into four classes and
ubiquitin-conjugating enzyme 2C (UBE2C) (also known as UbcH10) is a
class III E2 enzyme. The full length UBE2C protein contains 179
amino acids and the first 28 residues comprise an N-terminal
extension with various motifs which may contribute to target
protein recognition and conjugation (9). UBE2C is located on chromosome 20q13
and has a molecular weight of 19.65 kDa. Interestingly, UBE2C is
nearly undetectable in normal tissues, at both the RNA or protein
levels. In contrast, increasing evidence indicates that UBE2C
protein is overexpressed in many human tumor types, including brain
tumors (10), malignant breast
carcinomas (11), lung cancer
(12,13), anaplastic thyroid carcinomas
(14), esophageal adenocarcinomas
(15), hepatocellular carcinomas
(16) and colorectal cancer
(17–19), suggesting that UBE2C may play an
important role in tumorigenesis and progression. UBE2C
overexpression causes the centrosome duplication instability, and
variety of proteins can cause this phenomenon, including the
anaphase-promoting complex (APC/C) substrates cyclin B, AURKA and
PLK1 (20,21). However, its role in gastric cancer
carcinogenesis remains poorly defined.
Compared to conventional genome-wide association
studies GWAS revealed associations of single-nucleotide
polymorphisms (SNPs), gene expression genome-wide association
studies (eGWASs) aimed to discover more common expression and
similar to the SNP cell analysis (22–24).
In the previous study, we analyzed the 184 differentially expressed
genes in gastric cancer from the eGWAS and revealed AURKA is at the
core of the gastric cancer linkage network (25). AURKA is a serine/threonine kinase
that localizes to spindle poles, and ensures the correct assembly
of cellular components during mitosis in normal cells (26). In cancer cells, AURKA
overexpression results in the activation of several oncogenic
pathways, including those of PI3K/Akt, β-catenin, and NF-κB
(27,28). Importantly, we further found that
AURKA expression is closely related to UBE2C expression.
In our current report, we demonstrate that gastric
cancer malignancies are associated with high levels of UBE2C
expression. The knockdown of UBE2C decreases the levels of p-AURKA
and inhibits the development of gastric adenocarcinoma by
Wnt/β-catenin and PI3K/Akt signaling pathways. AURKA is the center
of the network.
Materials and methods
eGWAS analysis
To analyze the molecular signature of gastric
cancer, we collected 13 independent datasets from the NCBI Gene
Expression Omnibus using the public database eGWAS. The most
differentially expressed genes between tumors and non-tumors from
each databset were screened, P-values were calculated from the
number of positive/negative experiments for each gene, and Fisher's
exact test was used to determine significance. The Bonferroni
threshold (P=1.0×10−5) indicated that there were 184
gastric cancer susceptibility genes. These 184 genes were analyzed
for Gene Ontology (GO) using DAVID online tools, as well as pathway
analysis by Medusa. Additionally, we identified the top
differentially expressed genes and performed a KEGG pathway
analysis using GenMAPP v2.1, calculating the enrichment P-value.
Finally, we applied 13 differences of highest expression genes by
integrating the experiments, database and literature.
Cell culture
Human gastric cancer cell lines MGC-803 and SGC-7901
were purchased from China Academia Sinica Cell Repository
(Shanghai, China). Cells were cultured and maintained in RPMI-1640
medium supplemented with 10% fetal bovine serum (FBS; Hyclone,
Logan, UT, USA) under standard conditions. Cells were maintained at
37°C in a humidified atmosphere with 5% CO2.
Antibodies and other reagents
UBE2C, AURKA, β-catenin, AKT1, p-AKT1, GSK-3β,
p-GSK-3β, Slug, Snail and Twist antibodies were purchased from
Abcam (Cambridge, UK). E-cadherin, N-cadherin and p-AURKA
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). The GAPDH antibody was purchased from Zhongshan
Golden Bridge Biotechnology (Beijing, China). Western blot analysis
reagents were purchased from Sigma, PVDF membranes were purchased
from Millipore Corp. (Bedford, MA, USA) and RIPA lysis buffer was
purchased from Beijing Taike Biotechnology. Lentiviruses containing
an UBE2C and AURKA inhibitor sequences (Lenti-si-UBE2C and
Lenti-si-AURKA) or negative control (Lenti-NC) were obtained from
Shanghai GeneChem Co., Ltd. (Shanghai, China).
Transient transfection
The UBE2C and AURKA silencer siRNAs were synthesized
by Shanghai GenePharma Co., Ltd (Shanghai, China). The human
gastric cancer cells were seeded and grown in 6-well plates
overnight and transfected with negative control siRNA, siRNA
against UBE2C, or siRNA against AURKA using X-tremeGENE siRNA
transfection reagent (Roche) according to the manufacturer's
protocol. The primers for detecting UBE2C forward,
5′-GGATTTCTGCCTTCCCTGAA-3′ and reverse, 5′-GATAGCAGGGCGTGAGGAAC-3′;
the primers for detecting AURKA forward, 5′-AAAGAGCAAGCAGCCCCTGC-3′
and reverse, 5′-GAATTCAACCCGTGATATTCTT-3′; and the primers for
negative control forward, 5′-CCGGGAAACTGTGGCGTGATGG-3′ and reverse,
5′-AGGTGGAGGAGTGGGTGTCGCTGTT-3′. Briefly, 3 μl of siRNA was
added to 50 μl DMEM serum-free medium. In parallel, 3
μl of the transfection reagents was added to 50 μl
serum-free DMEM and mixed. Finally, after mixing and incubating the
siRNA and X-tremeGENE siRNA transfection reagents separately for 5
min, the reagents were combined, mixed, and placed at room
temperature (RT) for 20 min to form the siRNA/transfection
complexes. Subsequent experiments were then carried out 48 h after
transfection.
Western blot analysis
Protein extracts from siRNA-transfected MGC-803 and
SGC-7901 cells were collected, and after samples were adjusted to
equal protein concentration and volume, they were subjected to
SDS-PAGE on 10% SDS-acrylamide gels. Separated proteins were
transferred to PVDF membranes (Millipore Corp.) and then blocked.
Antibodies for UBE2C, AURKA, p-AURKA, AKT1, p-AKT1, GSK-3β,
p-GSK-3β, and β-catenin were used for western blot analysis, and
were incubated at a 1:1,000 dilution, followed by incubation with
their respective secondary antibodies (1:2,000 dilution).
Quantitative real-time PCR
Total RNA was extracted from cultured cells using
the TRIzol reagent method. Equal amounts (1 μg) of RNA were
converted into cDNA using oligo (dT) primer using a PrimeScript RT
Reagent kit (Takara Bio, Inc., Otsu, Japan). Real-time PCR analysis
involved 2 ng cDNA with 10 μl SYBR Green PCR Master Mix
(Applied Biosystems) and 2 μM primers in the ABI StepOne
Real-Time PCR System (Applied Biosystems). The PCR primer sequences
included those of UBE2C, AURKA, Twist and E-cadherin. The PCR
conditions were as follows: 30 cycles of 94°C for 30 sec, 56°C for
30 sec, 72°C for 90 sec, and a final extension at 72°C for 5
min.
