Introduction
Bladder cancer (BCa) is a common malignant tumor in
the urinary tract, ranking the first in urologic tumors and twelfth
in all cancers among the Chinese populations (1). BCa is diagnosed yearly in estimated
429,800 patients, of whom about 160,000 succumb to death (2). Other than age, a series of
environmental factors contributes to the onset of BCa, which
emphasizes the importance of prevention of the disease. Besides,
because of the recurrent feature of bladder cancer needing repeated
treatment and lifetime surveillance, thus imposing tremendous
economic burdens on both society and families. It is therefore
urgent to identify molecular candidates involved in the disease and
targets for gene therapy.
Protein phosphorylation and dephosphorylation not
only affect cell life in multiple ways but play an important part
during cancer progression (3).
Serine/threonine protein phosphatase 5 (PPP5C) as a member of the
protein serine/threonine phosphatase family is ubiquitously
expressed in mammalian cells (4).
Several studies have demonstrated that PPP5C is associated with
human cancers, among which liver and breast cancers are the most
studied (5,6). Moreover, constitutive expression of
PPP5C also accelerates tumor growth in mice (7). Despite the discovery of the role of
PPP5C in the development and progression of other cancers, whether
PPP5C participates in BCa remains unknown.
As a powerful tool in identifying the function of
candidate molecules, RNA interference (RNAi) system has been
utilized in cancer gene therapy (8,9).
Thus, the present study explored the biological function of PPP5C
in bladder cancer using ONCOMINE microarray datasets analysis and
lentivirus-mediated shRNA system.
Materials and methods
Cell culture
Human bladder cancer cell lines T24 and BT5637, and
human embryonic kidney cell line 293T were purchased from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China). BT5637
cells were cultured in RPMI-1640 medium (HyClone Laboratories,
Inc., Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco, Carlsbad, CA, USA). T-24 cells were cultured in
McCoy's 5A medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented
with 10% FBS (Gibco). 293T cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; HyClone Laboratories) supplemented
with 10% FBS. Cells were maintained at 37°C in a humidified
incubator with a constant air flow of 5% CO2.
Construction of lentivirus
Small interfering RNA (siRNA) targeting PPP5C
(NC_000019.10)
(5′-GAGACAGAGAAGATTACAGTACTCGAGTACTGTAATCTTCTCTGTCTCTTTTT-3′) and
the non-silencing control sequence
(5′-CTAGCCCGGTTCTCCGAACGTGTCACGTATCTCGAGATACGTGACACGTTCGGAGAATTTTTTTAAT-3′)
was transformed into short hairpin RNA (shRNA) and cloned into
pFH-L green fluorescent protein (GFP) vector (Shanghai Hollybio,
Shanghai, China) by double digestion with NheI and
PacI. The generated plasmid shPPP5C-pFH-L or shCon-pFH-L,
together with two pHelper plasmids pVSVG-I and pCMV△R8.92 (Shanghai
Hollybio) was transfected into 293T cells. After 72-h transfection,
the lentiviruses (Lv-shPPP5C or Lv-shCon) in the culture medium
were collected and purified by ultracentrifugation and the titer of
each lentivirus was determined.
Cell infection of lentivirus
T24 and BT5637 cells were seeded in 6-well plates
with a concentration of 5×104 and 4×104
respectively, and then infected with lentivirus Lv-shPPP5C or
Lv-shCon at a multiplicity of infection (MOI) of 25 and 35,
respectively. The percentage of GFP-positive cells represented the
infection efficiency.
Quantitative real-time PCR
After 120-h infection with lentivirus, T24 and
BT5637 cells were collected and total RNA was extracted with TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). Then, 2 µg total
RNA was reverse transcribed with Oligo (dT) primer and the M-MLV
Reverse Transcriptase (Promega, Madison, WI, USA), according to the
manufacturer's instructions. The complementary DNA was used for
real-time PCR and a final volume of 20 µl was adopted as
follows: 10 µl 2X SYBR Premix Ex Taq (Takara, Dalian,
China), 0.8 µl forward and reverse primers, 2.5 µM, 5
µl cDNA, 4.2 µl ddH2O. PCR and data
collection were performed on CFX Connect™ Real-Time PCR detection
system (Takara) and data analysis was operated with the
2−ΔΔCt method normalized to the endogenous control
β-actin. Primers used in real-time PCR are as follows: β-actin
forward, GTGGACATCCGCAAAGAC and β-actin reverse,
AAAGGGTGTAACGCAACTA; PPP5C forward, GGTGAGGTGAAGGCCAAGTA and PPP5C
reverse, TGT GGATCTGACCAGAGCAG.
Western blot assay
One hundred and twenty hours after infection, T24
and BT5637 cells were collected and lysed in 2X SDS lysis buffer
(100 mM Tris-HCl, pH 6.8, 10 mM EDTA, 4% SDS, 10% glycine). Cell
lysates were separated on SDS-PAGE gels and transferred onto
polyvinylidene difluoride membranes. The membranes were blocked in
TBST buffer with 5% milk. Then, the membranes were incubated with
the following primary antibodies: PPP5C (#:11715-1-AP; Proteintech
Group, Chicago, IL, USA), CDK4 (#:11026-1-AP; Proteintech Group),
c-Myc (#:sc-40; Santa Cruz Biotechnology, Santa Cruz, CA, USA), p27
(#:3686; Cell Signaling Technology, Danvers, MA, USA), BAD
(#:10435-1-AP; Proteintech Group), Beclin1 (#:3495; Cell Signaling
Technology) and GAPDH (#:10494-1-AP; Proteintech Group) for 12 h at
4°C. After that, horseradish peroxidase conjugated goat anti-rabbit
IgG (#:Sc-2054; Santa Cruz Biotechnology) was applied and incubated
for 1 h at 25°C. The dilution of each antibody was employed in
accordance with the manufacturer's guidelines. The signals of
proteins were detected with enhanced chemiluminescence.
Methylthiazoltetrazolium (MTT) cell
proliferation assay
BT5637 cells 3×103 and 2.5×103
T24 cells were cultured in 96-well plates for 1, 2, 3, 4 and 5
days, respectively, after infection with Lv-shCon and Lv-shPPP5C
lentivirus for 72 h. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT; Sigma-Aldrich) was applied to the cells
and after 4-h incubation, the supernatant was discarded. Cells were
incubated with acidic isopropanol (10% SDS, 5% isopropanol and 0.01
mol/l HCl) for 12 h at 37°C. The absorbance was detected at 595 nm
using a microplate reader. Each group had five duplicate and the
experiment was performed in triplicate.
Colony formation assay
Five hundred T24 cells and BT5637 cells were seeded
in 6-well plates and infected with the indicated lentivirus for 72
h. The supernatant was then discarded and culture medium was
replaced every 3 days. Subsequently, cells were cultured for
another 8 days, fixed with 4% para-formaldehyde and stained with
purple crystals. The number of colonies was analyzed statistically.
The experiment was performed three times and each group had three
repetitions.
