Introduction
Cervical cancer is the third most frequent cause of
cancer-related mortality among women worldwide (1). Over 500,000 cases of cervical cancer
were reported in 2015, with approximately one third of those cases
resulted in death (1). Advanced
cervical cancer is treated with surgery, radiotherapy,
chemotherapy, or a combination of these modalities; however, the
prognosis of patients with cervical cancer remains poor, and major
improvements in outcomes have not been reported over the past 30
years (2).
Cervical cancer generally develops from a
pre-cancerous cervical intraepithelial neoplasm (CIN) (3). At present, early-stage cervical
cancers, including high-grade CIN and early infiltrating cancer,
are only surgically treated using cervical conization and total
hysterectomy (4,5). Nonetheless, the incidence of
complications, such as infertility, miscarriage and premature
delivery, is high in patients treated with conization deu to the
shortening of the uterine cervix and sensitivity to intra-uterine
infection. Therefore, alternative modes of therapy are required in
order to avoid these complications (6).
Carcinogenesis of the cervix is triggered by
infection with high-risk human papillomavirus (HPV) (3,7,8).
High-risk HPV is detected in >90% of cervical cancer cases, and
HPV type 16 (HPV-16) accounts for approximately half of these
(9). When high-risk HPV
continuously infects basal cells of the cervical epithelium and is
integrated into the host chromosome, HPV oncoproteins E6 and E7 are
overexpressed and inactivate the tumor suppressor genes p53 and Rb,
thereby inducing cancer (10–12).
Therefore, cervical cancer may be resolved by inhibiting the
expression of E6 and E7. The expression of E6 and E7 is localized
in lesions, and these proteins are not expressed in normal tissues,
including the cervix (13),
thereby limiting the effects of specific inhibition of E6/E7 to
cancerous lesions.
RNA interference (RNAi) is a superior method of
inhibiting gene expression following transcription (14). Among various RNAi techniques, the
specificity of RNAi using short small interfering RNA (siRNA) for
the target gene is very high, and the inhibitory action of siRNA is
potent, but transient (15,16),
limiting its clinical application in the treatment of diseases
(17). Polymerase III
promoter-driven short hairpin RNA (shRNA) has recently been
developed as an alternative strategy capable of stably inhibiting
target gene expression for longer periods of time, and its effect
has been widely recognized (18).
Therefore, E6 and E7 of high-risk HPV may be strongly and
persistently inhibited using shRNA. However, the application of
this strategy in the treatment of cervical cancer would require a
vector that efficiently transfers the genes into target cervical
cancer cells.
Adeno-associated virus (AAV) vectors are derived
from non-pathogenic viruses and can transfer genes into
non-dividing cells (19). Many
serotypes have been developed as vectors, and specific tissue and
organ tropisms have been reported (20). Moreover, gene transfer into a broad
range of cells by direct administration and long-term protein
expression have been observed in humans; indeed, AAV vectors are
one of the most prominent viral vectors used to facilitate gene
therapy (21). Therefore, in the
present study, we aimed to develop a highly specific, novel
treatment for cervical cancer using an AAV vector containing hpV-16
E6/E7-targeting shRNA (AAV-shE6E7).
Materials and methods
Cell culture
The HPV-16-positive human cervical cancer cell
lines, BOKU and SKG-IIIa, and the human immortalized cell line,
293, were purchased from the Japanese Collection of Research
Bioresources Cell Bank (Osaka, Japan). The HPV-16-positive human
cervical cancer cell line, SiHa, was purchased from the American
Type Culture Collection (ATCC, Manassas, VA, USA). All cell lines
were authenticated by the above-mentioned cell banks by means of
short-tandem repeat-polymerase chain reaction (pCR) profiling. The
cells were cultured in Dulbecco’s modified Eagle’s medium/F12 (life
Technologies, Carlsbad, CA, USA) containing 10% inactivated fetal
bovine serum (Sigma-Aldrich, St. Louis, MO, USA) and 1%
penicillin/streptomycin (life Technologies) in 5% carbon dioxide at
37°C.
Efficiency of gene transfer into cervical
cancer cells using different serotypes of AAV vectors
The BOKU, SiHa and SKG-IIIa cells were seeded in
96-well plates at a density of 1×103 cells/well. After
12 h, the tumor cells were transduced with AAV vectors containing
the LacZ gene encoding β-galactosidase (AAV-CMV-LacZ) types 1–9
(22–25) at 3×105 viral genomes
(vg)/cell. After 48 h, β-galactosidase expression was measured
using a β-galactosidase staining kit (Cell Signaling Technology,
Danvers, MA, USA) according to the manufacturer’s instructions.
Construction of a plasmid vector
containing shE6E7
The U6 promoter and siRNA sequences were cut out
from the PvuII site of the pSilencer U6 vector (Thermo
Fisher Scientific, Waltham, MA, USA) and inserted into the blunted
MluI site of pAAV-hrGFP (Agilent Technologies, Santa Clara,
CA, USA) to prepare pu6si-CmV-green fluorescent protein (gFp). The
target site for the shRNA was the following 21-base sequence
(564–582) in HPV-16 E6/E7 mRNA, taken from a previously
published study (26): sense,
5′-GATCCGCATGGAGATACACCTACAttcaagagaTGTAGGTGTATCTCCATGCTTTTTTG-3′
and antisense,
5′-AATTCAAAAAAGCATGGAGATACACCTACAtctcttgaaTGTAGGTGTATCTCCATGCG-3′.
The two oligomers were heated at 90°C for 3 min, annealed by slow
cooling to 37°C over a 30-min period, and inserted into the
multi-cloning sites of pU6-MCS-CMV-GFP and the BamHI and
HindIII sites to prepare an shE6E7-targeting shRNA
expression plasmid vector (pU6-shE6E7-CMV-GFP). A control vector
(pU6-shNC-CMV-GFP) was prepared by inserting a 21-base sequence
(Thermo Fisher scientific) known to have no influence on gene
expression into pU6-shNC-CMV-GFP at the same sites.
Preparation of an AAV vector containing
shE6E7
shE6E7-containing and control AAV vectors were
prepared by transfecting the 293 cells with 3 plasmids, including
pU6-shE6E7-CMV-GFP or pU6-shNC-CMV-GFP, the helper plasmid for
adenovirus genes, and the helper plasmid for AAV (27,28).
Recombinant AAV vectors (AAV-shE6E7 and AAV-shNC) were collected by
repeated freezing and thawing of the cells 3 times. The vector
solutions were purified by density gradient ultracentrifugation,
and the vector titers were measured using real-time PCR, as
previously described (29).
Flow cytometry
Flow cytometry was used to measure the gene transfer
efficiency of the type 2 AAV vector (AAV2) according to GFP
expression, and cell cycle analysis was performed using Annexin V
(Annexin V-FITC apoptosis detection kit; BD Biosciences, San Jose,
CA, USA) and propidium iodide (PI) staining (BD Cycletest Plus DNA
Reagent kit; BD Biosciences) to determine the rate of cellular
apoptosis after AAV2-shE6E7 transduction. Briefly, the cells were
seeded in 12-well plates at 1×104 cells per well. After
12 h, the cells were transduced with 1×105 vg/cell of
AAV2-shE6E7 or AAV2-shNC. The cells were then collected by trypsin
treatment 48 h after transduction, washed twice with PBS, and
stained on ice with Annexin V-FITC for 15 min followed by BD
Cycletest for 10 min. The stained cells were measured using
fluorescence-activated cell sorting (FACs; BD Biosciences) with a
CellQuest software system (BD Biosciences).
Reverse transcription-quantitative PCR
(RT-qPCR)
Cellular mRNA was extracted using an RNeasy Mini kit
(Qiagen, Valencia, CA, USA) according to the manufacturer’s
instructions and was used as a template for cDNA synthesis by
reverse transcription (ReverTra Ace; Toyobo, Tokyo, Japan). RT-qPCR
was performed using a LightCycler System (Roche Diagnostics,
Mannheim, Germany) following the manufacturer’s instructions. pCR
was carried out using 40 cycles of heating at 95°C for 15 sec, 58°C
for 15 sec and 72°C for 20 sec. The mRNA levels of the target genes
were determined relative to the fluorescence signal level of
β-actin. Primer sequences are as follows: human β-actin forward,
5′-AGCCATGTACGTTGCTATCC-3′ and reverse, 5′-TTGGCGTACAGGTCTTTGC-3′;
HPV16E6: forward, 5′-TTACCACAGTTATGCACAGA-3′ and reverse,
5′-ACAGTGGCTTTTGACAGTTA-3′; and HPV16E7 forward,
5′-AGAAACCCAGCTGTAATCAT-3′ and reverse,
5′-TTATGGTTTCTGAGAACAGA-3′.
