Introduction
Progress in peri-operative management and adjuvant
therapy has led to the improved survival of patients with lung
cancer (1–3). It has been reported that adjuvant
radiation therapy is effective and is often performed to eliminate
lung cancer cells (4,5). However, radiation therapy often
induces radiation resistance and severe side-effects, including
radiation-induced lung disease (RILD), which may range from
treatable acute pneumonitis to lethal fibrosis (6–8).
Therefore, overcoming resistance to radiation without inducing
additional severe side-effects is vital for the treatment of
patients with refractory lung cancer.
Rapidly growing tumors located at a distance from
the supporting vasculature results in the characteristic tumor
microenvironment of low oxygen and nutrients (9,10).
The hypoxia-inducible factor-1α (HIF-1α) subunit is an important
regulator of cellular oxygen homeostasis and hypoxia adaptation
(11–13). In general, it has been reported
that cancer cells under hypoxic conditions acquire resistance to
radiation therapy, and to most types of chemotherapy in various
types of cancer, via the accumulation of HIF-1α (14–19).
High expression levels of HIF-1α have been reported
to be associated not only with radiation resistance, but also with
a poor prognosis of patients with lung cancer (20–23).
Shibamoto et al reported that the administration of a HIF-1
inhibitor, in adjunction with radiation, significantly suppressed
the proliferation of lung cancer cell lines in vivo
(24). However, some HIF-1
inhibitors may induce deleterious side-effects in non-cancerous
tissues. Therefore, a therapeutic HIF-1-targeting strategy without
side-effects may be an ideal radiation sensitizer for refractory
cancers which are resistant to radiation in clinical practice.
Strategies for the treatment of hypoxia to overcome
resistance to radiation have included the development of several
radiosensitizers, and methods for directly increasing blood
oxygenation, such as pure oxygen or carbogen breathing, ozone
therapy, hyperbaric oxygen therapy, hydrogen peroxide injections
and the administration of suspensions of oxygen carrier liquids,
including ultrafine oxygen nanobubble water (25). However, these experimental models
have shown limited success owing to unwanted side-effects and
insufficient efficacy. To improve the therapeutic significance of
HIF-1α targeting, we focused on the creation of oxygen nanobubbles
in a single-nanometer range. As previously indicated, smaller
bubbles are more stable and possibly more effective in penetrating
target cells (26).
In this study, we sought to validate a newly
developed method to create oxygen nanobubble water in the single
nanometer range, and to examine its effect on HIF-1α expression and
hypoxia-induced resistance to radiation across multiple cancer cell
lines.
Materials and methods
Formation of nanobubble by ΣPM-5
Oxygen nanobubble water was prepared by a nanobubble
water preparation device ΣPM-5 (bellows pump type) (27). In brief, oxygen and pure water were
mixed at 0.4 MPa and pushed out from the nozzle. The oxygenated
water collided at high velocity to create nanobubble.
Characterization of nanobubble using a
cryo-transmission electron microscope
The nanobubble water was diluted 100-fold for
measurement. Pure water with or without diluted nanobubble was
rapidly frozen using Vitrobot Mark IV (FEI Co., Ltd., Hillsboro,
OR, USA). The samples were embedded in amorphous ice for
observation. The sample thickness was 200 nm. Nanobubbles embedded
in amorphous ice at a sample temperature of about −193°C were
directly observed using a cryo-transmission electron microscope
Titan Krios (FEI Co., Ltd.). The electron beam used for observation
is approximately 20 electrons/Å2 by the low-dose
technique, and there is almost no increase in sample temperature
during photography.
Cell lines
The EBC-1 human lung cancer cell line was purchased
from the RIKEN Cell Resource Center of Biomedical Research
(Tsukuba, Japan), and the MDA-MB-231 human breast cancer cell line
and BEAS-2S non-cancerous human bronchial cell line were from the
American Type Culture Collection (Manassas, VA, USA). Baseline
culture medium was prepared using RPMI-1640 medium (Wako, Osaka,
Japan), which was dissolved in water with or without the oxygen
nanobubble. The cells were cultured in filtered (0.22 μm)
nanobubble or normal RPMI-1640 supplemented with 10% fetal bovine
serum and 1% penicillin and streptomycin antibiotics, and incubated
at 37°C and 5% CO2. For hypoxia, the cells were
incubated under hypoxic conditions (1% O2) using the
BIONIX-1 hypoxic culture kit (Sugiyamagen, Tokyo, Japan) for 24
h.
Analysis of cell viability under normoxic
and hypoxic conditions
Cell viability was analyzed using the Cell Counting
kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan). The cells
were seeded 4×103/100 μl per well in 96-well
plates. After 24 h, normal RPMI-1640 medium was changed with meda
with or without oxygen nanobubbles, and the cells were incubated
under normoxic (21% O2, room air) or hypoxic conditions
(1% O2). Following 24 h of incubation, the cells were
irradiated at 2, 6, 10, and 14 Gy doses using an X-ray machine
(Faxitron RX-650; Faxitron X-Ray LLC, Lincolnshire, IL, USA) with
100 kV, Al 0.3 mm filter. Following a 72-h incubation
post-radiation, 10 μl of CCK-8 solution were added to each
well and the plates were incubated at 37°C for 2 h. The absorbance
was detected at 450 nm using a plate reader (Bio-Rad, Hercules, CA,
USA).