Cell cycle analysis
T24 (1×105) and BT5637 cells were seeded
in 6-cm dishes and infected with Lv-shCon and Lv-shPPP5C lentivirus
for 3 days, followed by 40-h culture without the virus. The cells
were then collected and fixed with 75% ethanol for 24 h at 4°C.
Propidium iodide (20 mg/ml) (PI; Sigma-Aldrich) was used to treat
the cells and DNA content was recorded with a flow cytometer (BD
Biosciences, San Jose, CA, USA) according to the manufacturer's
instructions. The experiment was performed in triplicate.
Cell apoptosis analysis
T24 (1×105) and BT5637 cells were
cultured in 6-cm dishes and infected with Lv-shCon and Lv-shPPP5C
lentiviruses for 3 days. Then, the cells were reseeded on 6-cm
dishes. After 3 days, Annexin V/PI (BD Biosciences) double staining
was performed to analyze cell apoposis by flow cytometry. Each
experiment was repeated at least three times.
Animal experiments
Twelve six-week-old nude mice were randomly divided
into two groups and then were injected subcutaneously in the right
flank with 5×106 T24 cells pre-infected with Lv-shCon
and Lv-shPPP5C lentiviruses. Twenty days later, the tumor size was
measured every three days and calculated using the formula: (length
× width2)/2. At the end of the experiment, the mice were
euthanized and the tumors were removed from each nude mouse, imaged
and weighed.
Bioinformatic analysis of the ONCOMINE
microarray datasets
The expression level of PPP5C in BCa was analyzed
using the ONCOMINE microarray datasets (www.onocomine.org), which contain a great deal of
public and published micro-array datasets with tens of thousands of
samples. By using 'PPP5C', 'BCa' and 'mRNA' as the search terms, 17
datasets were acquired, of which 3 datasets with 7 subunits
included PPP5C expression in BCa and normal urothelial tissues.
Then, a meta-analysis was carried out to compare the level of the
PPP5C between the BCa and the normal tissue. Besides, we compared
the expression of the PPP5C from T stage and grade malignancy of
the BCa in the Blaveri Bladder 2 and Dyrskjot Bladder datasets.
Information on survival time in ALS Bladder was obtained for
survival analysis. All data are reported log2 Median-Centered
intensity in the ONCOMINE microarray datasets.
Statistical analysis
Data are presented as mean ± SD from at least three
experiments. Statistical comparison between the Lv-shCon and
Lv-shPPP5C was performed using Student's t-test. The PPP5C
expression and patient characteristics of the BCa were analyzed
using the Chi-square test in the BCa tissue microarray. In
addition, the Kaplan-Meier analysis was used to evaluate the
relation between the prognosis and the PPP5C expression in ALS
microarray datasets. The SPSS version 19 (IBM Corp., Armonk, NY,
USA) was used to calculate the statistics and P<0.05 was
considered statistically significant.
Results
PPP5C expression of the bladder cancer on
the ONCOMINE microarray datasets
To evaluate the expression level of the PPP5C in
BCa, the public online ONCOMINE microarray database was used to
perform meta-analysis for evaluation of PPP5C expression in BCa. A
total of 3 datasets including 7 groups comparing PPP5C expression
between the BCa and normal urothelial tissue were included in this
study, which comprised 343 BCa and 130 normal urothelial tissues.
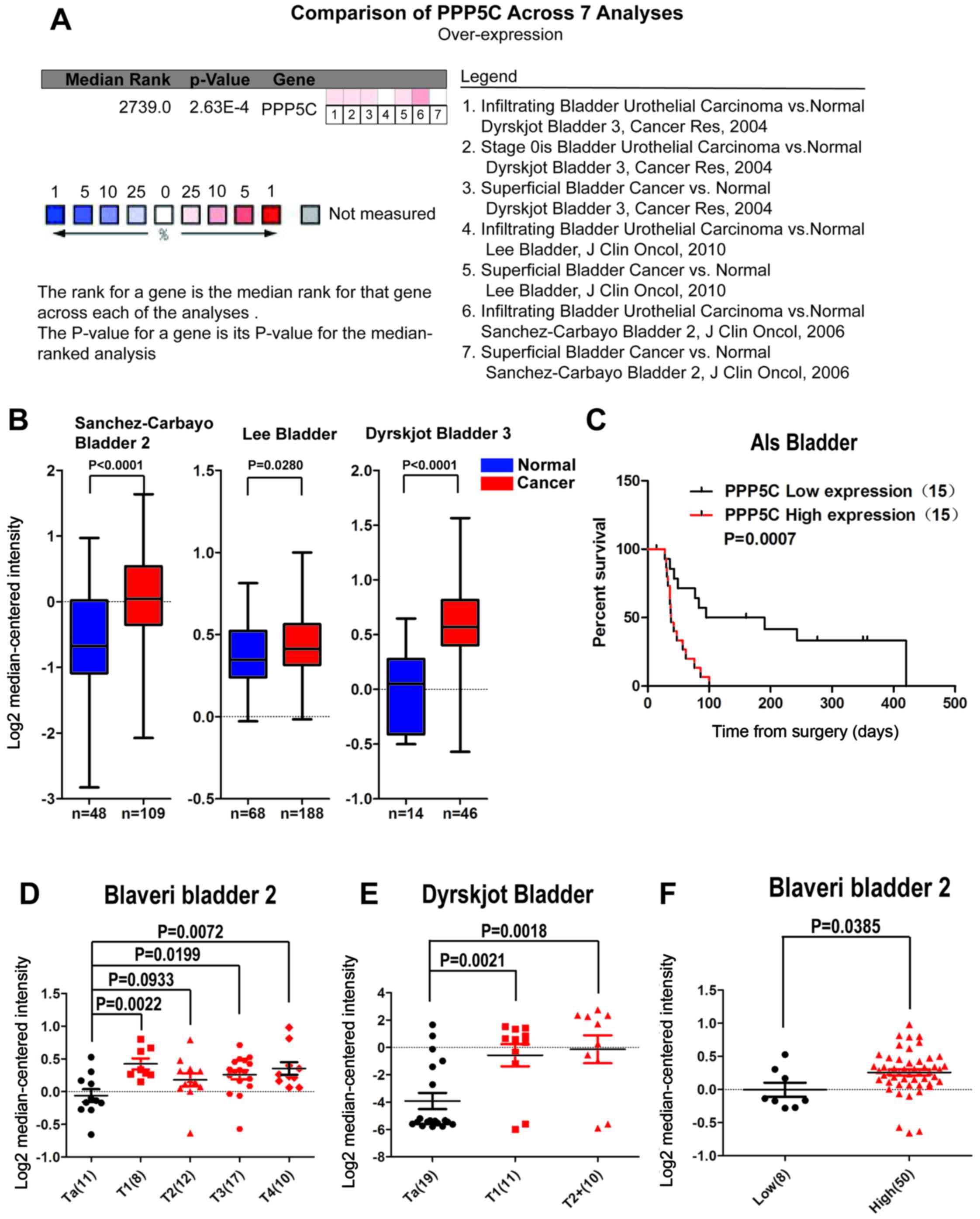
As shown in Fig. 1B, the
expression level of PPP5C in BCa was higher than that in the normal
tissue in all three datasets. Besides, the results of the
meta-analysis of the 7 groups in the 3 datasets also support that
the PPP5C was overexpressed in BCa (Fig. 1A), and the difference was
statistically significant between BCa and normal urothelial
tissues. Furthermore, the correlation between PPP5C expression and
T stage and malignant grade of BCa was analyzed. It was found that
PPP5C was highly expressed in T stages ≥T1 compared to that in Ta
of BCa (Fig. 1D and E), and in
high-grade BCa than that in low-grade BCa (Fig. 1F). Patients with low-level PPP5C
expression indicated a better prognosis in ALS Bladder microarray
dataset (Table I). Similarly,
Kaplan-Meier survival analysis of ALS dataset showed that overall
survival (OS) was poor in patients with upgraded PPP5C (Fig. 1C).