Western blot analysis
The cells were seeded in 6-well plates at
1×106 cells/well. After 12 h, the cells were transduced
with AAV2-shE6E7 or AAV2-shNC at 1×105 vg/cell. After 48
h, the cells were lysed using lysis buffer (1% NP-40, 150 mM NaCl,
50 mM Tris-HCl, pH 8.0), and protein was extracted. Protein samples
were mixed with 1% SDS sample buffer (10 mM Tris-HCl, pH 7.5, 150
mM NaCl, 1% SDS, EDTA-free protease inhibitor cocktail (Roche
Diagnostics), separated by 10% polyacrylamide gel electrophoresis,
and transferred onto polyvinylidene difluoride (pVDF) membranes
(Merck Millipore, Billerica, MA, USA). Membranes were placed in
PVDF Blocking Reagent for Can Get Signal (Toyobo) at room
temperature for 1 h and then reacted with anti-hpV-16/18 E6
(sc-460; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
anti-HPV-16 E7 (Cat. no. sc-6981; Santa Cruz Biotechnology),
anti-p53 (Cat. no. sc-126; Santa Cruz Biotechnology), anti-p21
(Cat. no. 2947S; Cell Signaling Technology), anti-p16 (Cat. no.
4824; Cell Signaling Technology), anti-Rb (Cat. no. 9313; Cell
Signaling Technology), anti-phosphorylated-Rb (p-Rb; Cat. no. 2181;
Cell Signaling Technology), or anti-β-actin monoclonal antibodies
(sc-1616-R; Santa Cruz Biotechnology) at 4°C overnight using Can
get Signal Immunoreaction Enhance Solution 1 (Toyobo). After
washing, the membranes were reacted with peroxidase-labeled
anti-mouse antibodies (GE Healthcare Japan, Tokyo, Japan) using Can
Get Signal Immunoreaction Enhance Solution 2 (Toyobo) at room
temperature for 1 h. Protein bands were detected using ECL Western
Blotting Detection Reagents (GE Healthcare Japan) and a cold CCD
system (LAS-4000mini; GE Healthcare Japan).
In vitro cell growth
The cells were seeded in 96-well plates at
1×103 cells per well. After 12 h, the cells were
transduced with AAV2-shE6E7 at 0–1×107 vg/cell or
AAV2-shNC at 1×105 vg/cell. After 72 h, 10 μl
premix WsT-1 reagent (Takara Bio, Otsu, Japan) was added, and the
absorbance at 450 nm was measured using a SpectraMax 190 microplate
reader (Molecular Devices, Sunnyvale, CA, USA) after 1 h.
Animal experiment
Five-week-old severe combined immunodeficiency
(sCID) nude mice (n=39) (Charles River Japan, Yokohama, Japan),
weighing 15–20 g, were inoculated with BOKu or siha cells, and
5-week-old BAlB/c nude mice (n=15) (Clea Japan, Tokyo, Japan),
weighing 15–20 g, were inoculated with SKG-IIIa cells. The animals
were maintained under specific pathogen-free conditions. The animal
experiments were performed according to the animal experimental
guidelines and following the approval of the Ethics Committee of
Jichi Medical University (Tochigi, Japan).
Subcutaneous tumors with 5- and 8-mm major axes were
investigated as early-stage and infiltrating cancer models,
respectively (30). Briefly, nude
mice were subcutaneously inoculated with 1×107 tumor
cells in the dorsal region to form a subcutaneous tumor, and
2.5×1011 vg/mouse AAV2-shE6E7 or AAV2-shNC was directly
injected once into the tumors after the tumors had reached the
target size for each purpose. The tumor size was measured twice
weekly using calipers, and the tumor volume was calculated as the
major axis x the minor axis2 × 1/2. The mice were
sacrificed when the tumor volume exceeded 500 mm3.
Multiple tumors were not observed in our study.
Immunohistochemical staining
The cancer-bearing mice were sacrificed by
decapitation, and the tumors were excised and embedded in paraffin.
TuNEl staining was performed using an in situ apoptosis
detection kit (Takara Bio) following the manufacturer’s
instructions. Briefly, paraffin-embedded tissue sections were
deparaffinized and treated with proteinase K (10–20 μg/ml,
15 min; Takara Bio). Endogenous peroxidase was blocked with 3%
H2O2 aqueous solution for 5 min. After
washing, 50 μl ice-cold labeling reaction solution was added
to the sections, and the samples were incubated in a moist
container at 37°C for 60 min. After washing, the sections were
reacted with 70 μl anti-FITC hRp conjugate (Takara Bio) at
37°C for 30 min, followed by color development with
DAB/h2O2 reaction solution (Takara Bio) at
room temperature for 10 min. The sections were then stained with
hematoxylin (Takara Bio), dehydrated, permeabilized, sealed and
observed under a light microscope (IX73, Olympus, Tokyo, Japan).
TUNEL-positive cells were counted in 5 visual fields at x40
magnification.
Statistical analysis
Student’s t-tests were used for between-group
comparisons, and differences with P-values <0.05 were considered
to indicate a statistically significant difference. Tukey’s tests
were used for multiple group comparisons, and differences with
P-values <0.05 were considered to indicate a statistically
significant difference.
Results
Optimization of AAV serotypes for gene
transfer into cervical cancer cells
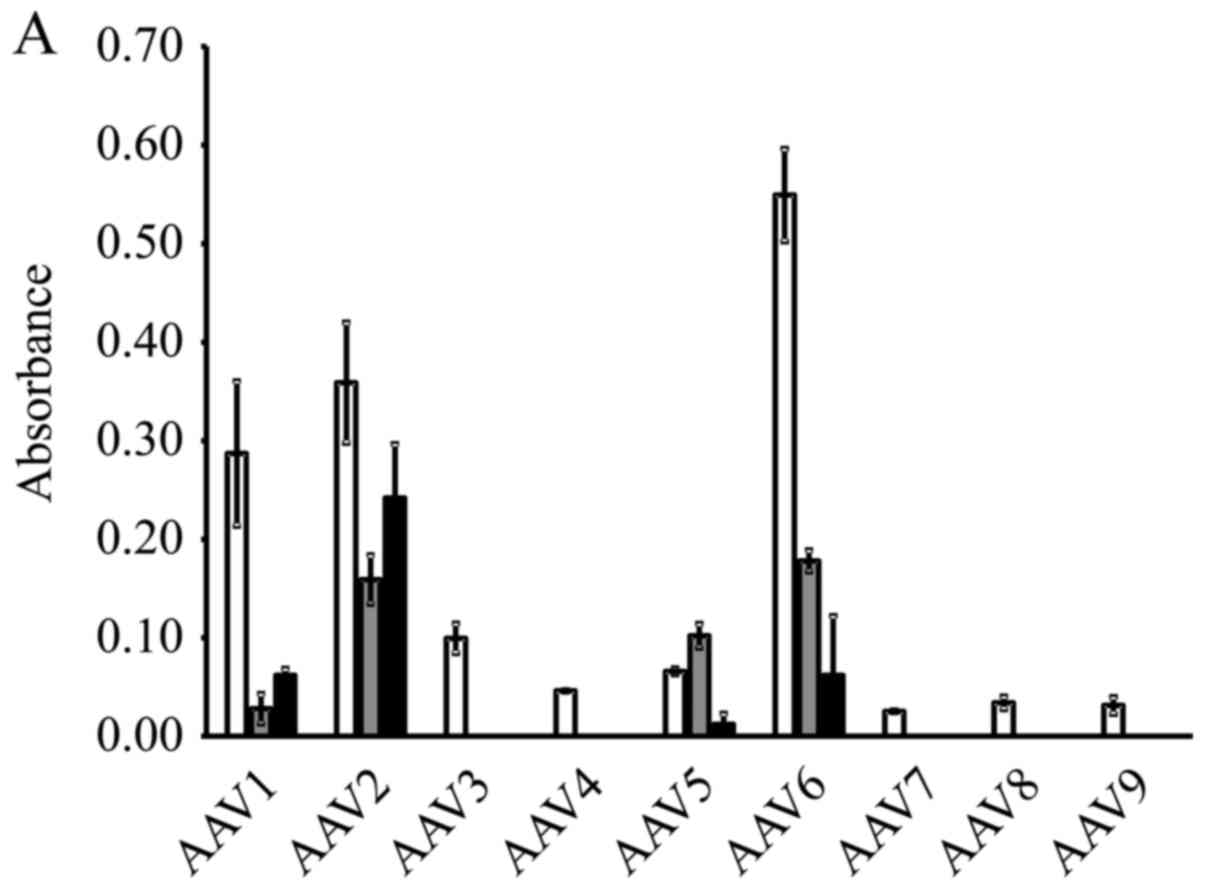
The results of β-galactosidase expression in the
cervical cancer cell lines are shown in Fig. 1A. A high expression level was
obtained by AAV serotype 2 in all 3 cell lines. Therefore, we
selected to perform the subsequent experiments using the AAV2-based
vectors. High levels of GFP expression were observed in the cells
that were transduced with AAV2-shNC, a GFP-encoding vector
(Fig. 1B).