Protein extraction and western blot
analysis
Protein extraction was performed using lysis buffer
[10% glycerol, 10 mM Tris-HCl (pH 7.5), 1 mM EDTA, 400 mM NaCl,
0.5% NP40, 4 μg/ml aprotonin, PMSF, proteasome inhibitor
MG-132 and 1 mM DTT]. Total protein (10 μg) was
electrophoresed on a 10% polyacrylamide gel, and then
electroblotted at 300 mA for 90 min on a nitrocellulose membrane
(Invitrogen, Carlsbad, CA, USA). Western blot analysis was used to
confirm the protein expression of HIF-1α and HSC70: These proteins
were detected using anti-HIF-1α rabbit polyclonal antibody
(1:1,000) (Cell Signaling Technology, Cat. no. 3716) and anti-HSC70
mouse monoclonal antibody (1:1,000) (Santa Cruz Biotechnology, Cat.
no. sc-7298). HSC70 expression was used as a loading control. The
signals were detected using the ECL Select Western Blotting
Detection System (GE Healthcare Life Sciences, Pittsburgh, PA, USA)
and Image Quant LAS 4000 software (GE Healthcare Life
Sciences).
Radiation treatment under normoxic and
hypoxic conditions
Following a 24-h pre-incubation, the EBC-1 and
MDA-MB231 cells in 96-well plates were exposed to hypoxic (1%
O2) and normoxic conditions with normal or oxygen
nanobubble medium for 6 and 24 h, and then treated with radiation.
The O2 concentration was continuously evaluated using
O2 concentration measuring devices (Oxy-M O2
monitor, Jikco) in the bags. Cell viability was evaluated by CCK-8
assay after 72 h of incubation under normoxic conditions. An X-ray
machine (Faxitron RX-650; Faxitron X-Ray LLC) with 100 kV, Al 0.3
mm filter was used as the radiation source for treatment.
Clonogenic assay under normoxic and
hypoxic conditions
The cells plated into 6-well plates with RPMI-1640
normal medium and incubated at 37°C in humidified 5% CO2
for 24 h. Follwing a medium change, using the medium with or
without oxygen nanobubble, the hypoxic plates were directly
incubated under hypoxic conditions (1% O2). After 24 h,
the cells were irradiated at various doses (0, 2, 6, 10, and 14
Gy). After 72 h, the medium was changed to normal RPMI-1640 medium
and the cells were monitored every 3 days until colonies were
visible. The plates were rinsed with phosphate-buffered saline
(PBS), and the colonies were fixed with 99.5% ethanol and stained
with 0.5% crystal violet (Sigma-Aldrich, St. Louis, MO, USA). The
colonies counted up to at least 50 cells after staining. The
surviva fraction (SF) was calculated as the mean (number of
colonies counted/number of cells plated)/plating efficiency.
Statistical analysis