 | Table IPPP5C expression and patient
characteristics of ALS Bladder dataset. |
Table I
PPP5C expression and patient
characteristics of ALS Bladder dataset.
| No. of
patients | PPP5C
| P-value |
|---|
| Low | High |
|---|
| Overall, n (%) | 30 (100.0) | 15 | 15 | |
| Mean patient age,
years (range) | 60.4±7.3 | 58.9±6.8 | 61.9±7.7 | 0.277a |
| Bergkvist grade, n
(%) | | | | 1.000b |
| Grade 1 and 2 | 3 | 1 | 2 | |
| Grade 3 and 4 | 27 | 14 | 13 | |
| T stage, n (%) | | | | 1.000b |
| ≤T2b | 3 | 2 | 1 | |
| ≥T3a | 27 | 13 | 14 | |
| M stage, n (%) | | | | 0.466b |
| M0 | 15 | 9 | 6 | |
| M1 | 15 | 6 | 9 | |
| Overall survival
status, n (%) | | | | 0.042b |
| Alive | 5 | 5 | 0 | |
| Dead | 25 | 10 | 15 | |
Lentivirus-mediated interference
downregulates PPP5C expression in bladder cancer cells
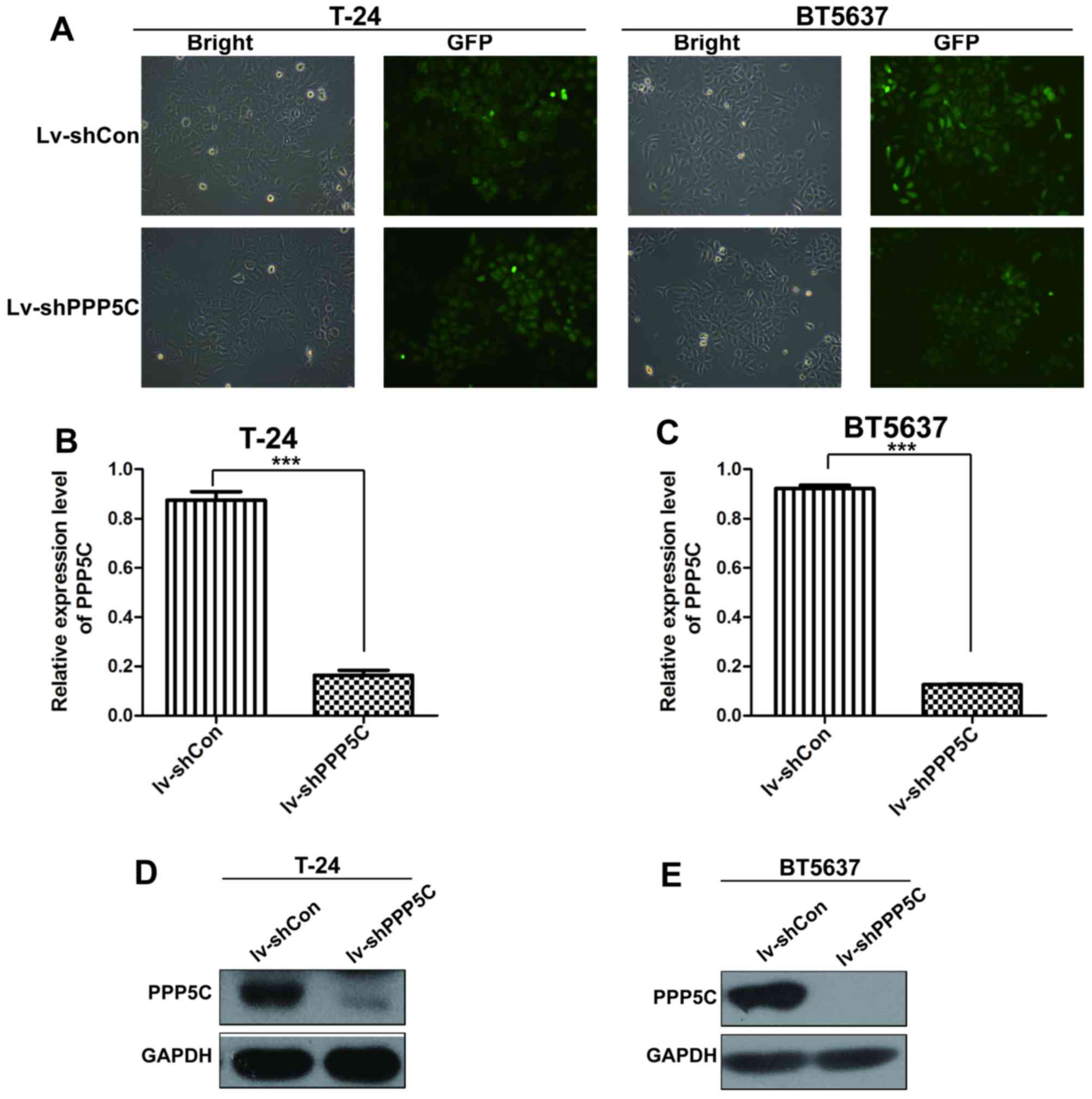
To determine the role of PPP5C in BCa, a
lentivirus-mediated shRNA system was used to silence PPP5C in two
human BCa cell lines T24 and BT5637. The cells were infected with
Lv-shPPP5C or the control lentivirus Lv-shCon, with an infection
efficiency of >90% identified by GFP expression.
The mRNA and protein levels of PPP5C in
Lv-shPPP5C-infected T24 and BT5637 was significantly reduced in day
3 and 5 post-transduction, respectively (Fig. 2B–E). These findings suggest that
lentivirus-mediated RNAi efficiently downregulated the endogenous
PPP5C expression in urinary BCa cell lines.