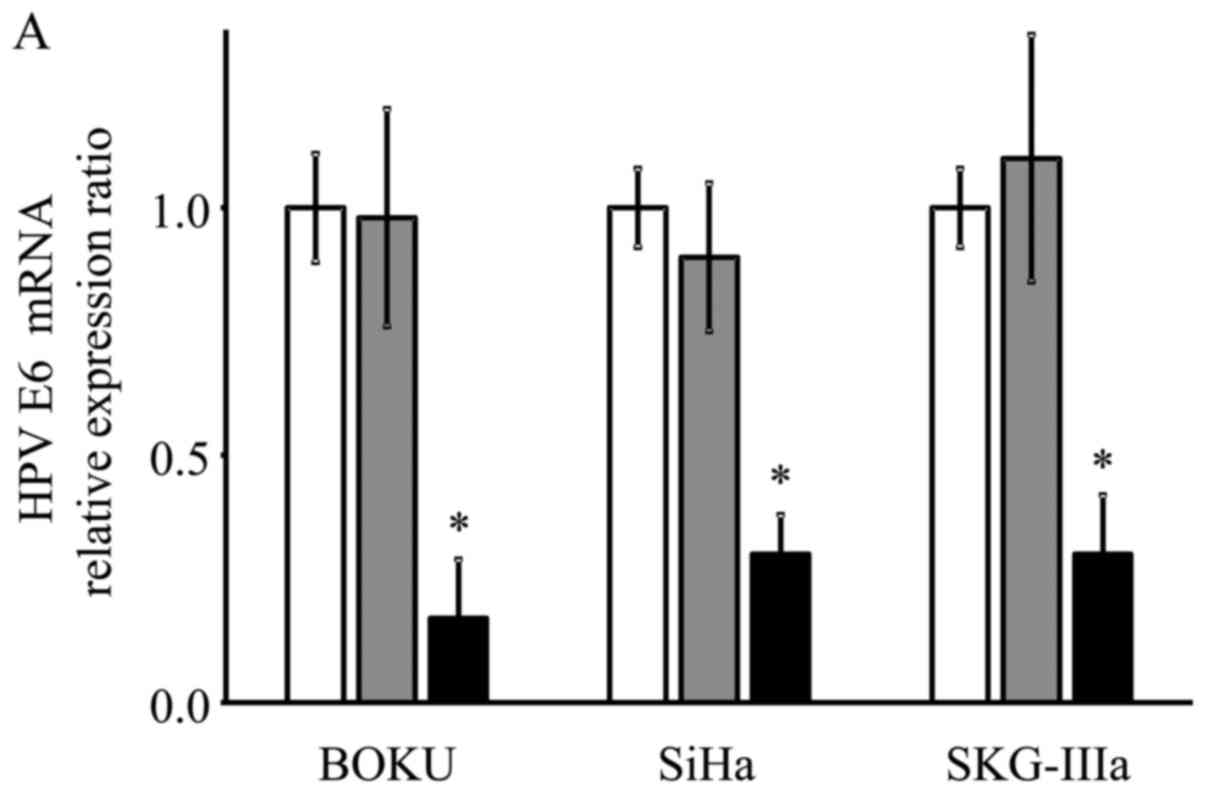
HPV-16 E6/E7 mRNA expression following
AAV2-shE6E7 transduction
As shown using RT-qPCR, the mRNA expression levels
of E6 and E7 were significantly decreased in the
AAV2-shE6E7-transduced cervical cancer cells compared with the
control cells (E6: 13, 29 and 21% vs. that of the control for the
BOKU, SiHa and SKG-IIIa cells, respectively; and E7: 12, 33 and 34%
vs. of the control for the BOKU SiHa SKG-IIIa, respectively;
Fig. 2).
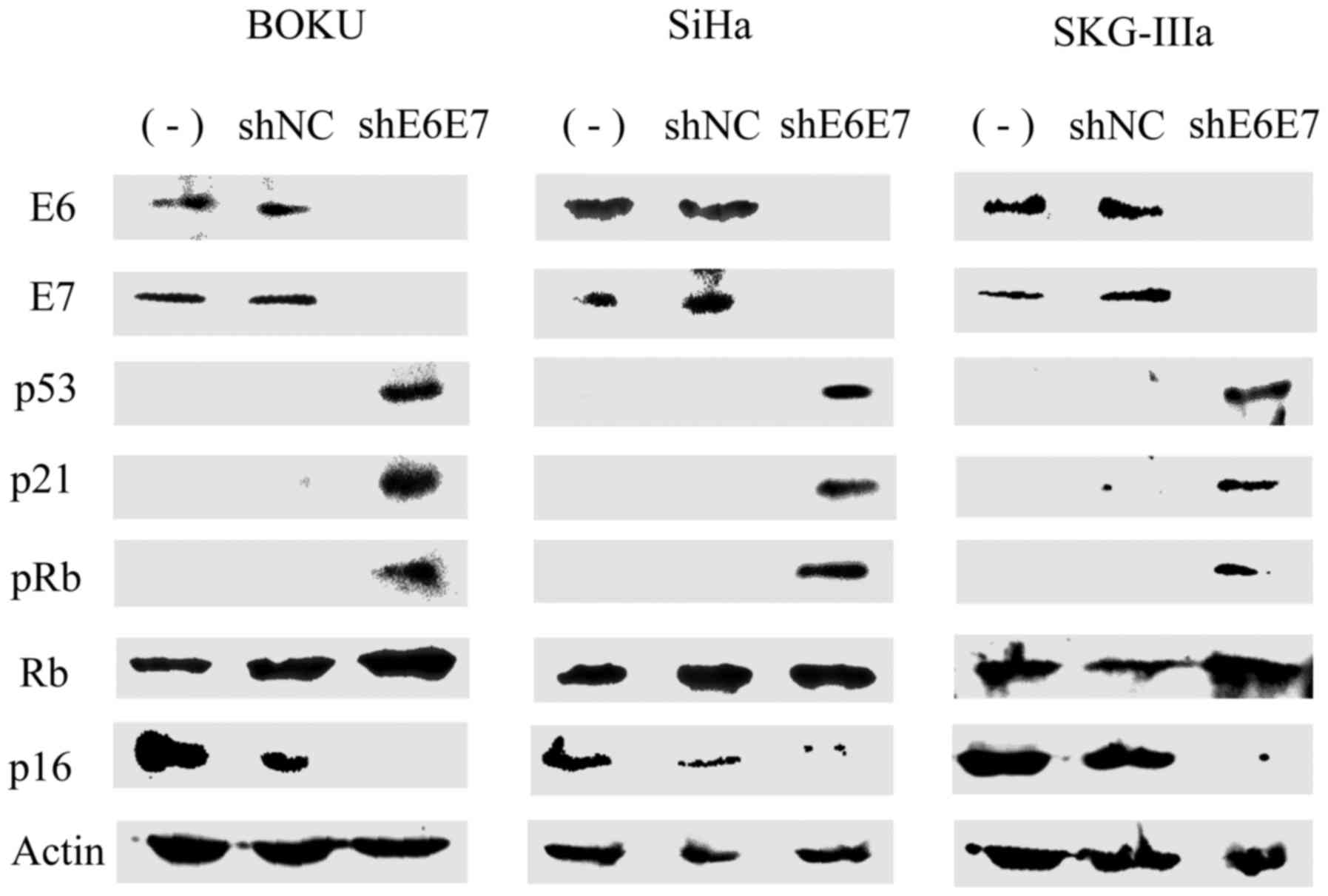
Protein expression following AAV2-shE6E7
transduction
Subsequently, western blot analysis was used to
measure protein expression in the cervical cancer cells following
transduction with AAV2-shE6E7 or AAV2-shNC. In all cell lines, the
expression levels of E6, E7 and p16 were decreased, while the
expression levels of p53, p21 and pRb were markedly increased in
the AAV2-shE6E7-transduced cells compared with those in the control
cells (Fig. 3).