For continuous variables, the data are expressed as
the means ± standard deviation. Cell viability between the
treatment groups was analyzed using JMP software (SAS Institute,
Cary, NC, USA). A Student’s t-test was used to compare the oxygen
nanobubble group with the control group. A probability P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of ΣPM-5
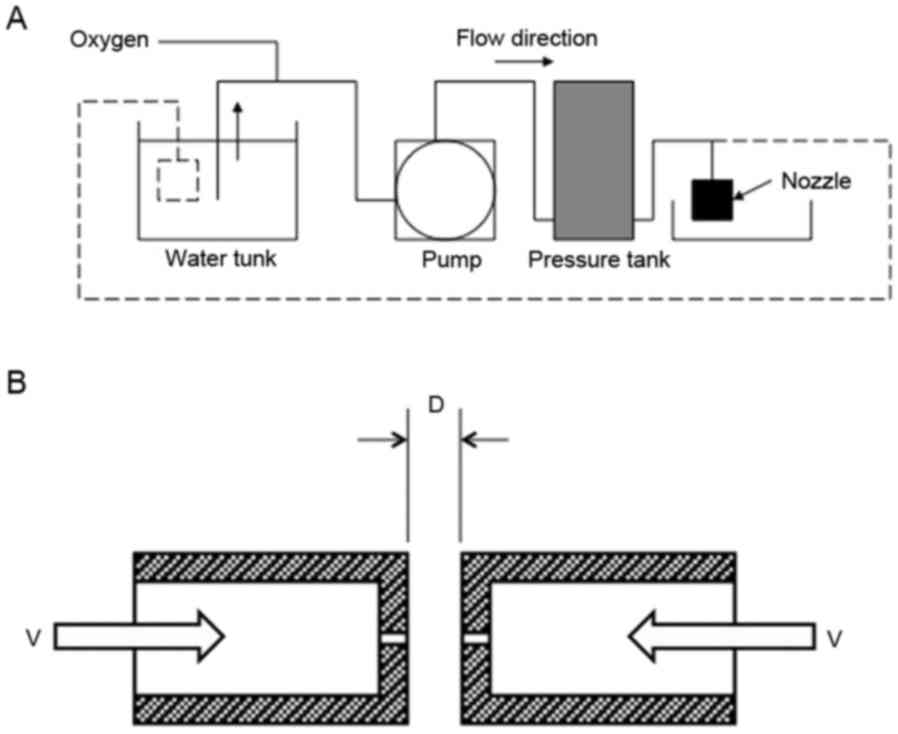
The schematic representation of ΣPM-5 is shown in
Fig. 1A. The oxygen was mixed with
the water in the pressurized tank at 0.4 MPa. The oxygenated water
was then pumped out from the small hole (diameter, 0.3–0.6 mm) in
the nozzle (Fig. 1B). The velocity
of water through the hole was calculated based on the flow rate
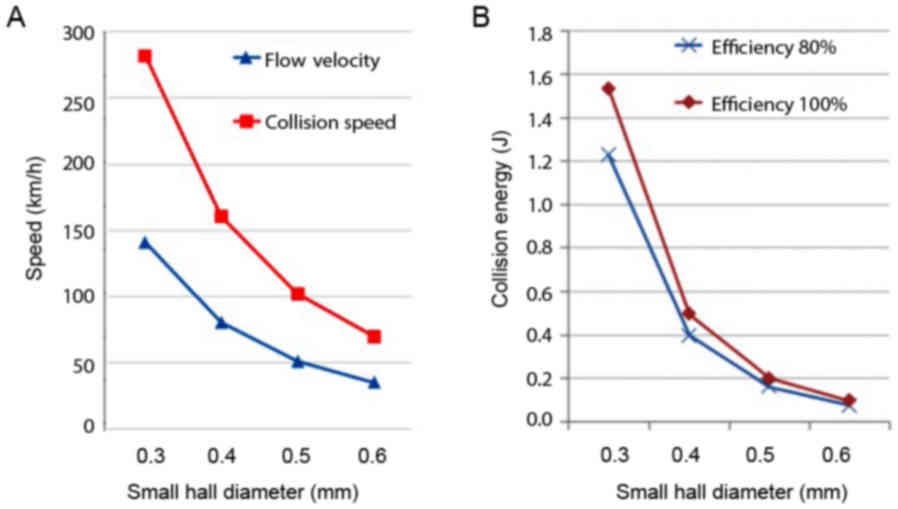
measurement (Fig. 2A).
The high velocity water through the small hole will
collide with the water from the other small hole placed
horizontally. The energy of water collision was calculated as
follows: 1/2 mV2 + 1/2 mV2 = mV2
(m, mass). The impact force was then calculated as follows: F =
mV2/½ D (D, distance between the small hole). The
collision energy force with the distance adjusted at 2 mm is shown
in Fig. 2B.
Characterization of nanobubbles
The oxygen nanobubble-containing water was created
by ΣPM-5 from pure water and oxygen. The nanobubble water was then
diluted to 1:100 and embedded in amorphous ice. The samples were
then observed using a cryo-transmission electron microscope. Pure
water was used as the control. As shown in Fig. 3A, no particles appeared in
amorphous ice prepared from pure water. As shown in Fig. 3B, the amorphous ice prepared from
the oxygen nanobubble-containing water contained oxygen
nanobubbles. In the area encircled by red broken lines, a dark
contrast originating from the isolated nanobubbles was observed.
The mean size was approximately 2–3 nm in diameter. On the other
hand, in the area encircled by yellow broken lines, a necklace-like
dark contrast originating from linear arrangement of nanobubbles
was recognized. This result indicated that the nanobubbles partly
aggregated. The volume of amorphous ice used for measurement was
1.8×10−14 ml (300×300×200 nm thickness) and contained
around 360 bubbles inside. Since the nanobubble water sample was
diluted 100-fold, the particle number of oxygen nanobubbles was
calculated to be 2×1018 particles/ml.
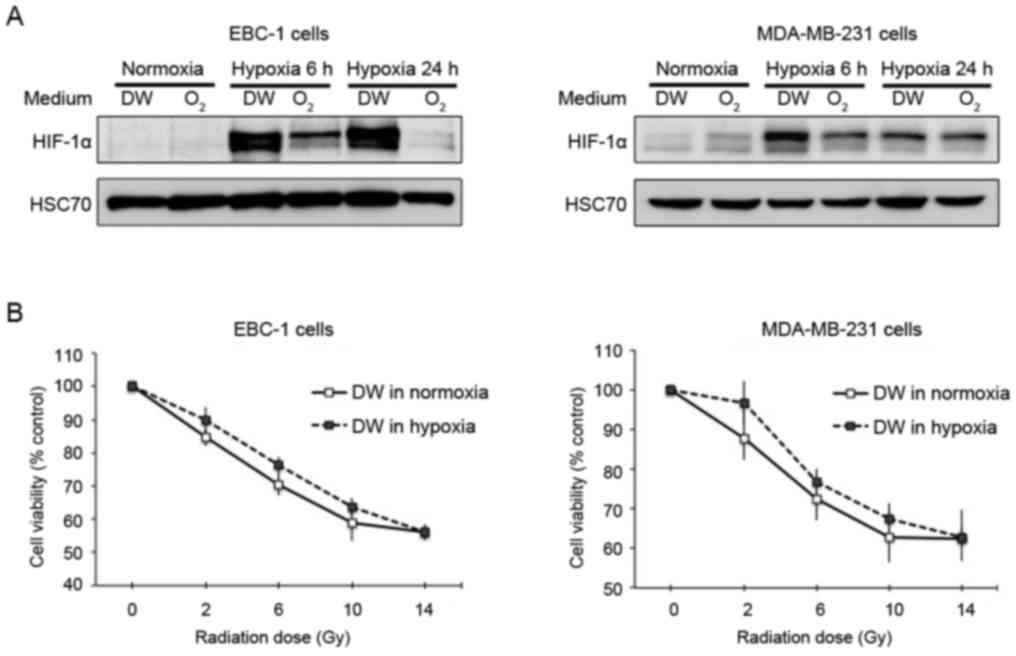
Oxygen nanobubble medium suppresses
hypoxia-induced HIF-1α expression
The overexpression of HIF-1α correlates with the
resistance of cells to radiation (21,22).