Colony formation and cell proliferation
assay
Tumor cells transfected with UBE2C siRNAs were
cultured at 2,000 cells/well in 6-well plates. The cells were
allowed to grow for 14 days, and the medium was changed every 3
days. Upon termination of the experiment, colonies were fixed with
paraformaldehyde (4% w/v), stained with crystal violet (0.5% w/v)
and counted. For cell proliferation, cells were assayed using the
Cell Counting kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan)
according to the manufacturer's protocol. To determine the effect
of UBE2C siRNA on cell proliferation, the tumor cells transfected
with UBE2C siRNAs were seeded into each well of a 96-well plate at
a density of 1×103 cells in 100 μl of culture
medium containing 10% FBS and were grown overnight. All experiments
were performed in triplicate. The medium was then replaced with 100
μl fresh medium with 10% CCK-8 reagent, and the cells were
incubated for 3.5 h at 37°C. At 0, 24, 48, 72 and 96 h, the
absorbance was measured at 450 nm using an EL×800 micro-plate
reader (BioTek, USA).
Matrigel invasion and Transwell cell
migration assay
The cell invasion capability was assessed using a
Matrigel invasion chamber (Becton-Dickenson, Bedford, USA),
incubated with 60 μl of 1 mg/ml Matrigel in a 24-well plate
(Corning Costar, Cambridge, MA, USA) at 37°C for 1 h to allow gel
polymerization. In the cell migration assay, the top chamber was
without matrigel. Transfected cells were trypsinized and washed
with the appropriate serum-free medium; then, 200 μl of the
cell suspension containing 5×104 cells was added to top
chamber, followed by the addition of 500 μl of the
appropriate medium containing 10% FBS to the well underneath the
insert. The cells were incubated at 37°C for 48 h and gently rinsed
with phosphate buffered saline (PBS) before, fixing with 4%
paraformaldehyde at RT for 15 min. Cells were stained with 0.25%
crystal violet for 5 min, rinsed again, and then allowed to dry.
The inserts were then viewed under the microscope at ×200
magnification (Olympus Corporation Tokyo, Japan) and the number of
cells/field in three random fields were counted.
Wound healing assay
Gastric cancer cells were cultured in 6-well plates
overnight. The cell monolayers were scratched in a line across the
well using a 200-μm standard pipette tip. The 'wound' was
then washed twice with serum-free media to remove cell debris, and
the plated cells were incubated in RPMI-1640 medium supplemented
with 2.5% FBS (Hyclone). The cell-free wound area was photographed
at the indicated times with use of a digital camera connected to an
inverted microscope (Nikon TE200; Nikon Corp., Tokyo, Japan). The
resulting images were analyzed by the use of Image J software.
Wound healing was calculated as the proportion of remaining
cell-free area compared with the initial wound area.
Flow cytometric analysis of the cell
cycle and apoptosis
The MGC-803 and SGC-7901 cells were transfected with
UBE2C siRNA for 48 h, harvested and then fixed with 70% ethanol at
-20°C. The fixed cells were then incubated with 50 mg/ml propidium
iodide (PI; Sigma, St. Louis, MO, USA), and 1 mg/ml RNAse A for 30
min at RT. The DNA content was then analyzed using a FACSCalibur
system (Becton Dickinson). The distribution of cells in each cell
cycle phase was determined using ModFit software (Becton
Dickinson). For apoptosis analysis, the cells were transfected with
si-UBE2C for 48 h and harvested, the phosphatidylserine exposure
level was determined by staining the cells with a human Annexin
V-FITC kit (MBL, Nagoya, Japan), according to the manufacturer's
instructions. At least 1×106 stained cells were analysed
by the FACS Calibur for each determination.
Immunofluorescence staining
Twenty-four hours after transfection, cells were
plated on glass cover slips and 48 h post-transfection the cover
slips were washed extensively in PBS and fixed with 4%
paraformaldehyde in PBS. After additional washing, the cells were
permeabilized with 1% Triton X-100 in PBS for 5 min. The cover
slips were then washed and blocked with 1% BSA for 30 min. Cells
were incubated with AURKA primary antibodies overnight at 4°C. The
samples were then washed and incubated with species specific
secondary rhoda-mine-labeled antibodies (TRITC) in PBS (1:100
dilution) for 2 h. Nuclei were stained with DAPI at RT for 10 min
and cover slips were mounted with antifade solution prior to
imaging on a confocal microscope (Olympus FV1000S).
Subcutaneous tumor model
Four-week-old immune-deficient nude mice (BALB/c-nu)
were purchased from the animal center of the Cancer Institute and
Hospital of Chinese Academy of Medical Sciences, bred at the
facility of laboratory animals, Tianjin Institute of Biological
Engineering. The mice were injected subcutaneously with MGC-803
cells which were stably expressed Lenti-si-UBE2C or Lenti-NC. The
cells were suspended with the concentration of 2×106/ml.
Mice were monitored daily and all the mice formed tumor
subcutaneously. The weight of the mice was measured every 3 days,
and the tumor weight was surveyed at the endpoint of the study.
Immunohistochemistry
Immunostaining was performed on paraffin sections of
Lenti-si-AURKA tumor specimens and Lenti-NC specimens using the
avidin-biotin complex (ABC)-peroxidase method. The sections were
incubated with primary antibodies against UBE2C overnight at 4°C.
They were then treated with biotinylated secondary antibody (1:100)
for 1 hour at RT, followed by incubation with ABC-peroxidase for
another 1 hour. Protein expression was detected by coloration with
diaminobenzidine (DAB) buffer. After washing with Tris buffer, the
sections were incubated with 3,3′-diaminobenzidine (DAB, 30 mg
dissolved in 100 ml PBS) for 5 min, rinsed in water and
counterstained with hematoxylin.
Statistical analysis
The significance of Kaplan-Meier statistics was
tested using the log-rank test. Multivariate analyses were
performed using the multivariate Cox regression model. SPSS 16.0
(SPSS, Chicago, IL, USA) was used for all of the calculations. All
of the data are represented by the mean ± SD. The statistical
significance was set at P<0.05.