Lentivirus-mediated knockdown of PPP5C
suppresses the viability and proliferation of bladder cancer cells
in vitro
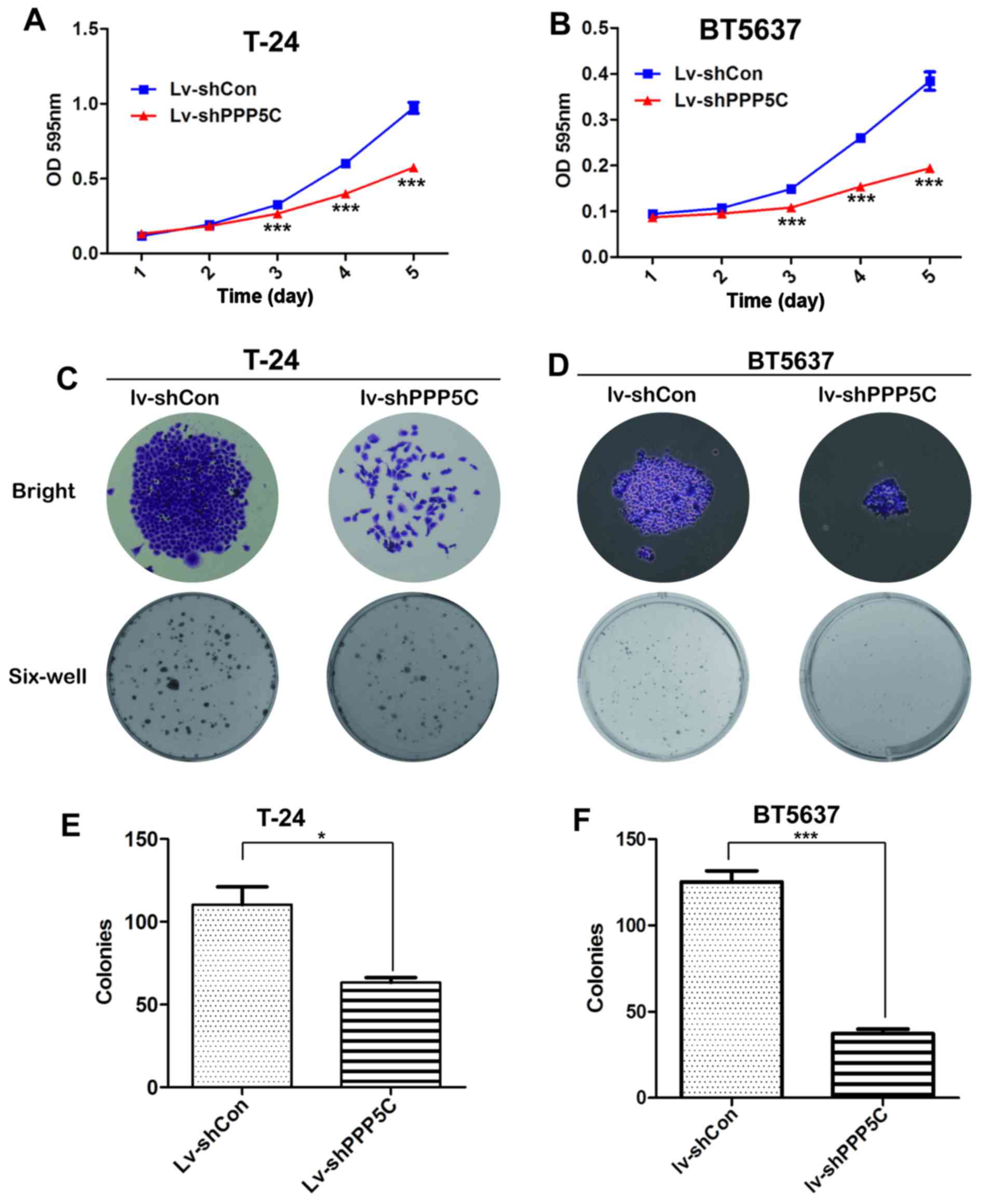
To identify whether PPP5C played a role in
tumorigenesis, MTT and colony formation assay were adopted.
Compared with Lv-shCon-infected T24 cells, the viability of cells
infected with Lv-PPP5C and Lv-shPPP5C was significantly reduced
(Fig. 3A and B).
To further elucidate the role of PPP5C in cancer
cell proliferation, colonies formed in T24 cells after PPP5C
knockdown were analyzed. As shown in Fig. 3C, colony formation in
Lv-shPPP5C-infected cells was obviously impaired with respect to
morphology and quantity, as compared with Lv-shCon-infected cells.
In addition, the number of colonies in Lv-shPPP5C-infected cells
was reduced by nearly 50% (Fig.
3E). Similar result was also observed in BT5637 cells (Fig. 3D and F). In summary, our data
demonstrate that PPP5C expression was of importance for the
viability and proliferation of BCa cells.
Knockdown of PPP5C inhibits bladder
cancer cell growth in vivo
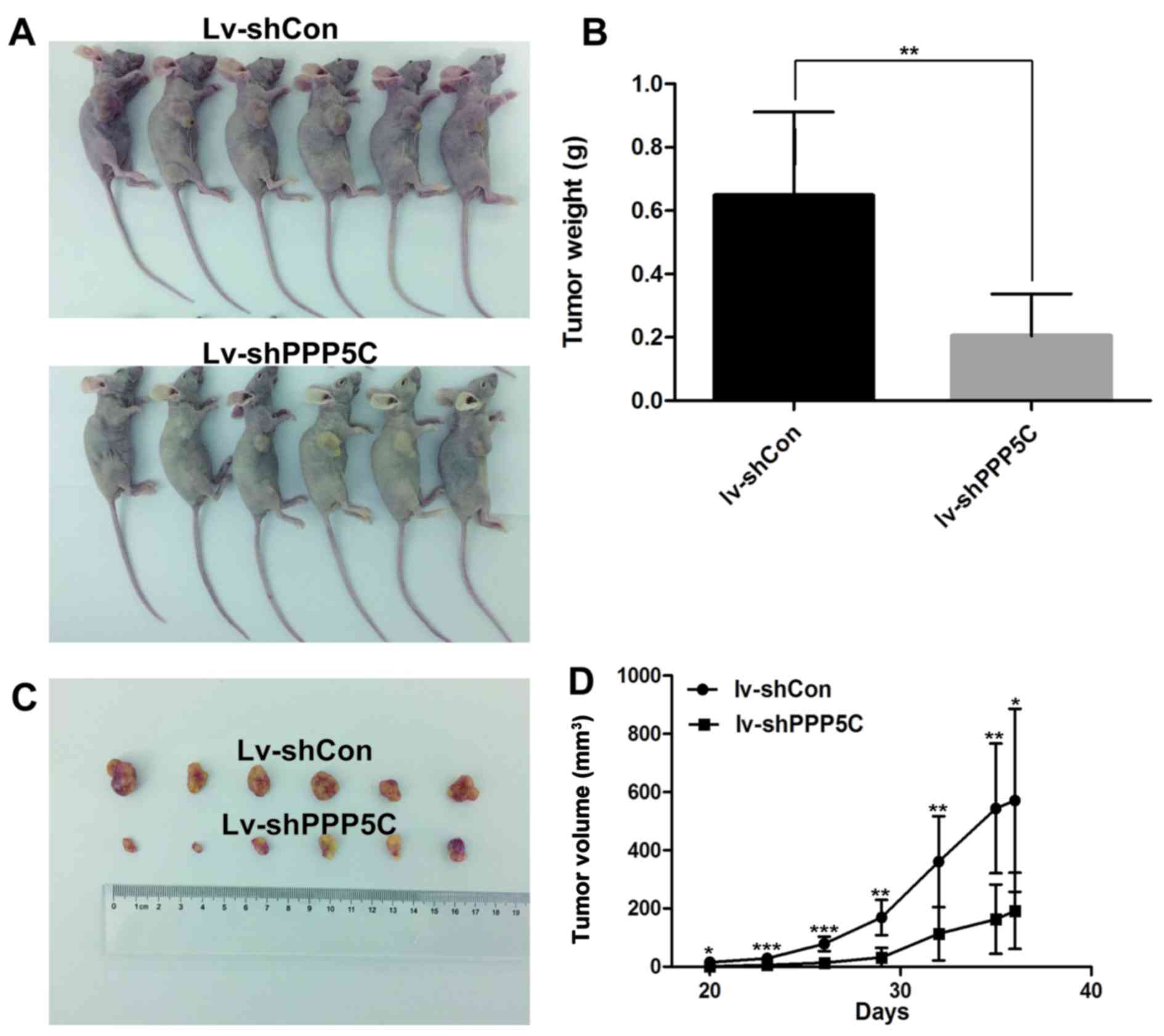
To further validate the oncogenic function of PPP5C
in vivo, Lv-shPPP5C-infected and Lv-shCon-infected T24 cells
were injected subcutaneously to the six-week-old nude mice, and the
tumor volume and weight were measured after tumor formation. As
shown in Fig. 4, tumor size and
weight in Lv-shPPP5C-infected mice were significantly reduced as
compared with those in mice treated with Lv-shCon-infection. These
results further support that PPP5C functions as an oncogene in
vivo.