Induction of apoptosis following
AAV2-shE6E7 transduction
Transduction with AAV2-shE6E7 increased the rates of
apoptosis in all 3 cell lines as compared with the control and
AAV2-shNC transduction, with much more evident increases observed
in the BOKU, SiHa and SKG-IIIa cells (Fig. 4A). Thus, a marked induction of
apoptosis was noted in all 3 cervical cancer cell lines transduced
with AAV2-shE6E7.
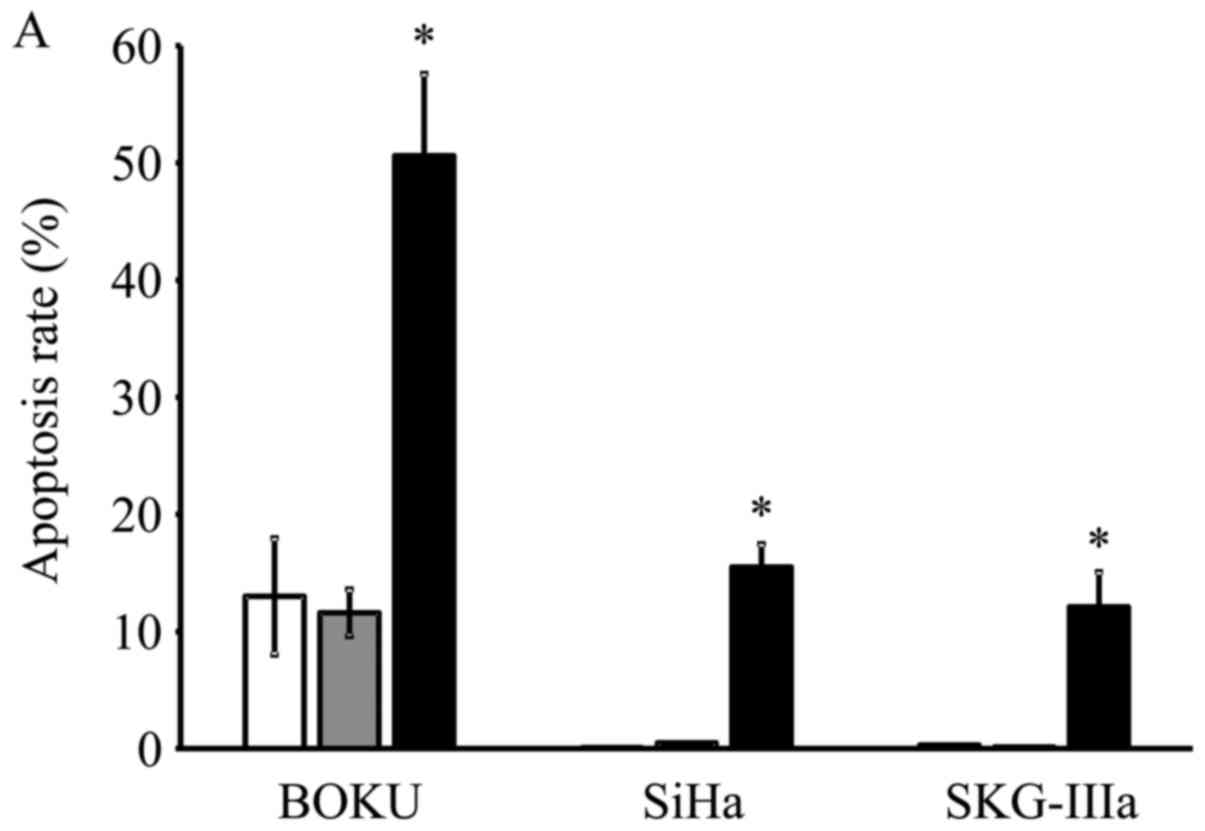 | Figure 4In vitro apoptosis and cell
cycle analysis following transduction with AAV2-shE6E7. (A)
Apoptosis-positive rates using Annexin V as an index 48 h following
AAV2-shE6E7 or AAV-shNC infection by flow cytometry (white bars,
uninfected control cells; gray bars, AAV2-shNC-infected group;
black bars, AAV2-shE6E7-infected group). The induction of apoptosis
was noted in the cells following AAV2-shE6E7 infection in all 3
cell lines. *P<0.05 as compared to the
AAV2-shNC-infected group. The results are presented as the means ±
SD. (B) Flow cytometric analysis of the cell cycle at 48 h
following AAV2-shE6E7 or AAV-shNC infection using PI staining as an
index (white, G0/g1 phase; black, S phase;
gray, G2M phase). The numbers of BOKU cells in the
G0/g1 phase were 57, 58 and 63% in the
control, AAV2-shNC-infected and AAV2-shE6E7-infected groups,
respectively, and those of the cells in the S phase were 14, 11 and
5%, respectively, showing that cells in the S phase decreased in
the AAV2-shE6E7-infected group. |
Changes in cell cycle distribution
following AAV2-shE6E7 transduction
Subsequently, the BOKU cells were transduced with
AAV2-shE6E7 or AAV2-shNC, and changes in the cell cycle
distribution were analyzed using PI staining. The results are shown
in Fig. 4B. As shown this band
graph, the proportions of BOKU cells in the
G0/g1 phase in the control group, the
AAV2-shNC infected group and the AAV2-shE6E7-infected group were
57, 58 and 63%, respectively, and the proportions in the S phase
were 14, 11 and 5%, respectively. The proportion of cells in the
G0/g1 phase increased, and the proportion of
cells in the S phase decreased in the AAV2-shE6E7-infected
group.