Results
eGWAS identifies UBE2C and AURKA as
functional genes for gastric cancer
After analyzing 13 independent microarray
experiments from the eGWAS in gastric cancer and 679 samples from
public repositories, we took 184 genes which repeated differential
expression shared the highest ranking. Among them, by integrating
the experiments, database and literature, we focused on 13 genes
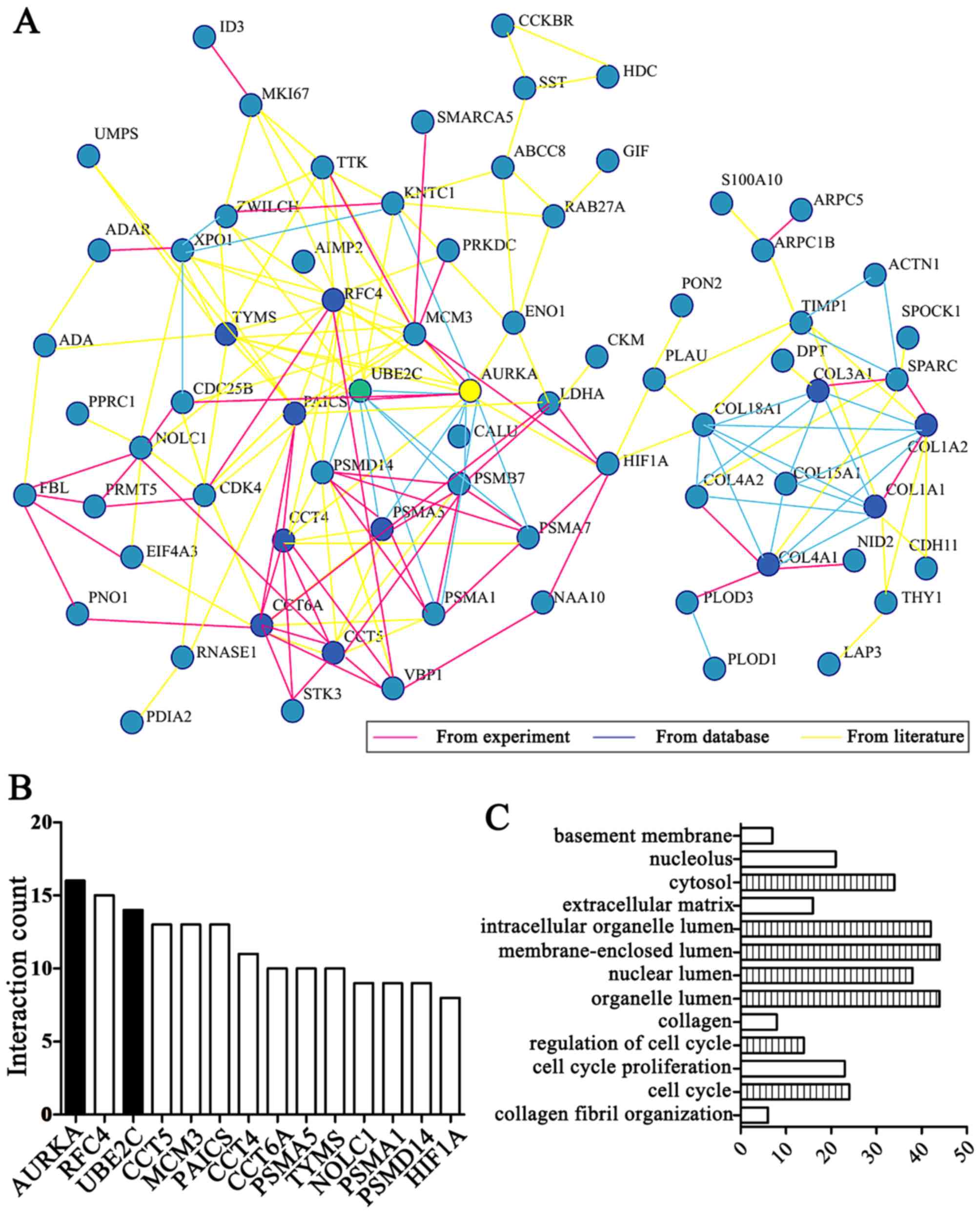
ranking behind AURKA (Fig. 1A and
B) and the results demonstrated that AURKA is a hub gene based
on linkage analysis. In addition, we found 'cell cycle' functions
were the most implicated of the UBE2C and AURKA genes in enrichment
of GO terms by DAVID online tool analysis (Fig. 1C).
Importantly, UBE2C was markedly differentially
expressed second to AURKA in gastric cancer compared with normal
gastric mucosa. UBE2C is located on chromosome 20q13 and belongs to
the E2 gene family and the first 28 residues comprise an N-terminal
extension with various motifs, and the remaining residues form the
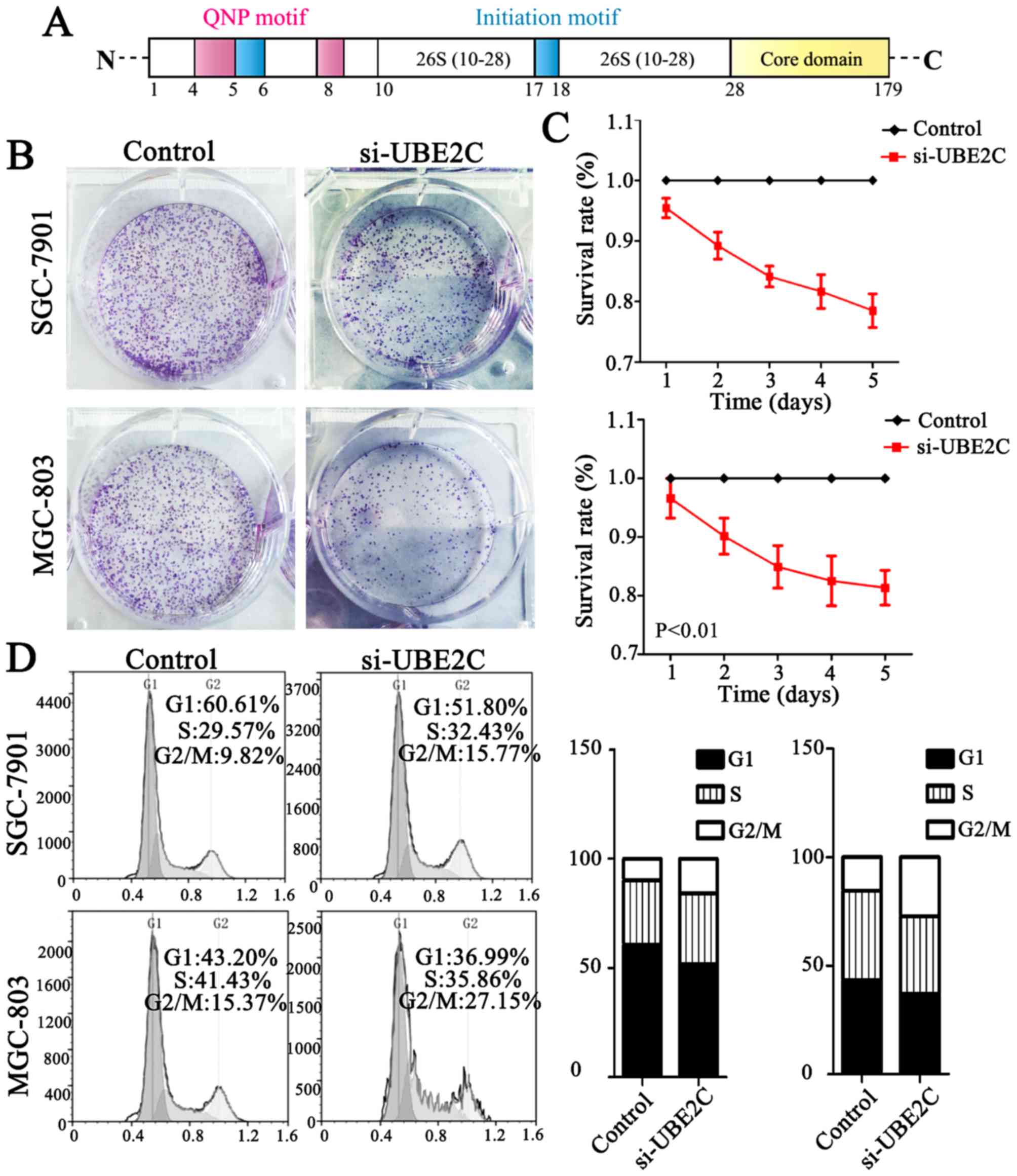
core domain (Fig. 2A). Taken
together UBE2C is frequently overexpressed in gastric cancer and is
associated with a worse overall survival.