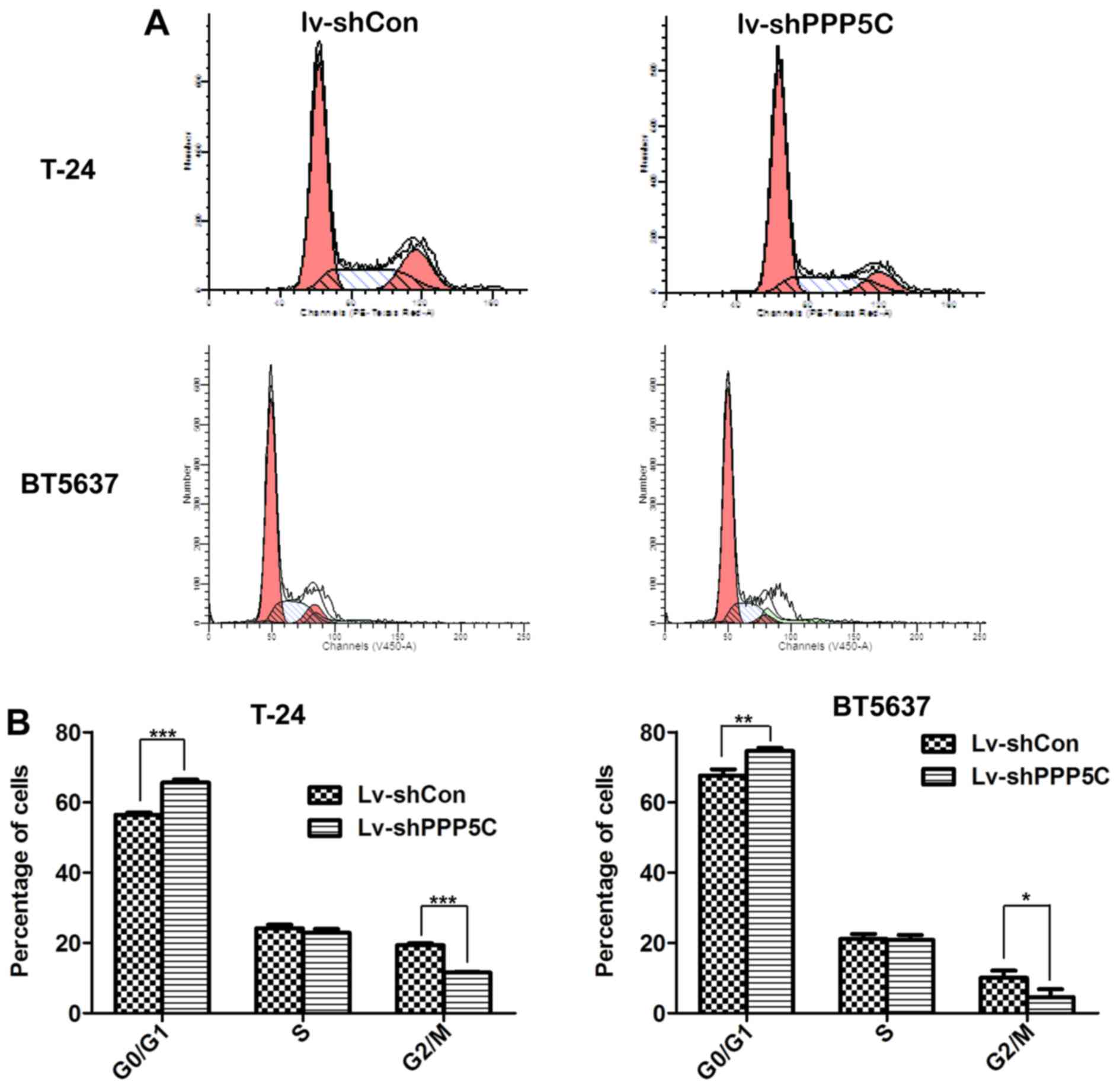
Disruption of PPP5C expression leads to
cell cycle arrest of the bladder cancer cells
Tumorigenesis largely depends on accelerated or at
least normal cell cycle of cancer cells. Knowing that PPP5C played
an important role in BCa cell proliferation, flow cytometric
analysis of cell cycle distribution through PI staining to see
whether PPP5C participated in the regulation of cancer cell cycle
showed that after infection with Lv-shPPP5C, T24 cells were
arrested in G0/G1 phase compared with Lv-shCon cells (65.61±0.86
vs. 56.49±0.57%), and the number of cells in G2/M phase was
decreased (11.54±0.24 vs. 19.37±0.49%) (Fig. 5A and B). Similarly, the proportion
of BT5637 cells in G1/G0 phase in Lv-shPPP5C group was increased
(74.6±0.88 vs. 67.66±1.74%) and the number of cells in G2/M phase
was reduced significantly (4.55±2.28 vs. 10.08±2.03%). These
findings suggest that knockdown of PPP5C could cause T24 and BT5637
cell cycle arrest in G0/G1 phase.
