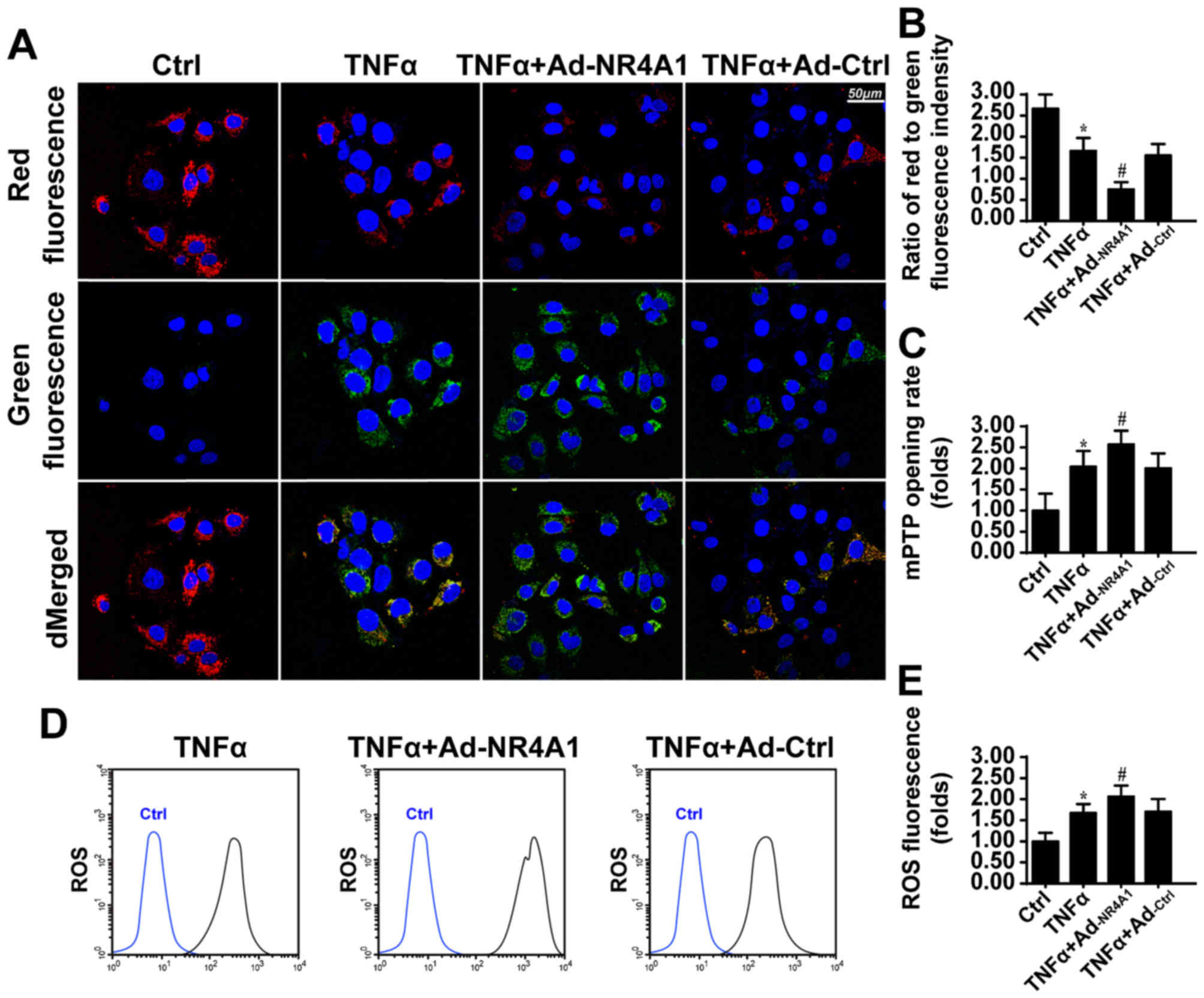
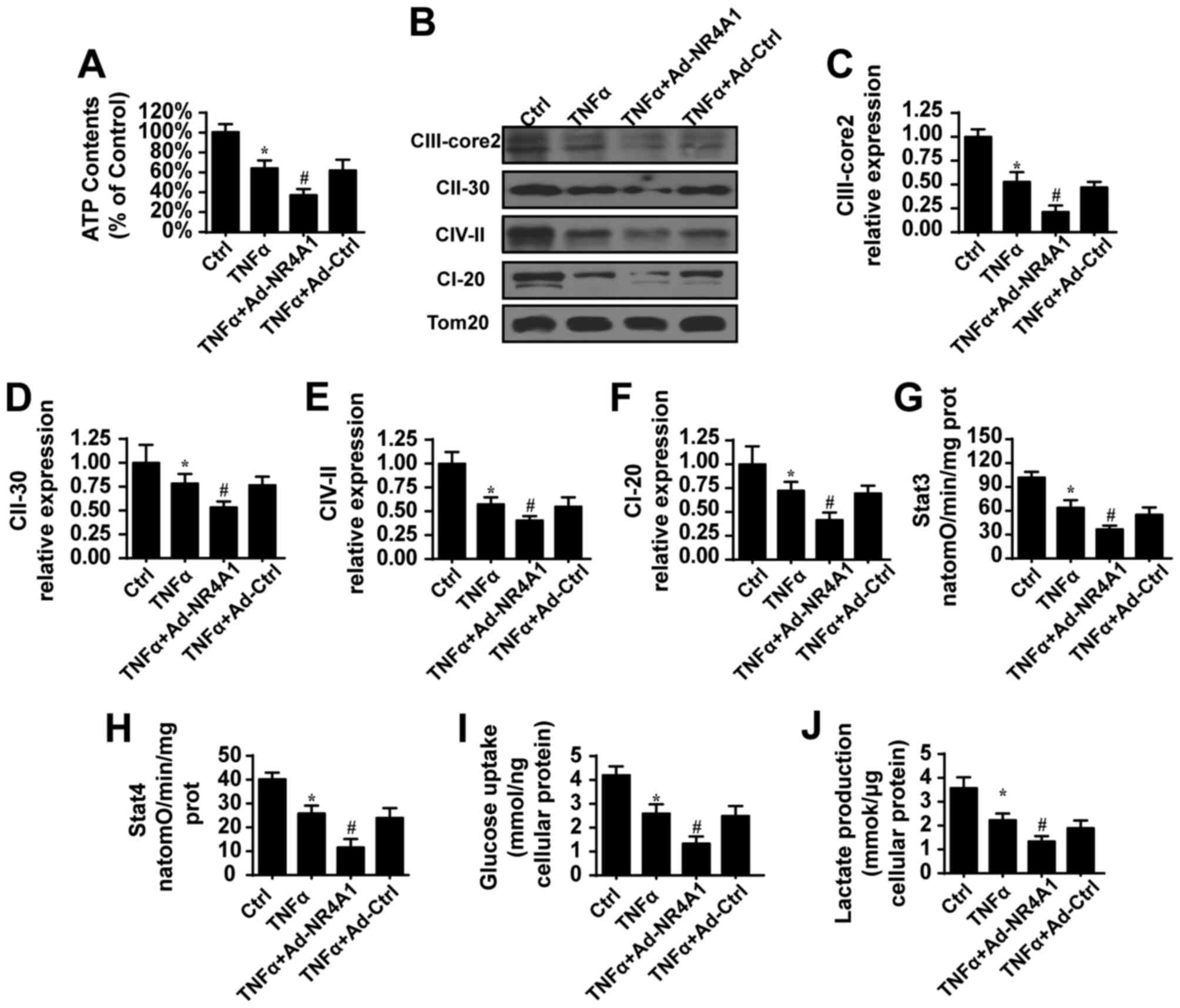
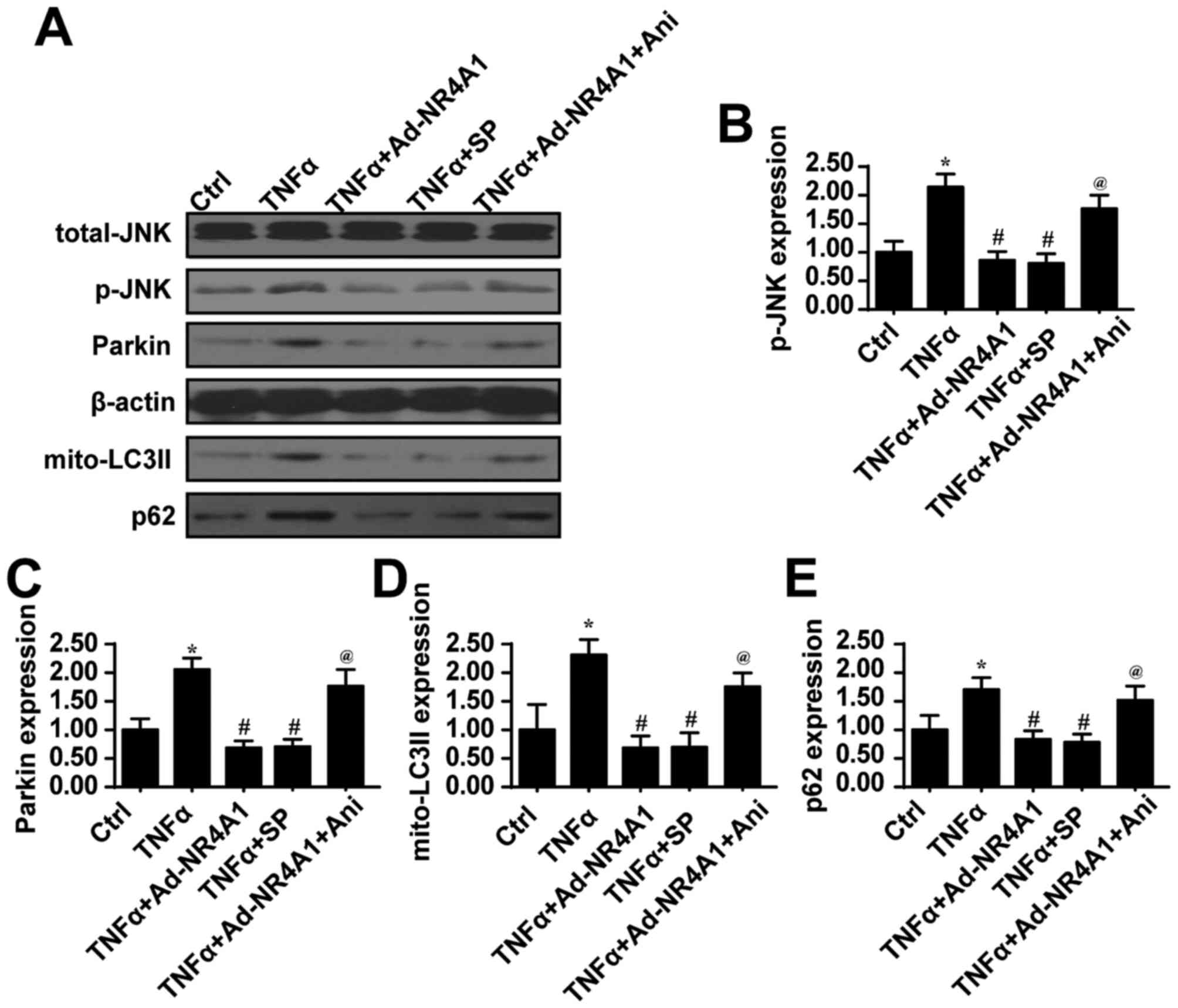
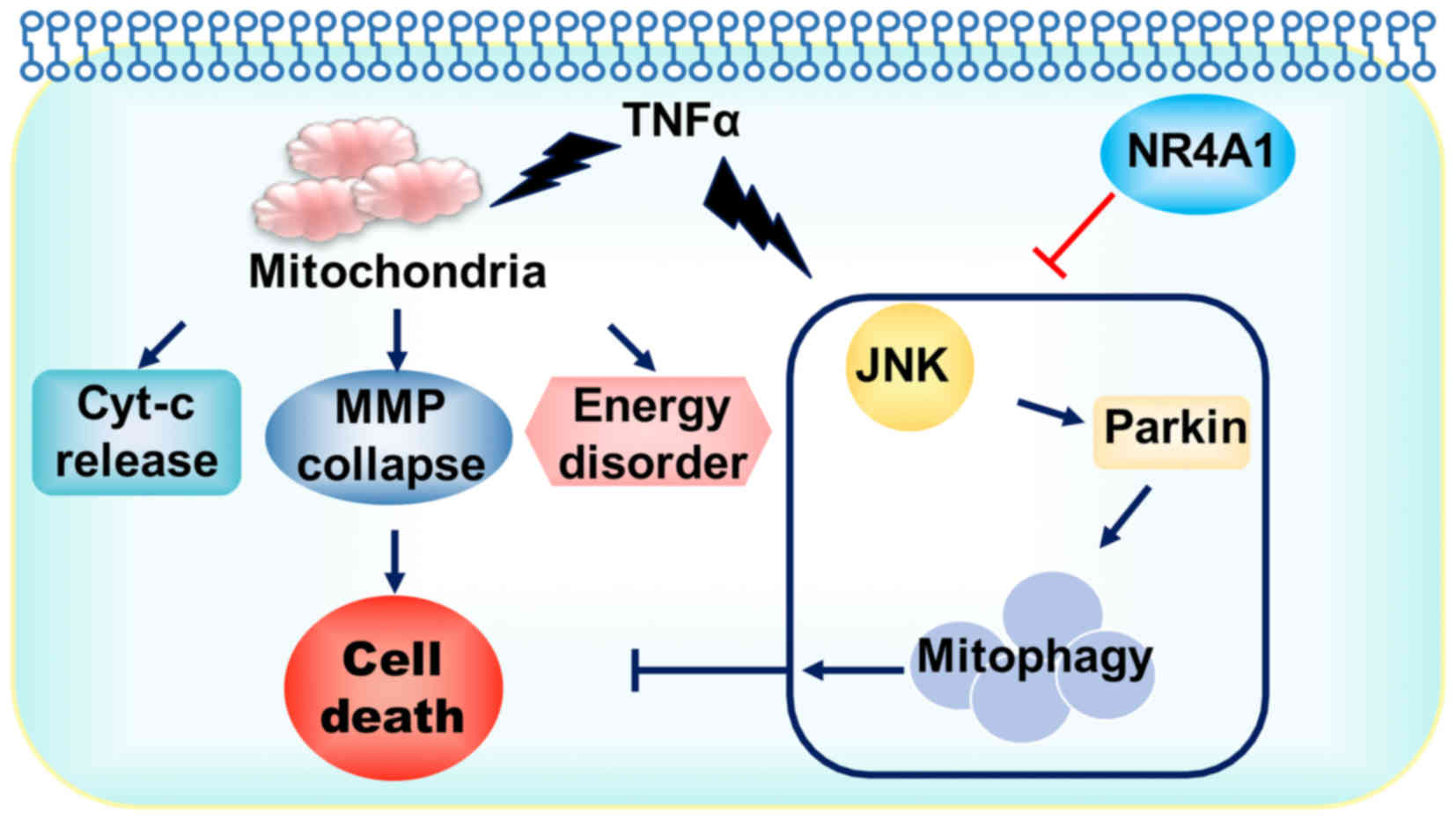
|
1
|
Balakrishnan M, George R, Sharma A and
Graham DY: Changing trends in stomach cancer throughout the world.
Curr Gastroenterol Rep. 19:362017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suzuki H and Mori H: World trends for H.
pylori eradication therapy and gastric cancer prevention strategy
by H. pylori test-and-treat. J Gastroenterol. Nov 14–2017.Epub
ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee HS, Kim WH, Kwak Y, Koh J, Bae JM, Kim
KM, Chang MS, Han HS, Kim JM, Kim HW, et al Gastrointestinal
Pathology Study Group of Korean Society of Pathologists; Molecular
Pathology Study Group of Korean Society of Pathologists: Molecular
testing for gastrointestinal cancer. J Pathol Transl Med.
51:103–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paoletti X, Oba K, Burzykowski T, Michiels
S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M,
et al GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research
International Collaboration) Group: Benefit of adjuvant
chemotherapy for resectable gastric cancer: A meta-analysis. JAMA.