Knockdown of UBE2C causes G2/M phase
arrest in the cell cycle and suppresses tumor formation
UBE2C is highly expressed in gastric cancer, but
whether the downregulation of UBE2C represses tumorigenesis is
unkown. Therefore, we knocked down UBE2C using siRNA in MGC803 and
SGC7901 cells, and examined the effects on colony formation, cell
proliferation and cell cycle distribution. As is shown in Fig. 2B, MGC803 and SGC7901 cells
transfected with si-UBE2C exhibited a significant reduction in
colony formation after 2 weeks (40.00%), compared with the control
group (56.33%). Furthermore, the CCK-8 analysis showed that the
survival rate of cells with si-UBE2C treatment was inhibited by 30%
(Fig. 2C). In addition, the
percentage of SGC7901 and MGC803 cells in the G2/M phase was 9.82
and 15.37% for the control cells, whereas the cells treated with
si-UBE2C was significantly increased to 15.77 and 27.15%
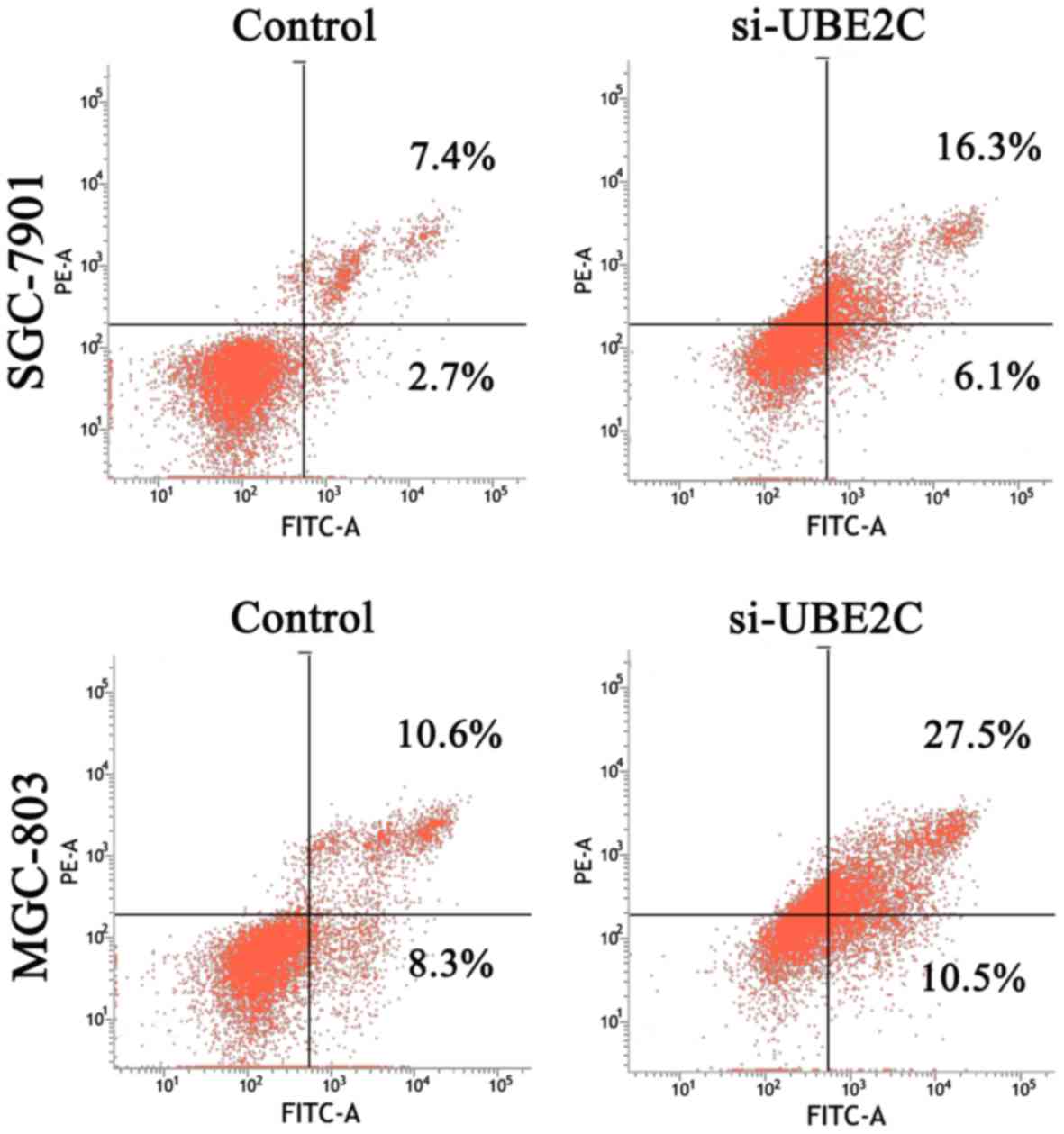
respectively (Fig. 2D). The extent
of siRNA-mediated cell death was analysed in these cell types using
an Annexin V assay. The results (Fig.
3) revealed that 22.4 and 38.0% of the UBE2C siRNA-treated
SGC7901 and MGC803 cells were Annexin V positive, whereas only 11.1
and 18.9% of the cells treated with control siRNAs showed this
positivity at 48 h post-transfection. These results suggested that
the suppression of UBE2C expression inhibited the proliferation and
induced apoptosis of gastric adenocarcinoma cells in
vitro.
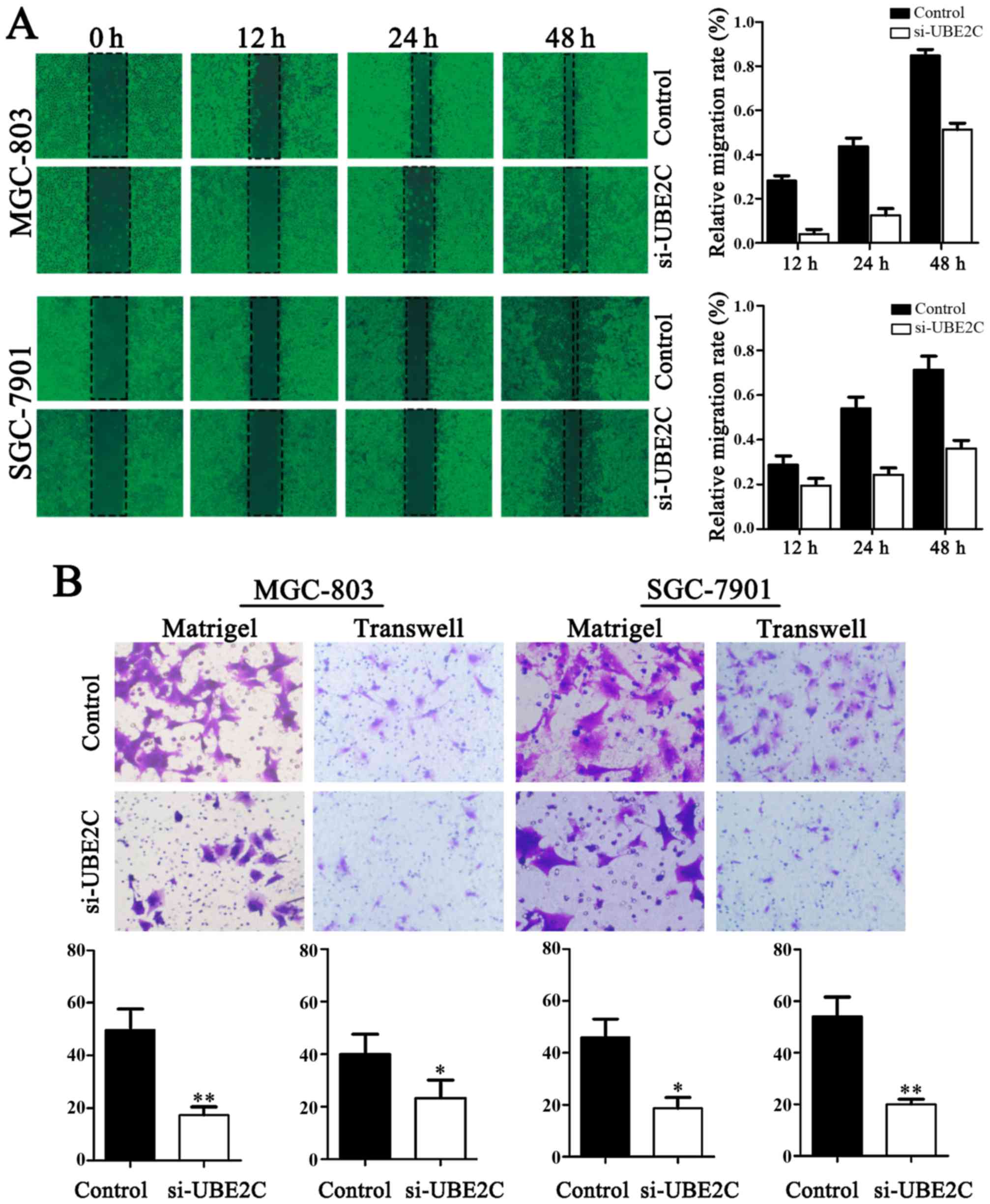
Downregulation of UBE2C inhibits the
migration and invasion of gastric adenocarcinoma cells
The migration of cells to the wound area was
analyzed at 12, 24 and 48 h after injury, and the results revealed
that the knockdown of UBE2C led to a marked inhibition of wound
healing, compared with the negative control. Specifically, the
relative migration rates of si-UBE2C in MGC803 and SGC7901 cells
were 19.60, 32.53 and 37.25%, as well as 2.16, 10.87 and 52.17%
after 12, 24 and 48 h, respectively. Conversely, in the negative
control the migration rates were 28.57, 53.06 and 71.43%, as well
as 27.08, 43.75 and 85.42%, respectively. As shown in Fig. 4, the number of cells invading
through the matrigel in MGC803 si-UBE2C-group was significantly
decreased (20.7±4.1), compared to control (52.1±2.2). The invasion
activity was inhibited in si-UBE2C group (18.7±1.2), compared to
the control (49.3±4.1). Collectively, these data indicate that
UBE2C play a role in gastric adenocarcinoma cell migration and
invasion in vitro.