We thus examined whether treatment with oxygen nanobubble medium
can overcome the hypoxia-induced resistance of human cancer cells
to radiation. The EBC-1 and MDA-MB-231 cells were pre-incubated in
medium with or without oxygen nanobubbles for 24 h, and were then
analyzed for HIF-1α expression after 6 and 24 h of exposure to
hypoxic conditions. We found that the oxygen nanobubble water
clearly suppressed hypoxia-induced HIF-1α expression in the EBC-1
and MDA-MB-231 cells (Fig. 4A). We
validated that this hypoxic condition induced the resistance of
both the EBC-1 and MDA-MB231 cells to radiation (Fig. 4B).
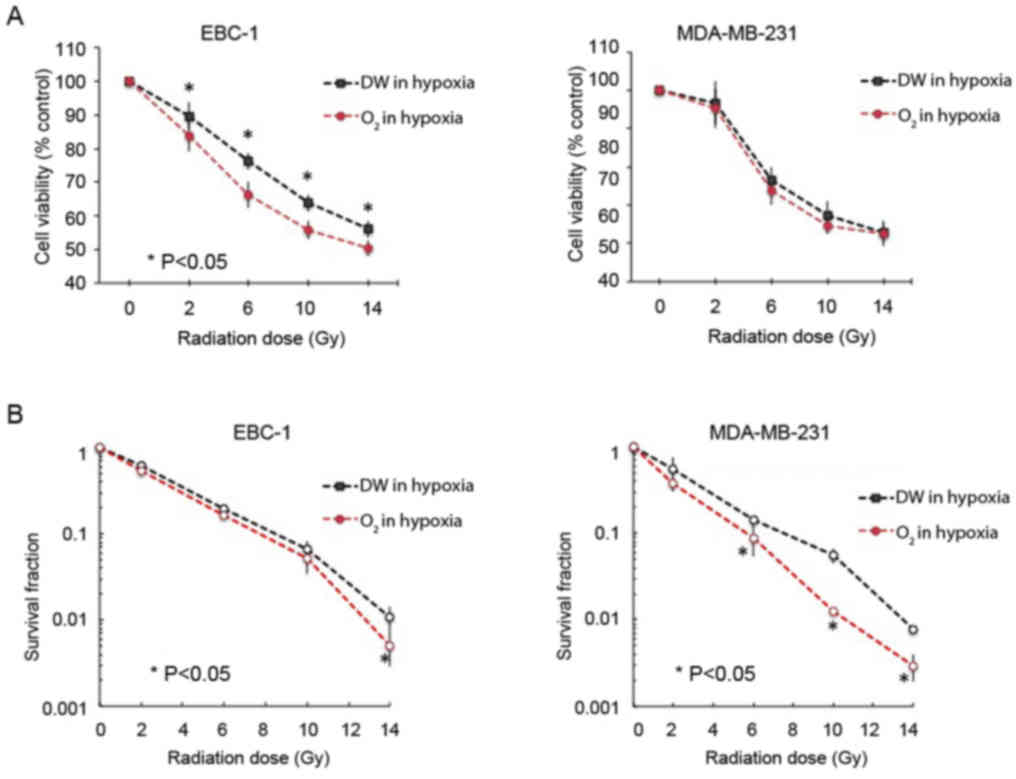
Oxygen nanobubble medium alters cancer
cell viability upon exposure to hypoxic conditions and radiation
treatment
We treated the EBC-1 and MDA-MB-231 cells with
radiation at doses of 0, 2, 6, 10, and 14 Gy to examine their
sensitivities to radiation under both normoxic and hypoxic
conditions (Fig. 5). Under hypoxic
conditions, we demonstrated that our oxygen nanobubble medium
enhanced the sensitivity of the EBC-1 cells to radiation compared
to the normal medium at 2, 6, 10, and 14 Gy doses of radiation. as
evidenced by a decreased cell viability upon oxygen nanobubble
treatment (Fig. 5A, left panel).
This effect was not significant in the MDA-MB-231 cells; however,
we observed a similar tendency in these cells as in the EBC-1 cells
(Fig. 5A, right panel). On the
other hand, colony formation assay revealed that the oxygen
nanobubble medium significantly suppressed the hypoxia-induced
resistance of both the EBC-1 and MDA-MB231 cells to radiation
compared to the normal medium at 2, 6, 10, and 14 Gy doses of
radiation (Fig. 5B).