303:1729–1737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Procaccio L, Schirripa M, Fassan M,
Vecchione L, Bergamo F, Prete AA, Intini R, Manai C, Dadduzio V,
Boscolo A, et al: Immunotherapy in gastrointestinal cancers. BioMed
Res Int. 2017:43465762017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bie Q, Jin C, Zhang B and Dong H: IL-17B:
A new area of study in the IL-17 family. Mol Immunol. 90:50–56.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Du LC and Gao R: Role of TNFα -308G/A gene
polymorphism in gastric cancer risk: A case control study and
meta-analysis. Turk J Gastroenterol. 28:272–282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong JT, Son DJ, Lee CK, Yoon DY, Lee DH
and Park MH: Interleukin 32, inflammation and cancer. Pharmacol
Ther. 174:127–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abozeid M, Rosato A and Sommaggio R:
Immunotherapeutic strategies for gastric carcinoma: A review of
preclinical and clinical recent development. BioMed Res Int.
2017:57912622017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grierson P, Lim KH and Amin M:
Immunotherapy in gastrointestinal cancers. J Gastrointest Oncol.
8:474–484. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Procaccio L, Schirripa M, Fassan M,
Vecchione L, Bergamo F, Prete AA, Intini R, Manai C, Dadduzio V,
Boscolo A, et al: Immunotherapy in gastrointestinal cancers. BioMed
Res Int. 2017:43465762017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsujimoto H, Ono S, Ichikura T, Matsumoto
Y, Yamamoto J and Hase K: Roles of inflammatory cytokines in the
progression of gastric cancer: Friends or foes? Gastric Cancer.
13:212–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carrasco-Pozo C, Tan KN, Reyes-Farias M,
De La Jara N, Ngo ST, Garcia-Diaz DF, Llanos P, Cires MJ and Borges
K: The deleterious effect of cholesterol and protection by
quercetin on mitochondrial bioenergetics of pancreatic β-cells,
glycemic control and inflammation: In vitro and in vivo studies.
Redox Biol. 9:229–243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kakimoto PA and Kowaltowski AJ: Effects of
high fat diets on rodent liver bioenergetics and oxidative
imbalance. Redox Biol. 8:216–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kheradpezhouh E, Barritt GJ and Rychkov
GY: Curcumin inhibits activation of TRPM2 channels in rat
hepatocytes. Redox Biol. 7:1–7. 2016. View Article : Google Scholar :
|
|
16
|
Kleszczyński K, Zillikens D and Fischer
TW: Melatonin enhances mitochondrial ATP synthesis, reduces
reactive oxygen species formation, and mediates translocation of
the nuclear erythroid 2-related factor 2 resulting in activation of
phase-2 antioxidant enzymes (γ-GCS, HO-1, NQO1) in ultraviolet
radiation-treated normal human epidermal keratinocytes (NHEK). J
Pineal Res. 61:187–197. 2016. View Article : Google Scholar
|
|
17
|
Ni HM, Williams JA and Ding WX:
Mitochondrial dynamics and mitochondrial quality control. Redox
Biol. 4:6–13. 2015. View Article : Google Scholar :
|
|
18
|
Xu S, Pi H, Zhang L, Zhang N, Li Y, Zhang
H, Tang J, Li H, Feng M, Deng P, et al: Melatonin prevents abnormal
mitochondrial dynamics resulting from the neurotoxicity of cadmium
by blocking calcium-dependent translocation of Drp1 to the
mitochondria. J Pineal Res. 60:291–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quijano C, Trujillo M, Castro L and
Trostchansky A: Interplay between oxidant species and energy
metabolism. Redox Biol. 8:28–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du K, Ramachandran A and Jaeschke H:
Oxidative stress during acetaminophen hepatotoxicity: Sources,
pathophysiological role and therapeutic potential. Redox Biol.
10:148–156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pawlak A, Strzadala L and Kalas W:
Non-genomic effects of the NR4A1/Nur77/TR3/NGFIB orphan nuclear
receptor. Steroids. 95:1–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wenzl K, Troppan K, Neumeister P and
Deutsch AJ: The nuclear orphan receptor NR4A1 and NR4A3 as tumor
suppressors in hematologic neoplasms. Curr Drug Targets. 16:38–46.