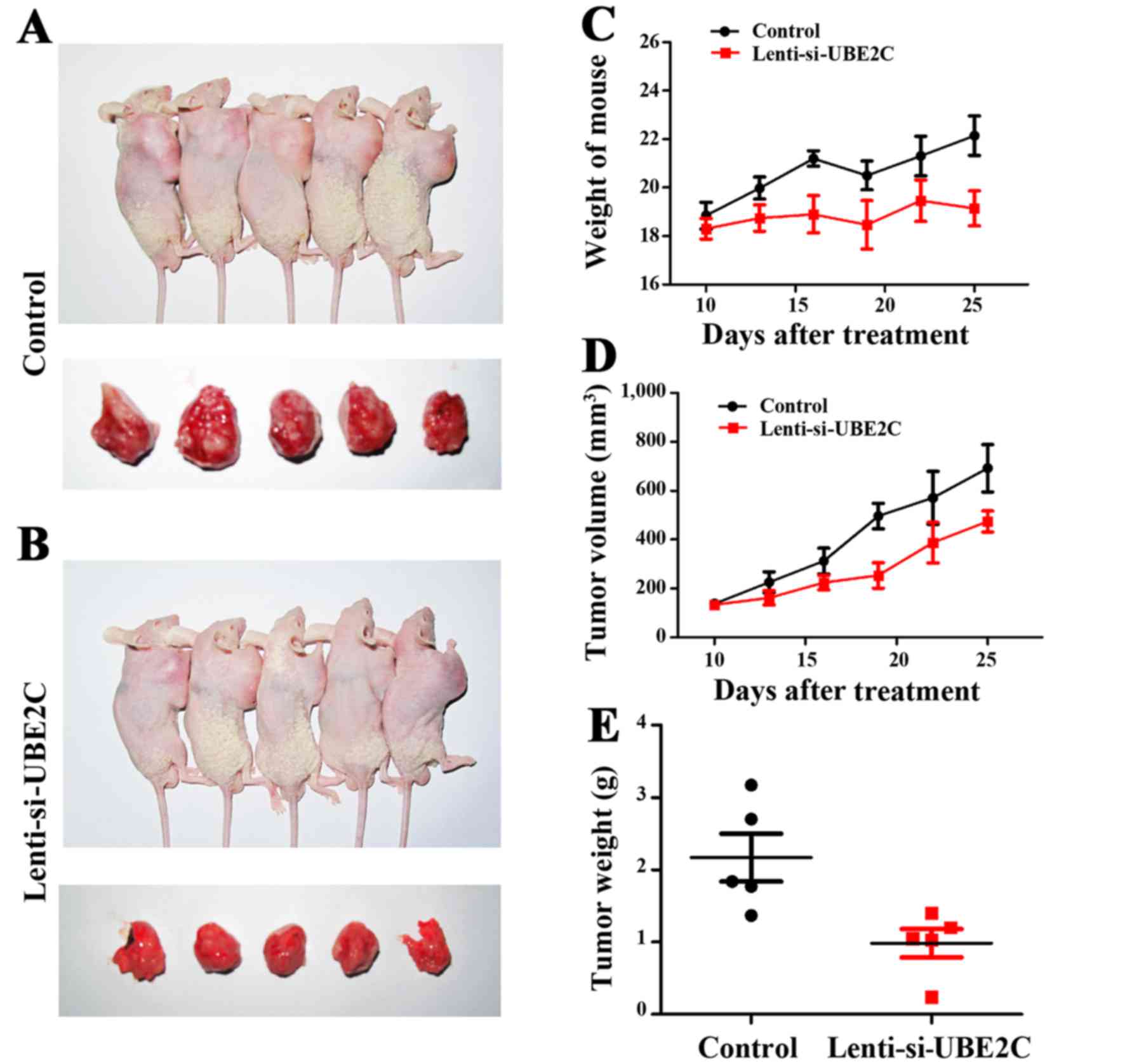
Inhibition of UBE2C suppresses gastric
cancer growth in xenograft models
UBE2C suppresses gastric cancer cell migration and
invasion in vitro. To verify the role of UBE2C in
vivo, the subcutaneous gastric carcinoma model using MGC-803
cells as described previously were established (Fig. 5A and B). The mouse and tumor
weights were measured, and the tumor volume was measured using
calipers to measure the tumor length and width (Fig. 5C and D). Compared with the
Lenti-NC-treated MGC-803 cells, the Lenti-si-UBE2C-treated tumor
was suppressed significantly (Fig.
5E). The results confirmed that UBE2C play a role in gastric
cancer growth in vivo.