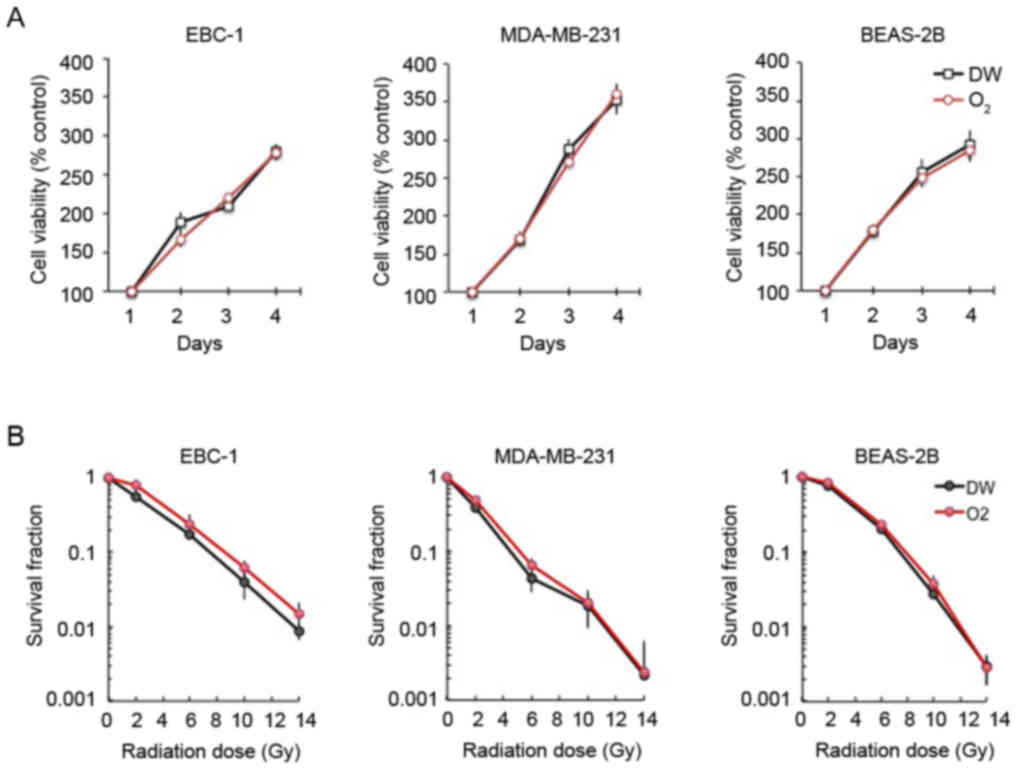
Normoxic application of oxygen nanobubble
medium does not affect the survival or radiosensitivity of lung
cancer, breast cancer, or non-cancer cell lines
The viability of the EBC-1 lung cancer, MDA-MB-231
breast cancer and non-cancerous BEAS-2B bronchial cells was not
affected by treatment with oxygen nanobubble medium under normoxic
conditions (Fig. 6A). Furthermore,
the sensitivity of the EBC-1, MDA-MB231 and BEAS-2B cells to
radiation was not affected by oxygen nanobubble treatment under
normoxic conditions (Fig. 6B).
Discussion
In this study, we produced oxygen nanobubble water
at a single nanometer size using the nanobubble water preparation
device, ΣPM-5. The characterization of the oxygen nanobubbles using
a cryo-transmission electron microscope verified that the
nanobubbles were at the single-nanometer range. Moreover, the
results of in vitro experiments demonstrated that the oxygen
nanobubble water significantly enhanced the the sensitivity of
human lung cancer and breast cancer cell lines to radiation under
hypoxic conditions via the suppression of HIF-1α expression.
Nanobubble water refers to a liquid containing small
bubbles typically with <200 nm in diameter (26). Unlike larger sized microbubbles
which disappear relatively quickly, nanobubbles remain stable in
water for a long period of time (28). Khier et al utilized oxygen
gas filled particles to efficiently oxygenate human blood ex vivo
without complement activation or hemolysis (29). Recently, Owen et al reported
the delivery of oxygen through the oral administration of oxygen
nanobubbles (25). Both research
groups utilized lipid or surfactant to create and stabilize oxygen
nanobubbles in a 50–200 nm range. In this study, we reported a
newly developed method to create oxygen containing nanobubbles in
the single-nanometer range. By utilizing a novel water hammer
method, in which the pressurized oxygen saturated water collides in
a high velocity, we produced oxygen-containing nanobubble water
without any additives. The results from cryo-transmission electron
microscopy measurement revealed stable oxygen nanobubbles in the
single-nanometer range. To the best of our knowledge, this is the
first study to demonstrate the creation and characterization of
single nanometer-sized nanobubbles.
Resistance to radiation causes serious complications
for patients with lung cancer. This resistance has been reported to
be induced by several pathways, including those associated with
hypoxia, tyrosine kinase receptors, AKT serine/threonine kinases,
DNA damage repair, developmental pathways, adhesion pathways and
inflammation (30,31). The decreased oxygen concentration,
and subsequent increase in HIF-1α activity, is known to be
associated with resistance to radiation, cell survival,
angiogenesis and the proliferative activity of cancer cells
(32–34). Therefore, in this study, we focused
on the use of oxygen nanobubbles as a method to reoxygenate hypoxic
cells and downregulate HIF-1α activity. Several downstream targets
of HIF, such as mediators of angiogenesis, including vascular
endothelial growth factor (VEGF), cell survival regulators
including insulin-like growth factor (IGF)-related factors, and
cell proliferation regulators, such as c-MYC and insulin-like
growth factor 2 (IGF2), are strongly associated with resistance to
radiation and cancer aggressiveness under hypoxic conditions
(35). In this study, we
demonstrated the ability of our oxygen nanobubble preparation to
significantly reduce HIF-1α activity under hypoxic conditions;
therefore, it may also modulate several of the important downstream
mediators of radiation resistance and malignancy previously
described.