2015. View Article : Google Scholar
|
|
23
|
Wu H, Bi J, Peng Y, Huo L, Yu X, Yang Z,
Zhou Y, Qin L, Xu Y, Liao L, et al: Nuclear receptor NR4A1 is a
tumor suppressor down-regulated in triple-negative breast cancer.
Oncotarget. 8:54364–54377. 2017.PubMed/NCBI
|
|
24
|
Beard JA, Tenga A and Chen T: The
interplay of NR4A receptors and the oncogene-tumor suppressor
networks in cancer. Cell Signal. 27:257–266. 2015. View Article : Google Scholar
|
|
25
|
Alonso-González C, González A,
Martínez-Campa C, Gómez-Arozamena J and Cos S: Melatonin sensitizes
human breast cancer cells to ionizing radiation by downregulating
proteins involved in double-strand DNA break repair. J Pineal Res.
58:189–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu H, Jin Q, Li Y, Ma Q, Wang J, Li D,
Zhou H and Chen Y: Melatonin protected cardiac microvascular
endothelial cells against oxidative stress injury via suppression
of IP3R-[Ca(2+)] c/VDAC-[Ca(2+)]m axis by activation of MAPK/ERK
signaling pathway. Cell Stress Chaperones. Jul 1–2017.Epub ahead of
print. View Article : Google Scholar
|
|
27
|
Lin C, Chao H, Li Z, Xu X, Liu Y, Hou L,
Liu N and Ji J: Melatonin attenuates traumatic brain injury-induced
inflammation: A possible role for mitophagy. J Pineal Res.
61:177–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin YW, Lee LM, Lee WJ, Chu CY, Tan P,
Yang YC, Chen WY, Yang SF, Hsiao M and Chien MH: Melatonin inhibits
MMP-9 transactivation and renal cell carcinoma metastasis by
suppressing Akt-MAPKs pathway and NF-κB DNA-binding activity. J
Pineal Res. 60:277–290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Zhou H, Wu W, Shi C, Hu S, Yin T,
Ma Q, Han T, Zhang Y, Tian F, et al: Liraglutide protects cardiac
microvascular endothelial cells against hypoxia/reoxygenation
injury through the suppression of the SR-Ca(2+)-XO-ROS axis via
activation of the GLP-1R/PI3K/Akt/survivin pathways. Free Radic
Biol Med. 95:278–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Luxán-Delgado B, Potes Y,
Rubio-González A, Caballero B, Solano JJ, Fernández-Fernández M,
Bermúdez M, Rodrigues Moreira, Guimarães M, Vega-Naredo I, Boga JA,
et al: Melatonin reduces endoplasmic reticulum stress and autophagy
in liver of leptin-deficient mice. J Pineal Res. 61:108–123. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
King AL, Mantena SK, Andringa KK,
Millender-Swain T, Dunham-Snary KJ, Oliva CR, Griguer CE and Bailey
SM: The methyl donor S-adenosylmethionine prevents liver hypoxia
and dysregulation of mitochondrial bioenergetic function in a rat
model of alcohol-induced fatty liver disease. Redox Biol.
9:188–197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mailloux RJ, Craig Ayre D and Christian
SL: Induction of mitochondrial reactive oxygen species production
by GSH mediated S-glutathionylation of 2-oxoglutarate
dehydrogenase. Redox Biol. 8:285–297. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang JW, Hong JM and Lee SM: Melatonin
enhances mitophagy and mitochondrial biogenesis in rats with carbon
tetrachloride-induced liver fibrosis. J Pineal Res. 60:383–393.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li QX, Ke N, Sundaram R and Wong-Staal F:
NR4A1, 2, 3 - an orphan nuclear hormone receptor family involved in
cell apoptosis and carcinogenesis. Histol Histopathol. 21:533–540.