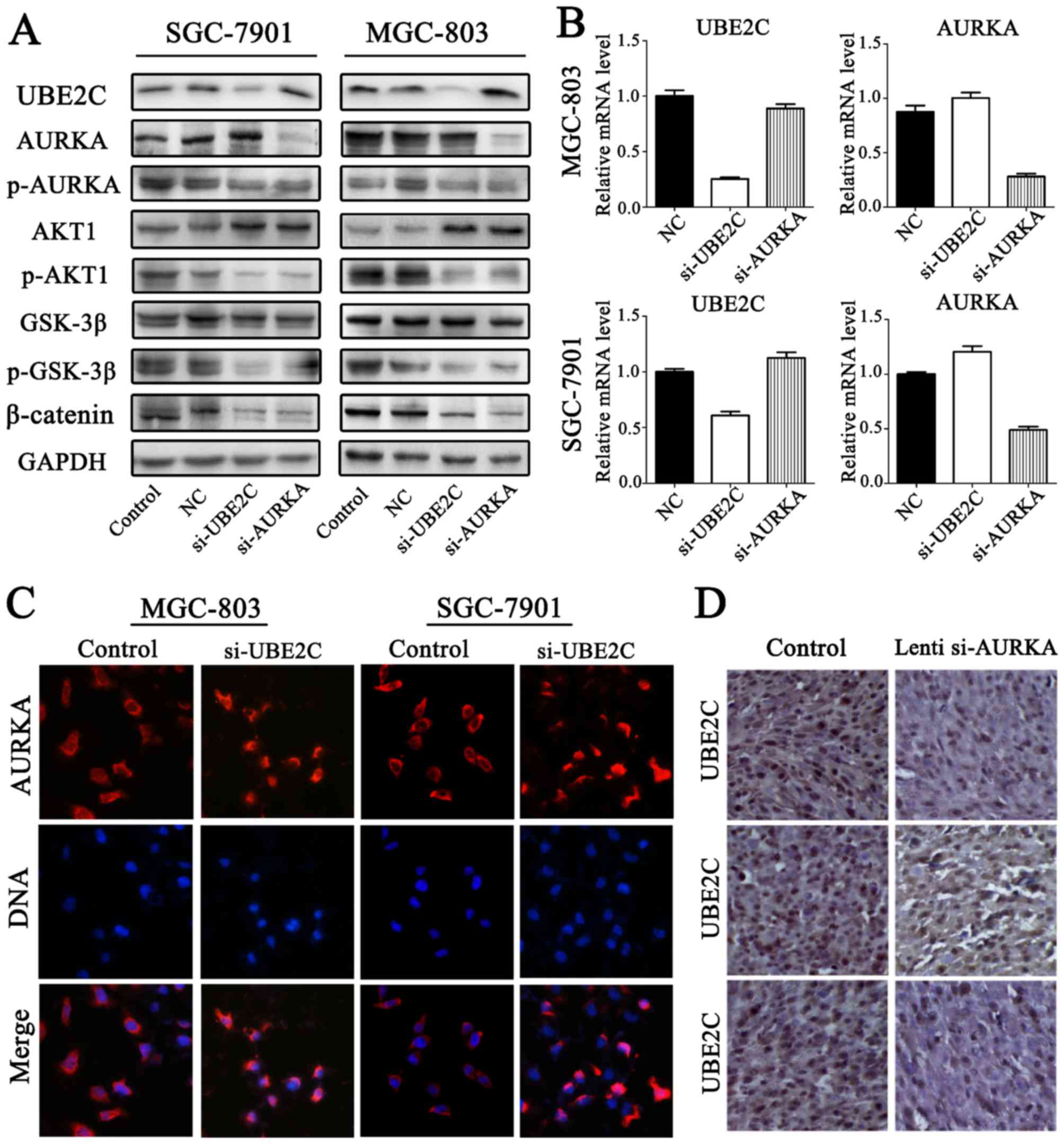
UBE2C deficiency inhibits p-AURKA
expression in gastric adenocarcinoma
To accurately determine AURKA and UBE2C expression
in gastric adenocarcinoma, the expression levels of p-AURKA were
determined through western blot analysis (Fig. 6A and B). The results confirmed that
p-AURKA expression was significantly decreased in si-UBE2C
treatment group. Furthermore, the levels of AURKA were increased
through qt-PCR and western blot analysis. Immunofluorescent
microscope analysis suggested that si-UBE2C treatment in MGC803 and
SGC7901 cell lines resulted in the increased expression of AURKA in
the cytoplasm (Fig. 6C). This was
determined by the observation of a significantly greater
fluorescent signal intensity in the cytoplasm compared with the
control groups, suggesting that the expression of AURKA was
negatively correlated with UBE2C. However, immunostaining analysis
revealed the UBE2C and AURKA protein expression in gastric cancer,
and the expression levels of UBE2C were slightly less in
Lenti-si-AURKA treatment compared with control groups (Fig. 6D). The results confirmed that
si-UBE2C inhibits the expression of p-AURKA in contrast to inducing
AURKA inactivation.
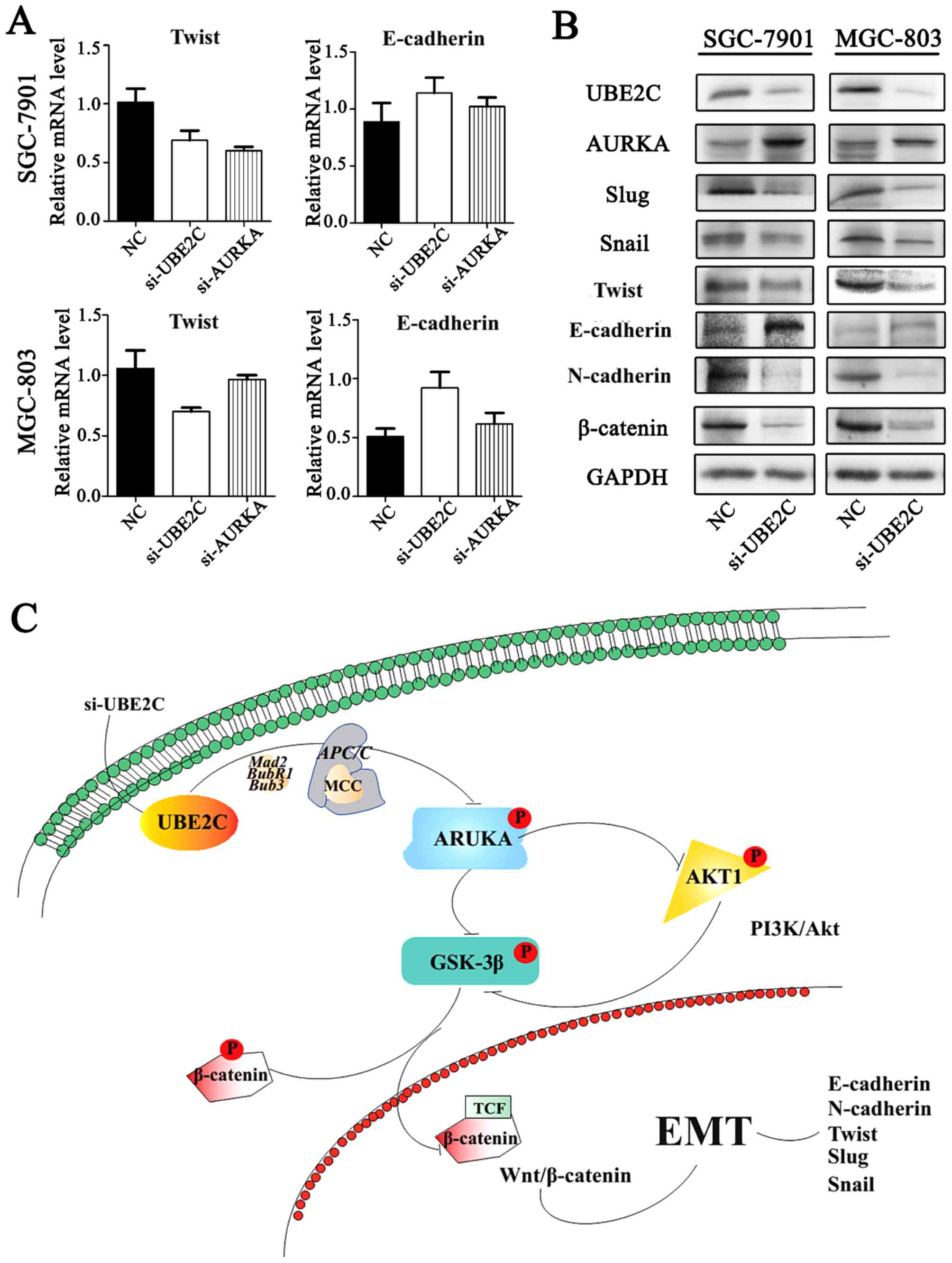
Inhibition of UBE2C represses
epithelial-mesenchymal tran- sition (EMT) through Wnt/β-catenin and
PI3K/Akt signaling pathways
Our previous studies have shown that AURKA induces
EMT through the Wnt/β-catenin and PI3K/Akt signaling pathways.