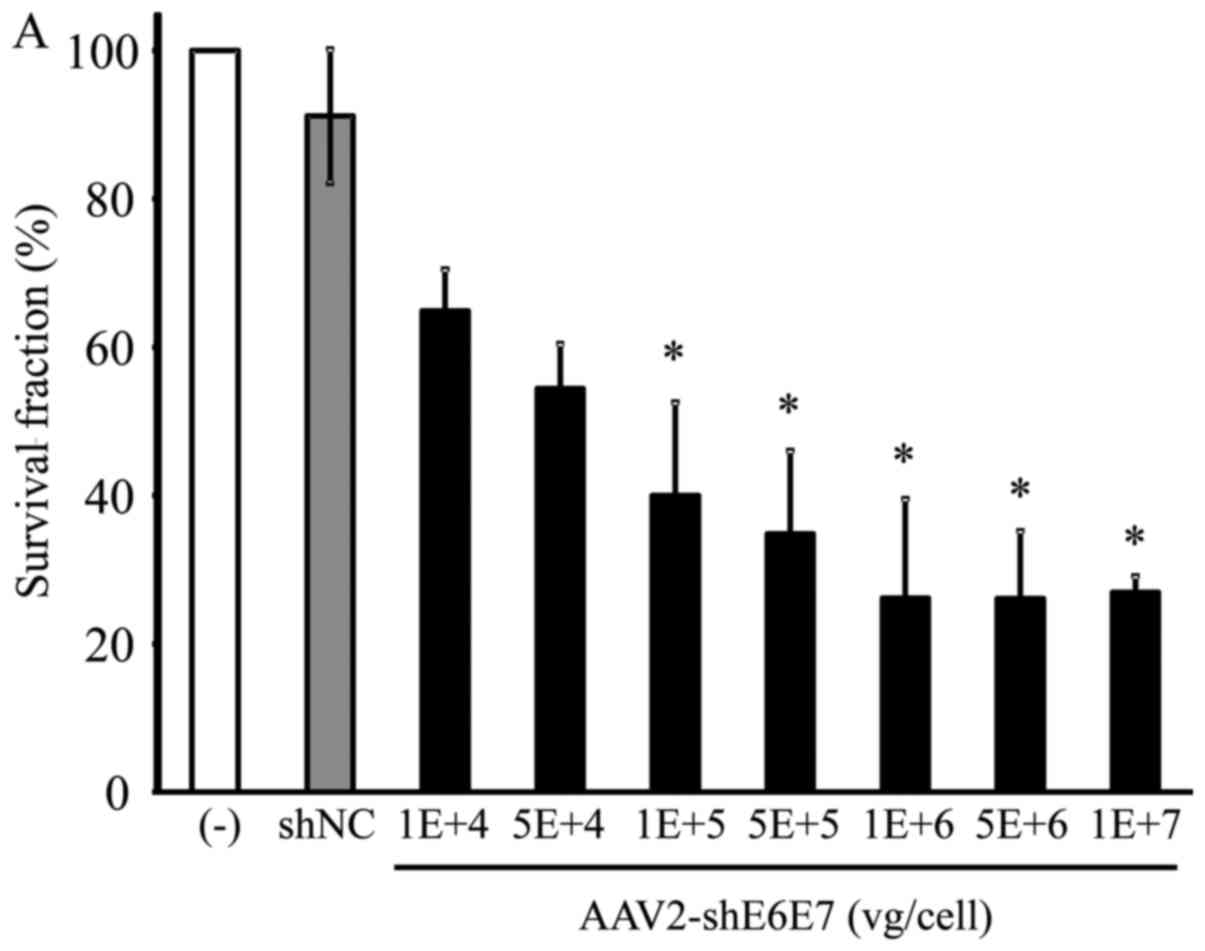
Changes in cell growth following
transduction with AAV2-shE6E7
The BOKU, SiHa, SKG-IIIa and 293 cells were
transduced with AAV2-shE6E7 or AAV2-shNC, and the viable cells were
counted. In all 3 cervical cancer cell lines, the numbers of viable
cells were decreased following transduction with AAV2-shE6E7 in a
concentration-dependent manner. By contrast, AAV2-shE6E7 had no
effect on the growth of 293 cells (Fig. 5).
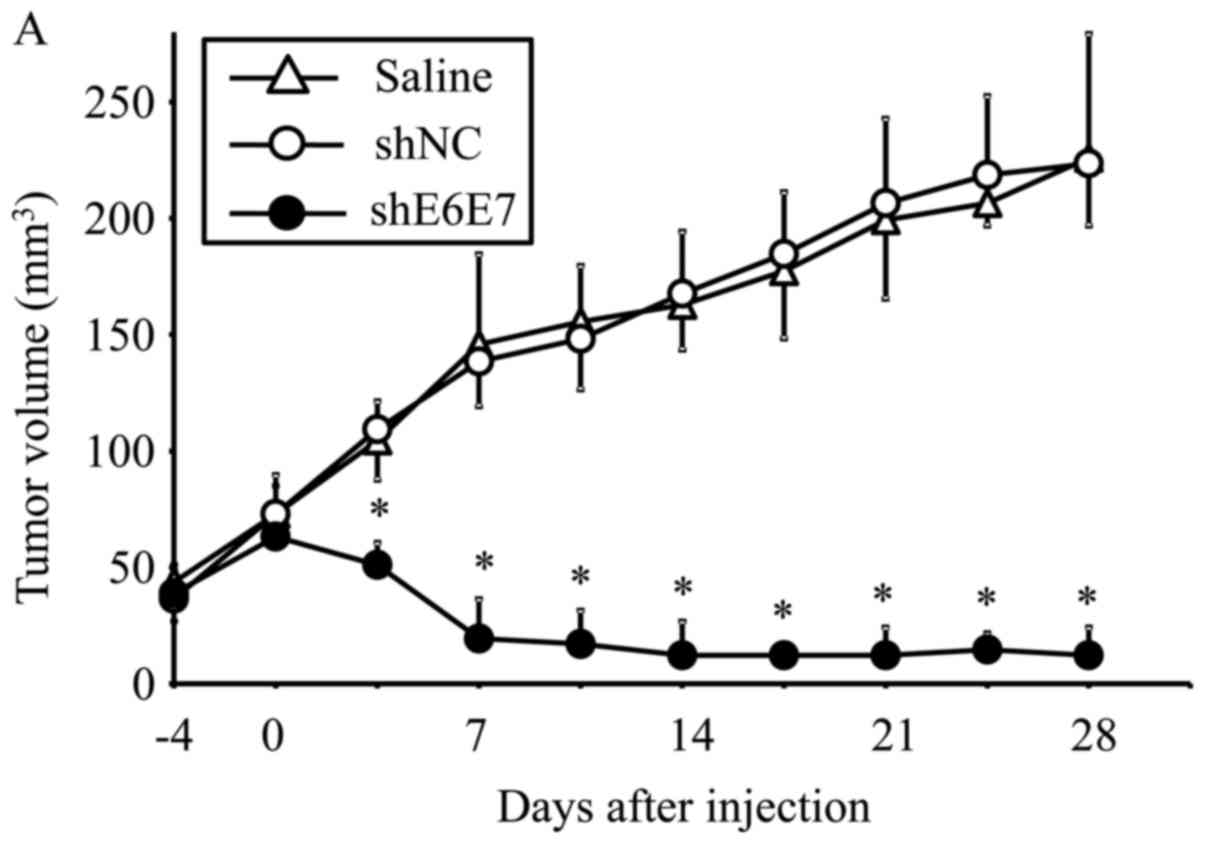
Eradication of in vivo xenograft tumors
following AAV2-shE6E7 transduction
AAV2-shE6E7, AAV2-shNC, or saline was injected in a
single dose into tumors formed by the subcutaneous transplantation
of BOKU, SiHa, or SKG-IIIa cells into mice, and tumor growth was
observed. First, we analyzed the efficacy of E6 and E7 knockdown
using smaller tumors (with a 5-mm major axis). The mean volume of
BOKU-derived tumors at 28 days after administration (n=4) was
significantly smaller than that of tumors that formed in mice
treated with saline (n=4) or AAV2-shNC (n=4) (p<0.01; Fig. 6A). Similarly, the mean volume of
SiHa-derived tumors at 14 days after administration was
significantly lower in the AAV2-shE6E7 group (n=4) than in the
saline (n=4) and AAV2-shNC groups (n=4) (p<0.01; Fig. 6B and C).
The efficacy of this treatment was then tested in
larger tumors (with an 8-mm major axis). At 42 days after
administration, the mean tumor volume was significantly decreased
in the AAV2-shE6E7-injected mice (n=5) with siha-derived tumors
compared with those in the saline (n=5) and AAV2-shNC groups (n=5)
(p<0.01; Fig. 6D and F).
Notably, no regrowth was observed in all tumors during the test
period of 8 weeks. Similar results were obtained in the mice with
SKG-IIIa-derived tumors (P<0.01 for the AAV2-shE6E7 group (n=5)
compared with those in the saline (n=5) and the AAV2-shNC group
(n=5); Fig. 6E).