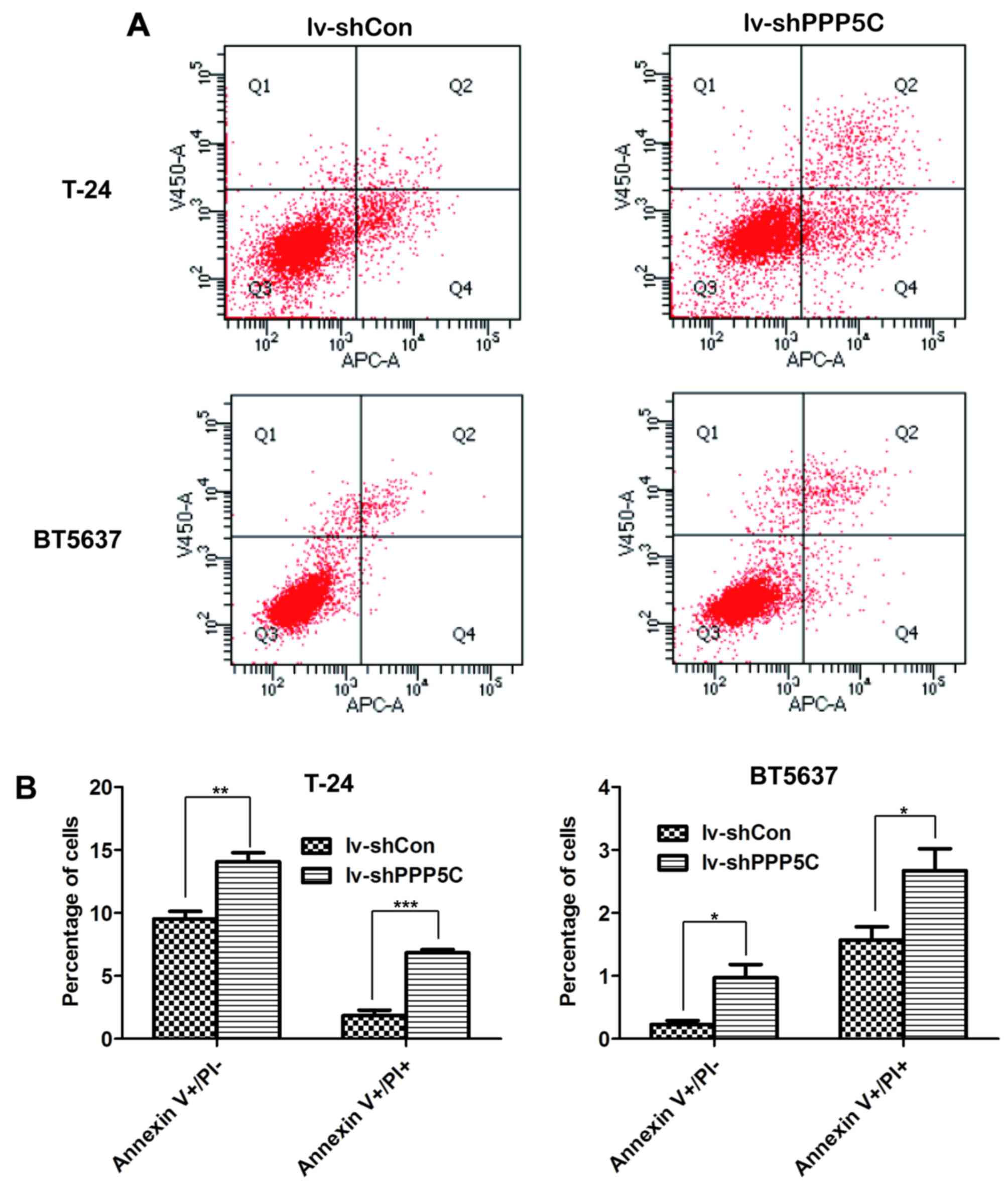
Downregulation of PPP5C expression
induces apoptosis of the BCa cells
To further confirm the effect of PPP5C on BCa cell
apoptosis, flow cytometric analysis through Annexin V/PI double
staining was performed in T24 and BT5637 cells after lentivirus
infection. The four domains divided by Annexin V and PI plots
represent the different states of cells. Viable cells were located
in Annexin V−/PI−, necrotic cells in Annexin
V−/PI+, early apoptotic cells in Annexin
V+/PI−, and late apoptotic cells in Annexin
V+/PI+ (Fig.
6A). As shown in Fig. 6B,
early and late apoptosis were increased significantly in lv-shPPP5C
as compared with lv-shCon both in T24 and BT5637 cells. Above all,
PPP5C silencing promoted apoptosis of BCa cells.
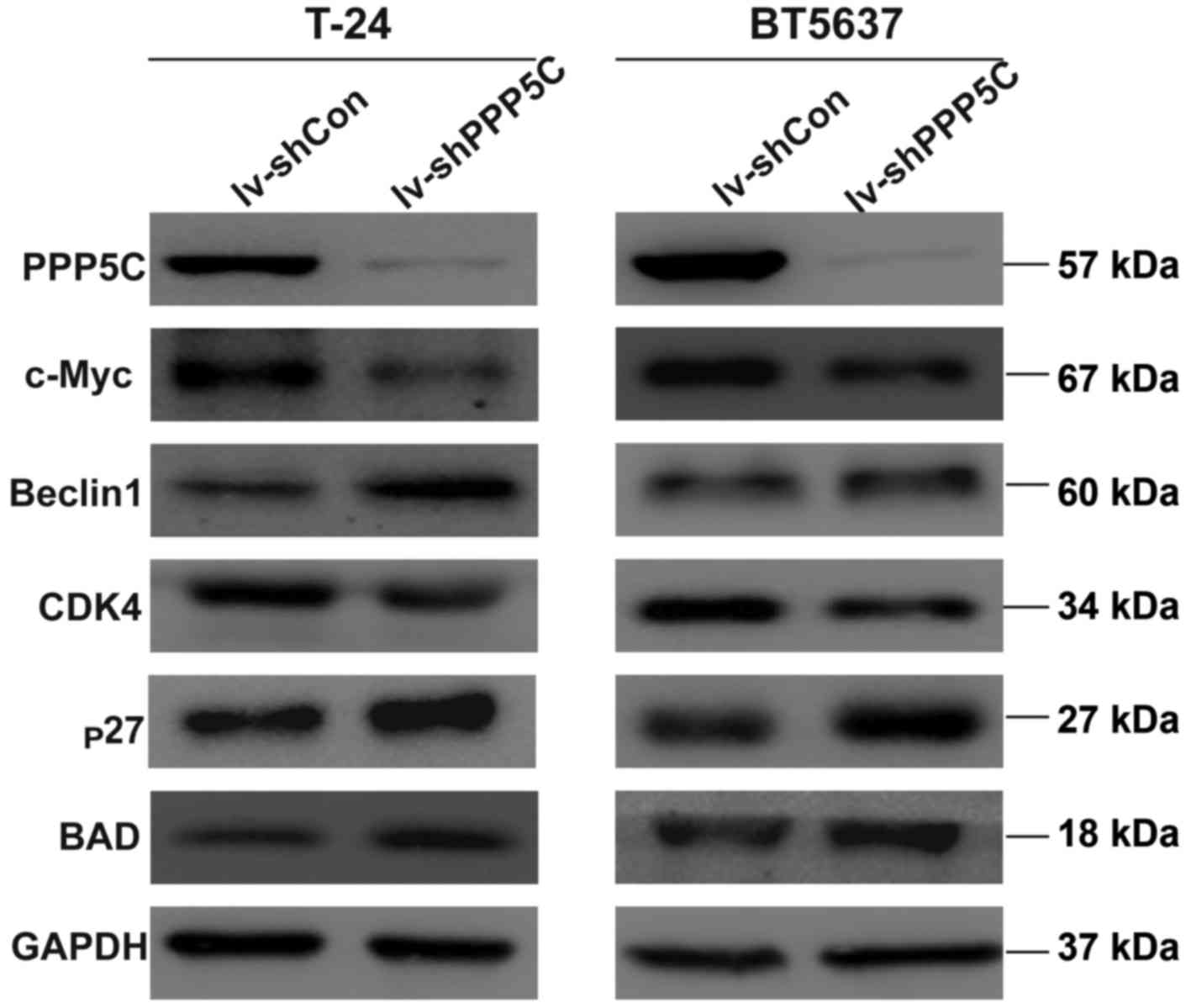
shRNA-mediated depression of PPP5C
regulates the protein level of CDK4, c-Myc, p27, BAD and
Beclin1
To further clarify the mechanism of the PPP5C in
regulating the biological function of the bladder cancer, the
expression of downstream signaling molecule were analyzed by
western blot analysis. The level of CDK4 and c-Myc was decreased
whereas the level of p27, BAD and Beclin1 was increased (Fig. 7). These results provide evidence
that interfering with the expression of PPP5C could regulate
apoptosis, cell cycle and other biological functions.