AURKA activity was inhibited by si-UBE2C, it was hypothesized that
the loss of UBE2C might also inhibit EMT. To examine this
hypothesis, the expression of E-cadherin, N-cadherin and their
interrelated genes were analyzed following si-UBE2C treatment. The
qt-PCR analysis revealed that the knockdown of UBE2C in MGC803 and
SGC7901 cells resulted in increased expression of the epithelial
marker E-cadherin and decreased expression of the mesenchymal
marker Twist (Fig. 7A). Consistent
with these results, western blot analysis results demonstrated a
lower protein expression of UBE2C, Twist, Snail, Slug and
N-cadherin, whereas E-cadherin was increased (Fig. 7B); these results were consistent
with the PCR results.
Discussion
In our gastric cancer eGWAS, we analyzed 184 genes
with differential expression sharing the highest ranking in gastric
cancer from independent datasets. The results indicated that AURKA
was a hub gene based on linkage analysis (25). Considering previous studies, we
hypothesized that there may exist some genes that are co-activated
with AURKA in gastric cancer. We used the DAVID online tools to
analyze the ontology of the 13 genes and identified UBE2C as being
significantly involved in similar processes with AURKA and it is
overexpressed in several primary tumors.
Much effort has been spent on the study of the
overexpression of UBE2C biological mechanisms in gastric cancer
(29,30). This E2 protein catalyzes the
ubiquitination and degradation of cyclins A and B, and acts
together with the E3 ligase of the APC/C to participate in the
regulation of the spindle assembly checkpoint (31). Furthermore, several previous
studies have demonstrated that UBE2C can release Mad2, BubR1, Bub3
and other mitotic complexes (MCC) to control the activity of APC/C
to induce tumorigenesis (7,32,33).
However, little is known about the precise mechanism by which UBE2C
expression is downregulated. The first 28 residues of UBE2C
comprise an N-terminal extension with various motifs, and the
remaining residues form the core domain. The core domain may
promote cancer cell proliferation and may play an oncogenic role in
gastric cancer (9). The knockdown
of UBE2C expression by siRNA resulted in a significant loss of cell
proliferation and viability in gastric adenocarcinoma cells. In the
current study, we showed that knockdown of UBE2C could reduce
gastric cancer tumori-genesis.
In our present analyses, we also investigated the
possible link between UBE2C and AURKA in the proliferation of
gastric cancer cells. UBE2C may insulate CDC20 from MCC by a
mechanism not involving ubiquitin and inhibit the activity of
APC/C, thereby effecting the levels of p-AURKA (7,32).
The results from our studies have demonstrated that the knockdown
of UBE2C decreases the level of p-GSK-3β, p-AKT1, β-catenin and
p-AURKA. In addition, knockdown of AURKA by Lenti-siRNA led to a
partial reduction in UBE2C expression. Moreover, we demonstrated
that AURKA and UBE2C regulate the Wnt/β-catenin and PI3K/Akt
signaling pathways in gastric adenocarcinoma. Collectively, UBE2C
may play a role in regulating AURKA activity.
Furthermore, we discovered that UBE2C interacts with
AURKA, which prevents EMT. Therefore, upon downregulation of UBE2C
expression with siRNA, there may be multiple mechanisms that
control Wnt/β-catenin and PI3K/Akt signaling pathways, which led to
inhibition of EMT (34,35). The suppression of p-AKT1 prevented
the inhibitory phosphorylation of p-GSK3β (36), thereby maintaining the degradation
of β-catenin. In addition, the downregulation of UBE2C was able to
suppress the expression of N-cadherin (37) and increase the expression of
E-cadherin (38). Similarly, the
expression of Slug (39), Twist
and Snail (40) were also
decreased, which are also crucial genes in the development of the
embryo and stimulate the process of EMT.
In summary, our data suggest that UBE2C may be a
promising target for gastric cancer. Although no specific UBE2C
inhibitors are currently available for clinical use, proteasome
inhibitors form a novel class of chemotherapeutic agents that lead
to cell cycle arrest and cell death. Tumor cells are more
susceptible to proteasome inhibition due to their rapid division
and disordered regulatory pathways. The mechanism of UBE2C in
gastric cancer needs further study, and the commissural inhibition
of UBE2C and AURKA may be a potential therapy for the treatment of
gastric adenocarci-noma. We hope this study can help other
researchers to further understand the role of UBE2C.
Acknowledgments
This research was supported by funds from the
National High Technology Research and Development Program 863
(2014AA021102 and 2016YFC0902502) and China National Natural
Scientific Fund (81372703 and 81172356). We would like to thank the
members of the Laboratory of Neuro-Oncology, Tianjin Neurological
Institute for their technical assistance.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamura G: Genetic and epigenetic
alterations of tumor suppressor and tumor-related genes in gastric
cancer. Histol Histopathol. 17:323–329. 2002.PubMed/NCBI
|
|
5
|
Zhong JL and Huang CZ: Ubiquitin
proteasome system research in gastrointestinal cancer. World J
Gastrointest Oncol. 8:198–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hao Z, Zhang H and Cowell J:
Ubiquitin-conjugating enzyme UBE2C: Molecular biology, role in
tumorigenesis, and potential as a biomarker. Tumour Biol.
33:723–730. 2012. View Article : Google Scholar
|
|
7
|
Xie C, Powell C, Yao M, Wu J and Dong Q:
Ubiquitin-conjugating enzyme E2C: A potential cancer biomarker. Int
J Biochem Cell Biol. 47:113–117. 2014. View Article : Google Scholar
|
|
8
|
Wagner KW, Sapinoso LM, El-Rifai W,
Frierson HF, Butz N, Mestan J, Hofmann F, Deveraux QL and Hampton
GM: Overexpression, genomic amplification and therapeutic potential
of inhibiting the UbcH10 ubiquitin conjugase in human carcinomas of
diverse anatomic origin. Oncogene. 23:6621–6629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wing SS and Jain P: Molecular cloning,
expression and characterization of a ubiquitin conjugation enzyme
(E217kB) highly expressed in rat testis. Biochem J.
305:125–132. 1995. View Article : Google Scholar
|
|
10
|
Jiang L, Huang CG, Lu YC, Luo C, Hu GH,
Liu HM, Chen JX and Han HX: Expression of ubiquitin-conjugating
enzyme E2C/UbcH10 in astrocytic tumors. Brain Res. 1201:161–166.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chou CP, Huang NC, Jhuang SJ, Pan HB, Peng
NJ, Cheng JT, Chen CF, Chen JJ and Chang TH: Ubiquitin-conjugating
enzyme UBE2C is highly expressed in breast microcalcification
lesions. PLoS One. 9:e939342014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perrotta I, Bruno L, Maltese L, Russo E,
Donato A and Donato G: Immunohistochemical analysis of the
ubiquitin-conjugating enzyme UbcH10 in lung cancer: A useful tool
for diagnosis and therapy. J Histochem Cytochem. 60:359–365. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pallante P, Malapelle U, Berlingieri MT,
Bellevicine C, Sepe R, Federico A, Rocco D, Galgani M, Chiariotti
L, Sanchez-Cespedes M, et al: UbcH10 overexpression in human lung
carcinomas and its correlation with EGFR and p53 mutational status.