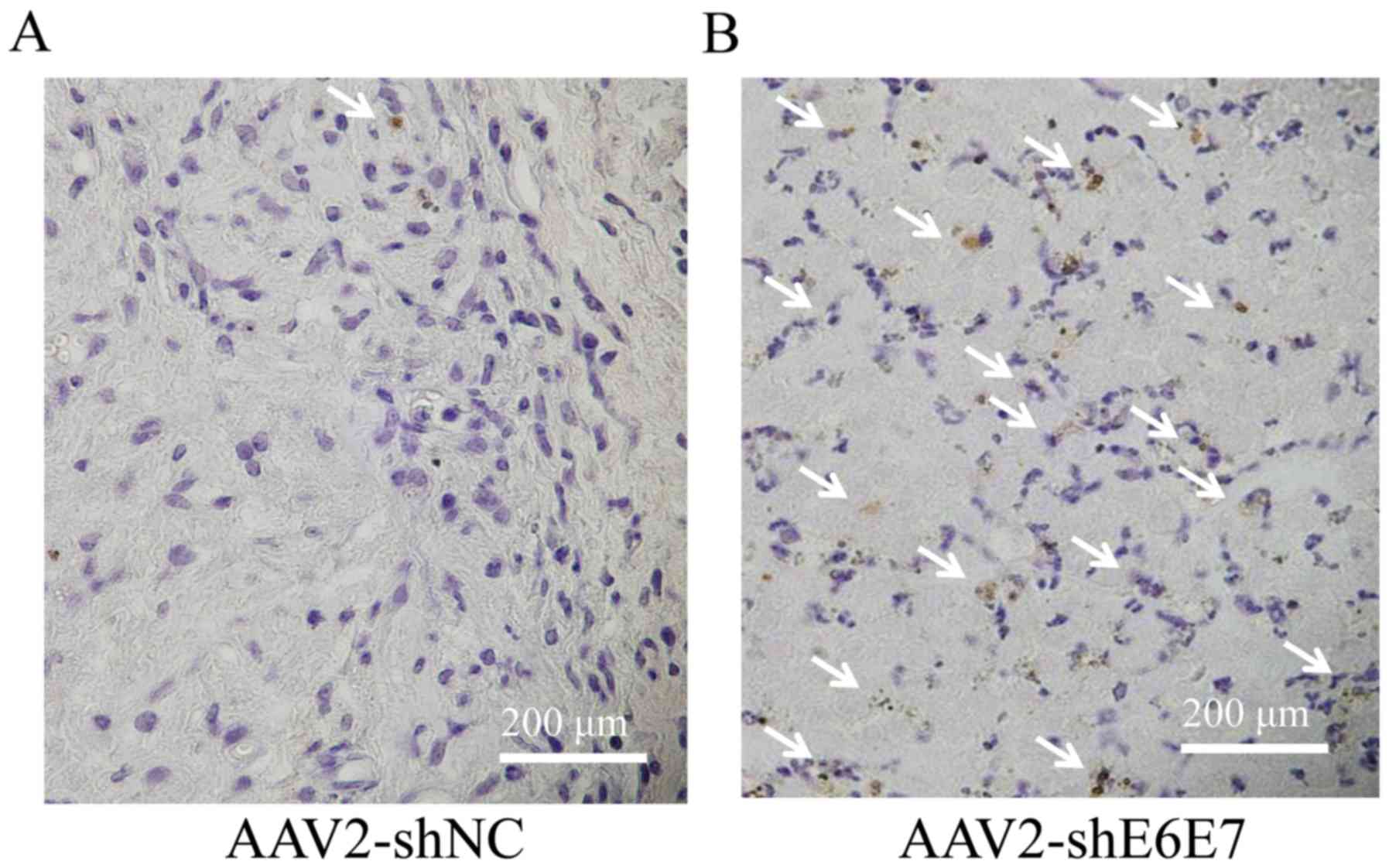
The results of TUNEL staining of the SiHa-derived
tumor tissues are shown in Fig. 7A and
B. The mean TUNEL-positive cell rate was significantly higher
in the AAV2-shE6E7 group than in the AAV2-shNC group (P<0.01;
Fig. 7C). No macroscopic changes,
including edema or inflammation, were noted at the sites of vector
inoculation, nor were there any differences in body weights among
the groups (data not shown).
Discussion
In this study, a basic analysis was performed aiming
at developing a novel, highly specific cervical cancer treatment
method using AAV-shE6E7. Firstly, the serotype of an AAV vector
with the highest transfer efficiency for cervical cancer cells was
screened, and the gene transfer efficiency of the AAV2 vector was
found to be the highest. AAV vectors are known to be highly
organ-specific, and serotypes with high transfer efficiency for
specific organs are known. For example, the transfer efficiencies
of serotypes 1 and 7 for skeletal muscles, serotypes 2 and 3 for
nerves, serotype 5 for the retina, and serotype 8 for the liver are
high (20). Our study clarified
that type 2 most efficiently transfers genes into cervical cancer
cells.
As regards HPV, >120 types have been reported due
to differences in the L1 gene base sequence (31). Approximately 20 types are high-risk
HPV causing cervical cancer (32),
and type 16 is most frequently detected in cervical cancer
(32). Thus, in this study, we
examined type 16. Since cancer genes of HPV16, E6 and E7, are
encoded by a single mRNA (33,34),
both E6 and E7 expression levels can be inhibited with one type of
shRNA (35). An shRNA sequence
that efficiently inhibits HPV16 E6 and E7 has been reported
(26), and we prepared an AAV2
vector (AAV2-shE6E7) containing this sequence. The GFP-encoding
gene was also contained in AAV2-shE6E7 and AAV2-shNC as a reporter
gene driven by the CMV promoter. When the 3 types of cervical
cancer cell were infected with AAV2-shNC in vitro, high gene
transfer efficiency (approximately 90%) was observed, and this was
comparable to that of AAV vectors for 293 cells known to show very
high efficiency. The gene transfer efficiency was also almost 100%
on macroscopic measurement using a hemocytometer in all cell lines
(data not shown). The treatment strategy targeted in this study is
local therapy directly transferring a gene into cancer cells, for
which high gene transfer efficiency is essential. It was
demonstrated that the AAV2 vector transfers genes into cervical
cancer at a high efficiency.
To confirm the effect of AAV2-shE6E7, the mRNA
expression levels of the direct targets, E6 and E7, were measured
by RT-qPCR. In the 3 types of cervical cancer cell infected with
AAV2-shE6E7 in vitro, the E6 and E7 mRNA expression levels
decreased to 1/3–1/10 of those in the control. Subsequently, the
expression levels of E6, E7 and related factors were examined by
western blot analysis. In all 3 types of cervical cancer cell cells
infected with AAV2-shE6E7, the E6, E7 and p16 expression levels
were decreased, whereas those of p53, p21, and pRb were increased.
Following the investigation of apoptosis using Annexin V as an
index, a marked induction of apoptosis was observed in all 3 types
of cervical cancer cell infected with AAV2-shE6E7. Following the
analysis of the cell cycle using flow cytometry, the numbers of
cells in the g1 phase increased following AAV2-shE6E7
infection, i.e., G1 arrest occurred. In cervical cancer,
E6 degrades p53, which results in the inhibition of downstream p21
expression, leading to the inhibition of apoptosis induction
(36). E7 inactivates pRb, which
causes the dysfunction of the G1 checkpoint and avoids
G1 arrest (36). To
adjust these abnormalities in the cell cycle, p16 expression is
enhanced for emergency avoidance of the condition (36). The inhibitory effect of AAV2-shE6E7
on E6 and E7 expression may have resolved these abnormalities and
each factor may have been corrected to function normally, leading
to apoptosis and normal functioning of the G1
checkpoint.
Subsequently, we investigated the actual inhibitory
effect of AAV2-shE6E7 on cervical cancer cell growth in
vitro. AAV2-shE6E7 showed a growth-inhibitory effect on all 3
types of cervical cancer cell in a concentration-dependent
manner.
Based on the in vitro experimental results,
the effect of AAV2-shE6E7 was investigated in in vivo animal
experiments. Mice were subcutaneously inoculated with cervical
cancer cells. After macroscopically confirming tumor formation,
AAV2-shE6E7 was subcutaneously injected around the tumor, and
changes in the tumor were observed. Firstly, the experiment was
performed with tumors with a 5-mm major axis (volume, approximately
60 mm3) as an early cancer model. In all tumors formed
by the 3 types of cervical cancer cells, a marked tumor size
reduction was noted after AAV2-shE6E7 administration, and the
tumors were mostly resolved. The experiment was then performed with
tumors with an 8-mm major axis (volume, approximately 250
mm3) as an advanced cancer model. Similarly, a marked
decrease in tumor size reduction was noted following the injection
of AAV2-shE6E7 in all tumors formed by the 3 types of cervical
cancer cells, and the tumors were mostly resolved. In addition, no
tumor re-growth was noted over long-term observation. Many
apoptotic bodies were observed in the tumors injected with
pAAV2-shE6E7, clarifying that AAV2-shE6E7 reduced the size of and
resolved the tumor by inducing apoptosis.
Several animal experiments with siRNA targeting E6
and E7 in cervical cancer have been reported (37–39).