Discussion
Compared with other solid cancers, much less is
known about the molecular mechanism of BCa. Despite a variety of
recent genome-wide profiling studies, there is still no valid
strategy for early diagnosis or therapeutic intervention of BCa
(10–12). Therefore, the confirmation of
critical molecular signatures is badly needed for the effective
treatment of the malignancy.
PPP5C is a ubiquitously-expressed protein with only
one form rather than two or more isoforms of other phosphoprotein
phosphatase members (13,14). The biological function of PPP5C
remains largely unknown. Some studies have reported its role in
cancer development. Elevation of PPP5C has been found both in human
cancer tissues and human breast cancer cell line MCF-7 (5). PPP5C is also associated with other
cancers such as liver cancer and several other cellular signaling
cascades (6,14,15).
However, few studies have focused on the role of PPP5C in BCa
progression. Given the severe consequences and economic burden
brought by BCa, we aimed to find out whether PPP5C was its
candidate biomarker and provide a novel therapeutic target for
BCa.
The ONCOMINE microarray datasets is a cancer
microarray database and integrated data-mining platform (16) that can be used to analyze
differential expressions between cancer and paired normal tissues.
Besides, the relation between gene expression and various clinical
data with respect to pathology, prognosis, metastasis and drug
resistance can also be analyzed using the ONCOMINE microarray
dataset. Increased numbers of studies have begun utilizing the
datasets to validate and explore their research results (17–19).
Using the ONCOMINE microarray dataset, the present study found that
PPP5C was upregulated in BCa, which was correlated with T stage,
malignant grade and survival of BCa patients (Fig. 1). The data that we obtained from
the ONCOMINE microarray dataset suggest that PPP5C may play a role
in the development and progression of BCa.
To confirm the oncogenic function of PPP5C in BCa,
we used a lentivirus-mediated RNA interference system to interfere
with the expression of PPP5C in BCa cells. Due to its ability to
integrate into the genome of host cells, lentiviruses have been
extensively employed as vectors for shRNA expression (22). Even in clinical trials,
lentivirus-delivered shRNA have been utilized without notable
side-effects (21,22). Lentivirus-mediated knockdown of
PPP5C revealed that PPP5C was crucial for the viability and
proliferation of bladder cancer in vitro and in
vivo.
Cell proliferation is closely related to cell cycle
progression. In G0/G1 phase, cells undergo DNA amplification and
nutrient accumulation, with no cell differentiation (23). Disturbance of the cell cycle will
impair cell proliferation. It was found in the present study that
PPP5C knockdown induced cancer cell arrest in G0/G1 phase, with
fewer cells in G2/M phase, indicating that PPP5C played a role in
regulating bladder cell cycle. Western blot analysis showed that
the expression of c-Myc and CDK4 was decreased and the expression
of p27 was increased, indicating a relationship between PPP5C and
cell cycle arrest. Being the most important subtype of cyclin
dependent kinases, CDK4 can guarantee cells to complete DNA
replication and pass through the G1 check point into S phase of the
cell cycle. Besides, c-Myc, a vital transcriptional regulatory
factor, also plays an important role in regulating G1 phase via
multiple mechanisms including transcriptional activation of CDK4
directly (24). Thus, decrease in
CDK4 may be a main reason for G0/G1 phase arrest. In addition, p27
as an important member of the cyclin-dependent kinase inhibitor
family can inhibit the activity of the cyclin/CDK complex and
negatively regulate the cell cycle (25). Hence, the increased expression of
p27 was related to G0/G1 phase arrest.
Although we have demonstrated the biological
function of PPP5C in bladder cancer cells, the exact molecular
mechanism needs to be further explored. The MAPK (mitogen-activated
protein kinases), a conserved family of enzymes, comprising several
major components (JNK, P38 and ERK) play an important role in the
cellular biological processes, such as proliferation, apoptosis,
stress response and metabolism (26–30).
In addition, regulation of MAPK cascade phosphorylation plays an
important role in its function. Thus, as a kind of the phosphatase,
PPP5C may affect the function of MAPK through dephosphorylation,
such as regulating the phosphorylation of ERK and JNK by
interfering with cell proliferation and apoptosis (17,31–33).
In conclusion, we demonstrated that
lentivirus-mediated shRNA targeting PPP5C played a crucial role in
viability, proliferation, apoptosis and migration of BCa cells,
suggesting that PPP5C may prove to be a potential therapeutic
target for the treatment of urinary BCa.
Acknowledgments
The present study is supported by grants from the
National Natural Science Foundation of China for Youths (81502211),
the Shanghai Committee of Science and Technology General Program
for Medicine (15ZR413900) and the Shanghai Changzheng Hospital
Grant for Youth (2015CZQN05).
Glossary
Abbreviations
Abbreviations:
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
GFP
|
green fluorescence protein
|
|
MOI
|
multiplicity of infection
|
|
MTT
|
methylthiazole tetrazolium
|
|
PI
|
propidium iodide
|
|
PPP5C
|
serine/threonine protein phosphatase
5
|
|
shRNA
|
short hairpin RNA
|
|
BCa
|
bladder cancer
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen P: The role of protein
phosphorylation in human health and disease. The Sir Hans Krebs
Medal Lecture. Eur J Biochem. 268:5001–5010. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hinds TD Jr and Sánchez ER: Protein
phosphatase 5. Int J Biochem Cell Biol. 40:2358–2362. 2008.