This study utilized our oxygen nanobubble water as a
modulator of radiation sensitivity under hypoxic conditions. This
nanobubble water included only water and single nanometer-range
oxygen bubbles, with an average size of 2–3 nm, without any
chemical compounds. Small size bubbles have some advantages,
including high stability and high oxygen occupancy compared to
larger ones. On the other hand, the continuous administration of a
high oxygen concentration is known to induce oxygen toxicity due to
the production of reactive oxygen species (36,37).
However, our nanobubble water did not affect the viability of human
lung cancer, breast cancer, or non-cancerous bronchial cells under
normoxic conditions. Additionally, the O2
concentration-measuring devices demonstrated that the low oxygen
concentration of our experimental hypoxia bags was not altered by
the oxygen nanobubble medium during exposure to hypoxia. Therefore,
our data suggests that our nanobubble water functions to modulate
intracellular hypoxia in cancer cells via the suppression of
hypoxia-induced HIF-1α expression in spite of continuous hypoxic
culture conditions.
Neo-adjuvant radiation and chemoradiotherapy (CRT)
have been considered as effective therapeutic tools to accomplish
radical resection and down-staging in patients with lung cancer
(38,39). Complete response is estimated as
14–40% by CRT (40,41); on the other hand, patients with
lung cancer that develop disease refractory to these therapies
often have poor outcomes (42).
Radiation is known to cause severe side-effects, including lethal
radiation fibrosis. However, modern radiation methodologies
(including proton beam, heavy ion, stereotactic ablative body and
intensity-modulated radio-therapies) are able to deliver precisely
focused radiation to protect surrounding non-cancerous tissues, and
effectively target refractory-prone hypoxic areas of tumors
(43–45). Our data demonstrated that oxygen
nanobubbles enhanced the sensitivity of human lung cancer cells and
breast cancer cells to radiation under hypoxic conditions. In this
study, we validated the efficacy of our oxygen nanobubble, across
multiple tumor types, against radiation resistance and HIF-1α
accumulation under hypoxic conditions. Therefore, oxygen nanobubble
water may serve as a sensitizing adjuvant when administered in
combination with low dose radiation, which may enhance the efficacy
of treatment, without increasing toxicity, in cancer patients.
In conclusion, in this study, we developed and
characterized pure oxygen nanobubble water, in the single nanometer
range, without any additives other than water and oxygen. In our
human cancer cell-based experiments, oxygen nanobubble water
demonstrated the ability to protect against hypoxia-induced
radiation resistance through the suppression of HIF-1α. Our
additive-free single nanometer-range oxygen nanobubble water may
prove to be a promising modulator against hypoxia/HIF-1α-mediated
radiation refractory cancers, although further studies are required
in order to test its safety and effectiveness. Future studies are
warranted to examine the preventative and therapeutic potential of
our nanobubble water in mouse tumor models of radiation resistance.
Additionally, an important challenge for future experiments will be
to validate the stability of nanobubbles in vivo. Recently,
Bandhari et al demonstrated the ability to detect oxygen
nanobubbles via hyperspectral dark-field microscopy in live cells
in vitro and tumor tissue ex vivo, and via ultrasound
imaging of in vivo tumors (46,47).
Thus, we aim to employ these previously validated techniques for
the detection of oxygen nanobubbles in tissue to examine the
stability our oxygen nanobubbles in vivo. In addition, our
in vitro experiments were performed in the presence of
serum, therefore, the possibility exists that serum-specific
cellular responses were elicited. However, we consider that serum
may not be a critical factor to evaluate the relationship of
hypoxia-induced radiation resistance and radiation sensitizers due
to previous studies that have examined this relationship using
serum-containing media (48–50).
Nevertheless, to rule out any potential artifactual responses in
our experiments due to the presence of serum, additional
experiments are warranted under serum-free conditions.
Acknowledgments
This study was supported in part by the Japan
Society for Promotion of Science (JSPS) Grant-in Aid for Scientific
Research (Grant nos. 15K10085 and 22591450). A part of this study
was supported by Osaka University Microstructural Characterization
Platform as a program of ‘Nanotechnology Platform’ of the Ministry
of Education, Culture, Sports, Science and Technology (MEXT),
Japan. The authors are grateful to Mr. M. Nagata and Mr. K.
Yoshiura in P.D.C.A. Inc. Japan for opening up new markets.
Notes
[1] Competing
interests
The authors M.I., N.G., T.Y., T.A., T.N., T.A.,
R.K., K.A., H.Y., H.K. and M.K., declare that they have no
competing interests. Y.T., K.T., and K.H. are co-inventors of a
patent (Tachibana Y, Tachibana K, Harada K, Sasajima S, Tamahashi
K, Honma K, Matsumoto Y: METHOD, A BUBBLE GENERATING NOZZLE, AND AN
APPARATUS FOR GENERATING MICRO-NANO BUBBLES JP.5555892,
JP/PCT/2013/066902, Filed August 29, 2013; issued July 23, 2014),
which has been filed in relation to the formulation of nanobubble
described in the study. Y.T., K.T. and D.Y. are co-inventors of a
patent (Yamannouchi D, Tachibana Y, Tachibana K: AQUEOUS SOLUTION
CAPABLE OF BEING ADMINISTERED TO LIVING BODY, AND METHOD FOR
PRODUCING SAME. WO 2017195852. JP/PCT2017/017779, Filed November
05, 2017) (Yamanouchi D, Tachibana Y, Tchibana K: AQUEOUS SOLUTION
CONTAINING OZONE NANOBUBBLES, METHOD FOR PRODUCING SAME AND
UTILIZATION OF AQUEOUS SOLUTION CONTAINING OZONE NANOBUBBLES. WO
2017199827. JP/PCT/2017/017781. Filed November 05, 2017.), which
has been filed in relation to the formulation and application of
nanobubble described in the study.