In these studies, experiments were conducted in which siRNA
targeting HPV E6E7 was administered under the protection of
atelocollagen (37,38). All of these results were limited in
tumor growth suppression despite multiple administrations. By
contrast, in our study using AAV2-shE6E7, tumor growth was
completely inhibited by only a single administration. These
differences in the effects may have reflected a difference in the
gene transfer efficiency for cancer cells between the siRNA
protected by atelocollagen and AAV2-shE6E7. An ex vivo study
using an AAV vector encoding E6E7 antisense RNA also reported
significant tumor growth inhibitory effect (40).
Recently, attempts to knock out E6 or E7 in cervical
cancer using genome editing technology have been reported (41–43).
In these studies, the effects in vitro or ex vivo
were confirmed. In order to obtain sufficient antitumor effect
in vivo, the combination with the AAV vector may be
effective as in this study. Recently, a CRIspR/Cas 9 derived from
Staphylococcus aureus that can be encoded to an AAV vector
was reported (44). We are
currently conducting fundamental research on cervical cancer
treatment applying this new genome editing technology.
As regards adverse effects, no weight loss or
abnormalities at the administration site was noted in mice treated
with AAV2-shE6E7, and these may have been due to the following 2
reasons: Firstly, AAV vectors have no toxicity, and secondly,
shE6E7 does not affect normal tissue as E6 and E7 are expressed
only in cervical cancer cells, not in normal tissue. It was thus
suggested that treatment with AAV-shE6E7 is safe.
AAV Rep 78 protein is known to inhibit the promoter
site of several oncogenes and viral genes. It has been reported
that E6 of cervical cancer can be suppressed using Rep 78 protein
(45). However, the AAV vector
used in our study does not express Rep 78 protein. Therefore, the
effect observed in our study is not the same as that of the Rep 78
protein.
HPV vaccine development using HPV L1 antigen has
become practically applied, and clinical administration is now
widespread (46,47). The reduction of the incidence of
cervical cancer by lowering the prevalence of HPV infection is
expected. On the other hand, no curative treatment method targeting
HPV has been established for tens of millions of individuals
already infected with high-risk HPV worldwide and patients with CIN
and cervical cancer. Treatment with AAV2-shE6E7 developed by us
efficiently inhibited E6 and E7 expression of high-risk HPV and
reduced the size of the lesion within a short time, for which a
long-term effect can be expected. In clinical application,
high-grade CIN and early infiltrating cancer may be firstly
treated. This treatment strategy is very likely to be the most
effective for these because lesions are localized. High-level
safety is essential as the incidence of these diseases is high in
young persons and those wishing to become pregnant. No adverse
effect of AAV2-shE6E7 was observed; however, further investigations
are required aiming at clinical application in the future.
For advanced cervical cancer, the effect of this
treatment strategy for broad infiltration and metastasis is
limited, and curative treatment cannot be expected. However, it was
effective for tumors of a certain size, suggesting that the
treatment may be applicable to reduce a bulky tumor size by
increasing the dose. It may be a substitute for neoadjuvant
chemotherapy (48,49) performed to increase the curability
of surgery through size reduction of bulky tumors and increase the
effect of radiotherapy. It may also be applicable for some local
recurrent carcinomas.
We performed this study using HPV type 16, but
cervical cancer caused by type 16 accounts for 50% of all cases
(32). To expand the indications
of this treatment strategy, it is necessary to develop an
shRNA-containing AAV vector effective for high-risk HPV other than
type 16. Since the base sequence of E6E7 is not homologous among
high-risk HPV types, to treat infection with several high-risk HPV
types, it is necessary to design shRNA for individual types.
However, the individual preparation of an AAV vector is
unnecessary. Since the U6 promoter and shRNA base sequences are
comprised of approximately 400 bases in total, theoretically,
approximately 10 pairs can be inserted into one AAV vector. If an
AAV vector containing shRNAs corresponding to E6E7 of 7 types: HPV
type 16, 18, 33, 45, 31, 58 and 52, can be developed, it may be
effective for approximately 90% of cervical cancer cases. On the
whole, as our data indicated, AAV2-shE6E7 exerted a marked
inhibitory effect on cervical cancer for a prolonged period of
time. A novel treatment strategy for cervical cancer using
AAV2-shE6E7 was thus suggested.
Acknowledgments
The authors would like to thank Mrs. Miyoko Mitsu,
Mrs. Satomi Fujiwara and Mrs. Michiko Ohashi for providing
excellent technical assistance. This study was partly supported by
the Ministry of Education, Culture, Sports, Science and Technology
(MEXT)-Supported Program for the Strategic Research Foundation at
Private Universities, 2013–2017.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Fitzmaurice C, Allen C, Barber RM,
Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O,
Dandona R, Dandona L, et al Global Burden of Disease Cancer
Collaboration: Global, regional, and national cancer incidence,
mortality, years of life lost, years lived with disability, and
disability-adjusted life-years for 32 cancer groups, 1990 to 2015:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 3:524–548. 2017. View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Woodman CB, Collins SI and Young LS: The
natural history of cervical HPV infection: Unresolved issues. Nat
Rev Cancer. 7:11–22. 2007. View
Article : Google Scholar
|
|
4
|
Sevin BU, Nadji M, Averette HE, Hilsenbeck
S, Smith D and Lampe B: Microinvasive carcinoma of the cervix.
Cancer. 70:2121–2128. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Massad LS, Einstein MH, Huh WK, Katki HA,
Kinney WK, Schiffman M, Solomon D, Wentzensen N and Lawson HW; 2012
ASCCP Consensus Guidelines Conference: 2012 updated consensus
guidelines for the management of abnormal cervical cancer screening
tests and cancer precursors. Obstet Gynecol. 121:829–846. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bevis KS and Biggio JR: Cervical
conization and the risk of preterm delivery. Am J Obstet Gynecol.
205:19–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boshart M, Gissmann L, Ikenberg H,
Kleinheinz A, Scheurlen W and zur Hausen H: A new type of
papillomavirus DNA, its presence in genital cancer biopsies and in
cell lines derived from cervical cancer. EMBO J. 3:1151–1157.
1984.PubMed/NCBI
|
|
8
|
Dürst M, Gissmann L, Ikenberg H and zur
Hausen H: A papillomavirus DNA from a cervical carcinoma and its
prevalence in cancer biopsy samples from different geographic
regions. Proc Natl Acad Sci USA. 80:3812–3815. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muñoz N, Bosch FX, de Sanjosé S, Herrero
R, Castellsagué X, Shah KV, Snijders PJ and Meijer CJ;
International Agency for Research on Cancer Multicenter Cervical
Cancer Study Group: Epidemiologic classification of human
papillomavirus types associated with cervical cancer. N Engl J Med.
348:518–527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dyson N, Howley PM, Münger K and Harlow E:
The human papilloma virus-16 E7 oncoprotein is able to bind to the
retinoblastoma gene product. Science. 243:934–937. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moody CA and Laimins LA: Human
papillomavirus oncoproteins: Pathways to transformation. Nat Rev
Cancer. 10:550–560. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Werness BA, Levine AJ and Howley PM:
Association of human papillomavirus types 16 and 18 E6 proteins
with p53. Science. 248:76–79. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Da Silva DM, Eiben GL, Fausch SC,
Wakabayashi MT, Rudolf MP, Velders MP and Kast WM: Cervical cancer
vaccines: Emerging concepts and developments. J Cell Physiol.
186:169–182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: potent and specific genetic interference by
double- stranded RNA in Caenorhabditis elegans. Nature.
391:806–811. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caplen NJ, Parrish S, Imani F, Fire A and
Morgan RA: Specific inhibition of gene expression by small
double-stranded RNAs in invertebrate and vertebrate systems. Proc
Natl Acad Sci USA. 98:9742–9747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elbashir SM, Harborth J, Lendeckel W,
Yalcin A, Weber K and Tuschl T: Duplexes of 21-nucleotide RNAs
mediate RNA interference in cultured mammalian cells. Nature.