Eur J Cancer. 49:1117–1126. 2013. View Article : Google Scholar
|
|
14
|
Pallante P, Berlingieri MT, Troncone G,
Kruhoffer M, Orntoft TF, Viglietto G, Caleo A, Migliaccio I,
Decaussin-Petrucci M, Santoro M, et al: UbcH10 overexpression may
represent a marker of anaplastic thyroid carcinomas. Br J Cancer.
93:464–471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin J, Raoof DA, Wang Z, Lin MY, Thomas
DG, Greenson JK, Giordano TJ, Orringer MB, Chang AC, Beer DG, et
al: Expression and effect of inhibition of the
ubiquitin-conjugating enzyme E2C on esophageal adenocarcinoma.
Neoplasia. 8:1062–1071. 2006. View Article : Google Scholar
|
|
16
|
Ieta K, Ojima E, Tanaka F, Nakamura Y,
Haraguchi N, Mimori K, Inoue H, Kuwano H and Mori M: Identification
of overexpressed genes in hepatocellular carcinoma, with special
reference to ubiquitin-conjugating enzyme E2C gene expression. Int
J Cancer. 121:33–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bavi P, Uddin S, Ahmed M, Jehan Z, Bu R,
Abubaker J, Sultana M, Al-Sanea N, Abduljabbar A, Ashari LH, et al:
Bortezomib stabilizes mitotic cyclins and prevents cell cycle
progression via inhibition of UBE2C in colorectal carcinoma. Am J
Pathol. 178:2109–2120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li SZ, Song Y, Zhang HH, Jin BX, Liu Y,
Liu WB, Zhang XD and Du RL: UbcH10 overexpression increases
carcinogenesis and blocks ALLN susceptibility in colorectal cancer.
Sci Rep. 4:69102014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen S, Chen Y, Hu C, Jing H, Cao Y and
Liu X: Association of clinicopathological features with UbcH10
expression in colorectal cancer. J Cancer Res Clin Oncol.
136:419–426. 2010. View Article : Google Scholar
|
|
20
|
van Ree JH, Jeganathan KB, Malureanu L and
van Deursen JM: Overexpression of the E2 ubiquitin-conjugating
enzyme UbcH10 causes chromosome missegregation and tumor formation.
J Cell Biol. 188:83–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukasawa K: Oncogenes and tumour
suppressors take on centrosomes. Nat Rev Cancer. 7:911–924. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu N, Wang Z, Song X, Wei L, Kim BS,
Freedman ND, Baek J, Burdette L, Chang J, Chung C, et al:
Genome-wide association study of gastric adenocarcinoma in Asia: A
comparison of associations between cardia and non-cardia tumours.
Gut. 65:1611–1618. 2016. View Article : Google Scholar
|
|
23
|
Oh S and Oh S: Epidemiological and
genome-wide association study of gastritis or gastric ulcer in
korean populations. Genomics Inform. 12:127–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saeki N, Ono H, Sakamoto H and Yoshida T:
Genetic factors related to gastric cancer susceptibility identified
using a genome-wide association study. Cancer Sci. 104:1–8. 2013.
View Article : Google Scholar
|
|
25
|
Liu X, Li Z, Song Y, Wang R, Han L, Wang
Q, Jiang K, Kang C and Zhang Q: AURKA induces EMT by regulating
histone modification through Wnt/β-catenin and PI3K/Akt signaling
pathway in gastric cancer. Oncotarget. 7:33152–33164.
2016.PubMed/NCBI
|
|
26
|
Mahankali M, Henkels KM, Gomez-Cambronero
J and Speranza F: A non-mitotic role for Aurora kinase A as a
direct activator of cell migration upon interaction with PLD, FAK
and Src. J Cell Sci. 128:516–526. 2015. View Article : Google Scholar :
|
|
27
|
Do TV, Xiao F, Bickel LE, Klein-Szanto AJ,
Pathak HB, Hua X, Howe C, O'Brien SW, Maglaty M, Ecsedy JA, et al:
Aurora kinase A mediates epithelial ovarian cancer cell migration
and adhesion. Oncogene. 33:539–549. 2014. View Article : Google Scholar
|
|
28
|
Tong T, Zhong Y, Kong J, Dong L, Song Y,
Fu M, Liu Z, Wang M, Guo L, Lu S, et al: Overexpression of Aurora-A
contributes to malignant development of human esophageal squamous
cell carcinoma. Clin Cancer Res. 21:7304–7310. 2004. View Article : Google Scholar
|
|
29
|
Zhang Y, Han T, Wei G and Wang Y:
Inhibition of microRNA-17/20a suppresses cell proliferation in
gastric cancer by modulating UBE2C expression. Oncol Rep.
33:2529–2536. 2015.PubMed/NCBI
|
|
30
|
Shuliang S, Lei C, Guangwu J and Changjie
L: Involvement of ubiquitin-conjugating enzyme E2C in proliferation
and invasion of prostate carcinoma cells. Oncol Res. 21:121–127.
2013. View Article : Google Scholar
|
|
31
|
Rape M, Reddy SK and Kirschner MW: The
processivity of multiubiquitination by the APC determines the order
of substrate degradation. Cell. 124:89–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alfieri C, Chang L, Zhang Z, Yang J,
Maslen S, Skehel M and Barford D: Molecular basis of APC/C
regulation by the spindle assembly checkpoint. Nature. 536:431–436.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garvanska DH, Larsen MS and Nilsson J:
Synergistic inhibition of the APC/C by the removal of APC15 in
HCT116 cells lacking UBE2C. Biol Open. 5:1441–1448. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vasiljevic A, Champier J,
Figarella-Branger D, Wierinckx A, Jouvet A and Fevre-Montange M:
Molecular characterization of central neurocytomas: Potential
markers for tumor typing and progression. Neuropathology.
33:149–161. 2013. View Article : Google Scholar
|
|
35
|
Gheldof A and Berx G: Cadherins and
epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci.
116:317–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dar AA, Belkhiri A and El-Rifai W: The
aurora kinase A regulates GSK-3β in gastric cancer cells. Oncogene.
28:866–875. 2009. View Article : Google Scholar
|
|
37
|
Radice GL: N-cadherin-mediated adhesion
and signaling from development to disease: Lessons from mice. Prog
Mol Biol Transl Sci. 116:263–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee JY and Kong G: Roles and epigenetic
regulation of epithelial-mesenchymal transition and its
transcription factors in cancer initiation and progression.
Cellular and molecular life sciences. Cell Mol Life Sci.
73:4643–4660. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Phillips S and Kuperwasser C: SLUG:
Critical regulator of epithelial cell identity in breast
development and cancer. Cell Adhes Migr. 8:578–587. 2014.
View Article : Google Scholar
|
|
40
|
Zhang P, Hu P, Shen H, Yu J, Liu Q and Du
J: Prognostic role of Twist or Snail in various carcinomas: A
systematic review and meta-analysis. Eur J Clin Invest.
44:1072–1094. 2014. View Article : Google Scholar : PubMed/NCBI
|