References
|
1
|
Nakagawa K, Watanabe SI, Kunitoh H and
Asamura H; The Lung Cancer Surgical Study Group of the Japan
Clinical Oncology Group: The Lung Cancer Surgical Study Group of
the Japan Clinical Oncology Group: Past activities, current status
and future direction. Jpn J Clin Oncol. 47:194–199. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arriagada R, Auperin A, Burdett S, Higgins
JP, Johnson DH, Le Chevalier T, Le Pechoux C, Parmar MK, Pignon JP,
Souhami RL, et al NSCLC Meta-analyses Collaborative Group: Adjuvant
chemotherapy, with or without postoperative radiotherapy, in
operable non-small-cell lung cancer: Two meta-analyses of
individual patient data. Lancet. 375:1267–1277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fiteni F, Westeel V and Bonnetain F:
Surrogate endpoints for overall survival in lung cancer trials: A
review. Expert Rev Anticancer Ther. 17:447–454. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shafiq J, Hanna TP, Vinod SK, Delaney GP
and Barton MB: A Population-based Model of Local Control and
Survival Benefit of Radiotherapy for Lung Cancer. Clin Oncol (R
Coll Radiol). 28:627–638. 2016. View Article : Google Scholar
|
|
5
|
Yang CF, Chan DY, Speicher PJ, Gulack BC,
Wang X, Hartwig MG, Onaitis MW, Tong BC, D’Amico TA, Berry MF, et
al: Role of adjuvant therapy in a population-based cohort of
patients with early-stage small-cell lung cancer. J Clin Oncol.
34:1057–1064. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giridhar P, Mallick S, Rath GK and Julka
PK: Radiation induced lung injury: Prediction, assessment and
management. Asian Pac J Cancer Prev. 16:2613–2617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bradley J and Movsas B: Radiation
pneumonitis and esophagitis in thoracic irradiation. Cancer Treat
Res. 128:43–64. 2006. View Article : Google Scholar
|
|
8
|
Marks LB, Yu X, Vujaskovic Z, Small W Jr,
Folz R and Anscher MS: Radiation-induced lung injury. Semin Radiat
Oncol. 13:333–345. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bertout JA, Patel SA and Simon MC: The
impact of O2 availability on human cancer. Nat Rev
Cancer. 8:967–975. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kunz M and Ibrahim SM: Molecular responses
to hypoxia in tumor cells. Mol Cancer. 2:232003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Semenza GL: Hypoxia-inducible factors:
Mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Keith B, Johnson RS and Simon MC: HIF1α
and HIF2α: Sibling rivalry in hypoxic tumour growth and
progression. Nat Rev Cancer. 12:9–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wilson WR and Hay MP: Targeting hypoxia in
cancer therapy. Nat Rev Cancer. 11:393–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harrison LB, Chadha M, Hill RJ, Hu K and
Shasha D: Impact of tumor hypoxia and anemia on radiation therapy
outcomes. Oncologist. 7:492–508. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brown JM and Wilson WR: Exploiting tumour
hypoxia in cancer treatment. Nat Rev Cancer. 4:437–447. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shannon AM, Bouchier-Hayes DJ, Condron CM
and Toomey D: Tumour hypoxia, chemotherapeutic resistance and
hypoxia-related therapies. Cancer Treat Rev. 29:297–307. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimoda LA and Semenza GL: HIF and the
lung: Role of hypoxia-inducible factors in pulmonary development
and disease. Am J Respir Crit Care Med. 183:152–156. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goudar RK and Vlahovic G: Hypoxia,
angiogenesis, and lung cancer. Curr Oncol Rep. 10:277–282. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Wang P, Guo F, Wang X, Wang J, Xu
J, Yuan D, Zhang J and Shao C: Autophagy enhanced the
radioresistance of non-small cell lung cancer by regulating ROS
level under hypoxia condition. Int J Radiat Biol. 93:764–770. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren W, Mi D, Yang K, Cao N, Tian J, Li Z
and Ma B: The expression of hypoxia-inducible factor-1α and its
clinical significance in lung cancer: A systematic review and
meta-analysis. Swiss Med Wkly. 143:w138552013.
|
|
22
|
Yang SL, Ren QG, Wen L and Hu JL:
Clinicopathological and prognostic significance of
hypoxia-inducible factor-1 alpha in lung cancer: A systematic
review with meta-analysis. J Huazhong Univ Sci Technolog Med Sci.
36:321–327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hung JJ, Yang MH, Hsu HS, Hsu WH, Liu JS
and Wu KJ: Prognostic significance of hypoxia-inducible
factor-1alpha, TWIST1 and Snail expression in resectable non-small
cell lung cancer. Thorax. 64:1082–1089. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shibamoto Y, Sugie C, Ito M and Ogino H:
The Japanese experiences with hypoxia-targeting
pharmacoradiotherapy: From hypoxic cell sensitisers to
radiation-activated prodrugs. Expert Opin Pharmacother.