411:494–498. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scherr M and Eder M: Gene silencing by
small regulatory RNAs in mammalian cells. Cell Cycle. 6:444–449.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paddison PJ and Hannon GJ: RNA
interference: The new somatic cell genetics? Cancer Cell. 2:17–23.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mueller C and Flotte TR: Clinical gene
therapy using recombinant adeno-associated virus vectors. Gene
Ther. 15:858–863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Z, Asokan A and Samulski RJ:
Adeno-associated virus serotypes: Vector toolkit for human gene
therapy. Mol Ther. 14:316–327. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grieger JC and Samulski RJ:
Adeno-associated virus vectorology, manufacturing, and clinical
applications. Methods Enzymol. 507:229–254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao G, Vandenberghe LH, Alvira MR, Lu Y,
Calcedo R, Zhou X and Wilson JM: Clades of Adeno-associated viruses
are widely disseminated in human tissues. J Virol. 78:6381–6388.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao GP, Alvira MR, Wang L, Calcedo R,
Johnston J and Wilson JM: Novel adeno-associated viruses from
rhesus monkeys as vectors for human gene therapy. Proc Natl Acad
Sci USA. 99:11854–11859. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rutledge EA, Halbert CL and Russell DW:
Infectious clones and vectors derived from adeno-associated virus
(AAV) serotypes other than AAV type 2. J Virol. 72:309–319.
1998.PubMed/NCBI
|
|
25
|
Takeda S, Takahashi M, Mizukami H,
Kobayashi E, Takeuchi K, Hakamata Y, Kaneko T, Yamamoto H, Ito C,
Ozawa K, et al: Successful gene transfer using adeno-associated
virus vectors into the kidney: Comparison among adeno-associated
virus serotype 1–5 vectors in vitro and in vivo. Exp Nephrol.
96:e119–e126. 2004. View Article : Google Scholar
|
|
26
|
Sima N, Wang W, Kong D, Deng D, Xu Q, Zhou
J, Xu G, Meng L, Lu Y, Wang S, et al: RNA interference against
HPV16 E7 oncogene leads to viral E6 and E7 suppression in cervical
cancer cells and apoptosis via upregulation of Rb and p53.
Apoptosis. 13:273–281. 2008. View Article : Google Scholar
|
|
27
|
Hata K, Mizukami H, Sadakane O, Watakabe
A, Ohtsuka M, Takaji M, Kinoshita M, Isa T, Ozawa K and Yamamori T:
DNA methylation and methyl-binding proteins control differential
gene expression in distinct cortical areas of macaque monkey. J
Neurosci. 33:19704–19714. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsushita T, Elliger S, Elliger C,
Podsakoff G, Villarreal L, Kurtzman GJ, Iwaki Y and Colosi P:
Adeno-associated virus vectors can be efficiently produced without
helper virus. Gene Ther. 5:938–945. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yagi H, Ogura T, Mizukami H, Urabe M,
Hamada H, Yoshikawa H, Ozawa K and Kume A: Complete restoration of
phenylalanine oxidation in phenylketonuria mouse by a
self-complementary adeno-associated virus vector. J Gene Med.
13:114–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adhim Z, Otsuki N, Kitamoto J, Morishita
N, Kawabata M, Shirakawa T and Nibu K: Gene silencing with siRNA
targeting E6/E7 as a therapeutic intervention against head and neck
cancer-containing HPV16 cell lines. Acta Otolaryngol. 133:761–771.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fehrmann F and Laimins LA: Human
papillomaviruses: Targeting differentiating epithelial cells for
malignant transformation. Oncogene. 22:5201–5207. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clifford GM, Smith JS, Plummer M, Muñoz N
and Franceschi S: Human papillomavirus types in invasive cervical
cancer worldwide: A meta-analysis. Br J Cancer. 88:63–73. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sherman L and Alloul N: Human
papillomavirus type 16 expresses a variety of alternatively spliced
mRNAs putatively encoding the E2 protein. Virology. 191:953–959.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smotkin D, Prokoph H and Wettstein FO:
Oncogenic and nononcogenic human genital papillomaviruses generate
the E7 mRNA by different mechanisms. J Virol. 63:1441–1447.
1989.PubMed/NCBI
|
|
35
|
Yoshinouchi M, Yamada T, Kizaki M, Fen J,
Koseki T, Ikeda Y, Nishihara T and Yamato K: In vitro and in vivo
growth suppression of human papillomavirus 16-positive cervical
cancer cells by E6 siRNA. Mol Ther. 8:762–768. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View Article : Google Scholar
|
|
37
|
Fujii T, Saito M, Iwasaki E, Ochiya T,
Takei Y, Hayashi S, Ono A, Hirao N, Nakamura M, Kubushiro K, et al:
Intratumor injection of small interfering RNA-targeting human
papillomavirus 18 E6 and E7 successfully inhibits the growth of
cervical cancer. Int J Oncol. 29:541–548. 2006.PubMed/NCBI
|
|
38
|
Yamato K, Yamada T, Kizaki M, Ui-Tei K,
Natori Y, Fujino M, Nishihara T, Ikeda Y, Nasu Y, Saigo K, et al:
New highly potent and specific E6 and E7 siRNAs for treatment of HP
V16 positive cervical cancer. Cancer Gene Ther. 15:140–153. 2008.
View Article : Google Scholar
|
|
39
|
Jonson AL, Rogers LM, Ramakrishnan S and
Downs LS Jr: Gene silencing with siRNA targeting E6/E7 as a
therapeutic intervention in a mouse model of cervical cancer.
Gynecol Oncol. 111:356–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu S, Meng L, Wang S, Wang W, Xi L, Tian
X, Chen G, Wu Y, Zhou J, Xu G, et al: Reversal of the malignant
phenotype of cervical cancer CaSki cells through adeno-associated
virus-mediated delivery of HPV16 E7 antisense RNA. Clin Cancer Res.
12:2032–2037. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kennedy EM, Kornepati AV, Goldstein M,
Bogerd HP, Poling BC, Whisnant AW, Kastan MB and Cullen BR:
Inactivation of the human papillomavirus E6 or E7 gene in cervical
carcinoma cells by using a bacterial CRIspR/Cas RNA-guided
endonuclease. J Virol. 88:11965–11972. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhen S, Hua L, Takahashi Y, Narita S, Liu
YH and Li Y: In vitro and in vivo growth suppression of human
papillomavirus 16-positive cervical cancer cells by CRIspR/Cas9.
Biochem Biophys Res Commun. 450:1422–1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu Z, Ding W, Zhu D, Yu L, Jiang X, Wang
X, Zhang C, Wang L, Ji T, Liu D, et al: TALEN-mediated targeting of
HPV oncogenes ameliorates HPV-related cervical malignancy. J Clin
Invest. 125:425–436. 2015. View Article : Google Scholar :
|
|
44
|
Ran FA, Cong L, Yan WX, Scott DA,
Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS, et
al: In vivo genome editing using Staphylococcus aureus Cas9.
Nature. 520:186–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ahn WS, Bae SM, Lee HJ, Kim YW, Lee JM,
Namkoong SE, Kim CK, Kim YW and Jin HS: Development of anticancer
gene vaccine interact with human papillomavirus oncoprotein
inhibition. Int J Gynecol Cancer. 16:270–276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Paavonen J, Jenkins D, Bosch FX, Naud P,
Salmerón J, Wheeler CM, Chow SN, Apter DL, Kitchener HC,
Castellsague X, et al HPV pATRICIA study group: Efficacy of a
prophylactic adjuvanted bivalent L1 virus-like-particle vaccine
against infection with human papillomavirus types 16 and 18 in
young women: An interim analysis of a phase III double-blind,
randomised controlled trial. Lancet. 369:2161–2170. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Romanowski B: Long term protection against
cervical infection with the human papillomavirus: Review of
currently available vaccines. Hum Vaccin. 7:161–169. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sardi JE, Giaroli A, Sananes C, Ferreira
M, Soderini A, Bermudez A, Snaidas L, Vighi S, Gomez Rueda N and di
Paola G: long-term follow-up of the first randomized trial using
neoadjuvant chemotherapy in stage Ib squamous carcinoma of the
cervix: The final results. Gynecol Oncol. 67:61–69. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Benedetti-Panici P, Greggi S, Colombo A,
Amoroso M, Smaniotto D, Giannarelli D, Amunni G, Raspagliesi F,
Zola P, Mangioni C, et al: Neoadjuvant chemotherapy and radical
surgery versus exclusive radiotherapy in locally advanced squamous
cell cervical cancer: Results from the Italian multicenter
randomized study. J Clin Oncol. 20:179–188. 2002. View Article : Google Scholar : PubMed/NCBI
|