2006.PubMed/NCBI
|
|
35
|
Dan Dunn J, Alvarez LA, Zhang X and
Soldati T: Reactive oxygen species and mitochondria: A nexus of
cellular homeostasis. Redox Biol. 6:472–485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang J: Teaching the basics of autophagy
and mitophagy to redox biologists–mechanisms and experimental
approaches. Redox Biol. 4:242–259. 2015. View Article : Google Scholar
|
|
37
|
Mukherjee D, Ghosh AK, Dutta M, Mitra E,
Mallick S, Saha B, Reiter RJ and Bandyopadhyay D: Mechanisms of
isoproterenol-induced cardiac mitochondrial damage: Protective
actions of melatonin. J Pineal Res. 58:275–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xin Z, Jiang S, Jiang P, Yan X, Fan C, Di
S, Wu G, Yang Y, Reiter RJ and Ji G: Melatonin as a treatment for
gastrointestinal cancer: A review. J Pineal Res. 58:375–387. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nacarelli T, Azar A and Sell C:
Mitochondrial stress induces cellular senescence in an
mTORC1-dependent manner. Free Radic Biol Med. 95:133–154. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bernardini JP, Lazarou M and Dewson G:
Parkin and mitophagy in cancer. Oncogene. 36:1315–1327. 2017.
View Article : Google Scholar
|
|
41
|
Jankowski M: The role of JNK pathway in
familial Parkinson's disease. Postepy Biochem. 53:297–303. 2007.In
Polish.
|
|
42
|
Lordick F and Janjigian YY: Clinical
impact of tumour biology in the management of gastroesophageal
cancer. Nat Rev Clin Oncol. 13:348–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ishimoto T, Baba H, Izumi D, Sugihara H,
Kurashige J, Iwatsuki M and Tan P: Current perspectives toward the
identification of key players in gastric cancer microRNA
dysregulation. Int J Cancer. 138:1337–1349. 2016. View Article : Google Scholar
|
|
44
|
Scartozzi M, Bittoni A, Pistelli M,
Galizia E, Berardi R, Giampieri R, Faloppi L and Cascinu S: Toward
molecularly selected chemotherapy for advanced gastric cancer:
State of the art and future perspectives. Cancer Treat Rev.
35:451–462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Myint ZW and Goel G: Role of modern
immunotherapy in gastrointestinal malignancies: A review of current
clinical progress. J Hematol Oncol. 10:862017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gao L, Zhao YC, Liang Y, Lin XH, Tan YJ,
Wu DD, Li XZ, Ye BZ, Kong FQ, Sheng JZ, et al: The impaired
myocardial ischemic tolerance in adult offspring of diabetic
pregnancy is restored by maternal melatonin treatment. J Pineal
Res. 61:340–352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rizzo NR, Hank NC and Zhang J: Detecting
presence of cardiovascular disease through mitochondria respiration
as depicted through biophotonic emission. Redox Biol. 8:11–17.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Doskey CM, Buranasudja V, Wagner BA,
Wilkes JG, Du J, Cullen JJ and Buettner GR: Tumor cells have
decreased ability to metabolize H2O2:
Implications for pharmacological ascorbate in cancer therapy. Redox
Biol. 10:274–284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eirin A, Ebrahimi B, Kwon SH, Fiala JA,
Williams BJ, Woollard JR, He Q, Gupta RC, Sabbah HN, Prakash YS, et
al: Restoration of mitochondrial cardiolipin attenuates cardiac
damage in swine renovascular hypertension. J Am Heart Assoc.
5:e0031182016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang W, Ren H, Xu C, Zhu C, Wu H, Liu D,
Wang J, Liu L, Li W, Ma Q, et al: Hypoxic mitophagy regulates
mitochondrial quality and platelet activation and determines
severity of I/R heart injury. eLife. 5:e214072016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang W, Siraj S, Zhang R and Chen Q:
Mitophagy receptor FUNDC1 regulates mitochondrial homeostasis and
protects the heart from I/R injury. Autophagy. 13:1080–1081. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mouton-Liger F, Jacoupy M, Corvol JC and
Corti O: PINK1/Parkin-dependent mitochondrial surveillance: From
pleiotropy to Parkinson's disease. Front Mol Neurosci. 10:1202017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chourasia AH and Macleod KF: Tumor
suppressor functions of BNIP3 and mitophagy. Autophagy.
11:1937–1938. 2015. View Article : Google Scholar : PubMed/NCBI
|