View Article : Google Scholar
|
|
5
|
Golden T, Aragon IV, Rutland B, Tucker JA,
Shevde LA, Samant RS, Zhou G, Amable L, Skarra D and Honkanen RE:
Elevated levels of Ser/Thr protein phosphatase 5 (PP5) in human
breast cancer. Biochim Biophys Acta. 1782:259–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shirato H, Shima H, Nakagama H, Fukuda H,
Watanabe Y, Ogawa K, Matsuda Y and Kikuchi K: Expression in
hepatomas and chromosomal localization of rat protein phosphatase 5
gene. Int J Oncol. 17:909–912. 2000.PubMed/NCBI
|
|
7
|
Golden T, Aragon IV, Zhou G, Cooper SR,
Dean NM and Honkanen RE: Constitutive over expression of
serine/threonine protein phosphatase 5 (PP5) augments
estrogen-dependent tumor growth in mice. Cancer Lett. 215:95–100.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren W, Wang X, Gao L, Li S, Yan X, Zhang
J, Huang C, Zhang Y and Zhi K: MiR-21 modulates chemosensitivity of
tongue squamous cell carcinoma cells to cisplatin by targeting
DCD4. Mol Cell Biochem. 390:253–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu J, Luo JH, Pang J, Cao JZ, Wu RH, Tong
ZT, Chen W and Xie D: Lentivirus-mediated RNA interference of
clusterin enhances the chemosensitivity of EJ bladder cancer cells
to epirubicin in vitro. Mol Med Rep. 6:1133–1139. 2012.PubMed/NCBI
|
|
10
|
Peter S, Borkowska E, Drayton RM, Rakhit
CP, Noon A, Chen W and Catto JW: Identification of differentially
expressed long noncoding RNAs in bladder cancer. Clin Cancer Res.
20:5311–5321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuda K, Takahashi A, Middlebrooks CD,
Obara W, Nasu Y, Inoue K, Tamura K, Yamasaki I, Naya Y, Tanikawa C,
et al: Genome-wide association study identified SNP on 15q24
associated with bladder cancer risk in Japanese population. Hum Mol
Genet. 24:1177–1184. 2015. View Article : Google Scholar
|
|
12
|
Draaken M, Knapp M, Pennimpede T, Schmidt
JM, Ebert AK, Rösch W, Stein R, Utsch B, Hirsch K, Boemers TM, et
al: Genome-wide association study and meta-analysis identify ISL1
as genome-wide significant susceptibility gene for bladder
exstrophy. PLoS Genet. 11:e10050242015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Swingle MR, Honkanen RE and Ciszak EM:
Structural basis for the catalytic activity of human
serine/threonine protein phosphatase-5. J Biol Chem.
279:33992–33999. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Golden T, Swingle M and Honkanen RE: The
role of serine/threonine protein phosphatase type 5 (PP5) in the
regulation of stress-induced signaling networks and cancer. Cancer
Metastasis Rev. 27:169–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morita K, Saitoh M, Tobiume K, Matsuura H,
Enomoto S, Nishitoh H and Ichijo H: Negative feedback regulation of
ASK1 by protein phosphatase 5 (PP5) in response to oxidative
stress. EMBO J. 20:6028–6036. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malik R, Khan AP, Asangani IA, Cieślik M,
Prensner JR, Wang X, Iyer MK, Jiang X, Borkin D, Escara-Wilke J, et
al: Targeting the MLL complex in castration-resistant prostate
cancer. Nat Med. 21:344–352. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Y, Pan XW, Li L, Chen L, Liu X, Lu
JL, Zhu XM, Huang H, Yang QW, Ye JQ, et al: Overexpression of USP39
predicts poor prognosis and promotes tumorigenesis of prostate
cancer via promoting EGFR mRNA maturation and transcription
elongation. Oncotarget. 7:22016–22030. 2016.PubMed/NCBI
|
|
19
|
Pan XW, Chen L, Hong Y, Xu DF, Liu X, Li
L, Huang Y, Cui LM, Gan SS, Yang QW, et al: EIF3D silencing
suppresses renal cell carcinoma tumorigenesis via inducing G2/M
arrest through downregulation of Cyclin B1/CDK1 signaling. Int J
Oncol. 48:2580–2590. 2016.PubMed/NCBI
|
|
20
|
ter Brake O, Konstantinova P, Ceylan M and
Berkhout B: Silencing of HIV-1 with RNA interference: A multiple
shRNA approach. Mol Ther. 14:883–892. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bank A, Dorazio R and Leboulch P: A phase
I/II clinical trial of beta-globin gene therapy for
beta-thalassemia. Ann NY Acad Sci. 1054:308–316. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Manilla P, Rebello T, Afable C, Lu X,
Slepushkin V, Humeau LM, Schonely K, Ni Y, Binder GK, Levine BL, et
al: Regulatory considerations for novel gene therapy products: A
review of the process leading to the first clinical lentiviral
vector. Hum Gene Ther. 16:17–25. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Menon SG and Goswami PC: A redox cycle
within the cell cycle: Ring in the old with the new. Oncogene.
26:1101–1109. 2007. View Article : Google Scholar
|
|
24
|
Amati B, Alevizopoulos K and Vlach J: Myc
and the cell cycle. Front Biosci. 3:d250–d268. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abukhdeir AM and Park BH: P21 and p27:
Roles in carcinogenesis and drug resistance. Expert Rev Mol Med.
10:e192008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dickinson RJ and Keyse SM: Diverse
physiological functions for dual-specificity MAP kinase
phosphatases. J Cell Sci. 119:4607–4615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rovida E and Stecca B: Mitogen-activated
protein kinases and Hedgehog-GLI signaling in cancer: A crosstalk
providing therapeutic opportunities? Semin Cancer Biol. 35:154–167.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang YL and Dong C: MAP kinases in immune
responses. Cell Mol Immunol. 2:20–27. 2005.PubMed/NCBI
|
|
30
|
Low HB and Zhang Y: Regulatory Roles of
MAPK phosphatases in cancer. Immune Netw. 16:85–98. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shah BH and Catt KJ: Protein phosphatase 5
as a negative key regulator of Raf-1 activation. Trends Endocrinol
Metab. 17:382–384. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
von Kriegsheim A, Pitt A, Grindlay GJ,
Kolch W and Dhillon AS: Regulation of the Raf-MEK-ERK pathway by
protein phosphatase 5. Nat Cell Biol. 8:1011–1016. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han X, Xu B, Beevers CS, Odaka Y, Chen L,
Liu L, Luo Y, Zhou H, Chen W, Shen T, et al: Curcumin inhibits
protein phosphatases 2A and 5, leading to activation of
mitogen-activated protein kinases and death in tumor cells.
Carcinogenesis. 33:868–875. 2012. View Article : Google Scholar : PubMed/NCBI
|