5:2459–2467. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Owen J, McEwan C, Nesbitt H,
Bovornchutichai P, Averre R, Borden M, McHale AP, Callan JF and
Stride E: Reducing tumour hypoxia via oral administration of oxygen
nanobubbles. PLoS One. 11:e01680882016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Agarwal A, Ng WJ and Liu Y: Principle and
applications of microbubble and nanobubble technology for water
treatment. Chemosphere. 84:1175–1180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tachibana Y, Tachibana K, Harada K,
Sasajima S, Tamahashi K, Honma K and Matsumoto Y: Method, a bubble
generating nozzle, and an apparatus for generating micro-nano
bubbles. Sigma Technology, Inc, JP, PCT/JP2013/066902. Filed.
August 29–2013, issued July 23, 2014.
|
|
28
|
Takahashi M, Chiba K and Li P:
Free-radical generation from collapsing microbubbles in the absence
of a dynamic stimulus. J Phys Chem B. 111:1343–1347. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kheir JN, Polizzotti BD, Thomson LM,
O’Connell DW, Black KJ, Lee RW, Wilking JN, Graham AC, Bell DC and
McGowan FX: Bulk manufacture of concentrated oxygen gas-filled
microparticles for intravenous oxygen delivery. Adv Healthc Mater.
2:1131–1141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vaupel P, Thews O and Hoeckel M: Treatment
resistance of solid tumors: Role of hypoxia and anemia. Med Oncol.
18:243–259. 2001. View Article : Google Scholar
|
|
31
|
Kim BM and Hong Y, Lee S, Liu P, Lim JH,
Lee YH, Lee TH, Chang KT and Hong Y: Therapeutic implications for
overcoming radiation resistance in cancer therapy. Int J Mol Sci.
16:26880–26913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chan DA, Krieg AJ, Turcotte S and Giaccia
AJ: HIF gene expression in cancer therapy. Methods Enzymol.
435:323–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xia Y, Choi HK and Lee K: Recent advances
in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem.
49:24–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Semenza GL: Hypoxia-inducible factor 1 and
cancer pathogenesis. IUBMB Life. 60:591–597. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Poprac P, Jomova K, Simunkova M, Kollar V,
Rhodes CJ and Valko M: Targeting free radicals in oxidative
stress-related human diseases. Trends Pharmacol Sci. 38:592–607.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gào X and Schöttker B: Reduction-oxidation
pathways involved in cancer development: A systematic review of
literature reviews. Oncotarget. 8:51888–51906. 2017.PubMed/NCBI
|
|
38
|
Daly BD, Cerfolio RJ and Krasna MJ: Role
of surgery following induction therapy for stage III non-small cell
lung cancer. Surg Oncol Clin N Am. 20:721–732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu YP, Li B, Xu XL and Mao WM: Is there a
survival benefit in patients with stage IIIA (N2) non-small cell
lung cancer receiving neoadjuvant chemotherapy and/or radiotherapy
prior to surgical resection: A systematic review and meta-analysis.
Medicine (Baltimore). 94:e8792015. View Article : Google Scholar
|
|
40
|
Baker S, Dahele M, Lagerwaard FJ and Senan
S: A critical review of recent developments in radiotherapy for
non-small cell lung cancer. Radiat Oncol. 11:1152016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Campbell BA, Ball D and Mornex F:
Multidisciplinary lung cancer meetings: Improving the practice of
radiation oncology and facing future challenges. Respirology.
20:192–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Scagliotti GV, Novello S, Rapetti S and
Papotti M: Current state-of-the-art therapy for advanced squamous
cell lung cancer. Am Soc Clin Oncol Educ Book. 33:354–358. 2013.
View Article : Google Scholar
|
|
43
|
Haasbeek CJ, Slotman BJ and Senan S:
Radiotherapy for lung cancer: Clinical impact of recent technical
advances. Lung Cancer. 64:1–8. 2009. View Article : Google Scholar
|
|
44
|
Cascales A, Martinetti F, Belemsagha D and
Le Pechoux C: Challenges in the treatment of early non-small cell
lung cancer: What is the standard, what are the challenges and what
is the future for radiotherapy? Transl Lung Cancer Res. 3:195–204.
2014.
|
|
45
|
Huo M, Gorayski P, Pinkham MB and Lehman
M: Advances in radiotherapy technology for non-small cell lung
cancer: What every general practitioner should know. Aust Fam
Physician. 45:805–809. 2016.PubMed/NCBI
|
|
46
|
Bhandari P, Wang X and Irudayaraj J:
Oxygen nanobubble tracking by light scattering in single cells and
tissues. ACS Nano. 11:2682–2688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bhandari PN, Cui Y, Elzey BD, Goergen CJ,
Long CM and Irudayaraj J: Oxygen nanobubbles revert hypoxia by
methylation programming. Sci Rep. 7:92682017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Luo H, Wang L, Schulte BA, Yang A, Tang S
and Wang GY: Resveratrol enhances ionizing radiation-induced
premature senescence in lung cancer cells. Int J Oncol.
43:1999–2006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Millet P, Granotier C, Etienne O and
Boussin FD: Radiation-induced upregulation of telomerase activity
escapes PI3-kinase inhibition in two malignant glioma cell lines.
Int J Oncol. 43:375–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lechtman E and Pignol JP: Interplay
between the gold nanoparticle sub-cellular localization, size, and
the photon energy for radiosensitization. Sci Rep. 7:132682017.
View Article : Google Scholar : PubMed/NCBI
|